Abstract
Background
Evidence on the use of aspirin in women with chronic hypertension has indicated low effectiveness in preventing superimposed preeclampsia. The possible cause has not been well explored. Many researchers have attributed it to inadequacies in dosages, timing of therapy initiation, timing of intake and adherence. Investigations into these aspects have yielded conflicting results. Previous research has mainly focused on the dosage and gestational timing, yet the timing the drug is taken and adherence are also important aspects in evaluating drug efficacy. Therefore, an appraisal of the combination of these parameters was necessary.
Aim
This review aimed to explore the theoretical explanations for aspirin's low effectiveness in reducing the incidence of superimposed preeclampsia among women with chronic hypertension and propose therapy modifications to boost aspirin. We also aimed to synthesize evidence on the effectiveness of aspirin at different doses, gestation timing, timing of ingestion, and adherence in this population.
Methods
The review followed the Whittemore and Knafl procedure which involves problem identification, literature search, data extraction/evaluation, data analysis and presentation of findings. 17 studies were evaluated in the integrative literature review obtained from international databases including PubMed, Hinari and Google Scholar. We searched for articles that pertained to the effectiveness of aspirin regarding dosage, gestational age at initiation, timing of ingestion and adherence.
Results
On exploration of the possible explanation for the low effectiveness of aspirin on superimposed preeclampsia, it was noted that the pathogenesis of this condition in chronic hypertension has various pathways. However, aspirin targets only one of them, thereby leaving a gap for the other pathways to advance. After synthesizing the studies included in this review, it was noted that aspirin doses ≥ 150 mg were safer and more effective in the majority of the populations. Overall, there was an optimistic trend between higher doses (≥ 150 mg), initiated before 12 weeks of gestation, taken over 90% of the time at night/bedtime.
Conclusion
Owing to the variety of pathways through which preeclampsia develops in women with preexisting hypertension, this review recommends weight-adjusted multi-component pharmacological combinations such as aspirin and L-arginine, or aspirin and S-nitroglutathione to enhance aspirin’s effectiveness in this population. We also noted that the effectiveness of aspirin largely depends on early identification and control of hypertension, doses ≥ 150 mg, initiation of the therapy before the 12th week of gestation and taking the drug more than 90% of the time at night/bedtime. We recommend further research in this area, consistent follow-up of women prescribed aspirin and use of combined methods to measure adherence.
Keywords: Preeclampsia, Superimposed preeclampsia, Effectiveness aspirin and superimposed preeclampsia, Efficacy aspirin prophylaxis, Chronic hypertension during pregnancy
Why the review
What is known: low-dose aspirin has low efficacy in preventing preeclampsia that is superimposed on chronic hypertension.
What this study adds: this study uncovers the possible reasons for the low effectiveness of aspirin in women with chronic hypertension and suggests therapy modifications that can enhance its efficacy
Introduction
Preeclampsia (PE) is among the most dreaded pregnancy conditions as it leads to life-threatening problems for the mother and the unborn baby [56]. It is a progressive multisystem disease that usually develops in pregnant women after the 20th week of pregnancy, characterized by hypertension (diastolic blood pressure of 90 mmHg) and evidence of end-organ dysfunction [49]. Preeclampsia is the primary cause of maternal deaths worldwide and complicates up to 15% of all pregnancies each year [47, 71]. This is mainly due to complications such as pulmonary oedema, stroke and kidney failure among others [20, 44].
The cause of preeclampsia is unknown, and its pathophysiology is not well understood. However, evidence links its origin to a discrepancy in antigenic and antiangiogenic proteins, which results from inadequate trophoblastic invasion [8]. This leads to systemic inflammation, maternal endothelial damage, accelerated platelet adhesion, increased thrombotic events and placental ischemia [60].
No particular treatment has been identified for this condition except delivery of the baby and the placenta regardless of the gestational age [9]. However, aspirin (acetylsalicylic acid) at low doses (below 300 mg) has proved to suppress the synthesis of thromboxane which is the main culprit in causing vasoconstriction and platelet aggregation seen in preeclampsia [60]. Nonetheless, some aspirin-treated women still develop preeclampsia, also referred to as superimposed preeclampsia (SIP). The women in this category mainly include those with chronic hypertension, pre-existing diabetes, multiple pregnancies, and obesity [19, 21, 39, 45]. There is still an unmet need for these women. More so, the reasons this drug acts well in some individuals but not in others remain unclear.
