Abstract
Background
This study aimed to compare the characteristics of anterior and posterior human Tenon’s capsule fibroblasts and determine the effect of matrix metalloproteinase (MMP) inhibition on cultured human Tenon’s capsule fibroblasts.
Methods
Tissues obtained from the anterior and posterior Tenon’s capsules were partially incised, cultured, and subjected to microarray analysis. Electrophoresis was performed on the cells treated with Ilomastat (MMP-9 inhibitor), and collagen 1, 3, and 6. Antibodies against transforming growth factor-beta (TGF-β) and vimentin were used as probes to compare the expression levels.
Results
There were no differences between anterior and posterior Tenon’s capsule fibroblasts in the microarray. Western blot analysis showed similar expression of collagen 1, 3, and 6, TGF-β, and vimentin between anterior and posterior Tenon’s capsule fibroblast. Ilomastat did not affect the proliferation of cultured human Tenon's capsule.
Conclusions
No differences in characteristics were observed between anterior and posterior Tenon’s capsule fibroblasts, and inhibition of MMPs did not affect the proliferation of cultured human Tenon’s capsule fibroblasts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-025-04258-7.
Keywords: Antimetabolite, Tenon’s capsule fibroblast, Matrix metalloproteinase
Background
Glaucoma is marked by distinct structural changes along with related visual field problems leading to irreversible vision loss [1]. Treatment of glaucoma mainly involves lowering intraocular pressure (IOP), which has been proven to be the most effective method of management [1]. Lowering IOP decreases the chance of developing glaucoma in people without optic nerve damage and slows down new damage in those already affected by glaucoma [2–4]. To lower IOP, the initial method for treating glaucoma involves using topical medications [5]. When medication alone is insufficient to control IOP, incisional surgery, such as trabeculectomy or glaucoma drainage implant is indicated [5]. Even though the surgery is considered effective in managing uncontrolled glaucoma, it can be associated with serious complications [1]. Efforts to increase the success rate of glaucoma surgery have been attempted so far [6–9]. Studies related to understanding the postoperative wound healing response, which is pointed out as the biggest cause of surgical failure, are being reported [10–12].
The introduction of antimetabolites, such as mitomycin C (MMC) and 5-fluorouracil (5-FU) has played an important role in improving the postoperative success of glaucoma surgery [8, 13–15]. MMC and 5-FU act as agents to decrease postoperative fibrosis, which is the most common cause of filtration surgery failure [13, 15]. MMC has improved the success rate of trabeculectomy by inhibiting the proliferation of Tenon’s capsule fibroblast [16]. Nevertheless, the use of MMC has been linked to issues such as conjunctival epithelial damage, and it is characterized by non-healing leaking blebs and the risk of an avascular bleb, bleb infection, endophthalmitis, corneal epithelial defects, and hypotony [15, 17–21]. The 5-FU is an antimetabolite that inhibits fibroblast proliferation and prevents the conjunctiva from scarring down onto the sclera [13, 22]. However, 5-FU may cause corneal toxicity, bleb leaks, and damage of the cells that are placed at the front of the eye, with wound leak leading to postoperative hypotony, hypotony maculopathy, late endophthalmitis, shallow anterior chamber, and expulsive hemorrhage [6, 13, 22–25].
Unsuccessful surgery in certain high-risk patients despite antiproliferative treatment has led to new research on alternative targets in anti-scarring therapy [9]. Matrix metalloproteinases (MMPs) are enzymes that cleave the extracellular matrix (ECM) and are regulated at the transcriptional, activation, and proteolytic activity inhibition levels [26, 27]. When cells are damaged, membrane-type MMPs are secreted by various cells such as neutrophils, macrophages, endothelial cells, keratinocytes, and fibroblasts, and are involved in cell invasion and migration [9, 28, 29]. Previous studies on MMPs have suggested their potential significance in the postoperative wound healing process, which has led to their surgical application in glaucoma treatment [16, 30–33]. Ilomastat, a broad-spectrum MMP inhibitor, reduces scar formation and cellularity at filtration surgery sites, thereby preventing the failure of glaucoma surgery [9, 34].
The two representative glaucoma surgeries–filtration surgery and Ahmed valve insertion–have a similar context for creating an outflow tract; however, their surgical sites are different. Trabeculectomy involves a surgical opening of the sclera within 2–3 mm of the limbus and creates a flow of the aqueous humor. A filter site for Ahmed valve implantation is formed in the area close to the limbus and spreads toward the posterior part of the eye. The main body locating 9–10 mm away from the limbus during valve implantation, and a subconjunctival space is created in this area; therefore, unlike trabeculectomy, it is somewhat posterior [35]. Creation of the subconjunctival space begins at the posterior part, away from the limbus. Because the encapsulated bleb formed from the two surgeries differs in size and location, some studies have been conducted to determine the histopathological features of Tenon’s cysts (encapsulated blebs) on trabeculectomy and Ahmed valve implantation [36, 37].
