Abstract
Background
Management of pulmonary atresia with ventricular septal defect and ductal-dependent pulmonary circulation (PA/VSD/PDA) varies according to pulmonary artery morphology and institutional surgical strategy. We adopted a range of initial palliative surgical options for patients with PA/VSD/PDA and evaluated the effectiveness of our management strategy.
Methods
Twenty-five patients with PA/VSD/PDA were enrolled between May 2015 and July 2023. Patients with major aortopulmonary collateral arteries were excluded. The mean age at initial surgery was 14.9 ± 13.7 days, and the mean weight was 3.17 ± 0.35 kg. Twenty-two (88%) patients were neonates. Nineteen patients underwent initial palliative systemic-to-pulmonary shunt, while six underwent an initial definitive Rastelli operation depending on the main pulmonary artery morphology and branch pulmonary arteries size.
Results
One patient died of postoperative brain hemorrhage following the initial definitive Rastelli operation. The mean follow-up duration was 58.5 ± 28.4 months. During follow-up, one patient died suddenly two months after the initial central shunt procedure. All surviving patients with a shunt, except two, underwent biventricular repair: ten with the Rastelli operation and six with the transannular patch (TAP) procedure. The staged TAP group demonstrated a significantly larger freedom from reoperation rate than that of the initial Rastelli operation group (p = 0.022) and a significantly lower catheter-based reintervention rate than that of the other two management groups (p = 0.011).
Conclusions
A management strategy using an initial definitive Rastelli operation or systemic-to-pulmonary shunt based on main pulmonary artery development and branch pulmonary arteries size is safe and effective for PA/VSD/PDA treatment. The staged TAP procedure could be a viable option for patients with PA/VSD/PDA and a well-developed main pulmonary artery segment.
Trial registration
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-025-03555-y.
Keywords: Pulmonary atresia, Tetralogy of fallot, Ductal-dependent pulmonary circulation
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-025-03555-y.
Background
Management of pulmonary atresia with ventricular septal defect and ductal-dependent pulmonary circulation (PA/VSD/PDA) varies according to the morphology of the pulmonary arteries and the surgical strategy employed by each institution [1–5]. We have implemented various initial palliative surgical options for these complex cases and published articles detailing our institutional experiences and outcomes [6, 7]. Informed by these experiences and two preceding studies, we advocate for recommended surgical strategies, such as early complete repair and palliative shunt procedure followed by definitive biventricular repair using the Rastelli operation or transannular patch (TAP) procedure, depending on the development of the main pulmonary artery and branch pulmonary arteries size. In the present study, we report our institutional experience based on this rationale and provide insights into our management strategy for patients with PA/VSD/PDA.
Methods
Ethical statement
This study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 55-2024-043; April 15, 2024). The need for informed consent was waived due to the retrospective nature of the study.
Patients’ profiles
From May 2015 to July 2023, 27 consecutive patients with PA/VSD/PDA underwent surgical management at the Pusan National University Yangsan Hospital. Among these patients, one underwent initial right ventricle-to-pulmonary artery conduit formation and another a neonatal réparation à l’étage ventriculaire (REV)-type operation, each in the early period, resulting in their exclusion. Ultimately, 25 patients (11 males, 44%) were enrolled. Patients with significant major aortopulmonary collateral arteries (MAPCAs) were excluded. The clinical records of the patients were retrospectively reviewed for perioperative and follow-up data. The diameters of the left and right pulmonary arteries immediately before their respective upper lobe branches were assessed using computed tomography (CT). To determine the Nakata index, larger measurements were obtained by calculating the sum of the cross-sectional areas of the two pulmonary arteries and dividing them by the patient’s body surface area [8]. The mean age at the initial operation was 14.9 ± 13.7 (range, 2–61) days, and the mean weight was 3.17 ± 0.35 (range, 1.87–4.04) kg. Twenty-two (22/25, 88%) patients were neonates. Two patients presented with CATCH 22, and one presented with an imperforate anus. Three patients were born prematurely (< 37 weeks gestational age).
