Summary
Background
Despite numerous randomized controlled trials (RCTs) on cancer vaccines, systematic evaluations of their efficacy and safety for patients with advanced non-small cell lung cancer (NSCLC) following first-line therapy remain lacking.
Methods
In this systematic review and meta-analysis (PROSPERO, CRD42024568178), PubMed, Cochrane Library, and Embase databases were searched from inception up to December 27, 2024. Published phase II or III RCTs reporting survival outcomes in advanced or metastatic NSCLC patients who received vaccine therapy after first-line therapy were included. Data were independently extracted by two reviewers. The primary outcome was overall survival (OS), with progression-free survival (PFS) as secondary outcome. Treatment-related adverse events (TRAEs) was the major safety outcome. Random-effects model was employed in this meta-analysis. Risk of bias was evaluated with RoB 2.
Findings
Eleven RCTs comprising 3228 patients (67% male, n = 2162) were included, without high risk of bias. The included studies involved employing cancer vaccines as first-line maintenance therapy, second-line therapy, or third-line therapy. In general, Cancer vaccines associated with improved OS (HR = 0.85, 95% CI, 0.78–0.92, P < 0.001) but did not significantly improve PFS (HR = 0.91, 95% CI, 0.79–1.05, P = 0.195). Subgroup analyses indicated better OS for patients with ECOG = 1, first-line chemotherapy, squamous cell carcinoma, stable disease after first-line therapy, stage IV, and smoking history. Squamous cell carcinoma (HR = 0.74, 95% CI, 0.61–0.90, P = 0.003) responded better to vaccine therapy than adenocarcinoma (HR = 0.83, 95% CI, 0.55–1.26, P = 0.377). The pooled OR for TRAEs was 1.5 (95% CI, 0.63–3.61, P = 0.361). Exploratory analysis indicated that immune response to cancer vaccines may serve as a predictive biomarker for vaccines effect. Besides, consistent efficacy and safety results were obtained when the meta-analysis was specific to first-line maintenance therapy based on seven trials.
Interpretation
This meta-analysis demonstrated the clinical efficacy and safety of cancer vaccines in advanced NSCLC patients after first-line therapy, especially in those with squamous cell carcinoma. Immune response was identified as a predictive biomarker for vaccines effect. This study provides the state-of-the-art evidence for the clinical application of cancer vaccines in advanced NSCLC patients after first-line therapy.
Funding
20240484580, 20230484314, 2023YFF0723500, 2021RU002, 82173386, RZ2022-04.
Keywords: Cancer vaccines, Advanced non-small cell lung cancer, Post-first-line therapy, Systematic review, Meta-analysis
Research in context.
Evidence before this study
Despite advances in first-line therapies, the prognosis for patients with advanced NSCLC remains poor. Cancer vaccines are emerging as a therapeutic approach due to the high heterogeneity and mutational burden associated with NSCLC. Randomized controlled trials (RCTs) have investigated the safety and efficacy of these vaccines, but outdated meta-analyses have focused on the effectiveness of cancer vaccines across various treatment scenarios rather than specifically on their use after first-line therapy. We conducted a systematic review and meta-analysis of RCTs, performing comprehensive literature searches in the PubMed, Cochrane Library, and Embase databases up to December 27, 2024 using search terms “NSCLC”, “cancer vaccine”, “vaccination”, “chemotherapy”, “radiotherapy”, “chemoradiotherapy”, and “immunotherapy”. This meta-analysis specifically addresses the efficacy and safety of cancer vaccines in NSCLC patients with advanced disease after first-line therapy, addressing a significant knowledge gap in the current literature.
Added value of this study
To our best knowledge, this is the first systematic review and meta-analysis based on RCTs to investigate the efficacy and safety of cancer vaccines for advanced NSCLC after first-line therapy. Results from the meta-analyses demonstrated significant clinical efficacy and favorable safety profile for therapeutic vaccination in these patients. Furthermore, additional evidences from subgroup analyses revealed that patients with squamous cell carcinoma experienced better survival outcomes, indicating its potential as an independent prognostic factor. Immune response also showed promising survival predictive association, further supporting its potential role as a biomarker.
Implications of all the available evidence
The findings of meta-analyses offer critical data on the added value of utilizing cancer vaccines following first-line therapy for advanced NSCLC. The results of subgroup analyses informed the enrollment of patients in future studies. Additionally, detecting immune responses during treatment may offer early predictions of vaccine efficacy, allowing for potential adjustments to treatment regimens. These findings provide crucial evidence and guidance for the clinical application of cancer vaccines, highlighting their potential to improve survival in patients with advanced NSCLC.
Introduction
According to GLOBOCAN estimates 2022, lung cancer is the most common malignant tumor and the leading cause of cancer-related deaths worldwide, accounting for over 1.8 million fatalities.1 Approximately 85% of patients are classified as non-small cell lung cancer (NSCLC), of which more than 70% are diagnosed at an advanced stage.2 For patients with advanced disease, chemoradiotherapy, targeted therapy and immunotherapy are the principled first-line treatments. Nonetheless, despite these interventions, the majority of patients still experience disease progression, resulting in poor survival outcomes.3 Consequently, there is an urgent need for new therapeutic strategies to prolong survival, particularly after first-line therapy. Cancer vaccines, as an active immunotherapy, activate the immune system by promoting antigen cross-presentation, which leads to tumor killing. The high genetic heterogeneity and mutational burden of NSCLC make it a promising candidate for vaccine-based therapies.4 Randomized controlled trials (RCTs) have investigated the safety and efficacy of cancer vaccines in NSCLC. Meta-analyses have shown that vaccination can improve overall survival (OS) without significantly increasing adverse events (AEs) in NSCLC patients.5,6 However, the efficacy of cancer vaccines as monotherapy is modest, with limited survival benefits for patients with advanced disease. Over the past decades, numerous clinical trials have investigated the application of cancer vaccines as post-first-line therapy in advanced NSCLC, such as maintenance therapy or second-line therapy. Recent RCTs have also begun to explore the combinations of vaccines with chemotherapy or immunotherapy as first-line therapy for advanced NSCLC patients.7,8 With the development of neoantigen identification and mRNA technology, a growing number of vaccines clinical trials are being registered, focusing on advanced NSCLC patients.9 Nevertheless, the clinical outcomes of cancer vaccines as post-first-line therapy in these patients have not been systemically review and analyzed, highlighting the need for higher-level evidence to support their application in clinical practice.