In this study, we investigated the differences in characteristics between anterior and posterior Tenon’s fibroblasts through direct cell culture and various analytical methods to confirm the effect of MMP inhibition on fibroblast proliferation to understand the wound healing response to glaucoma surgery.
Materials and methods
Material acquisition and cell culture
Primary cultures of human Tenon fibroblasts were established from subconjunctival Tenon biopsies dissected from patients who underwent glaucoma filtration surgery. Immediately after dissection, the tissues were cut into thin pieces (1 mm) by chopping with a sterile scalpel. The minced tissues with varied sizes were plated on a T25 culture flask containing Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island NY), penicillin (100 U/mL), and streptomycin (100 ug/mL). The pieces were allowed to settle and incubated at 37 °C in a humidified chamber with 5% CO2 without any disturbance until they adhered firmly to the bottom of the flask. Fibroblasts usually begin to grow beneath tissue pieces and propagate to confluence within 2 weeks. The cells were subcultured by trypsinization at a split ratio of 1:4, and passages 5–7 were used for the subsequent experiments.
Microarray
Total RNA was extracted from Tenon’s cells ( ) using the TRIzol reagent (Gibco) according to the manufacturer’s instructions. The cDNA was reverse-transcribed from the purified RNA (200 ng), amplified, and transcribed into cRNA. Sense-strand cDNA was generated from the cRNA using random primers, followed by fragmentation and labeling. Biotinylated sense-stranded DNA was hybridized to Affymetrix GeneChip Human 2.0 ST Array (Affymetrix, High Wycombe, UK) for 16 h. The arrays were washed, stained using Fluidics Station 450, and scanned using Scanner 3000 7G (Affymetrix).
) using the TRIzol reagent (Gibco) according to the manufacturer’s instructions. The cDNA was reverse-transcribed from the purified RNA (200 ng), amplified, and transcribed into cRNA. Sense-strand cDNA was generated from the cRNA using random primers, followed by fragmentation and labeling. Biotinylated sense-stranded DNA was hybridized to Affymetrix GeneChip Human 2.0 ST Array (Affymetrix, High Wycombe, UK) for 16 h. The arrays were washed, stained using Fluidics Station 450, and scanned using Scanner 3000 7G (Affymetrix).
The data were summarized and normalized with a robust multi-average (RMA) method implemented in Affymetrix® Power Tools. We conducted gene-level RMA analysis to export the results and subsequently performed a differentially expressed gene (DEG) analysis. The statistical significance of the expression data was determined based on fold change. For the DEG set, we used hierarchical cluster analysis, with complete linkage and Euclidean distance as measures of similarity. Furthermore, we conducted Gene Enrichment and Functional Annotation analysis for the significant probe list, making use of Gene Ontology (http://geneontology.org) and Kyoto Encyclopedia of Genes and Genomes (http://kegg.jp). The analysis and visualization of differentially expressed genes were performed using R 3.3.2 (www.r-project.org).
Western blot analysis
Human Tenon's fibroblasts were seeded in a 24-well plate at approximately 70% confluence and cultured in the presence or absence of Ilomastat (an MMP inhibitor) for up to 7 days. At the end of the culture period, adherent cells were washed once and lysed with 0.4 mL of 1X Laemmli sample buffer. The cell lysates (10 ug) were boiled for 5 min, loaded onto a sodium dodecyl sulfate-polyacrylamide gel (10%), and electrophoresed at a constant current. The protein concentration was assessed using the RC DC protein assay kit (Bio-Rad, Hercules, CA, USA), with bovine serum albumin as the standard. The resolved proteins were subsequently transferred to a nitrocellulose membrane and blocked overnight using 5% skim milk solution in Tris-buffered saline containing 0.05% Tween-20. The membrane was incubated for 2 h with mouse monoclonal antibodies against collagen type I (Col-1) (Santa Cruz Biotechnology, CA, USA), collagen type III (Col-3) (Santa Cruz Biotechnology), collagen type VI (Col-6) (Santa Cruz Biotechnology), transforming growth factor beta (TGF-β; Santa Cruz Biotechnology), vimentin (Sigma-Aldrich, St Louis, USA), tubulin (Sigma-Aldrich, St Louis, USA), and beta-actin (b-actin; Santa Cruz Biotechnology, CA, USA), all of which were diluted 1000 folds. Following three washes with TTBS, the membrane was incubated for 2 h with peroxidase-conjugated anti-mouse antibodies (Amersham, Buckinghamshire, UK) diluted 2,000 fold in TTBS. The membrane was washed three times and probed with a chemiluminescence kit (Amersham, Piscataway, USA) according to the manufacturer’s protocol. Multiple bands representing the expression of antibodies (Col-1, Col-2, Col-6, TGF-β, and vimentin) were evident on the western blots of all anterior and posterior Tenon’s fibroblasts. Different concentrations (10 and 100 µM) of Ilomastat were applied and cultured for 1, 3, and 7 days before it was harvested.