Chronic hypertension is the most common high-level risk factor for preeclampsia among those named to be less responsive to aspirin [36, 58]. It is postulated that half of the women in this category develop SIP [29]. Moreover, SIP is not only associated with early-onset preeclampsia, but also severe forms of preeclampsia [13, 15, 36]. Therefore, this article will focus on preeclampsia that is superimposed on pre-existing hypertension and also provide some narratives on the possible reasons this drug does not give the desired effects in chronically hypertensive women.
Possible reasons for the low effectiveness of aspirin in SIP
Although several studies have been done in this area, there are no clear explanations on what contributes to the low efficacy of aspirin in chronically hypertensive women. Some theories attribute it to the prolonged damage on the maternal endothelial cells and blood vessels caused by persistent high blood pressure. In which case, chronic hypertension is said to be a precursor for abnormal placental embedment and development in the first trimester of pregnancy [1, 6, 70]. Chronic hypertension also causes inflammation of the endometrium before pregnancy, thereby changing the pattern of implantation and placentation [6]. However, the risk has been found to correspond with the chronicity of hypertension before a woman begins taking antihypertensive therapy. In settings where blood pressure is regularly checked and controlled, the risk is approximately six times greater than that of normotensive women [36], whilst it is 27 times higher in settings where hypertension is not controlled early [23, 73]. This creates a critical need for hypertension screening, diagnosis and treatment. Perhaps, free medical camps at worship places and public events could help reduce this gap, especially in low-resource settings where people are less likely to seek screening services.
The hypoxic environment in the placenta resulting from suboptimal placental invasion and pre-existing hypertension also increases the production of hydroxyeicosatetraenoic acids (20-HETEs). These metabolites of arachidonic acid are progressively produced in preeclampsia. They potentially mediate inflammation, induce vasoconstriction (hypertension) and enhance vascular dysfunction (features of preeclampsia). On the other hand, aspirin is not known to inhibit this process nor affect these metabolites [52]. However, the synthesis of 20-HETEs is invariably inhibited by nitric oxide [50]. Nitric oxide has antigenic and vasculogenic properties which facilitate cytotrophoblast endovascular invasion and placental development [34]. It also prevents inflammation, inhibits platelet aggregation within the endothelium, and plays an important role in the physiological vascular adaptations of normal pregnancy [2, 42]. In context, it decreases the vasopressor responsiveness while increasing the uteroplacental blood flow [42]. However, under stressful conditions, such as those in preeclampsia, nitric oxide synthase is redirected to produce superoxide anions [53]. The superoxide anions further degrade the available nitric oxide, resulting in proteinuria and hypertension (preeclampsia) [36, 48]. Aspirin is not known to enhance nitric oxide production, nor inhibit superoxide anion production.
Although inconclusive, studies which have used the nitric oxide precursor (L-arginine) or nitric oxide donors for preeclampsia prevention have shown promising outcomes. Two systematic reviews of 14 randomized controlled trials comprising 1,800 women evaluated L-arginine. The results indicated that the drug lowered blood pressure among the chronically hypertensive women and also reduced the incidence of preeclampsia among the high-risk women [17, 24]. Several other studies have also reported similar results [10, 37, 43, 46]. Most of these studies further noted that some women with pre-existing hypertension who were given L-arginine did not require other antihypertensive drugs. These studies used varying methodologies and sample sizes in different populations, and they all agreed that L-arginine reduced high blood pressure and the incidence of preeclampsia. Therefore, a combination of aspirin and L-arginine could produce better outcomes in pregnant women with chronic high blood pressure. Given the high safety record of L-arginine [43], it might also be a better substitute for antihypertensive medications in pregnancy since most of these drugs are contraindicated in pregnancy due to the possibility of fetotoxicity [41].
Some other theories attribute this SIP to the persistent renal hypoperfusion in chronic hypertension, which activates the renin–angiotensin–aldosterone system [78]. This system increases blood pressure, activates platelet activity, and facilitates endothelial dysfunction. This, creates another pathway for SIP besides those mentioned earlier. However, aspirin has not demonstrated the potential to inhibit the renin–angiotensin–aldosterone system effectively. Nonetheless, some nitric oxide donors, such as S-glutathione, have shown positive regulation of the renin-angiotensin system. S-nitrosoglutathione effectively reduces the production of angiotensin II, the hormone that predominantly increases blood pressure in chronic hypertension [78]. This drug enhances endothelial function, lowers blood pressure, prevents platelet activation, and improves uteroplacental blood flow. However, glutathione (a naturally occurring antioxidant in the body) is always abnormally reduced in women with preeclampsia [57]. Probably, exogenous glutathione could enhance the effects of aspirin in women with pre-existing hypertension.