Based on published data, we conducted this systematic review and meta-analysis of RCTs to assess the clinical efficacy and safety of cancer vaccines after first-line therapy in NSCLC patients with advanced disease. This study also investigates the impact of subgroup factors, including baseline characteristics, tumor histology, tumor stage, Eastern Cooperative Oncology Group Performance Status (ECOG PS), first-line therapy, initial treatment response. We exploratively analyzed the predictive biomarker for vaccines effect. Since most of the trials included treats vaccines as maintenance therapy after first-line treatment, we analyzed it separately. This study aims to provide solid evidence-based support for the application of cancer vaccines as post-first-line therapy in advanced NSCLC patients.
Methods
This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines10 and AMSTAR 2 guidelines (Assessing the Methodological Quality of Systematic Reviews).11 The study protocol was registered in PROSPERO database (CRD42024568178).
Literature search
A comprehensive literature search of PubMed, Cochrane Library, and Embase databases was conducted, and only full-text RCTs published in English up to December 27, 2024 were included. We using Medical Subject Headings (MeSH) terms such as NSCLC, cancer vaccine or vaccination, chemotherapy, radiotherapy, chemoradiotherapy, immunotherapy. The reference lists of acquired literatures were manually scrutinized for detailed information. Two independent reviewers conducted the search, with disagreements resolved by an advisory group of three senior authors. The representative search strategies are provided in Supplementary Material.
Study selection
The inclusion criteria were as follows: (1) RCTs; (2) full-length articles; (3) studies enrolling stage III/IV or metastatic NSCLC patients; (4) patients received vaccine therapy after first-line therapy; and (5) reporting survival outcomes. The exclusion criteria were as follows: (1) phase I trials; (2) reviews, case reports, comments, corresponding letters or editorials; (3) studies lacking endpoints data; (4) comparisons of vaccination versus other adjuvant therapies; and (5) non-English literatures. Two independent reviewers initially screened titles and abstracts, followed by a full-text review to exclude ineligible studies.
Data extraction
Data were independently extracted by two reviewers using a standardized form. The extracted items from included studies were as follow: first author, year of publication, journal of publication, country of study, clinical trial phase, masking method, main inclusion criteria, enrollment size, demographic characteristics (male or female ratio, median or mean age), disease stage, first-line treatment received, type of vaccines, intervention model, overall survival (OS), progression-free survival (PFS), AEs, clinical trial registration identifier. Any disagreements between two reviewers were resolved by the advisory group.
Quality assessment
The risk of bias of included studies was assessed using the Cochrane tool for assessing risk of bias in randomized trial (RoB 2).12 The assessment covered randomized sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases, with each item rated as having high, low, or unclear risk. The methodological quality of the meta-analyses was assessed employing AMSTAR 2 that consists of 16 items and seven critical domains, aiming to assess the quality of the systemic review throughout the process of issue selection, design, search, data extraction, statistical analysis, reporting and transparency. The evidence quality of meta-analyses was assessed by Grading of Recommendation Assessment, Development, and Evaluation (GRADE) system, scoring based on five downgrading factors (Study limitations, Indirectness of evidence, Inconsistency of results, Imprecision, Publication bias) and three upgrading factors (Large magnitude of effect, Dose response, Plausible confounding). GRADE assigns an overall rating of high, moderate, low, or very low quality. Two reviewers according to criteria independently rated the quality of included trials and meta-analyses, with any disagreements were resolved by the advisory group.
Outcome measures
The primary clinical outcome in this meta-analysis was OS, defined as the time from randomization to death from any cause or the last date of follow-up. The secondary clinical outcome was PFS, which was calculated from the date of randomization to the date of documented progression or death from any cause. The incidence of treatment-related adverse events (TRAEs) was defined as the primary safety outcome and was assessed using Common Terminology Criteria for Adverse Events (CTCAE).
Statistical analysis
Hazard ratios (HR) for OS and PFS, as well as odds ratios (OR) for adverse events, were extracted or calculated, and pooled with 95% confidence intervals (CI). When HR was unavailable, it was estimated through Kaplan–Meier curves using the method reported by Tierney et al.13 The incidence of AEs was extracted as the number of events and total patients in each group, allowing calculation of odds ratios (OR) and 95% CI. Heterogeneity among the included studies was assessed using Cochran's Q statistic and the I2 statistic, with I2 value > 50% indicating high heterogeneity.14 Random-effects model was used for all the pooled analyses in this meta-analysis. Publication bias was assessed through funnel plots and Egger's test. Sensitivity analysis were performed to evaluate the robustness and reliability of the results in meta-analysis. Subgroup analyses were conducted, and differences between subgroup pooled effects were reported. A two-sided P < 0.05 was considered statistically significant difference. All statistical analyses were performed using STATA version 12 software (Stata Corporation, College Station, TX, USA).
Role of funding source
The funders of this study had no involvement in the study design, data collection, data analysis, data interpretation, and writing of the report.
Results
Study selection
A total of 1422 literatures were retrieved through the initial search strategy, from which 223 duplicates were removed. After screening the title and abstract, 1155 literatures were excluded. The remaining 44 literatures were thoroughly assessed for eligibility, with 33 being excluded for different reasons (Table S1). Ultimately, 11 of full-text RCTs studies were included for meta-analysis (Fig. 1).
Fig. 1.
PRISMA flowchart for study identification and selection.
Characteristics of included trials and patients
In general, 11 of studies (10 RCTs) included 3228 patients diagnosed with advanced NSCLC and received either vaccine or control treatment after first-line treatment. The characteristics of the included RCTs are summarized in Table 1. All studies were double-arm RCTs: five studies17,20,21,24,25 were phase II, five studies16,18,19,22,23 were phase III, and one study15 was phase II/III. Of the 11 studies, six15,18, 19, 20, 21, 22 were placebo-controlled, three17,23,25 were best supportive care (BSC)-controlled, one16 was standard of care (SoC) chemotherapy-controlled, and one24 compared vaccine plus docetaxel with placebo plus docetaxel. The L-BLP25 vaccine was employed in four studies17,18,21,22 and CIMAvax-EGF used in two studies,23,25 and other vaccines including personalized peptide vaccine,24 Vx-001,20 Belagenpumatucel-L,19 OSE2101,16 Racotumomab-alum.15 All of these studies reported OS data, while four studies15,19,21,24 also reported PFS data. Additionally, safety outcomes were available in eight studies.15,16,18,19,21,23, 24, 25 Worthwhile, two of 11 studies reported the same RCT, with one18 reporting the safety and survival results and the later published one22 updating the survival data. Hence, we used the safety data18 and updated survival data22 for pooled analyses of safety outcomes and clinical outcomes respectively, where part of subgroup survival data18 were used for the subgroup analysis of OS. Among the 11 studies, eight15,17, 18, 19, 20, 21, 22, 23 involved vaccines as first-line maintenance therapy (the included patients did not progress after first-line treatment), two24,25 involved both second-line therapy and first-line maintenance therapy, and one16 involved second- or third-line therapy. Regarding the first-line therapy, 1074 patients in three trials20,23,25 received chemotherapy, 1411 patients in two trials21,22 received chemoradiotherapy, 929 patients in four trials15,17,19,24 received chemotherapy, or chemoradiotherapy. In addition, 36 patients received first-line immunotherapy combined with chemotherapy and 183 patients received sequential second-line immunotherapy in a study.16
Table 1.