Cell proliferation assay
Tenon cells were seeded in 96-well plates at a density of  cells/well and allowed to grow overnight until adherence. The next day, cells were washed and further cultured with 100 µL of culture media in the presence or absence of Ilomastat at different concentrations (0, 1, 10, and 100 µM). At the end of the culture, cells were added with 10 µL of reagent of Cell Counting Kit-8 (CCK-8; Dojindo, Rockville, MD) per each well at days specified (days 1, 3, and 7). CCK-8 utilizes a highly water-soluble tetrazolium salt, WST-8 [2-(2-methoxy-4-nitrophenyl)−3-(4-nitrophenyl)−5-(2,4-disulfophenyl)−2 H-tetrazolium, a monosodium salt], which is reduced by cellular dehydrogenases to produce a water-soluble formazan dye. This dye appears as an orange-colored product that is soluble in a tissue culture medium. Following a 1-h incubation period with shaking, cell viability was assessed by measuring the absorbance at 490 nm wavelength using an xMark microplate spectrophotometer (Bio-Ra, Hercules, CA).
cells/well and allowed to grow overnight until adherence. The next day, cells were washed and further cultured with 100 µL of culture media in the presence or absence of Ilomastat at different concentrations (0, 1, 10, and 100 µM). At the end of the culture, cells were added with 10 µL of reagent of Cell Counting Kit-8 (CCK-8; Dojindo, Rockville, MD) per each well at days specified (days 1, 3, and 7). CCK-8 utilizes a highly water-soluble tetrazolium salt, WST-8 [2-(2-methoxy-4-nitrophenyl)−3-(4-nitrophenyl)−5-(2,4-disulfophenyl)−2 H-tetrazolium, a monosodium salt], which is reduced by cellular dehydrogenases to produce a water-soluble formazan dye. This dye appears as an orange-colored product that is soluble in a tissue culture medium. Following a 1-h incubation period with shaking, cell viability was assessed by measuring the absorbance at 490 nm wavelength using an xMark microplate spectrophotometer (Bio-Ra, Hercules, CA).
Ethics statement
This study was approved by the Institutional Review Board of Hallym University Dongtan Sacred Heart Hospital (Aproval no.2021-09-013), and conducted in accordance with the Declaration of Helsinki. All the participants provided written informed consent.
Results
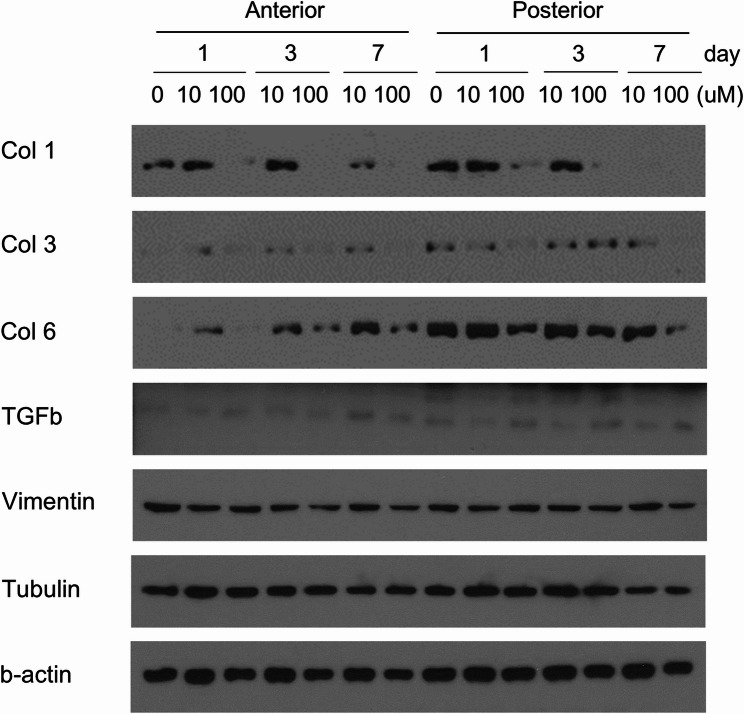
Western blot analysis of Ilomastat-induced changes in anterior and posterior Tenon’s fibroblast is shown in Fig. 1. TGF-β and vimentin showed similar expression between anterior and posterior Tenon’s fibroblast despite the concentration difference (0, 10, and 100 µM) and the number of days cultured (1, 3, and 7 days). Col-1, Col-3, and Col-6 presented a slight weakening of the band as the number of days passed (1, 3, and 7 days); however, the difference was not significant. There were no significant differences in Col-1, Col-3, and Col-6; vimentin; and tubulin in Ilomastat-induced changes between anterior and posterior Tenon’s fibroblasts.
Fig. 1.