Surgical management strategy
Based on our previous experiences and two preceding studies [6, 7], we advocate for recommended surgical strategies, such as early complete and staged definitive repairs following palliative systemic-to-pulmonary shunt procedure. Patients with PA/VSD/PDA were categorized into two types based on main pulmonary artery morphology: those with sizable main pulmonary artery segments potentially exhibiting membranous pulmonary atresia, and those without main pulmonary artery segments [9]. We hypothesized that, in PA/VSD/PDA, a sizable main pulmonary artery segment could be promoted to grow following a shunt procedure. Accordingly, when the main pulmonary artery segment was sizable or the branch pulmonary arteries were small, an initial palliative systemic-to-pulmonary shunt procedure was implemented. In contrast, when the main pulmonary artery segment was absent but the branch pulmonary arteries were of sufficient size, a definitive neonatal Rastelli operation was performed. After the initial systemic-to-pulmonary shunt operation, a staged definitive biventricular repair was performed using a staged Rastelli operation or TAP procedure, depending on main pulmonary artery and infundibular development (see Additional File 1: Figure S1). A systemic-to-pulmonary shunt procedure with or without cardiopulmonary bypass (CPB) was performed in 19 patients, and an initial definitive Rastelli operation in six. Among the patients who underwent the initial palliative shunt operation, a right modified Blalock–Taussig shunt (RMBTS) using a 3.5-mm expanded polytetrafluoroethylene (ePTFE) tube graft via midline sternotomy was performed in 18 patients, and a central shunt using a 3.5-mm ePTFE tube graft was performed in one. One patient underwent RMBTS banding with a 3.5-mm ePTFE tube graft strip to prevent excessive pulmonary blood flow after the operation.
Staged definitive correction using the TAP procedure
When the main pulmonary artery segment and infundibular area showed satisfactory growth after the initial palliative shunt procedure, a staged definitive correction was performed using the TAP procedure. A vertical incision was made from the main pulmonary artery to the right ventricular infundibulum crossing the pulmonary annulus remnant (Figs. 1a and b). When a membranous pulmonary valve leaflet was present, we attempted to save as much as possible to function as a pulmonary valve. A small right ventriculotomy was performed, and the infundibular hypertrophied muscle bundles were resected, similar to the tetralogy of Fallot repair. The atretic pulmonary annulus back wall was augmented with interrupted sutures between the resected right ventricular muscle epicardium and the nearby incised main pulmonary artery (Figs. 1c and d). The right ventricular outflow tract (RVOT) size after the augmentation was checked with a Hegar dilator with a size z-score of 0, and a glutaraldehyde-treated autologous pericardium patch angioplasty was subsequently performed (Fig. 1e). Usually, after surgery, a mild pressure gradient (PG) remains in the RVOT.
Fig. 1.
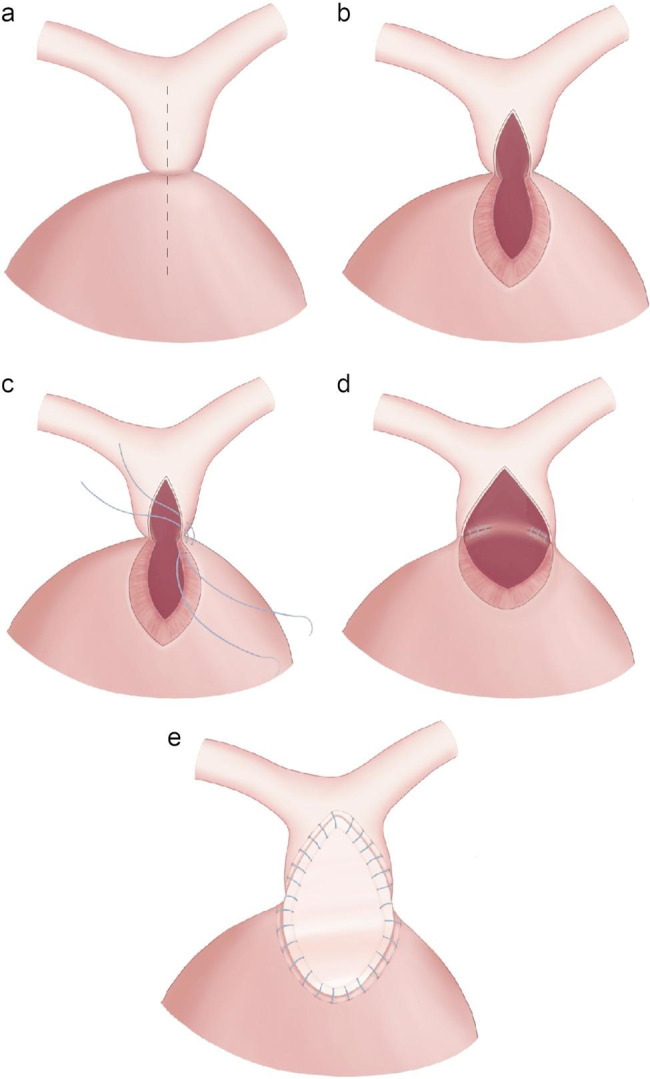
Transannular patch procedure. (a, b) A vertical incision was performed from the main pulmonary artery to the right ventricular infundibulum; (c, d) the atretic pulmonary annulus back wall was augmented with interrupted sutures; and (e) glutaraldehyde-treated autologous pericardium patch angioplasty completed the procedure
Statistical analysis
Data were collected and analyzed using Microsoft Excel 2021 (Microsoft Corp., Redmond, WA, USA), SPSS statistics 29.0 (IBM Corp., Armonk, NY, USA) and R software version 3.5.3 (R Foundation for Statistical Computing), and p-values < 0.05 were considered statistically significant. Descriptive statistics were calculated for each study population. Continuous variables are reported as mean ± standard deviation or median with interquartile range based on the distribution patterns, which were assessed using the Shapiro–Wilk test. Categorical variables are presented as counts and percentages. Continuous variables were compared using Student’s t-test, analysis of variance, or the Kruskal–Wallis test, as appropriate, and categorical variables were analyzed with the χ2 or Fisher’s exact test when expected cell counts were less than 5. The Kaplan–Meier curve was used to estimate survival rates and freedom from reoperation for RVOT management, and these were compared between groups using log-rank analysis.