Characteristics of included studies for vaccine therapy in advanced NSCLC patients after first-line therapy.
| Study | Country | Phase | Tumor stage | Sample, n | Male, n (%) | Age, y | First-line therapy | Intervention (sample, n) | Control (sample, n) | Extracted endpoint | Registration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alfonso 201415 | Cuba | II/III | IIIB/IV | 176 | 118 (67%) | – | Chemo/Chemoradio | Racotumomab-alum (87) | Placebo (89) | OS, PFS, AEs | RPCEC00000009 |
| Besse 202316 | France | III | Advanced/Metastatic | 219 | 155 (70.8%) | 65 (41–86) | Chemo/ICB | OSE2101 (139) | SoC (80) | OS, AEs | NCT02654587 |
| Butts 201117 | Canada | IIb | IIIB/IV | 171 | 95 (55.6%) | 59 | Chemo/Chemoradio | BLP25 + BSC (88) | BSC (83) | OS | – |
| Butts 201418,a | Canada | III | III | 1239 | 846 (68.3%) | 61 (19–89) | Chemoradio | BLP25 (829) | Placebo (410) | OS, AEs | NCT00409188 |
| Giaccone 201519 | USA | III | IIIA/IIIB/IV | 532 | 307 (58%) | 61 ± 8.5 | Chemo/Chemoradio | Belagenpumatucel-L (270) | Placebo (262) | OS, PFS, AEs | NCT00676507 |
| Gridelli 202020 | Italy | II | IV | 190 | 132 (69.5%) | – | Chemo | Vx-001 (89) | Placebo (101) | OS | NCT01935154 |
| Katakami 201721 | Japan | II | III | 172 | 145 (84.3%) | 63 (33–86) | Chemoradio | BLP25 (114) | Placebo (58) | OS, PFS, AEs | – |
| Mitchell 201522,a | Australia | III | III | 1239 | 846 (68.3%) | 61 (19–89) | Chemoradio | BLP25 (829) | Placebo (410) | OS | NCT00409188 |
| Rodriguez 201623 | Cuba | III | IIIB/IV | 378 | – | – | Chemo | CIMAvax-EGF (246) | BSC (132) | OS, AEs | RPCEC00000161 |
| Takayama 201624 | Japan | II | IIIB/IV/Recurrent | 50 | 41 (82%) | – | Chemo/Radio | PPV vaccine + Docetaxel (26) | Placebo + Docetaxel (24) | OS, PFS, AEs | UMIN000003521 |
| Vinageras 200825 | Cuba | II | IIIB/IV | 74 | – | – | Chemo | CIMAvax-EGF (37) | BSC (37) | OS, AEs | – |
Chemo: Chemotherapy; Chemoradio: Chemoradiotherapy; Radio: Radiotherapy; ICB: Immune checkpoint blockers; BSC: Best supportive care; SoC: Standard of care; OS: Overall survival; PFS: Progression-free survival; AEs: Adverse events.
Studies reported from the same RCT, Mitchell 2015 updated the survival data.
Quality assessment of included studies
The risk of bias assessment of the included studies is shown in Figure S1. Overall, no studies were classified as having a high risk of bias, and more than half of the studies were deemed to have a low risk of bias. For studies with some concerns, Mitchell et al.'s22 and Butts et al.'s18 studies raised concerns regarding the selection of reported results, Takayama et al.'s24 study had issues related to the randomization process, and Rodriguez et al.'s23 and Besse et al.'s16 studies raised concerns regarding deviations from intended interventions. Altogether, the included studies were generally considered to be at a low risk of bias.
Quality assessment of meta-analyses
A total of 25 meta-analyses were conducted to evaluate the clinical and safety outcomes of cancer vaccines in advanced NSCLC patients after first-line therapy (Table 2). In the assessment of methodological quality, all meta-analyses were rated as high quality according to AMSTAR 2, indicating no critical weaknesses. Regarding the quality of evidence for each outcome, the GRADE system demonstrated high quality evidence for 23 meta-analyses, and moderate quality evidence for two meta-analyses that were downgraded due to publication bias. Since all studies included were RCTs, the overwhelming majority of meta-analysis results were high quality evidence.
Table 2.
Results and quality assessment of meta-analyses for vaccine therapy after first-line therapy.