Methods
This review was done using Whittemore and Knafl’s [75] method. This method is widely used in integrative reviews. It involves five stages, which include problem identification, literature search, data extraction/evaluation, data analysis and presentation of findings.
Stage 1: Problem identification
This study attempted to answer the following questions:
What could be the possible reasons for the low effectiveness of aspirin in preventing SIP among women with chronic hypertension?
Which other drugs can enhance the efficacy of aspirin in preventing SIP among women with chronic hypertension?
What is the available evidence on the effectiveness of aspirin in preventing SIP among women with chronic hypertension?
Stage 2: Literature search
An online literature search was conducted from international databases including PubMed, Hinari and Google Scholar. We searched for articles that pertained to the effectiveness of aspirin regarding dosage, gestational age at initiation, timing of ingestion and adherence. We used the Boolean operators “OR” and “AND” to combine the search terms; preeclampsia* OR pregnancy-induced hypertension*, superimposed* preeclampsia on chronic* OR pre-existing hypertension, (effectiveness OR efficacy aspirin* OR low-dose aspirin*) AND (superimposed preeclampsia), aspirin prophylaxis* OR therapy* OR prevention during pregnancy, chronic* OR pre-existing hypertension during pregnancy*, (aspirin dosage* OR dose) AND (preeclampsia OR superimposed preeclampsia), aspirin OR low-dose aspirin initiation* OR administration* in pregnancy, (low-dose aspirin OR aspirin) AND (time of ingestion* or time taken), (aspirin OR low-dose aspirin) AND (adherence* OR compliance OR acceptance). All potential articles were imported into the EndNote software to identify and remove duplicate studies. We also manually searched cross-references of the relevant studies for any other eligible studies.
Stage 3: Data extraction
Inclusion and exclusion criteria
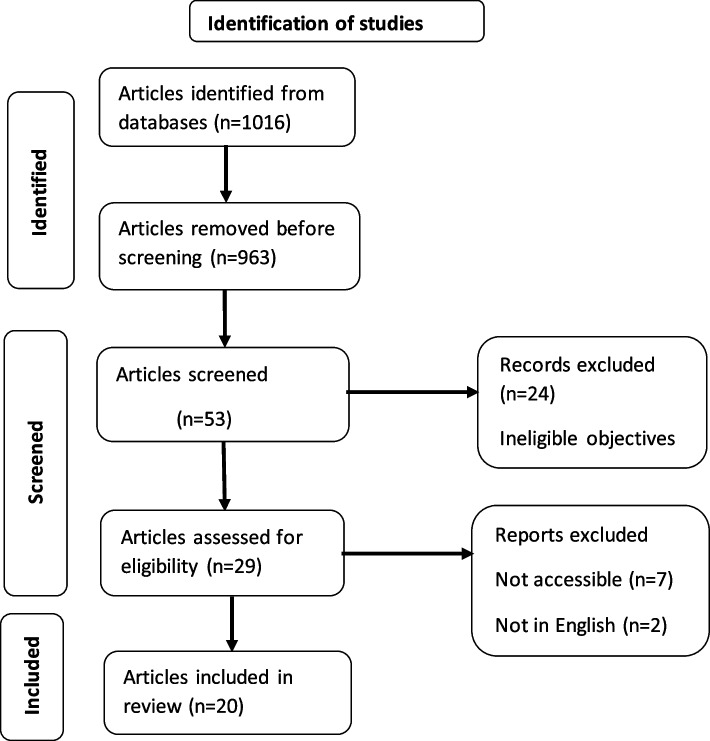
Studies were included if: i) they were original or systematic reviews and or meta-analyses written in English and ii) evaluated aspirin effectiveness in terms of dosage, gestational timing of initiation, time of ingestion and adherence, iii) the population studied included women with pre-existing hypertension. We excluded: i) studies that did not indicate chronic hypertension as one of the risk factors considered, ii) studies that combined aspirin with other drugs such as calcium supplements, iii) studies whose data was inaccessible, iv) commentaries, case reports, theses, dissertations, conference abstracts, textbooks, care guidelines and grey literature. We did not restrict the literature search to any period. Two researchers (i.e. the corresponding and the second author) independently assessed the extracted articles for eligibility. Identified discrepancies were discussed by the two reviewers and resolved by consensus. In the identification stage, the primary search generated 1,016 records, of which 963 were excluded because they were not relevant to the study topic. In the second stage, titles and abstracts of 53 records were screened, and 27 of them were removed because of ineligible objectives. Nine more were excluded due to inaccessibility (7) and not being in English (2). 17 studies qualified for inclusion after full text review. The flow of article selection is summarized in Fig. 1.