Results
Mortality and morbidity
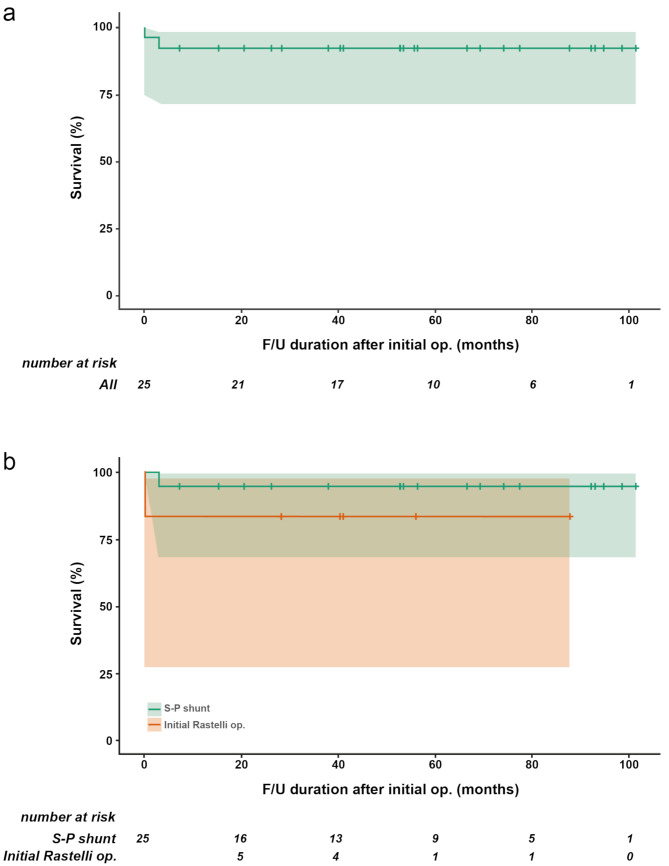
One in-hospital death (1/25, 4%) occurred due to brain hemorrhage two days after the initial definitive Rastelli operation. Four patients (4/25, 16%) required unplanned reoperation during hospital admission. All four patients were in the initial systemic-to-pulmonary shunt group, and reoperations included patent ductus arteriosus clipping due to pulmonary overcirculation (n = 1), RMBTS revision (n = 1), left pulmonary artery angioplasty (n = 1), and RMBTS revision plus left pulmonary artery angioplasty (n = 1). The mean follow-up duration was 58.5 ± 28.4 (range, 7.1–101.4) months. During the follow-up period, one sudden interstage death occurred two months after the initial central shunt procedure, which was potentially attributed to central shunt occlusion. Overall survival rates at 1, 5, and 8 years after the initial operation were all 92%. The survival rates for the two initial surgical treatment groups were 94.7% for shunt and 83.3% for initial Rastelli operation at 1 year and 94.7% for shunt and 83.3% for initial Rastelli operation at 8 years. No statistically significant difference was observed between the two groups (p = 0.349; Fig. 2).
Fig. 2.
Survival curves comparing the systemic-to-pulmonary shunt and initial Rastelli operation. (a) All patients and (b) each patient group. F/U, follow-up; op., operation; S-P, systemic-to-pulmonary
Patient characteristics in each surgical management group and postoperative details
All of the 18 surviving patients who underwent systemic-to-pulmonary shunt, except two, received biventricular repair, with 10 undergoing the Rastelli operation and six undergoing the TAP procedure. Among the remaining two patients, one continued to have a palliative right ventricle-to-pulmonary artery shunt without VSD closure due to small branch pulmonary arteries, and the other was waiting for a definitive operation. Figure 3 shows a flowchart summarizing the surgical management of each cohort.
Fig. 3.