| Outcomes | Indicator | Sample and study | Effect (95% CI) | GRADE quality assessment |
GRADE quality | AMSTAR 2 rating | Figure | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Limitation | Indirectness | Inconsistency | Imprecision | Publication bias | Large effect | Dose response | Plausible confounding | |||||||
| Meta-analysis of clinical outcomes | ||||||||||||||
| Survival | OS | 320115, 16, 17,19, 20, 21, 22, 23, 24, 25 | 0.85 (0.78–0.92) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 2A |
| PFS | 93015,19,21,24 | 0.91 (0.79–1.05) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 2B | |
| Meta-analysis of safety outcomes | ||||||||||||||
| Safety | AEs | 222815,18,21,23 | 1.66 (0.91–3.00) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | S5A |
| SAEs | 222815,18,21,23 | 1.03 (0.73–1.45) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | S5B | |
| TRAEs | 244015,16,18,21,23 | 1.50 (0.63–3.61) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | S5C | |
| TRSAEs | 244015,16,18,21,23 | 1.09 (0.38–3.11) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | S5D | |
| Subgroup meta-analysis of OS | ||||||||||||||
| ECOG PS | ECOG = 0 | 60315,16,19,21,23 | 0.73 (0.53–1.00) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3A |
| ECOG = 1 | 60315,16,19,21,23 | 0.80 (0.68–0.95) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| ECOG = 2 | 4219,23 | 1.24 (0.54–2.85) | Not serious | Not serious | Not serious | Not serious | Suspected | No | No | No | ⊕⊕⊕○ (Moderate) | High | ||
| Tumor stage | IIIA | 58918,19,21 | 0.91 (0.72–1.15) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3B |
| IIIB | 136015,18,19,21,23 | 0.80 (0.62–1.04) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| IV | 76815,16,19,23 | 0.83 (0.70–0.98) | Not serious | Not serious | Not serious | Not serious | Suspected | No | No | No | ⊕⊕⊕○ (Moderate) | High | ||
| First-line therapy | Chemo | 81815,20,23,25 | 0.78 (0.66–0.93) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3C |
| Chemoradio | 157219,21,22 | 0.85 (0.71–1.03) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| First-line response | SD | 73715,16,18,21,23 | 0.78 (0.67–0.92) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3D |
| OR | 113415,16,18,21 | 0.73 (0.49–1.07) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| Histology | Adeno | 60315,19,21,23 | 0.83 (0.55–1.26) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3E |
| Squamous | 48415,16,19,23 | 0.74 (0.61–0.90) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| Smoking | Never | 9016,21,23 | 0.91 (0.56–1.49) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3F |
| Former | 43315,16,23 | 0.73 (0.56–0.97) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| Current | 19315,16,23 | 0.61 (0.46–0.81) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| Age | <65 years | 74016,19,21,23 | 0.86 (0.71–1.04) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3G |
| ≥65 years | 48816,19,21,23 | 0.86 (0.69–1.08) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | ||
| Vaccine | L-BLP25 | 158217,21,22 | 0.87 (0.77–0.99) | Not serious | Not serious | Not serious | Not serious | Undetected | No | No | No | ⊕⊕⊕⊕ (High) | High | 3H |
| Exploratory meta-analysis of correction between immune response and OS | ||||||||||||||
| OS | Immune response | 16315,20,24,25 | 0.34 (0.24–0.50) | Not serious | Not serious | Not serious | Not serious | Undetected | Yes | No | No | ⊕⊕⊕⊕ (High) | High | 4A |
OS: Overall survival, PFS: Progression-free survival, AEs: Adverse events, SAEs: Serious adverse events, TRAEs: Treatment-related adverse events, TRSAEs: Treatment-related serious adverse events, ECOG PS: Eastern Cooperative Oncology Group Performance Status, Chemo: Chemotherapy, Chemoradio: Chemoradiotherapy, SD: Stable disease, OR: Objective response, Adeno: Adenocarcinoma, Squamous: Squamous cell carcinoma.
Clinical outcomes of OS and PFS
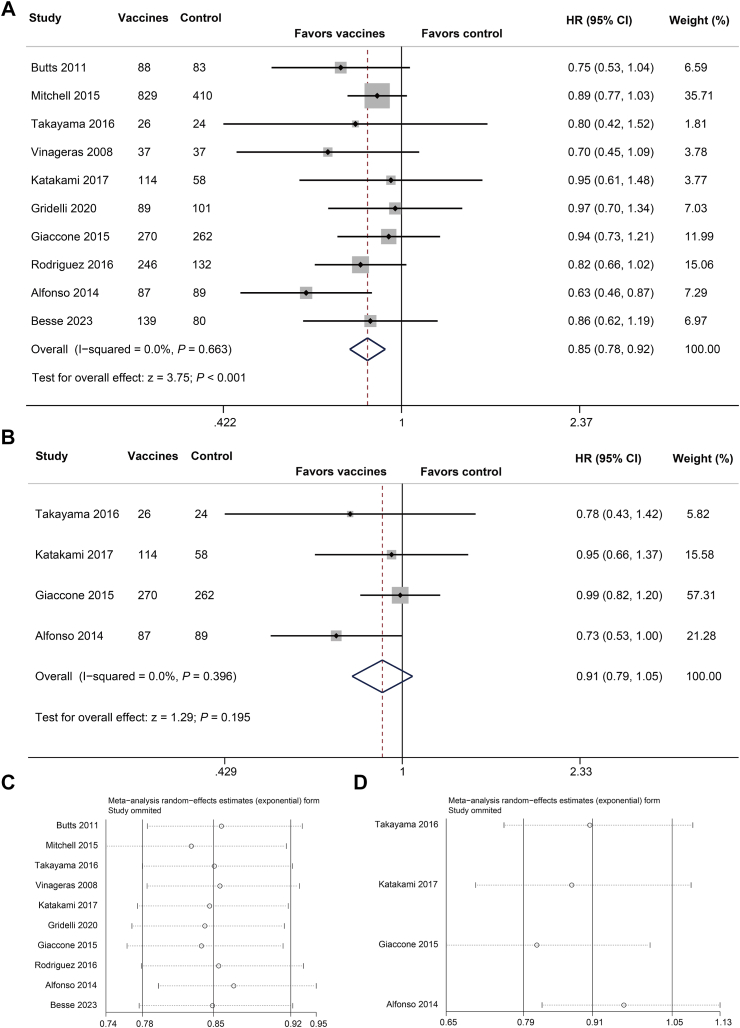
The follow-up time of each study was showed in Table S2. In the pooled analysis, cancer vaccine therapy exhibited favorable survival outcomes in patients with advanced NSCLC after first-line therapy. Based on 10 RCTs involving 3201 patients (Fig. 2A), the quantitative analysis of OS demonstrated significant better outcomes in the vaccines group (HR = 0.85, 95% CI, 0.78–0.92, P < 0.001), with low heterogeneity (I2 = 0, P = 0.663). Egger's test was used to assess potential publication bias in survival outcomes analysis. As shown in funnel plot (Figure S2A) and Egger regression asymmetry plots (Figure S2B), no publication bias was detected (Egger's test, P = 0.342). Sensitivity analysis showed that sequentially excluded each study utilizing the leave-one-out method did not significantly alter the analysis results, confirming the robustness of the pooled OS results (Fig. 2C). However, the pooled analysis of PFS based on four RCTs15,19,21,24 comprising 930 patients, showed non-significant improvement in the vaccines group (Fig. 2B, HR = 0.91, 95% CI, 0.79–1.05, P = 0.195), without significant heterogeneity (I2 = 0, P = 0.396) and publication bias (Figure S2C and D, Egger's test, P = 0.406). Sensitivity analysis showed stable results (Fig. 2D). Specific to vaccines as first-line maintenance therapy, pooled analysis of OS based on seven RCTs also indicated significant improved survival in the vaccines group (HR = 0.86, 95% CI, 0.78–0.94, P = 0.001), with no publication bias and robustness of pooled results (Figure S3). The pooled result for PFS based on three RCTs of cancer vaccines as maintenance therapy (Figure S4, HR = 0.90, 95% CI, 0.75–1.09, P = 0.284) was also consistent with those as post-first-line therapy. Overall, this meta-analysis demonstrated the clinical efficacy of cancer vaccines after first-line therapy, and similarly when employed only as first-line maintenance therapy.