Fig. 1.
Flow chart for literature search
Quality assessment
For randomized controlled trials, the RoB2 criteria was used to assess the risk of bias [31]. For systematic reviews, we used the ROBIS criteria [74] and for the non-randomized studies, we used the Risk Of Bias In Non-Randomized Studies—of interventions (ROBINS-I) criteria [35]. Here, we assessed for randomization, deviations from the intervention indicated, missing of outcome data, and selective reporting. Assessment of these studies is included in the tables of the selected studies; 1, 2, 3 and 4.
Stage 4: Data analysis
After verifying the selected articles for inclusion, four data extraction sheets were generated to guide the information required from each study. These were about the aspirin dose, gestational timing of initiation, timing of ingestion and adherence. The variables of interest included the author, year, population, aim, method, findings and risk of bias. The data extraction sheets were read and agreed upon by the corresponding author and a co-author (PA and GN). Information in each article was extracted and analyzed separately by the two researchers. The information gathered on the sheets was then read by both researchers, who discussed and agreed on the relevant and significant information to retain on the sheets. The similarities and differences in the studies were examined, and the data were synthesized. Finally, conclusions were drawn and verified to ensure that they answered the research questions of this review. The selected articles are summarized in Tables 1, 2, 3, and 4.
Table 1.
Effectiveness of different aspirin doses on superimposed preeclampsia in women with chronic hypertension
| Author | year | Population | Purpose | Design | Main findings | Risk of bias |
|---|---|---|---|---|---|---|
| Sibai, Mirro et al [67] | 1989 | 774 women at high risk for PE | To evaluate the efficacy of aspirin 60 mg in preventing preeclampsia | Double blind, randomized, placebo-controlled trial | No significant differences were noted in the treatment and placebo groups. The incidence of preeclampsia was 26% in the aspirin group and 25% in the placebo group (p = 0.66) | Moderate |
| Sinha, Singh et al [68] | 2023 | 116 high-risk pregnant women | To compare the efficacy of 75 mg versus 150 mg aspirin in preventing PE in high-risk pregnant women | Randomized Controlled clinical trial | 33.92% of the women in the 75 mg group developed preeclampsia (33.92%) (OR = 5.341, 95% CI = 1.829- 15.594) compared to 8.77% (p = 0.001) in the 150 mg group | Moderate |
| Ashraf, Ali et al [3] | 2024 | 156 women at high risk for preeclampsia | To determine the efficacy of aspirin 150 mg vs 75 mg in the prevention of SIP | Parallel arm randomized control open-label study | The incidence of superimposed preeclampsia was lower in the 150 mg aspirin group compared to the 75 mg group | Low |
| Demuth, Pellan et al [16] | 2024 | 13,981 women at risk for preterm preeclampsia | To estimate the efficacy of a daily dose of aspirin 75 or 81 mg with a placebo in preventing pre-term PE | Meta-analyses of 11 randomized controlled trials | A pooled analysis showed no significant reduction in preeclampsia between aspirin (75 to 81 mg) and the placebo | High |
| Hu, Chen et al [32] | 2024 | 10,547 women at high risk for PE | To determine the optimal dose of aspirin among < 80 mg, 80 mg, 100 mg, and > 100 mg for preventing PE in high-risk pregnant women | Network meta-analysis of data from 23 randomized controlled trials | Doses between 80 and 100 mg were found to be more effective than those > 100 and < 80 mg in reducing the risk of preeclampsia | Low |
| Richards, Giorgione et al [58] | 2023 | 2150 women with stage 1 hypertension | To determine the efficacy of aspirin 60 mg, 75 mg, 100 mg, and 150 mg in reducing the risk of superimposed pre-eclampsia in women with chronic hypertension | Systematic review and meta-analysis of nine (three retrospective cohort studies and six randomized trials) | Low-dose aspirin 60 mg, 75 mg, 100 mg, 150 mg did not reduce the odds of pre-eclampsia (OR 0.91, 95% CI 0.64–1.29 | Moderate |
| Poon, Wright et al [55] | 2017 | 1592 high-risk women included in the Aspirin for Evidence-Based Preeclampsia Prevention trial | To examine the effect of 150 mg aspirin in the prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history | Secondary analysis of data | 150 mg of aspirin did not cause a reduction in the incidence of preeclampsia among women with chronic hypertension | High |
