Abstract
Introduction
Esophageal carcinosarcoma (ECS) is a rare malignant tumor with a polypoid configuration. It is biphasic and composed of epithelial carcinomatous and mesenchymal sarcomatous components.
Case presentation
We present a 74-year-old Persian woman with a known case of hypertension and chronic renal insufficiency who presented with dysphagia one month ago. On presentation she was oriented, with normal ranged Vital signs. The vital signs were within normal ranges. Her physical examination revealed pale conjunctiva. Gastrointestinal endoscopy showed a 29*25 mm ulcerative polypoid lesion in the distal part of the esophagus which several biopsies were taken. Microscopic examination revealed biphasic mesenchymal and epithelial malignant tumors compatible with carcinosarcoma.
Discussion
Dysphagia is the most common symptom of ECS. Immunohistochemistry is the diagnostic gold standard for ECS. The markers for the carcinomatous elements are CEA, EMA, pancreatin, chromogranin A, CD56, and synaptophysin, and for the sarcomatous elements, desmin, p63, vimentin, and smooth muscle/sarcomeric actin.
Conclusion
ECS is a biphasic tumor and the most effective treatment option for ECS is surgical resection.
Keywords: Carcinosarcoma, Esophagus, Tumor
1. Introduction
Esophageal carcinosarcoma (ECS) is a rare malignant tumor with an incidence of approximately 1.3 % of all esophageal neoplasms, although almost 90 % of esophageal tumors are squamous cell carcinomas or adenocarcinomas [1]. In 1904, Hansmann reported the first case of esophageal carcinosarcoma in the literature [2].
ECS is characterized by a large, bulky intraluminal polypoid growth pattern without esophagus wall deep infiltration [3]. The risk factors of ECS include smoking, alcohol consumption, and advanced age. The most common sites of ECS are the middle and lower esophageal sections of the esophagus [4]. Symptoms of ECS include dysphagia, chest pain, and weight loss [5]. ECS is a biphasic tumor composed of epithelial carcinomatous and mesenchymal sarcomatous components [6].
The gold standard diagnostic method for carcinosarcoma is immunohistochemistry. The markers of the carcinomatous component include CEA, EMA, pancreatin, chromogranin A, CD56, and synaptophysin, and for the sarcomatous elements are desmin, p63, vimentin and smooth muscle/sarcomeric actin [3]. The most effective treatment option for ECS is surgical resection [7]. This work has been reported in line with the SCARE criteria [8].
Here, we present a 74-year-old Persian woman with dysphagia one month ago. Gastrointestinal endoscopy revealed an esophageal polypoid lesion that histologically confirmed the diagnosis of carcinosarcoma.
2. Case presentation
We present a 74-year-old Persian woman with a known case of hypertension and chronic renal insufficiency who presented with dysphagia, anorexia, nausea, and non-bloody vomiting from one month ago. The dysphagia is progressive with solid food. There was an absence of bloody stool, melena, constipation, fever, weakness, and weight loss.
Her social and family history included no significant of note. Her social and family history included no significant of note. Her drug history is metoprolol due to hypertension.
On presentation, the patient was oriented and her vital signs were within normal limits. On physical examination, she had a pale conjunctiva. On chest examination, auscultation revealed unilateral fine crackles in the lungs and heart sounds were normal. On abdominal examination, the abdomen was soft, without organomegaly, tenderness or rebound tenderness. Shifting dullness and fluid wave tests were negative.
The patient was nil per os (NPO) and was continuously monitored by pulse oximetry and a cardiac monitoring device. Intravenous fluids, ondansetron, and pantoprazole were administered. Laboratory tests were requested for the patient, the results of which are shown in Table 1.
Table 1.
Laboratory findings.