Fig. 2.
Meta-analysis of survival outcomes. (A) Forest plot and (C) sensitivity analyses of OS. (B) Forest plot and (D) Sensitivity analysis of PFS.
Safety outcomes
The treatment duration of each study was showed in Table S2. Pooled analyses were conducted to assess the safety of cancer vaccines in advanced NSCLC patients after first-line therapy, focusing on AEs, serious adverse events (SAEs), TRAEs, and treatment-related serious adverse events (TRSAEs), as reported across various trials. According to four trials15,18,21,23 with 2228 patients, the pooled OR of overall AEs (Figure S5A) and SAEs (Figure S5B) were 1.66 (95% CI, 0.91–3.00, P = 0.098; significant heterogeneity, I2 = 69.7%, P = 0.020) and 1.03 (95% CI, 0.73–1.45, P = 0.877; non-significant heterogeneity, I2 = 49.6%, P = 0.114), respectively. No significant differences were observed between the vaccines and control groups for overall AEs and SAEs. As for TRAEs (Figure S5C) and TRSAEs (Figure S5D), pooled analyses of five trials comprising 2344 patients also demonstrated non-significant safety difference between two groups. For TRAEs, OR was 1.50 (95% CI, 0.63–3.61, P = 0.361) with significant heterogeneity (I2 = 88.8%, P < 0.001); For TRSAEs, OR was 1.09 (95% CI, 0.38–3.11, P = 0.873) with significant heterogeneity (I2 = 67.7%, P = 0.015). Pooled analyses of individual TRAEs reported by at least three trials, across eight studies15,16,18,19,21,23, 24, 25 consisting of 3099 patients (Table 3), demonstrated that most TRAEs did not show significant differences between the vaccine and control groups, except for headache and injection site reactions that likely due to the immunogenicity of the injected vaccines. In addition, meta analyses focused on first-line maintenance therapy also showed no significant difference on AEs (Figure S5A), SAEs (Figure S5B), TRAEs (Figure S6A) and TRSAEs (Figure S6B). The pooled results of individual TRAEs for first-line maintenance therapy were displayed in Table S3. Overall, cancer vaccines did not significantly increase the incidence of TRAEs compared to the control group. This meta-analysis showed the favorable safety profile of vaccine therapy in advanced NSCLC patients following first-line therapy, as well as when cancer vaccines employed as maintenance therapy.
Table 3.
Pooled results of individual TRAEs for vaccine therapy after first-line therapy.
| TRAEs | Study and sample | Pooled analysis |
Heterogeneity |
||
|---|---|---|---|---|---|
| OR (95% CI) | P value | I2 (%) | P value | ||
| Cough | 220815,18,19 | 1.18 (0.96–1.46) | 0.132 | 5.7 | 0.346 |
| Dyspnoea | 205415,18,23 | 1.03 (0.82–1.28) | 0.810 | 0 | 0.819 |
| Fatigue | 262316,18,19,23 | 0.97 (0.65–1.46) | 0.880 | 57.2 | 0.072 |
| Asthenia | 84515,16,23,25 | 0.87 (0.38–1.99) | 0.739 | 75.2 | 0.007 |
| Body or extremity pain | 224516,18,19 | 1.40 (0.87–2.27) | 0.169 | 61.4 | 0.075 |
| Musculoskeletal pain | 129715,16,19,23 | 1.10 (0.77–1.59) | 0.596 | 5.9 | 0.346 |
| Headache | 266615,18,19,23,25 | 1.75 (1.02–3.00) | 0.041 | 69.6 | 0.010 |
| Nausea | 270316,18,19,23,25 | 1.37 (0.87–2.16) | 0.178 | 56.8 | 0.055 |
| Decreased appetite or anorexia | 284815,16,18,19,23,24 | 1.00 (0.63–1.60) | 0.998 | 54.9 | 0.050 |
| Vomiting | 67016,23,25 | 2.66 (0.41–17.41) | 0.308 | 83.6 | 0.002 |
| Arthralgia | 287815,16,18,19,23,25 | 1.14 (0.68–1.91) | 0.628 | 56.1 | 0.044 |
| Injection site reaction | 159815,16,19,21,23, 24, 25 | 14.97 (3.18–70.40) | 0.001 | 90.1 | <0.001 |
| Fever | 137715,16,19,23,25 | 2.47 (0.99–6.18) | 0.054 | 80.2 | <0.001 |
| Skin symptoms | 137715,16,19,23,25 | 1.35 (0.27–6.66) | 0.714 | 88.3 | <0.001 |
Subgroup meta-analysis of OS
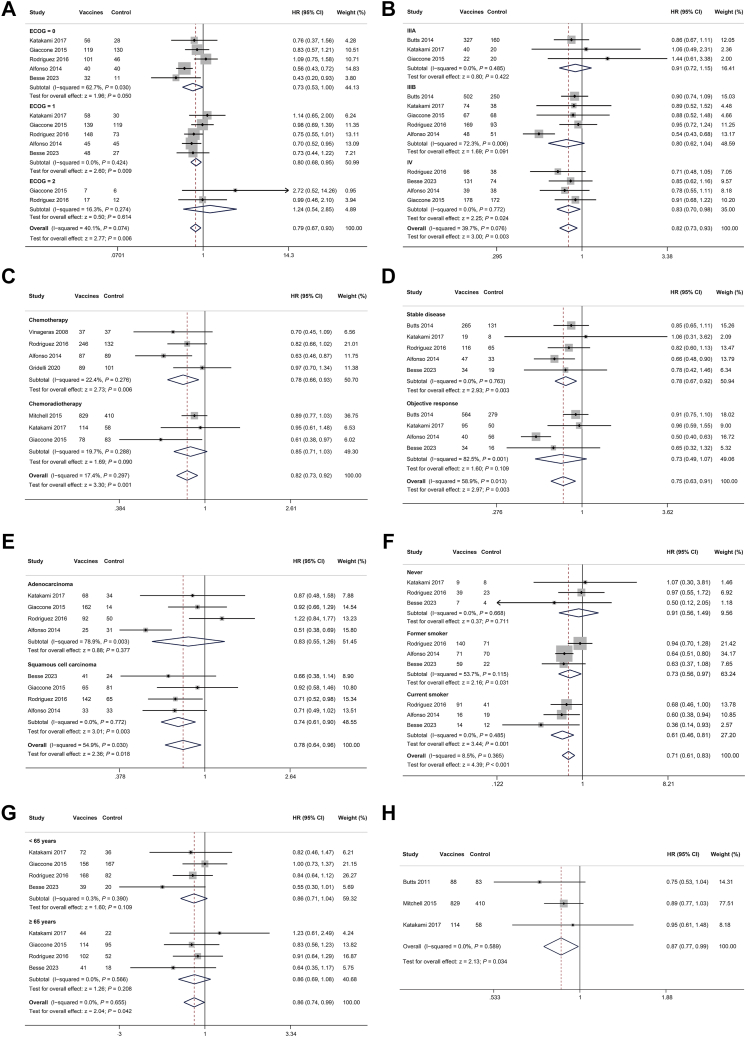
The subgroup meta-analyses were conducted to investigate the potential association between OS and factors such as ECOG PS, tumor stage, first-line therapy, response to first-line therapy, tumor histology, smoking history, and age. The subgroup pooled results of post-first-line therapy were showed in Fig. 3. Regarding ECOG PS (Fig. 3A), the pooled analysis for the ECOG = 0 subgroup demonstrated a marginally significant improvement in OS for the vaccines group (HR = 0.73, 95% CI, 0.53–1.00, P = 0.05), and the ECOG = 1 subgroup demonstrated significantly better OS in the vaccines group (HR = 0.80, 95% CI, 0.68–0.95, P = 0.009). In contrast, the ECOG = 2 subgroup exhibited no significant difference in OS between the vaccines group and control group (HR = 1.24, 