Western blot analysis of Ilomastat effects on fibrosis-related protein expression in anterior, posterior Tenon’s fibroblasts. Cells treated with Ilomastat are harvested and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by probing with the indicated antibodies. Separate membranes are examined for the expression of collagen types I (Col-1), III (Col-3), and VI (Col-6). The same membranes are stripped and re-probed with antibodies against transforming growth factor-beta (TGFb) or vimentin. Tubulin and b-actin serve as loading controls. Full-length, unprocessed Western blot images are provided in Supplementary Figure S1–S5. Original, unprocessed images for Col-3 and TGFb could not be retrieved due to data loss; however, the cropped images shown here were derived from the original experiment and are consistent with the overall findings
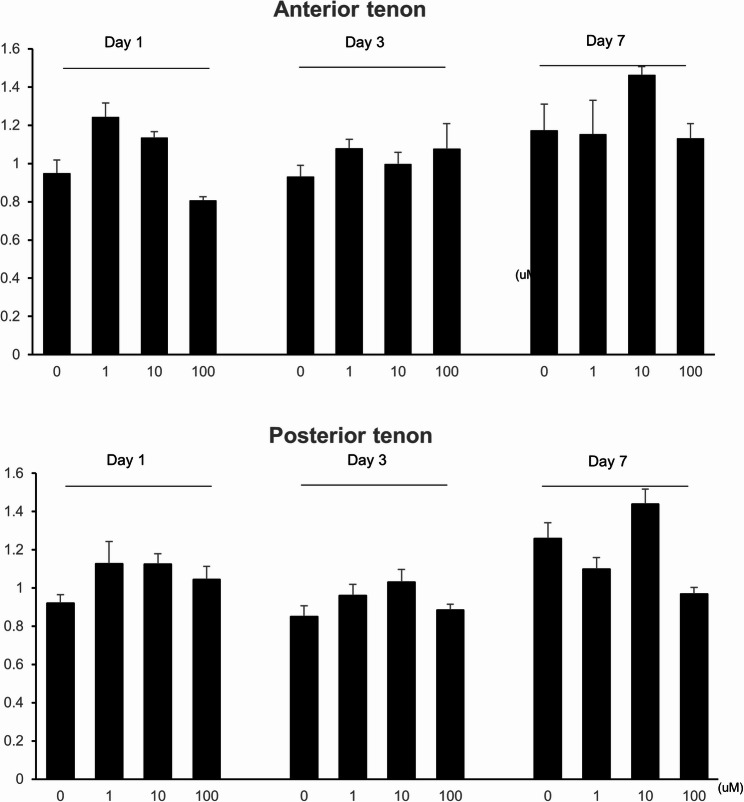
Ilomastat-treated anterior and posterior Tenon’s fibroblast cell proliferation, measured using ultraviolet absorbance, is depicted in Fig. 2. When CCK-8 reagent was added on day 1, anterior Tenon’s fibroblast showed an increase in cell proliferation with the Ilomastat concentration of 1–10 µM (1.243 with 1 µM, 1.136 with 10 µM), and a decrease of proliferation with 100 µM of Ilomastat (0.807). The posterior Tenon’s fibroblast depicted a similar pattern, 1.128 with 1 µM, 1.126 with 10 µM, and 1.046 with 100 µM of Ilomastat. CCK-8 added at day 3 exhibited a rather nonspecific pattern, showing 0.931 with 0 µM, 1.08 with 1 µM, 0.998 with 10 µM, and 1.078 with 100 µM of Ilomastat for the anterior Tenon, and 0.85 with 0 µM, 0.96 with 1 µM, 1.032 with 10 µM, and 0.885 with 100 µM. CCK-8 added at day 7 presented as 1.173 with 0 µM, 1.153 with 1 µM, 1.463 with 10 µM, and 1.132 with 100 µM of Ilomastat for the anterior tenon, and 1.26 with 0 µM, 1.1 with 1 µM, 1.44 with 10 µM, and 0.968 with 100 µM. None of the changes were significant.
Fig. 2.
Ilomastat-induced changes in the cell proliferation between anterior and posterior Tenon’s fibroblasts. Anterior and posterior Tenon’s fibroblasts treated with Ilomastat at concentrations as indicated added with Cell Counting Kit-8 (CCK-8) reagent at days as indicated. Cell proliferation evaluated by measuring the absorbance at 490 nm wavelength spectrophotometrically
Microarray analysis was performed to explore potential differences in gene expression profiles between anterior and posterior Tenon’s capsule fibroblasts.
Discussion
Wound healing is crucial for successful glaucoma surgery [38]. Wound contraction and scarring can block aqueous flow beyond the sclerostomy site and cause inadequate IOP control [39, 40]. The postoperative wound healing response begins with an increase in the vascular permeability and infiltration of inflammatory cells, formation of granulation tissue by fibroblast migration, and formation of scar tissue by collagen cross-linking. These steps are not separate or deterministic but rather overlap each other [7, 41, 42].