| Test | Result (/μL) | Reference range (/μL) |
|---|---|---|
| WBC (white blood cells) ×10⁹/L | 6 | 4.0 - 11.0 |
| RBC /μL | 4,220,000 | 4,700,000 - 6,100,000 (Male) / 4,200,000 - 5,400,000 (Female) |
| HB (Hemoglobin) g/dL | 10 | 13 - 16 (Male) / 12 - 15 (Female) |
| HCT (Hematocrit) % | 35 | 38 - 50 (Male) / 35 - 45 (Female) |
| MCV (Mean Corpuscular Volume) fL | 84 | 80 - 100 |
| MCH (Mean Corpuscular Hemoglobin) pg | 25 | 27 - 33 |
| MCHC (Mean Corpuscular Hemoglobin Concentration) g/dL | 30 | 32 - 36 |
| RDW-CV % | 16 | 11.5 - 14.5 |
| PLT (Platelets) ×10³/μL | 218 | 150 - 450 |
| MPV (Mean Platelet Volume) fL | 10 | 7.4 - 10.4 |
| PDW (Platelet Distribution Width) % | 13 | 9 - 17 |
| PMN % | 75 | 50 - 70 |
| Lymph % | 11 | 20 - 40 |
| Mix % | 12 | 2 - 10 |
| Urea mg/dL | 84 | 11 - 55 |
| Cr (Creatinine) mg/dL | 2 | 0.6 - 1.3 |
| AST (Aspartate Aminotransferase) U/L | 15 | Up to 37 |
| ALT (Alanine Aminotransferase) U/L | 7 | Up to 41 |
| ALP (Alkaline Phosphatase) U/L | 139 | 100 - 360 |
| Bili T (Total Bilirubin) mg/dL | 0 | 0.3 - 1.2 |
| Bili D (Direct Bilirubin) mg/dL | 0 | 0.0 - 0.3 |
| PT (Prothrombin Time) sec | 12 | 12 - 14 |
| PTT (Partial Thromboplastin Time) sec | 25 | 25 - 45 |
| INR (International Normalized Ratio) | 1 | 0.8 - 1.2 |
| BS (Blood Sugar) mg/dL | 122 | <140 Normal, 140-199 Prediabetes, >=200 Diabetes |
| Potassium mmol/L | 3 | 3.5 - 5 |
| Sodium mmol/L | 142 | 135 - 145 |
| Magnesium mg/dL | 3 | 1.7 - 2.2 |
| Calcium mg/dL | 8 | 8.5 - 10.5 |
| Phosphorus mg/dL | 4 | 2.5 - 4.5 |
| Retic count % | 1 | <1 |
| TIBC (Total Iron-Binding Capacity) μg/dL | 236 | 250 - 450 |
| Fe (Iron) μg/dL | 54 | 60 - 170 |
| Ferritin ng/mL | 24 | 30 - 400 (Male) / 13 - 150 (Female) |
| Albumin g/dL | 3 | 3.5 - 5.2 |
We requested abdominopelvic ultrasonography, which showed a normal-sized liver with mild heterogeneous parenchymal echogenicity and periportal cough. Intrahepatic bile duct without dilatation. The CBD diameter was 8.5 mm in the Porto-hepatic region. The gallbladder, spleen, pancreas, both kidneys and urinary bladder were normal.
Because of the dysphagia, we also requested a gastrointestinal endoscopy under anesthesiologic care; the finding was a 29*25 mm ulcerative polypoid lesion in the distal part of the esophagus, from which several biopsies were taken for histopathologic examination. The mucosa of the stomach was erythematous and congested. The mucosa of the bulb and the second part of the duodenum (D2) were normal.
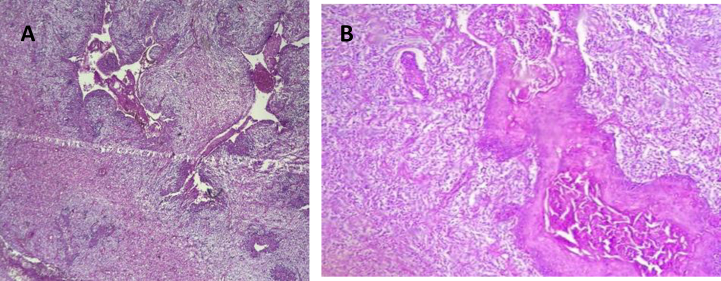
H&E staining and immunohistochemistry were performed. Microscopic examination of the polypoid lesion of the esophagus revealed superficial ulceration with atypical mesenchymal cells with fascicular pattern admixed with epithelioid squamous cells with keratin production and gland formation with high mitotic rate (more than 5 per HPF) and foci of necrosis. Biphasic mesenchymal and epithelial malignant tumor compatible with carcinosarcoma (Fig. 1).
Fig. 1.
A and B shows atypical mesenchymal cells with fascicular pattern admixed with epithelioid squamous cells with keratin production, high mitotic rate (more than 5 per HPF) and foci of necrosis compatible with carcinosarcoma.