Surgical management results of each cohort. d/t, due to; op., operation; PA/VSD/PDA, pulmonary atresia with ventricular septal defect and ductal-dependent pulmonary circulation; REV, réparation à l’étage ventriculaire; RMBT, right modified Blalock–Taussig; RV-to-PA, right ventricle-to-pulmonary artery; TAP, transannular patch
Overall, six patients (24%) underwent staged definitive repair using TAP, 10 (40%) underwent a staged Rastelli operation, and six (24%) underwent an initial Rastelli operation. The characteristics of the patients in the three treatment groups before the initial operation are listed in Additional File 2: Table S1. No differences were found in age, sex, weight, incidence of premature birth, or associated anomalies between groups. A non-significant trend toward a larger Nakata index was observed in the initial Rastelli operation group (p = 0.110; Fig. 4a). The main pulmonary artery size in the initial Rastelli operation group was significantly smaller than the other two treatment groups (p < 0.001). As shown in Fig. 4b and c, all patients undergoing the initial Rastelli operation had a right pulmonary artery size greater than 4 mm and a left pulmonary artery size greater than 3.5 mm, which were represented by a right pulmonary artery z-score greater than − 0.5 (see Additional File 1: Figure S2a) and a left pulmonary artery z-score greater than − 1.0 (see Additional File 1: Figure S2b). Moreover, the initial main pulmonary artery sizes in the staged TAP group were all greater than 3.5 mm (Fig. 4d), which corresponded with an initial main pulmonary artery z-score greater than − 7.5 (see Additional File 1: Figure S2c).
Fig. 4.
The initial Nakata index and pre-operative pulmonary artery sizes in each treatment group. (a) Nakata index; (b) right pulmonary artery size; (c) left pulmonary artery size, and (d) main pulmonary artery size. LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery; TAP, transannular patch
The postoperative details after definitive repair for each treatment group are summarized in Additional File 2: Table S2. The staged TAP group showed a significantly larger immediate postoperative RVOT PG (14.67 ± 5.50 mmHg) than the other two treatment groups (p = 0.006). Moreover, a higher grade of the last follow-up pulmonary regurgitation (PR) was noticed in the staged TAP group (p = 0.001). Other parameters, including follow-up duration after the definitive operation, last follow-up RVOT PG, and last follow-up right ventricular dilation on echocardiography, did not differ among the three treatment groups. The right ventricle-to-pulmonary artery conduit for the Rastelli operation was selected with our homemade ePTFE tricuspid valved conduit [10], bicuspid valved conduit [11], or monocuspid valved conduit [12]. The conduits used in the staged Rastelli operation were larger than those used in the initial Rastelli operation.
Elective reoperations and catheter-based interventions after definitive operation
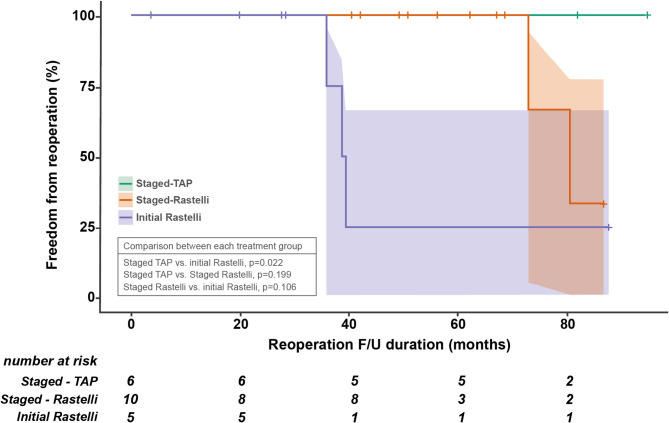
Overall, 21 patients achieved biventricular repair and were discharged from the hospital (details in Additional File 2: Table S2). Among these patients, elective reoperations after definitive repair were performed in six patients (6/21, 28.6%): two patients with right ventricle-to-pulmonary artery conduit change in the staged Rastelli operation, three patients with right ventricle-to-pulmonary artery conduit change and one patient with tracheoplasty in the initial Rastelli operation. Kaplan–Meier curves displaying RVOT reoperation after definitive repair are shown in Fig. 5. The staged TAP group showed a significantly larger freedom from reoperation rate than the initial Rastelli operation group (p = 0.022). Catheter-based interventions were performed in one patient (1/6, 16.7%; right pulmonary artery balloon angioplasty) in the staged TAP group, nine (9/10, 90%; RVOT balloon valvuloplasty in five, left pulmonary artery balloon angioplasty in four, and right pulmonary artery and left pulmonary artery balloon angioplasty in two) in the staged Rastelli operation group, and four (4/5, 80%; RVOT balloon valvuloplasty in three, left pulmonary artery balloon angioplasty in two, and right pulmonary artery and left pulmonary artery balloon angioplasty in two) in the initial Rastelli operation group. The most common catheter-based intervention was branch pulmonary artery balloon angioplasty. The staged TAP group showed a significantly lower catheter-based intervention rate than the other two management groups (p = 0.011).