Strong antimetabolic agents such as MMC and 5-FU have been important in the surgical management of glaucoma [8, 15, 20]. However, complications induced by agents, such as thin leaky blebs, have led to a search for alternative antimetabolic agents [8, 43]. Ilomastat, a broad-spectrum synthetic MMP inhibitor, reduces tissue damage [9, 12, 34, 44]. It has a significant inhibitory effect on a variety of fibroblast-mediated functions, such as collagen contraction, cell migration, and collagen production, with less toxic effects on the rabbit cornea, conjunctiva, and ciliary body than MMC [9, 45]. In 2003, Daniels et al. reported an in vitro study stating that MMPs are generated during Tenon’s capsule fibroblast-mediated collagen lattice contraction and that matrix contraction and production can be inhibited through MMP inhibition [9]. In an experimental animal study in 2003 by Wong et al., it was reported that when an MMP inhibitor was used after glaucoma filtration surgery, the duration of the filtration bleb was longer and IOP was lower than that of the control group [12]. Despite previous studies showing a significant reduction in matrix contraction and production, this study showed no significant effect of MMP inhibitors on cultured human Tenon’s fibroblast [9]. Most of the previous studies performed with Ilomastat were animal studies, and few studies used in vitro cultured human Tenon’s fibroblasts to observe the reaction, as in our study [9, 19, 34, 45–47]. An in vitro study using human Tenon’s fibroblast by Daniels et al. concluded that MMP inhibition (1–100 µM) reduced the proteolytic activity, and Ilomastat did not affect fibroblast proliferation [9]. Several factors may contribute to this discrepancy. First, the in vitro environment may not fully replicate the complex wound healing environment, where Ilomastat uptake efficiency and interactions with other extracellular matrix components could differ significantly from in vivo conditions. Second, donor variability, such as differences in patient age, health status, or tissue characteristics, may have introduced heterogeneity in fibroblast responses, potentially masking the effects of MMP inhibition.
Thieme et al. analyzed early Tenon’s cysts generated in a young age group that underwent Ahmed valve implantation [37]. A previous study reported that early Tenon’s cysts are composed of two layers. The inner layer had a smooth surface characterized by compressed collagen fibers and myofibroblasts accompanied by elastoid degeneration, and the outer layer was rich in blood vessels. According to the results of a study by Bae et al., when comparing Tenon’s cysts generated after trabeculectomy and Ahmed valve implantation, the Tenon’s cysts in the group that underwent Ahmed valve implantation were thicker than those in the group that underwent trabeculectomy. However, there was no difference between the two groups and histological findings [36].
Tenon’s capsule is connected to the sclera by fine collagenous trabeculae. The anterior Tenon’s capsule from the limbus to approximately 3 mm is firmly fixed to the sclera, preventing the conjunctiva from being easily picked, while the posterior capsules of the posterior part are firmly fixed to the sclera. It adheres loosely to the sclera and creates an episcleral space that does not contain fluids or endothelium.
Clinically, the shape of the filter site created after Ahmed valve insertion and bleb formed after trabeculectomy differs. The position of the reservoir formed during aqueous flow differed, resulting in variations in the flow and flow resistance of the aqueous humor between the two surgeries. In addition, the stimuli applied to fibroblasts naturally differ depending on the flow. Moreover, the insertion of a foreign body may cause this difference, as in the case of Ahmed valve surgery. All these factors contributed to the differences between the two surgeries, making it difficult to explain the distinction.
Based on the various facts mentioned above, this study investigated the differences in characteristics between anterior and posterior Tenon’s fibroblasts through human cell culture and various analysis methods and confirmed the effect of MMP inhibition on fibroblast proliferation.
This study revealed no significant difference between the anterior and posterior Tenon’s capsules and that MMP inhibitors did not affect human-cultured Tenon’s capsule fibroblast proliferation. We expect that identifying the site-specific characteristics of Tenon’s capsule fibroblasts will provide basic data for a better understanding of the wound healing response after glaucoma surgery. The success rate of glaucoma surgery can be increased by controlling the complicated postoperative wound healing process.