| Banala, Moreno et al. [6] | 2020 | 457 women with chronic hypertension | To evaluate the effectiveness of aspirin 81 mg for the prevention of superimposed preeclampsia in women with chronic hypertension before and after the ACOG guideline | Retrospective cohort study | No significant reduction in the incidence of preeclampsia after the implementation of aspirin 81 mg was noted | Moderate |
| Xiao, Ling et al [77] | 2023 | 266 Chinese women at high risk | To assess the efficacy of aspirin 75 mg in preventing preeclampsia in high-risk pregnancies | Retrospective real-world study | There was a significant reduction in the incidence of preeclampsia in women at high risk for preeclampsia, except those who had chronic hypertension | Low |
| Ayyash, Goyert et al [5] | 2024 | 1135 women with chronic hypertension | To examine the impact of aspirin 162 mg vs 81 mg on the prevention of superimposed PE | Retrospective chart review | No significant difference was noted in the incidence of SIP in the 162 mg, 81 mg and no treatment groups. However, 162 mg showed a lower incidence compared to 81 mg | Moderate |
Table 2.
Effectiveness of aspirin on superimposed preeclampsia in regard to gestation age at initiation
| Author | Year | Population | Purpose | Design | Main findings | Risk of bias |
|---|---|---|---|---|---|---|
| Sinha, Singh et al [68] | 2023 | 113 pregnant women at high risk | To compare the efficacy of aspirin 150 mg versus 75 mg in the prevention of PE if initiated between 12 and 16 weeks of gestation | Parallel, open-label, randomized control trial | There were five times greater odds of preeclampsia in those who received aspirin 75 mg compared to those who received 150 mg (cOR = 5.341, CI = 1.829–15.594) | Moderate |
| Ashraf, Ali et al [3] | 2024 | 156 pregnant women with moderate to high risk of PE | To determine the efficacy of aspirin 75 mg vs. 150 mg in moderate and high-risk women if initiated between 11 and 14 weeks | Parallel arm randomized control open-label study | Aspirin 150 mg in moderate and high-risk pregnancies starting at 11–14 weeks was more effective and safer than 75 mg in reducing the incidence of Preeclampsia | Low |
| Richards, Giorgione et al [58] | 2023 | 2150 pregnant women with chronic hypertension | To determine the efficacy of aspirin 60 mg, 75 mg, 100 mg, 150 mg in the prevention of SIP among women with stage 1 hypertension if initiated before 20 weeks | Systematic review and meta-analysis of nine (three retrospective cohort studies and six randomized trials) studies | Low-dose aspirin prophylaxis did not reduce the odds of pre-eclampsia (OR 0.91, 95% CI 0.64–1.29) | Moderate |
| Chaemsaithong, Cuenca-Gomez et al [14] | 2020 | 1426 pregnant women at high risk of PE | To determine whether aspirin initiated before 11 weeks’ gestation reduces the rate of preeclampsia | Systematic Review and meta-analysis of 8 randomized controlled trials | Low-dose aspirin initiated at < 11 weeks’ gestation was associated with a no significant reduction in the risk of preeclampsia (relative risk, 0.52; 95% confidence interval, 0.23–1.17, P =.115), gestational hypertension (relative risk, 0.49; 95% confidence interval, 0.20–1.21; P =.121) | Moderate |
| Shen, Martinez‐Portilla et al [66] | 2021 | 1592 women at risk of PE | To examine the efficacy of aspirin 150 mg in reducing the risk of PE initiated between 11 and 13 weeks | Secondary analysis of data from the ASPRE trial | The use of 150 mg aspirin in women with chronic hypertension did not have a significant effect compared to those without chronic hypertension | Moderate |
| Banala, Moreno et al. [6] | 2020 | 457 women with chronic hypertension | To assess the impact of the ACOG guideline regarding low-dose aspirin initiated between 12 and 16 weeks of gestation for prevention of SIP In Women With Chronic Hypertension | Retrospective cohort study | No significant decrease in SIP was noted after the implementation of aspirin 81 mg initiated between 12 to 16 weeks of gestation | Moderate |
| Huai, Lin et al [33] | 2021 | 397 women with stage 1 hypertension | To evaluate the preventive effect of aspirin 100 mg in women with stage 1 hypertension initiated before 16 weeks of gestation | Secondary analysis of data from a randomized controlled trial | The incidence of PE was not significantly reduced in the aspirin group compared to the control group | Moderate |
| Xiao, Ling et al [77] | 2023 | 266 women at high risk | To assess the efficacy of aspirin 75 mg in preventing PE in high-risk pregnancies | Retrospective chart review | A lower incidence of preeclampsia was observed among women who started taking aspirin before 16 weeks than those who started after 16 weeks (4.69%) vs (17.65%, p = 0.0239). Chronic hypertension was associated with a higher incidence of PE despite taking aspirin (p = 0.0001) | Low |
Table 3.