95% CI, 0.54–2.85, P = 0.614). In terms of tumor stage (Fig. 3B), patients with stage IV disease exhibited significantly improved OS in the vaccines group compared to the control group (HR = 0.83, 95% CI, 0.70–0.98, P = 0.024), while no such benefit was observed in stage III patients. The vaccines group also demonstrated significant better survival outcomes in patients received first-line chemotherapy (Fig. 3C, HR = 0.78, 95% CI, 0.66–0.93, P = 0.006), but not observed in patients received chemoradiotherapy (Fig. 3C, HR = 0.85, 95% CI, 0.71–1.03, P = 0.090). Moreover, patients who achieved stable disease (SD) showed significant better survival in the vaccines group compared to control group (HR = 0.78, 95% CI, 0.67–0.92, P = 0.003), while those with objective response showed non-significant better survival in vaccines group (Fig. 3D, HR = 0.73, 95% CI, 0.49–1.07, P = 0.109). Pooled results showed that tumor histology impacted OS outcomes. Patients with squamous cell carcinoma had better OS than adenocarcinoma when compared to control group (Fig. 3E). Specifically, the OS comparison between vaccines and control groups in adenocarcinoma was non-significant (HR = 0.83, 95% CI, 0.55–1.26, P = 0.377), whereas squamous cell carcinoma patients showed a significant benefit (HR = 0.74, 95% CI, 0.61–0.90, P = 0.003). Given the correlation between NSCLC histology and smoking status,26 patients were categorized into three cohorts: never smokers, former smokers, and current smokers. As shown in Fig. 3F, patients with a history of smoking had significant better OS outcomes in the vaccines group compared to control group. Age did not affect survival outcomes of vaccine therapy (Fig. 3G). Notably, in three trials17,21,22 that evaluated the same cancer vaccine (L-BLP25), pooled analysis demonstrated a significant improvement in OS for the vaccines group compared to control group (Fig. 3H, HR = 0.87, 95% CI, 0.77–0.99, P = 0.034). In summary, subgroup analyses revealed that ECOG PS, response to first-line therapy, tumor stage, tumor histology, and smoking history were significantly associated with OS in vaccine therapy after first-line therapy. These also observed in the subgroup analyses for first-line maintenance therapy (Table S4).
Fig. 3.
Subgroup meta-analysis of survival outcomes. (A) ECOG PS, (B) tumor stage, (C) first-line therapy, (D) response to first-line therapy, (E) tumor histology, (F) smoking history, (G) age, (H) L-BLP25 vaccine.
Exploratory analysis of predictive biomarker for vaccination clinical outcomes
The efficacy of cancer vaccines is closely tied to their immunogenicity to elicit robust immune responses induced in vivo.27, 28, 29 We exploratively analyzed the potential of vaccine-induced immune responses as predictive biomarker for vaccine efficacy in post-first-line therapy. Based on data from four trials with 163 patients in the vaccines group, the pooled analysis demonstrated that immune responders had a significant better OS than non-responders (Fig. 4A, HR = 0.34, 95% CI, 0.24–0.50, P < 0.001). The funnel plot (Figure S2E) and Egger regression asymmetry plots (Figure S2F, Egger's test, P = 0.034) showed some publication bias, but the sensitivity analysis confirmed the stability and reliability of the findings (Fig. 4B). Furthermore, this improved OS in immune responders also observed in the pooled analysis of first-line maintenance therapy (Figure S6C). This exploratory analysis indicates that immune response could serve as a predictive biomarker for vaccines efficacy during either post-first-line therapy or first-line maintenance therapy.
Fig. 4.
Exploratory analysis of correction between immune response and OS. (A) Forest plot and (B) Sensitivity analysis.
Discussion
With the accelerated development of mRNA vaccines in recent years, therapeutic cancer vaccines have emerged as an extremely promising immunotherapy for the treatment of NSCLC.9,30 However, the clinical efficacy and safety of cancer vaccines in advanced NSCLC patients after first-line therapy have not yet been comprehensively assessed. To the best of our knowledge, this is the first systematic review and meta-analysis focused specifically on the use of cancer vaccines in previously treated NSCLC patients. The findings hold important implications, providing high-level evidence-based guidance for the application of cancer vaccines as post-first-line therapy or first-line maintenance therapy in advanced NSCLC.