This study had a few limitations. First, our study was performed in vitro with cultured human cells; therefore, some in vivo reactions might have been excluded, which could have affected the results of the study. Second, the differences between the cells were defined only with the antibodies of specific agents, such as Col-1, Col-3, and Col-6; TGF-β; and vimentin. Third, the long-term efficacy of these drugs was not assessed. The study was based on cells that were cultured for a maximum of 7 days. Although it did not show a significant effect within 1 week, there is the possibility of a delayed effect. Additionally, we were unable to provide full-length, unprocessed Western blot images for Collagen Type III (Col-3) and TGF-β due to data loss during storage. While the cropped images for these proteins are presented in Fig. 1, the absence of original images may limit the ability to fully verify the data. However, the results for Col-3 and TGF-β are consistent with those of other proteins (Col-1, Col-6, Vimentin, and b-actin), we acknowledge that this does not fully substitute for comprehensive data. The consistency across multiple proteins supports the conclusion that anterior and posterior Tenon’s capsule fibroblasts exhibit similar characteristics under the tested conditions; however, the absence of full-length blots for Col-3 and TGF-β limits the robustness of these findings. We did not perform replicate experiments or employ alternative quantification methods, such as ELISA or qRT-PCR, to validate these results. To address this limitation in future studies, we propose using alternative approaches, such as qRT-PCR to quantify mRNA expression or ELISA to measure protein levels, to confirm the expression profiles of Col-3 and TGF-β and enhance the reliability of the data. Another limitation of our study is the absence of experiments simulating fibrotic conditions, such as pretreatment with TGF-β1, which could provide insights into the effects of MMP inhibition in a wound healing context more relevant to postoperative fibrosis. TGF-β1 is known to induce fibroblast activation and extracellular matrix production, mimicking the fibrotic environment following glaucoma surgery [48]. However, we did not conduct these additional experiments to explore the impact of Ilomastat under TGF-β1-induced conditions. This limitation may restrict the generalizability of our findings to clinical scenarios where fibrosis is a key factor. Future studies should incorporate TGF-β1 pretreatment or other models of fibrosis to investigate whether MMP inhibition modulates fibroblast behavior in a more physiologically relevant context, thereby enhancing the translational potential of these findings. Another limitation is the reliance on CCK-8 assays to assess the effects of MMP inhibition which primarily measure cell proliferation and do not capture other critical fibroblast functions, such as ECM remodeling and contractility. The absence of collagen contraction assays in our study restricts our ability to fully evaluate the impact of Ilomastat on these aspects of fibroblast behavior. Future studies should incorporate 3D collagen contraction assays or other models of ECM remodeling to comprehensively assess the effects of MMP inhibitors, thereby enhancing the understanding of their therapeutic potential in managing postoperative scarring. Finally, a significant limitation of our study is the absence of statistical analyses to assess differences in fibroblast proliferation across Ilomastat concentrations and time points due to limited sample size. While our study provides valuable insights into the characteristics of anterior and posterior Tenon’s capsule fibroblasts and the effects of MMP inhibition in vitro, we acknowledge the limitations of extrapolating these findings to clinical surgery outcomes. In vitro cell culture systems, while useful for controlled experiments, do not fully replicate the complex in vivo wound healing environment, where factors such as fibroblast contractility, interactions with the extracellular matrix, and drug bioavailability play critical roles. For instance, the in vivo postoperative environment involves dynamic interactions between multiple cell types, inflammatory mediators, and biomechanical forces, which may influence the efficacy of MMP inhibitors like Ilomastat in ways not captured in our study. Additionally, the bioavailability of Ilomastat in vivo may differ significantly from in vitro conditions due to factors such as tissue penetration and systemic clearance. Therefore, our findings should be interpreted as foundational data that contribute to understanding the role of MMP inhibition in Tenon’s fibroblasts.
Despite these limitations, this study holds significant value in advancing our understanding of Tenon’s capsule fibroblasts in the context of glaucoma surgery. A key strength of our study lies in the use of cultured human Tenon’s capsule fibroblasts, which enhances the translational relevance of our findings compared to animal models commonly used in prior studies [12, 34]. To our knowledge, this is the first study in Asia to investigate the characteristics of anterior and posterior Tenon’s fibroblasts and the effects of MMP inhibition using human cells, addressing a critical gap in regional research. By demonstrating that MMP inhibition with Ilomastat does not significantly affect fibroblast proliferation, our findings provide essential basic information that lay the groundwork for future investigations into postoperative wound healing. These insights are particularly valuable for developing targeted anti-scarring therapies, as they contribute foundational knowledge that can inform clinical strategies to improve the success rate of glaucoma surgery. This study thus serves as a pivotal step toward bridging basic research and clinical applications in glaucoma management.