Fig. 5.
Freedom from right ventricular outflow tract reoperation curve. F/U, follow-up; TAP, transannular patch
Pulmonary artery growth after initial palliative shunt procedure
Pulmonary artery measurements were evaluated using CT before the initial systemic-to-pulmonary shunt procedure and before the definitive operation. The mean Nakata indices were 135.20 ± 38.83 (range, 64.73–207.27) before systemic-to-pulmonary shunt procedure and 180.42 ± 55.37 (range, 95.28–273.63) before definitive operation for the mean interstage period of 8.36 ± 3.26 (range, 1.58–12.92) months. The mean Nakata index score before the definitive operation was significantly greater than that before the previous shunt procedure (p = 0.015; see Additional File 1: Figure S3a). The mean main pulmonary artery sizes were 3.69 ± 0.72 mm before the systemic-to-pulmonary shunt procedure and 7.06 ± 2.07 mm before definitive operation for patients with a visible main pulmonary artery before the shunt procedure. The mean main pulmonary artery before definitive operation was significantly larger than that before the shunt procedure (p < 0.001; see Additional File 1: Figure S3b). This growth pattern was maintained in the main pulmonary artery z-score changes (p = 0.002; see Additional File 1: Figure S2c).
Cardiothoracic ratio changes in surgical treatment groups
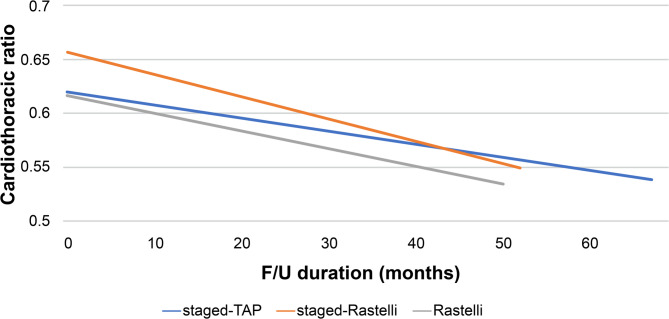
We investigated time-related cardiothoracic ratio changes in each surgical management group. The mean cardiothoracic ratios after definitive operation were 0.62 ± 0.03, 0.66 ± 0.04, and 0.62 ± 0.06 in the staged TAP, staged Rastelli, and initial Rastelli operation groups, respectively (p = 0.123). The mean cardiothoracic ratios at the last follow-up image were 0.54 ± 0.04, 0.55 ± 0.05, and 0.53 ± 0.05 in the staged TAP, staged Rastelli, and initial Rastelli operation groups, respectively, and did not significantly differ between these three groups (p = 0.805). All three surgical treatment groups showed decreased cardiothoracic ratios at the last follow-up image for the mean follow-up duration of 67.0 ± 22.7, 52.8 ± 29.2, and 50.6 ± 22.9 months in the staged TAP, staged Rastelli, and initial Rastelli operation groups, respectively (Fig. 6).
Fig. 6.
Time-related cardiothoracic ratio changes for each surgical management group. F/U, follow-up; TAP, transannular patch
Discussion
The management of PA/VSD/PDA presents considerable challenges owing to the complexity of the condition, the need for staged surgical interventions or early neonatal definitive operations, and variability in pulmonary artery morphology [1, 3–5, 13, 14]. Herein, we evaluated the management strategy employed at our center, using either palliative systemic-to-pulmonary shunt procedure or an initial definitive Rastelli operation in 25 patients with PA/VSD/PDA, excluding those with MAPCAs.
Our findings highlight the safety and efficacy of a tailored surgical approach based on the development of main and branch pulmonary arteries. For patients with a sizable main pulmonary artery segment or small-branch pulmonary arteries, a palliative systemic-to-pulmonary shunt was performed, followed by a staged Rastelli operation or a TAP procedure depending on the main pulmonary artery and infundibular development. In contrast, for patients without a main pulmonary artery segment and with sizable branch pulmonary arteries for the primary definitive Rastelli operation, an initial definitive Rastelli operation was performed. These strategies allow for personalized care, considering individual pulmonary artery development, and ensure optimal outcomes.