Our analysis included 11 studies involving 3228 patients, and the Cochrane assessment indicated a low risk of bias across these studies. Due to the variety of cancer vaccine types and formulations, there are currently no definitive conclusions regarding their overall efficacy in advanced NSCLC. With this background, pooled analyses based on different studies are indeed warranted for comprehensively assessing the efficacy of cancer vaccines in advanced patients after first-line therapy. All the 11 studies (10 RCTs) included in this meta-analysis reported OS as a primary endpoint, we pooled OS data from 10 trials15, 16, 17,19, 20, 21, 22, 23, 24, 25 for analysis as one18 of 11 studies provided outdated outcomes. The pooled analysis demonstrated a significant clinical benefit from vaccine therapy when as post-first-line therapy. Notably, a trial by Alfonso et al.15 showcased the most substantial benefit, where 87 patients in the vaccines group received five vaccination every two weeks followed by revaccination every four weeks, with a reported HR was 0.63 (95% CI, 0.46–0.87). In trials involving cancer vaccines for patients with advanced disease, OS is typically the preferred endpoint over PFS, given the difficulty in achieving progression-free outcomes in most advanced patients. However, four15,19,21,24 of 11 studies compared the PFS between the vaccines and control groups, showing better but not statistically significant PFS in vaccines group. First-line maintenance therapy is part of post-first-line therapy, the pooled results of OS15,17,19, 20, 21, 22, 23 and PFS15,19,21 in first-line maintenance therapy were consistent with post-first-line therapy. In addition to the different vaccines, pooled analysis of the same vaccine L-BLP25 also show clinical benefit. Comprehensively considering the pooled results of both OS and PFS, cancer vaccines is effective either as post-first-line therapy or first-line maintenance therapy for patients with advanced NSCLC.
After determining the overall efficacy of cancer vaccines, we further analyze the factors influencing the efficacy by performing a subgroup analysis on OS for post-first-line therapy. Beyond their inherent antitumor mechanisms, chemotherapy and radiotherapy are closely related to immune modulation.31 Chemotherapeutic agents and radiotherapy can induce immunogenic cell death, releasing tumor antigens that complement cancer vaccines and facilitate the cancer–immunity cycle.32,33 Our analysis found chemotherapy subgroup showed significant improved survival in vaccines group, while chemoradiotherapy improved without significance. The vast majority of included trials were implemented before immunotherapy became the standard practice for advanced NSCLC, thus those patients received first-line chemotherapy with or without radiotherapy. Subgroup analysis was not possible for first-line chemo-immunotherapy because there was only one study. Since none of the included studies enrolled patients who received radiotherapy alone, pooled results for vaccine therapy following radiotherapy were unavailable. Additionally, stage IV patients treated with vaccines experienced better survival benefits compared to stage III patients, while those with poor performance status did not benefit. The efficacy of first-line therapy impacts the success of subsequent vaccination, in patients achieving objective responses, cancer vaccines did not further improve survival, except in those with SD. Notably, smoking history and tumor histology significantly influenced survival after vaccination. Squamous cell carcinoma is strongly associated with smoking, our findings revealed that squamous cell carcinoma patients and smokers have better survival than adenocarcinoma and non-smokers. Previous meta-analyses have demonstrated that immune checkpoint inhibitors significantly improved survival in NSCLC patients with a history of smoking compared to non-smokers.34, 35, 36 This aligns with our findings regarding vaccine therapy. Based on our results, squamous cell carcinoma may be more responsive to cancer vaccines and could be a potential independent prognostic factor, which are able to direct the patient selection in clinical application. Further research is warranted to explore the tumor immune microenvironment in smokers and squamous cell carcinoma patients to better understand the mechanism underlying therapeutic benefits. For first-line maintenance therapy, the results of subgroup analyses were generally consistent with those for post-first-line therapy. In the performance status subgroup, only ECOG = 1 showed a significant better survival, and in smoking history subgroup only current smokers. This difference may be related to variation in study size.
Safety and tolerability are critical considerations in the clinical translation of cancer therapeutics. In our analyses, there were no significant differences between the vaccine and control groups in terms of AEs, SAEs, TRAEs and TRSAEs either in post-first-line therapy or first-line maintenance therapy. Notably, the incidence of injection site reactions and skin symptoms was significantly higher in vaccination group due to the immunogenicity of vaccines, although these reactions were transient. Overall, cancer vaccines are safe and well-tolerated in the treatment of advanced NSCLC.
Although there is currently no established predictive biomarker for the clinical effect of cancer vaccines, most studies have analyzed vaccine-induced immune responses (such as antibody titers and cellular immune responses) as potential biomarker. Our exploratory analyses for both post-first-line therapy and first-line maintenance therapy support the notion that immune response serves as a predictive biomarker for monitoring the efficacy of cancer vaccines, which could guide treatment decision during treatment. In summary, this systematic review and meta-analysis based on RCTs demonstrated both the safety and clinical efficacy of cancer vaccines in advanced NSCLC after first-line therapy, providing high-level evidence-based guidance for clinical practice.
This study has several limitations. First, only 11 RCTs were included, which may limit the statistical power and generalizability of the findings. Second, the vaccines used in the included studies were diverse, making it difficult to conduct pooled analyses of each cancer vaccines separately. Third, in the exploratory analysis of biomarkers, the limited data prevented us from performing subgroup analyses to differentiate between humoral and cellular immune responses, making it challenging to identify which immune response type may serve as the dominant predictive marker.
In conclusion, this systematic review and meta-analysis of 11 RCTs demonstrated that cancer vaccines significantly prolong survival and exhibit a favorable safety profile in patients with advanced NSCLC after first-line treatment, suggesting their potential as an effective post-first-line therapy or first-line maintenance therapy. Our results indicate that patients with squamous cell carcinoma may derive greater benefit from cancer vaccines, and vaccine-induced immune responses could serve as predictive biomarker for vaccines effect. These findings provide important evidence and guidance for the clinical application of cancer vaccines, which hold promise for further improving survival outcomes in advanced NSCLC patients.