In conclusion, no differences in characteristics were observed between anterior and posterior Tenon’s capsule fibroblasts, and inhibition of MMPs did not affect the proliferation of cultured human Tenon’s capsule fibroblasts. Further studies are necessary in the future for clinical applications.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
WS designed the study, acquired, validated, analyzed and interpreted the data. NHK interpreted the data and drafted the manuscript. SSS acquired the data. KEH, RMJ, CK revised the manuscript. All authors read and reviewed the final manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Hallym University Dongtan Sacred Heart Hospital (Aproval no.2021-09-013), and conducted in accordance with the Declaration of Helsinki. All the participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boland MV, Ervin AM, Friedman DS, Jampel HD, Hawkins BS, Vollenweider D, Chelladurai Y, Ward D, Suarez-Cuervo C, Robinson KA. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive services task force. Ann Intern Med. 2013;158(4):271–9. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, Singh K. Relationship between intraocular pressure and primary open angle glaucoma among white and black americans. The Baltimore eye survey. Arch Ophthalmol. 1991;109(8):1090–5. [DOI] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Gordon MO. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13. discussion 829 – 730. [DOI] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the early manifest Glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–79. [DOI] [PubMed] [Google Scholar]
- 5.Conlon R, Saheb H, Ahmed II. Glaucoma treatment trends: a review. Can J Ophthalmol. 2017;52(1):114–24. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood EJ, Parrish RK 2nd, Heuer DK, Skuta GL, Hodapp E, Palmberg PF, Gressel MG, Feuer W. Glaucoma filtering surgery with 5-fluorouracil. Ophthalmology. 1987;94(9):1071–8. [DOI] [PubMed] [Google Scholar]
- 7.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165(6):728–37. [DOI] [PubMed] [Google Scholar]
- 8.Rothman RF, Liebmann JM, Ritch R. Low-dose 5-fluorouracil trabeculectomy as initial surgery in uncomplicated glaucoma: long-term followup. Ophthalmology. 2000;107(6):1184–90. [DOI] [PubMed] [Google Scholar]
- 9.Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT. Matrix metalloproteinase Inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44(3):1104–10. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y-L, Tsou P-F, Tan GS, Perera SA, Ho C-L, Wong TT, Aung T. Postoperative complications after Glaucoma surgery for primary Angle-Closure Glaucoma vs primary Open-Angle Glaucoma. Arch Ophthalmol. 2011;129(8):987–92. [DOI] [PubMed] [Google Scholar]
- 11.Stamper RL, McMenemy MG, Lieberman MF. Hypotonous maculopathy after trabeculectomy with subconjunctival 5-fluorouracil. Am J Ophthalmol. 1992;114(5):544–53. [DOI] [PubMed] [Google Scholar]
- 12.Wong TT, Mead AL, Khaw PT. Matrix metalloproteinase Inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2003;44(3):1097–103. [DOI] [PubMed] [Google Scholar]
- 13.Green E, Wilkins M, Bunce C, Wormald R. 5-Fluorouracil for glaucoma surgery. Cochrane Database Syst Rev. 2014;2:Cd001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng JW, Cai JP, Li Y, Wei RL. Intraoperative mitomycin C for nonpenetrating glaucoma surgery: a systematic review and meta-analysis. J Glaucoma. 2011;20(5):322–6. [DOI] [PubMed] [Google Scholar]
- 15.Wolters JEJ, van Mechelen RJS, Al Majidi R, Pinchuk L, Webers CAB, Beckers HJM, Gorgels T. History, presence, and future of mitomycin C in glaucoma filtration surgery. Curr Opin Ophthalmol. 2021;32(2):148–59. [DOI] [PubMed] [Google Scholar]
- 16.Jampel HD. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human tenon’s capsule fibroblasts. Ophthalmology. 1992;99(9):1471–6. [DOI] [PubMed] [Google Scholar]
- 17.Smith S, D’Amore PA, Dreyer EB. Comparative toxicity of mitomycin C and 5-Fluorouracil in vitro. Am J Ophthalmol. 1994;118(3):332–7. [DOI] [PubMed] [Google Scholar]
- 18.Al Habash A, Aljasim LA, Owaidhah O, Edward DP. A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin Ophthalmol. 2015;9:1945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martorana GM, Schaefer JL, Levine MA, Lukowski ZL, Min J, Meyers CA, Schultz GS, Sherwood MB. Sequential therapy with saratin, bevacizumab and Ilomastat to prolong bleb function following Glaucoma filtration surgery in a rabbit model. PLoS ONE. 2015;10(9):e0138054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mearza AA, Aslanides IM. Uses and complications of mitomycin C in ophthalmology. Expert Opin Drug Saf. 2007;6(1):27–32. [DOI] [PubMed] [Google Scholar]
- 21.Shao T, Li X, Ge J. Target drug delivery system as a new scarring modulation after glaucoma filtration surgery. Diagn Pathol. 2011;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruderman JM, Welch DB, Smith MF, Shoch DE. A prospective, randomized study of 5-fluorouracil and filtration surgery. Trans Am Ophthalmol Soc. 1987;85:238–53. [PMC free article] [PubMed] [Google Scholar]