We used various surgical treatment options for these complex patients and previously published articles on our institutional experiences and outcomes [6, 7]. In a previous study [6], we conducted staged repairs on 44 patients with PA/VSD/PDA between June 1991 and October 2010, starting with a palliative shunt procedure including 31 neonates (70%). Unfortunately, ten patients (22.7%) died before definitive repair, and six deaths were attributed to shunt-related complications, indicating a high overall mortality rate. In that study, we recommended several surgical treatment strategies, including early complete repair, early second-stage definitive repair after the palliative shunt procedure to reduce interstage attrition, and alternative palliative right ventricle-to-pulmonary artery shunting. In 2011, we began utilizing the right ventricle-to-pulmonary artery shunt as an alternative palliative procedure for patients with PA/VSD/PDA to address the limitations of the systemic-to-pulmonary shunt procedure [7]. Between August 2011 and August 2015, we applied this approach to 13 patients. While a right ventricle-to-pulmonary artery shunt offers a viable option for initial palliation, significant complications, such as RVOT pseudoaneurysm, dehiscence of the shunt anastomosis site, shunt inflow stenosis, and persistent postoperative hypoxemia, warrant caution.
Informed by these experiences, we advocate for recommended surgical strategies, such as early definitive Rastelli operation and initial palliative systemic-to-pulmonary shunt procedure followed by staged Rastelli operation or TAP procedure, depending on the main pulmonary artery segment development and branch pulmonary arteries size. Although several centers perform primary one-stage repair for these challenging cases, many institutions still perform staged repair because of the technical ease of the operation and unavailability of a small extracardiac conduit for neonates [15, 16]. Small homograft conduits are currently unavailable in Korea. However, during the study period, we developed 10-mm ePTFE monocuspid valved conduit for neonates to overcome these problems [12]. Moreover, in the present study, we encountered four unplanned reoperations, all of which occurred in patients undergoing an initial palliative systemic-to-pulmonary shunt procedure. The main reasons for unplanned reoperation were related to the shunt operation itself or to left pulmonary artery problems. Therefore, for patients without a main pulmonary artery segment and with sizable branch pulmonary arteries requiring primary definitive repair, we performed an initial definitive Rastelli operation with reasonable results; sizable branch pulmonary arteries were defined as a right pulmonary artery size greater than 4 mm (z-score greater than − 0.5) and left pulmonary artery size greater than 3.5 mm (z-score greater than − 1.0) in this study (Fig. 4b and c; see Additional File 1: Figure S2a and S2b).
We hypothesized that, in patients with PA/VSD/PDA who had a sizable main pulmonary artery, the main pulmonary artery segment could grow following a systemic-to-pulmonary shunt procedure. Accordingly, definitive repair using the TAP procedure was performed in six patients (24%) utilizing the well-developed main pulmonary artery segment. In our study, the staged TAP procedure group had a main pulmonary artery greater than 3.5 mm (z-score greater than − 7.5) (Fig. 4d; see Additional File 1: Figure S2c), which demonstrated significant growth following the shunt procedure (see Additional File 1: Figure S3b and S3c). Therefore, an initial main pulmonary artery diameter of approximately ≥ 3.5 mm may be considered sufficient. However, as shown in Fig. 4d, three patients had a main pulmonary artery size greater than 3.5 mm in the staged Rastelli operation group. The reasons for selecting the staged Rastelli operation over the TAP procedure included abnormal coronary artery crossing the RVOT area in one patient and no infundibular development for the TAP procedure in two patients. When the staged TAP procedure is performed, a well-developed main pulmonary artery segment and preserved infundibular portion are essential.
A staged definitive surgery using TAP offers several advantages. During the TAP procedure, a relatively small right ventricle incision was made, and the infundibular hypertrophied muscle bundles were resected. This operation is similar to the tetralogy of Fallot repair. The RVOT size after the operation was the same as that required for a z-score “0” size Hegar dilator to pass through the RVOT. After the operation, a mild PG typically persists through RVOT, with an immediate postoperative RVOT PG of 14.67 ± 5.50 mmHg. We believe that this residual PG could play a role in protecting the RVOT from severe PR. This PG can function as a valve during the diastolic phase, and we were able to identify a decreased cardiothoracic ratio in the most recent follow-up image compared with the immediate postoperative finding (Fig. 6). Additionally, in the final follow-up echocardiography, none of the patients who underwent the staged TAP procedure exhibited more than mild right ventricular dilation (Additional File 2: Table S2). The staged TAP procedure group also showed significantly increased freedom from RVOT reoperation compared with the initial Rastelli operation. In the staged TAP procedure, we did not use a right ventricle-to-pulmonary artery conduit for definitive repair. Therefore, the risk of RVOT reoperation, such as right ventricle-to-pulmonary artery conduit changes, was decreased. Another advantage of the staged TAP procedure was the decreased catheter-based reintervention rate compared with the other two management groups. During the TAP procedure, we manipulated the branch pulmonary arteries less, which may have resulted in a decreased catheter-based intervention rate.