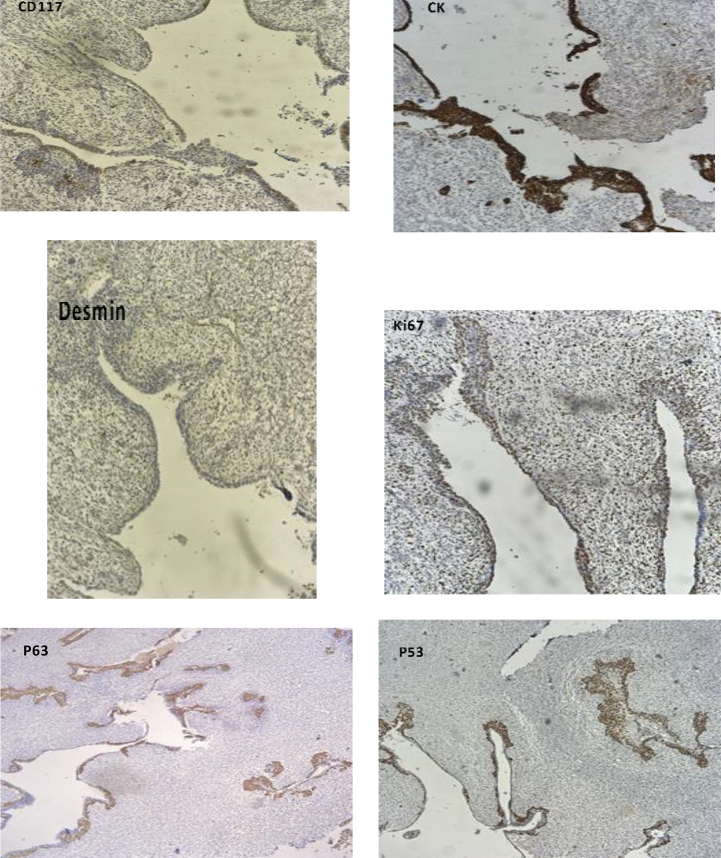
On immunohistochemical analysis, the atypical mesenchymal cells expressed p53 (70 %), p63 (10 %), and Ki-67 (40 %) and were negative for CD117, desmin, and cytokeratin. The atypical epithelial cells were positive for CK (50 %), p53 (40 %), p63 (90 %), and Ki-67 (40 %), while negative for desmin and CD117 (Fig. 2).
Fig. 2.
Immunohistochemical analysis shows the The mesenchymal atypical cells expressed p53 (70 %), p63 (10 %), and Ki-67 (40 %) and were negative for CD117, desmin, and cytokeratin. The atypical epithelial component was positive for CK (50 %), p53 (40 %), p63 (90 %), and Ki-67 (40 %), while negative for desmin and CD117.
Due to the size of the lesion, a distal esophagectomy and esophagogastrostomy with midline laparotomy were performed. Postoperative follow-up was conducted over a six-month period. During this time, the patient recovered well, with gradual improvement in oral intake and weight gain. No signs of recurrence or major complications were observed in clinical examinations or imaging studies.
The patient reported being satisfied with the treatment outcome, especially with the improvement in swallowing and daily functioning. She also mentioned feeling supported throughout the treatment process and expressed hope that sharing her case might help others in similar situations. This work has been reported in line with the SCARE criteria [8].
3. Discussion
Esophageal carcinosarcoma (ECS) is a rare malignant tumor with an incidence of about 1.3 % of esophageal neoplasms [1].
In 1904, Hansmann reported the first case of esophageal carcinosarcoma in the literature [2].
.ECS has a polypoid configuration, usually consisting of invasive and/or in situ squamous cells on the surface of an exophytic tumor and sarcomatous spindle cells that form the body of the polypoid mass [1]. The pathogenesis of tumors is not clear [3].
In the esophagus, the most common tumor site was the mid-esophagus (56.9 %), followed by the lower esophagus (23.5 %) and upper esophagus (19.6 %) [4].
Further studies also showed that ECS most often occurs in middle-aged men with a history of smoking and/or alcohol abuse [4].
The most prominent and frequent symptom of ECS is dysphagia similar to squamous cell carcinoma along with chest pain, and weight loss [5]. Patients with ECS generally have better survival outcomes than patients with typical squamous cell carcinoma of the same size because Symptoms due to intraluminal growth appear early and can Detect diseases earlier [7].
ECS is a biphasic tumor composed of epithelial carcinomatous and mesenchymal sarcomatous components with the same stem cell origin; the sarcomatous component is thought to have a monoclonal epithelial origin with sarcomatous differentiation [6].
The gold standard diagnostic method for carcinosarcoma is immunohistochemistry. The markers of the carcinomatous component include CEA, EMA, pancreatin, chromogranin A, CD56, and synaptophysin, and for the sarcomatous elements are desmin, p63, vimentin and smooth muscle/sarcomeric actin [3]. In the present case, the malignant cells in the carcinoid component were positive for p63 (90 %), whereas the malignant cells in the sarcomatous component were negative for p63 (10 %). This biological feature suggests that the sarcomatous component may have arisen from the carcinoid component. p53 is a distinct marker for the confirmation of high-grade neoplastic changes of different tissue that is highly positive in malignant spindle cells.