Effectiveness of aspirin on superimposed preeclampsia according to the time of drug ingestion
| Author | Year | Population | Purpose | Design | Main findings | Risk of bias |
|---|---|---|---|---|---|---|
| Hermida, Ayala et al [4] | 1999 | 240 pregnant women at risk of PE | To assess time-dependent effects of aspirin 100 mg in women at differing risk of PE | A double-blind, randomized, placebo-controlled trial | There was no effect of aspirin on the blood pressure of women who took aspirin on waking up. Aspirin significantly reduced blood pressure in those who took it 8 h after waking up. The effect was highly statistically significant among women who took aspirin before bedtime | Low |
| Ruan [62] | 2011 | 1245 patients with hypertension | To assess the effect of the administration of low-dose aspirin before bedtime on blood pressure in hypertensive patients | A systematic review of 6 Randomized controlled clinical trials | Compared to aspirin on waking up, aspirin intake before bedtime had a significant effect on lowering SBP (mean difference: − 6.29 mmHg, 95%CI: (− 8.01, − 4.58)), and DBP (mean difference: − 2.26 mmHg, 95%CI: (− 4.95, 0.43) | Moderate |
| Xiao, Ling et al [77] | 2023 | 266 pregnant women at high risk of PE | To assess the efficacy of aspirin 75 mg in high-risk pregnancies when taken at different times | Retrospective chart review | Aspirin taken at bedtime had a significantly lower incidence of PE than at other times. There was a higher occurrence of PE in those with chronic hypertension despite taking aspirin compared with those without hypertension (p = 0.0001) | Low |
| Sinha, Singh et al [68] | 2023 | 116 women at high risk of preeclampsia | To compare the efficacy of 150 mg versus 75 mg aspirin in the prevention of preeclampsia among high-risk pregnancies | Parallel, open-label, randomized control trial | Aspirin 150 mg once a day at bedtime was more effective than 75 mg once a day at bedtime in preventing PE | Moderate |
Table 4.
Effectiveness of aspirin on superimposed preeclampsia in regard to the level of adherence
| Author | Year | Population | Purpose | Design | Main findings | Risk of bias |
|---|---|---|---|---|---|---|
| Hauth, Goldenberg et al | 1993 | 600 high-risk pregnant women | To test the hypothesis that acetylsalicylate (aspirin) reduces the incidence or severity of pregnancy-associated hypertension | Randomized controlled clinical trial | At 94% adherence, daily ingestion of 60 mg of aspirin beginning at 24 weeks'gestation significantly reduced the occurrence of PE | Moderate |
| Poon, Wright et al [55] | 2017 | 1592 women at risk of PE | To examine whether there are differences in the efficacy of aspirin on the incidence of preterm preeclampsia at different adherence levels | Secondary analysis of data | At an adherence of < 90%, 90% and > 90%, aspirin 150 mg did not reduce the incidence of PE in women at risk. A history of chronic hypertension was significant in the prediction of preterm PE (0.042) despite aspirin use | High |
| Wright, Poon et al [76] | 2017 | 1620 women at high risk | To examine the influence of compliance on the beneficial effects of aspirin in preventing preterm preeclampsia | Secondary analysis of data | At > 90% adherence, aspirin 150 mg was positively associated with a significant reduction in the incidence of preeclampsia. Effectiveness correlated with the level of compliance | Moderate |
| Banala, Moreno et al. [6] | 2020 | 457 women with chronic hypertension | To evaluate the effectiveness of aspirin for the prevention of superimposed preeclampsia in women with chronic hypertension | Retrospective cohort study | At 57% adherence, the overall incidence of superimposed preeclampsia was not significantly different in the group before and after the revised ACOG guideline | Moderate |
Stage 5: Data presentation
In this review, 17 articles were included; five randomized controlled clinical trials [3, 26, 30, 67, 68] two systematic reviews and meta-analyses [14, 58], two meta-analyses [16, 32], one systematic review [62] and eight studies that analyzed secondary data [5, 6, 33, 55, 66, 76, 77].