We identified that the mean Nakata index before the definitive operation significantly increased after the preceding shunt procedure for a mean interstage period of 8.36 ± 3.26 months. Among the 18 patients who survived and underwent an initial palliative systemic-to-pulmonary shunt procedure, 16 (88.9%) underwent definitive biventricular repair through a staged Rastelli operation or staged TAP procedure. Only two remaining patients were still receiving a palliative right ventricle-to-pulmonary artery shunt due to small-branch pulmonary arteries or were waiting for a definitive operation.
Despite these favorable results, this study had some limitations that should be acknowledged. The small sample size and the retrospective single-center design restricted the generalizability of our findings. Furthermore, surgical decision-making was based on the surgeon’s discretion, taking into account the size of the main pulmonary artery segment and branch pulmonary arteries. The follow-up duration was insufficient for evaluating long-term right ventricle function. Comprehensive right ventricular functional assessments, including cardiac magnetic resonance imaging, were not performed, except for follow-up cardiothoracic ratio and echocardiography data. A more thorough evaluation is planned in future studies. Additionally, patients who underwent staged TAP procedure exhibited a higher PR grade. Therefore, despite the favorable outcomes observed over five-year follow-up period (mean follow-up duration, 67.0 ± 22.7 months; range, 27.7–94.7 months), close monitoring of PR severity and right ventricular function remains essential. Lastly, this study could not assess the role of different treatment approaches, such as initial patent ductus arteriosus stenting, as an alternative mode of palliation for PA/VSD/PDA, despite recent reports suggesting their utility [17–19].
Conclusions
In conclusion, our management strategy for patients with PA/VSD/PDA, which includes an initial palliative systemic-to-pulmonary shunt procedure or an early definitive Rastelli operation based on main pulmonary artery development and branch pulmonary arteries size, is a safe and effective surgical treatment option. In particular, the use of staged TAP procedure offers a viable alternative for patients with well-developed main pulmonary artery segment. Further studies with larger cohorts and longer follow-up periods are needed to refine these surgical strategies and improve long-term outcomes in patients with complex congenital heart defects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Editage (www.editage.com) for English language editing and journal submission support.
Abbreviations
- CPB
Cardiopulmonary bypass
- CT
Computed tomography
- ePTFE
Expanded polytetrafluoroethylene
- MAPCA
Major aortopulmonary collateral artery
- PA/VSD/PDA
Pulmonary atresia with ventricular septal defect and ductal-dependent pulmonary circulation
- PG
Pressure gradient
- REV
Réparation à l’étage ventriculaire
- RMBTS
Right modified blalock–taussig shunt
- RVOT
Right ventricular outflow tract
- TAP
Transannular patch
Author contributions
JHL conceptualized the study, curated the data, conducted the investigation, and wrote the first draft of the manuscript. HKim conceptualized the study, developed the methodology, analyzed the data, acquired funding, provided resources, prepared the visual presentation of the data, wrote the initial draft of the manuscript, and edited the manuscript. KHC curated the data. SCS developed the methodology, supervised the study, and revised the manuscript for intellectual content. HDL supervised the study. HKo conducted the investigation and provided resources. JHB conducted the investigation and provided resources. THK conducted the investigation and provided resources. MSY curated and analyzed the data and supervised the study. All authors read and approved the final manuscript.
Funding
This study was supported by a 2025 research grant from Pusan National University Yangsan Hospital (to author Hyungtae Kim). The funder had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 55-2024-043; April 15, 2024). The need for informed consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
This manuscript was presented at the 71st Annual Meeting of the Southern Thoracic Surgical Association held last November 7–10, 2024, at Hyatt Regency Lost Pines Resort & Spa in Austin, TX, USA.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alsoufi B, Mori M, McCracken C, Williams E, Samai C, Kogon B, et al. Results of primary repair versus shunt palliation in ductal dependent infants with pulmonary Atresia and ventricular septal defect. Ann Thorac Surg. 2015;100:639–46. 10.1016/j.athoracsur.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 2.Bradley SM, Erdem CC, Hsia TY, Atz AM, Bandisode V, Ringewald JM. Right ventricle-to-pulmonary artery shunt: alternative palliation in infants with inadequate pulmonary blood flow prior to two-ventricle repair. Ann Thorac Surg. 2008;86:183–8. 10.1016/j.athoracsur.2008.03.047. discussion 188. [DOI] [PubMed] [Google Scholar]