- 23.Ophir A, Ticho U. Encapsulated filtering bleb and subconjunctival 5-fluorouracil. Ophthalmic Surg. 1992;23(5):339–41. [PubMed] [Google Scholar]
- 24.Ophir A, Ticho U. A randomized study of trabeculectomy and subconjunctival administration of fluorouracil in primary glaucomas. Arch Ophthalmol. 1992;110(8):1072–5. [DOI] [PubMed] [Google Scholar]
- 25.Leyland M, Bloom P, Zinicola E, McAlister J, Rassam S, Migdal C. Single intraoperative application of 5-Fluorouracil versus placebo in low-risk trabeculectomy surgery: a randomized trial. J Glaucoma. 2001;10(6):452–7. [DOI] [PubMed] [Google Scholar]
- 26.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–4. [DOI] [PubMed] [Google Scholar]
- 27.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh Y. Membrane-type matrix metalloproteinases: their functions and regulations. Matrix Biol. 2015;44–46:207–23. [DOI] [PubMed] [Google Scholar]
- 29.Löffek S, Schilling O, Franzke C-W. Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38(1):191–208. [DOI] [PubMed] [Google Scholar]
- 30.Huang YL, Liu CJ, Chiu AW, Wang YC, Huan SK, Lee FL, Chen SJ, Hsu WM, Hsieh SL. A feasible tool to detect mRNA expression of matrix metalloproteinases and their tissue inhibitors in human tenon’s capsule. Ophthalmic Res. 2002;34(6):375–9. [DOI] [PubMed] [Google Scholar]
- 31.Chintala SK, Wang N, Diskin S, Mattox C, Kagemann L, Fini ME, Schuman JS. Matrix metalloproteinase gelatinase B (MMP-9) is associated with leaking glaucoma filtering blebs. Exp Eye Res. 2005;81(4):429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CJ, Huang YL, Chiu AW, Ju JP. Transcript expression of matrix metalloproteinases in the conjunctiva following glaucoma filtration surgery in rabbits. Ophthalmic Res. 2004;36(2):114–9. [DOI] [PubMed] [Google Scholar]
- 33.Shima I, Katsuda S, Ueda Y, Takahashi N, Sasaki H. Expression of matrix metalloproteinases in wound healing after glaucoma filtration surgery in rabbits. Ophthalmic Res. 2007;39(6):315–24. [DOI] [PubMed] [Google Scholar]
- 34.Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of Ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2005;46(6):2018–22. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MR, Mendis U, Smith SD, Paliwal A. Ahmed glaucoma valve implant vs trabeculectomy in the surgical treatment of glaucoma: a randomized clinical trial. Am J Ophthalmol. 2000;130(3):267–73. [DOI] [PubMed] [Google Scholar]
- 36.Bae K, Suh W, Kee C. Comparative study of encapsulated blebs following Ahmed Glaucoma valve implantation and trabeculectomy with Mitomycin-C. Korean J Ophthalmol. 2012;26(4):265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thieme H, Choritz L, Hofmann-Rummelt C, Schloetzer-Schrehardt U, Kottler UB. Histopathologic findings in early encapsulated blebs of young patients treated with the Ahmed Glaucoma valve. J Glaucoma. 2011;20(4):246–51. [DOI] [PubMed] [Google Scholar]
- 38.Kahook MY, Schuman JS. Chandler and Grant’s Glaucoma. SLACK Incorporated. 2020.
- 39.Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983;101(5):795–8. [DOI] [PubMed] [Google Scholar]
- 40.Fuller JR, Bevin TH, Molteno AC, Vote BJ, Herbison P. Anti-inflammatory fibrosis suppression in threatened trabeculectomy bleb failure produces good long term control of intraocular pressure without risk of sight threatening complications. Br J Ophthalmol. 2002;86(12):1352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett NT, Schultz GS. Growth factors and wound healing: part II. Role in normal and chronic wound healing. Am J Surg. 1993;166(1):74–81. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence WT. Physiology of the acute wound. Clin Plast Surg. 1998;25(3):321–40. [PubMed] [Google Scholar]
- 43.Ordan JL, Catey B, Melville MM, Vincenzo M, Peng TK, Richard W, Keith B. Risk factors for development of post-trabeculectomy endophthalmitis. Br J Ophthalmol. 2000;84(12):1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barletta JP, Angella G, Balch KC, Dimova HG, Stern GA, Moser MT, van Setten GB, Schultz GS. Inhibition of pseudomonal ulceration in rabbit Corneas by a synthetic matrix metalloproteinase inhibitor. Investig Ophthalmol Vis Sci. 1996;37(1):20–8. [PubMed] [Google Scholar]
- 45.Suh W, Han KE, Han JR. Safety of using matrix metalloproteinase inhibitor in experimental Glaucoma filtration surgery. J Korean Med Sci. 2017;32(4):666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherwood MB. A sequential, multiple-treatment, targeted approach to reduce wound healing and failure of glaucoma filtration surgery in a rabbit model (an American ophthalmological society thesis). Trans Am Ophthalmol Soc. 2006;104:478–92. [PMC free article] [PubMed] [Google Scholar]
- 47.Mohamed-Ahmed AHA, Lockwood A, Li H, Bailly M, Khaw PT, Brocchini S. An Ilomastat-CD eye drop formulation to treat ocular scarring. Invest Ophthalmol Vis Sci. 2017;58(9):3425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kottler UB, Jünemann AG, Aigner T, Zenkel M, Rummelt C, Schlötzer-Schrehardt U. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human tenon’s capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005;80(1):121–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.