Contributors
Concept and design: Shaoyi Chen, Jun Wang, Mantang Qiu; Acquisition, analysis, or interpretation of data: Shaoyi Chen, Peiyu Wang, Zewen Sun; Drafting of the manuscript: Shaoyi Chen, Mantang Qiu; Critical review of the manuscript for important intellectual content: Shaoyi Chen, Peiyu Wang, Kezhong Chen, Jun Wang, Mantang Qiu; Statistical analysis: Shaoyi Chen, Peiyu Wang; Obtained funding: Jun Wang, Mantang Qiu. Administrative, technical, or material support: Kezhong Chen, Yun Li, Fan Yang; Supervision: Mantang Qiu, Jun Wang. Shaoyi Chen, Peiyu Wang, and Mantang Qiu had accessed and verified the underlying data.
Data sharing statement
All data analyzed during this study are included in this article and its supplementary material.
Declaration of interests
We declare no competing interests.
Acknowledgements
This study was supported by Beijing Nova Program (20240484580 and 20230484314), National Key R&D Program of China (2023YFF0723500), Research Unit of Intelligence Diagnosis and Treatment in Early Non-small Cell Lung Cancer, Chinese Academy of Medical Sciences (2021RU002), National Natural Science Foundation of China (82173386), Peking University People's Hospital Research and Development Founds (RZ2022-04).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103369.
Contributor Information
Peiyu Wang, Email: 18339979852@163.com.
Kezhong Chen, Email: chenkezhong@pkuph.edu.cn.
Jun Wang, Email: jwangmd@pku.edu.cn.
Mantang Qiu, Email: qiumantang@163.com.
Appendix A. Supplementary data
References
- 1.Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3.Miller K.D., Nogueira L., Mariotto A.B., et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 4.Jia Q., Wu W., Wang Y., et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. 2018;9(1):5361. doi: 10.1038/s41467-018-07767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M., Cao J.X., Liu Y.S., et al. Evaluation of tumour vaccine immunotherapy for the treatment of advanced non-small cell lung cancer: a systematic meta-analysis. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding M., Yang J. Therapeutic vaccination for non-small-cell lung cancer: a meta-analysis. Med Oncol. 2014;31(4):928. doi: 10.1007/s12032-014-0928-1. [DOI] [PubMed] [Google Scholar]
- 7.Awad M.M., Govindan R., Balogh K.N., et al. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell. 2022;40(9):1010–1026.e11. doi: 10.1016/j.ccell.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Quoix E., Lena H., Losonczy G., et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212–223. doi: 10.1016/S1470-2045(15)00483-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen S., Cheng S., Cai J., et al. The current therapeutic cancer vaccines landscape in non-small cell lung cancer. Int J Cancer. 2024;155(11):1909–1927. doi: 10.1002/ijc.35088. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfonso S., Valdés-Zayas A., Santiesteban E.R., et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2014;20(14):3660–3671. doi: 10.1158/1078-0432.CCR-13-1674. [DOI] [PubMed] [Google Scholar]
- 16.Besse B., Felip E., Garcia Campelo R., et al. Randomized open-label controlled study of cancer vaccine OSE2101 versus chemotherapy in HLA-A2-positive patients with advanced non-small-cell lung cancer with resistance to immunotherapy: ATALANTE-1. Ann Oncol. 2023;34(10):920–933. doi: 10.1016/j.annonc.2023.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Butts C., Maksymiuk A., Goss G., et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137(9):1337–1342. doi: 10.1007/s00432-011-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butts C., Socinski M.A., Mitchell P.L., et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 19.Giaccone G., Bazhenova L.A., Nemunaitis J., et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 2015;51(16):2321–2329. doi: 10.1016/j.ejca.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Gridelli C., Ciuleanu T., Domine M., et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in patients with stage IV non-small cell lung cancer: final results of a randomised phase 2 clinical trial. Br J Cancer. 2020;122(10):1461–1466. doi: 10.1038/s41416-020-0785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katakami N., Hida T., Nokihara H., et al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer. 2017;105:23–30. doi: 10.1016/j.lungcan.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell P., Thatcher N., Socinski M.A., et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. 2015;26(6):1134–1142. doi: 10.1093/annonc/mdv104. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez P.C., Popa X., Martínez O., et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22(15):3782–3790. doi: 10.1158/1078-0432.CCR-15-0855. [DOI] [PubMed] [Google Scholar]
- 24.Takayama K., Sugawara S., Saijo Y., et al. Randomized phase II study of docetaxel plus personalized peptide vaccination versus docetaxel plus placebo for patients with previously treated advanced wild type EGFR non-small-cell lung cancer. J Immunol Res. 2016;2016 doi: 10.1155/2016/1745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neninger Vinageras E., de la Torre A., Osorio Rodríguez M., et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(9):1452–1458. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 26.Jha S.K., De Rubis G., Devkota S.R., et al. Cellular senescence in lung cancer: molecular mechanisms and therapeutic interventions. Ageing Res Rev. 2024;97 doi: 10.1016/j.arr.2024.102315. [DOI] [PubMed] [Google Scholar]
- 27.Jhunjhunwala S., Hammer C., Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 28.Blankenstein T., Coulie P.G., Gilboa E., Jaffee E.M. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12(4):307–313. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Q., Long Y., Yang Y., et al. Development and clinical applications of therapeutic cancer vaccines with individualized and shared Neoantigens. Vaccines (Basel) 2024;12(7):717. doi: 10.3390/vaccines12070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su S., Chen F., Xu M., Liu B., Wang L. Recent advances in neoantigen vaccines for treating non-small cell lung cancer. Thorac Cancer. 2023;14(34):3361–3368. doi: 10.1111/1759-7714.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galluzzi L., Guilbaud E., Schmidt D., Kroemer G., Marincola F.M. Targeting immunogenic cell stress and death for cancer therapy. Nat Rev Drug Discov. 2024;23(6):445–460. doi: 10.1038/s41573-024-00920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y., Zhu Y., Wang K., et al. Activation of autophagy by in situ Zn(2+) chelation reaction for enhanced tumor chemoimmunotherapy. Bioact Mater. 2023;29:116–131. doi: 10.1016/j.bioactmat.2023.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golden E.B., Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25(1):11–17. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Dai L., Jin B., Liu T., Chen J., Li G., Dang J. The effect of smoking status on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: a systematic review and meta-analysis. eClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S., Wang G., Xu Y., Cai S., Xiong J. Immunotherapeutic efficacy in NSCLC and patients' smoking status: a meta-analysis. J Clin Oncol. 2019;37(15_suppl):e20510. [Google Scholar]
- 36.Kim J.H., Kim H.S., Kim B.J. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget. 2017;8(54):93149–93155. doi: 10.18632/oncotarget.18703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.