- 3.Kim ER, Lee CH, Kim WH, Lim JH, Kim YJ, Min J, et al. Primary versus staged repair in neonates with pulmonary Atresia and ventricular septal defect. Ann Thorac Surg. 2021;112:825–30. 10.1016/j.athoracsur.2020.06.098 [DOI] [PubMed] [Google Scholar]
- 4.Lee WY, Kang SR, Im YM, Yun TJ. Surgical options for pulmonary Atresia with ventricular septal defect in neonates and young infants. Pediatr Cardiol. 2020;41:1012–20. 10.1007/s00246-020-02352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macalister SJ, Buratto E, Naimo PS, Ye XT, Fulkoski N, Weintraub RG, et al. Long-term outcomes of staged complete repair of pulmonary Atresia with ventricular septal defect. Ann Thorac Surg. 2023;115:445–51. 10.1016/j.athoracsur.2022.09.022 [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Sung SC, Chang YH, Jung W, Lee HD, Park JA, et al. Outcome of staged repair of tetralogy of fallot with pulmonary Atresia and a ductus-dependent pulmonary circulation: should primary repair be considered? Korean J Thorac Cardiovasc Surg. 2011;44:392–8. 10.5090/kjtcs.2011.44.6.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi KH, Sung SC, Kim H, Lee HD, Ban GH, Kim G, et al. Right ventricle-to-pulmonary artery shunt in pulmonary Atresia with a ventricular septal defect: A word of caution. Pediatr Cardiol. 2017;38:707–11. 10.1007/s00246-017-1570-4 [DOI] [PubMed] [Google Scholar]
- 8.Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, Nakazawa M, et al. A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. J Thorac Cardiovasc Surg. 1984;88:610–9. 10.1016/S0022-5223(19)38300-X [PubMed] [Google Scholar]
- 9.Castaneda AJ, Mayer JJ, Hanley F. Cardiac surgery of the neonate and infant. Philadelphia: W. B. Saunders Company 1994. [Google Scholar]
- 10.Choi KH, Sung SC, Kim H, Lee HD, Kim G, Ko H, et al. Simplified tricuspid polytetrafluoroethylene valved conduit: midterm results of multicenter study. Ann Thorac Surg. 2019;108:1228–33. 10.1016/j.athoracsur.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 11.Choi KH, Sung SC, Kim H, Lee HD, Kim G, Ko H. Late results of right ventricular outflow tract reconstruction with a bicuspid expanded polytetrafluoroethylene valved conduit. J Card Surg. 2018;33:36–40. 10.1111/jocs.13507 [DOI] [PubMed] [Google Scholar]
- 12.Choi KH, Kim H, Lee JH, Sung SC, Lee HD, Ko H, et al. A 10-mm monocusp expanded polytetrafluoroethylene valved conduit for right ventricular outflow tract reconstruction in neonates and young infants. World J Pediatr Congenit Heart Surg. 2025;16:530–6. 10.1177/21501351241306043 [DOI] [PubMed] [Google Scholar]
- 13.Farouk A, Zahka K, Siwik E, Erenberg F, Al-Khatib Y, Golden A, et al. Individualized approach to the surgical treatment of tetralogy of fallot with pulmonary Atresia. Cardiol Young. 2009;19:76–85. 10.1017/S1047951108003430 [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Kwak JG, Lee C. Primary repair of symptomatic neonates with tetralogy of fallot with or without pulmonary Atresia. Korean J Pediatr. 2014;57:19–25. 10.3345/kjp.2014.57.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elhedai H, Mohamed M, Mohammed SSS, Mustafa KHH, Seedahmed MHA, Mohamedahmed AYY. Comparison of staged repair versus single-stage complete repair for pulmonary Atresia with ventricular septal defect: A systematic review and meta-analysis. Indian J Thorac Cardiovasc Surg. 2022;38:5–16. 10.1007/s12055-021-01296-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meadows JJ, Bauser-Heaton H, Petit CJ, Goldstein BH, Qureshi AM, McCracken CE, et al. Comparison of treatment strategies for neonates with tetralogy of fallot and pulmonary Atresia. J Thorac Cardiovasc Surg. 2023;166:916–e256. 10.1016/j.jtcvs.2023.01.008 [DOI] [PubMed] [Google Scholar]
- 17.Glatz AC, Petit CJ, Goldstein BH, Kelleman MS, McCracken CE, McDonnell A, et al. Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: insights from the congenital catheterization research collaborative. Circulation. 2018;137:589–601. 10.1161/CIRCULATIONAHA.117.029987 [DOI] [PubMed] [Google Scholar]
- 18.Lemley BA, Wu L, Roberts AL, Shinohara RT, Quarshie WO, Qureshi AM, et al. Trends in ductus arteriosus stent versus Blalock-Taussig-Thomas shunt use and comparison of cost, length of stay, and short-term outcomes in neonates with ductal-dependent pulmonary blood flow: an observational study using the pediatric health information systems database. J Am Heart Assoc. 2023;12:e030575. 10.1161/JAHA.123.030575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong-Siegel JR, Petit CJ, Glatz AC. Pulmonary Atresia and ventricular septal defect without major aortopulmonary collateral arteries: diagnostic evaluation and the role of ductal stenting. World J Pediatr Congenit Heart Surg. 2025;16:197–202. 10.1177/21501351241269953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.