Molecular analysis has shown that the two components of carcinosarcoma have genetic mutations, mainly involving the P53, cyclin D1, P16, MDM2 and CDK4 genes. P53 gene mutations in both the sarcomatous and carcinomatous components is seen, but the type of mutation differs)3). Due to facility limitations, we were unable to perform molecular tests.
While surgical resection remains the mainstay of treatment for esophageal carcinosarcoma, it is important to acknowledge that other therapeutic strategies may be appropriate depending on tumor characteristics. For instance, endoscopic resection has been reported in the literature as a feasible option for smaller, localized lesions. Additionally, in cases demonstrating aggressive features—such as high p53 or Ki67 expression—adjuvant chemoradiotherapy may be considered to improve disease control [1]. In the present case, given the extent of invasion and histopathological findings and large size tumor; 29*25 mm surgery alone was selected as the most suitable approach, and no further adjuvant therapy was pursued.
4. Conclusion
Esophageal carcinosarcoma is a rare biphasic neoplasm, typically presenting with dysphagia due to its intraluminal polypoid growth. Accurate diagnosis relies on histopathological and immunohistochemical analysis, and surgical resection remains the primary treatment modality.
Authors' contributions
M.R. Kalantari, M.Karimi and F. Ramezani participated in the conception and design of the study and wrote the manuscript and evaluated the patients. M.R. Kalantari, M. Karimi, F. Ramezani and F. Farshidi did the microscopic examination of the specimens and wrote the pathology reports. R. Mashoufi wrote the radiology reports. All authors reviewed the manuscript and approved the final manuscript.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
Ethical Committee approved the study under the ethical code IR.HUMS.REC. 1402.246 and the study conforms with the Helsinki Declaration's statements.
Funding
The study did not receive any funding.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Mahmoud Reza Kalantari, Email: kalantarim@mums.ac.ir.
Mohadeseh Karimi, Email: m.karimi@hums.ac.ir.
Ramin Mashoufi, Email: raminemrama@gmail.com.
Farzaneh Ramezani, Email: Farzanermm@gmail.com.
Availability of data and materials
The data sets used during the current study are available from the corresponding author on reasonable request.
References
- 1.Schizas D., Mastoraki A., Bagias G., Ioannidi M., Kanavidis P., Moris D.…Liakakos T. Carcinosarcomas of the esophagus: a systematic review of a rare nosologic entity. J. BUON. 2018;23(5):1432–1438. [PubMed] [Google Scholar]
- 2.Jain V., Varshney P., Aggarwal D., Soni S.C., Varshney V.K., Selvakumar B., Agarwal L. Carcinosarcoma of the esophagus—a diagnostic challenge. Ochsner J. 2023;23(3):243–247. doi: 10.31486/toj.23.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu F., Zou W.B., Li X.P., Xu Y.M., Qi X.F., Hu L.H.…Yao D.K. Multiple carcinosarcomas of the esophagus and stomach. Oncol. Lett. 2013;5(3):1017–1021. doi: 10.3892/ol.2012.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakovich G., Ouellette D., Beauchamp G. An unusual tumor of the esophagus. J. Thorac. Cardiovasc. Surg. 2010;139(4):e91–e93. doi: 10.1016/j.jtcvs.2008.11.075. [DOI] [PubMed] [Google Scholar]
- 5.Gaur D.S., Kishore S., Saini S., Pathak V.P. Carcinosarcoma of the esophagus. Singapore Med. J. 2008;49:e283–e285. [PubMed] [Google Scholar]
- 6.Yoshimoto T., Kobayashi S., Karnataka K., Kobayashi K., Nagata Y., Morita M.…Eguchi S. Preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil for locally advanced esophageal carcinosarcoma: a case report and review of the literature. Surg. Case Rep. 2018;4:1–7. doi: 10.1186/s40792-018-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida H., Fujishima F., Onodera Y., Konno-Kumagai T., Maruyama S., Okamoto H.…Sasano H. Esophageal carcinosarcoma with basaloid squamous cell carcinoma: a case report and review of the literature. Tohoku J. Exp. Med. 2019;249(4):255–263. doi: 10.1620/tjem.249.255. [DOI] [PubMed] [Google Scholar]
- 8.Kerwan A., Al-Jabir A., Mathew G., Sohrabi C., Rashid R., Franchi T., Nicola M., Agha M., Agha R.A. Revised surgical CAse REport (SCARE) guideline: an update for the age of artificial intelligence. Premier Journal of Science. 2025;10 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used during the current study are available from the corresponding author on reasonable request.