Results
Dose of aspirin
Ten studies evaluated the effectiveness of different dosages of aspirin in preventing SIP in women with preexisting hypertension. Three RCTs, three systematic reviews, and three retrospective chart reviews as shown in Table 1. These studies produced varied outcomes. Two Randomized controlled studies (RCT) by [3, 68] compared 75 mg and 150 mg in 116 and 156 women at high risk, respectively. Both studies found lower incidences of SIP in the treatment groups that used 150 mg compared to the 75 mg groups. Another rigorous retrogressive chart review of 1135 women with chronic hypertension further affirmed that a 162 mg dose was superior to 81 mg in reducing SIP in this population [5]. Notably, all these results were not statistically significant, although higher doses (150 and 162 mg) seemed to have a better effect. This might be due to the small sample sizes used in the RCTs. The findings may support the theory that a 150 mg dosage is sufficient to regulate placental growth factor (PlGF) and cytokine production, thus reducing placental infarcts and improving trophoblastic function [51]. Although these RCTs used relatively small sample sizes, which limited their external validity, they both suggested a dose–response effect of aspirin. Given the high degree of precision of RCTs [12], these results were promising. However, conclusions based on three studies may not be sufficient. Therefore, further studies are needed to verify and validate these dosages. Although the risk of side effects is believed to increase with the dosage, evidence from studies that assessed the risk/benefit aspect found the 150 mg and 162 mg dosages safer compared to the risk [3, 5]. In contrast, a traditional and meta-analysis of data from 23 RCTs involving 10,547 pregnant women evaluated doses ranging from 50 to 160 mg and concluded that aspirin doses ranging between 80 to 100 mg were more effective than the others (Hu, Chen et al. 2024). Much as this was a large study with a low risk of bias (12 < 50%), sub-group analyses particularly for women with chronic hypertension was not reported. Therefore, the efficacy of these dosages in this sub-group could not be concluded. On the other hand, one meta-analysis of data from 13,981 women and a systematic review of data from 2,150 women found that dosages of 60 mg, 75 mg, 100 mg and 150 mg did not reduce the possibility of SIP in this category of women [16, 55, 58]. These two studies used large samples of data from clinical trials. However, they were characterized by marked risk of bias and significant heterogeneity, thus producing inconclusive results. Lower dosages have also been assessed in different populations, producing conflicting results. One retrospective cohort study evaluated a dose of 81 mg among 457 high-risk women [6], while another used 75 mg [77]. Neither of these studies observed significant effects in their participants. The latter study further noted that a dose of 75 mg was effective in all the other risky groups investigated, except those with chronic hypertension. This study was done among Chinese women. In support of these findings, evidence shows that doses less than 100 mg are insufficient to inhibit platelet aggregation in half of the women at risk [11, 18].
Gestational timing at initiation
Eight studies evaluated the effect of aspirin when initiated at different gestational ages. Two RCTs, two systematic reviews, and four retrospective chart reviews, as shown in Table 2. One systematic review meta-analyzed data from six RCTs and three retrospective cohort studies to assess the impact of aspirin (60 mg, 75 mg, 150 mg) introduced before 20 weeks of gestation [58]. This was done in 2,150 women with stage I hypertension. The results showed no significant reduction in the incidence of SIP. This study analyzed a large sample size, most of which was obtained from RCTs. However, it was defined by marked heterogeneity in terms of populations, definitions of hypertension/SIP, outcomes and dosages used. Moreover, some of the studies did not satisfactorily control for confounding or state the actual dosages used. Given that this analysis pooled up the results, this might have limited the ability to detect positive effects of the different dosages, even when they existed. Another retrospective chart review compared initiation of 75 mg aspirin before and after 16 weeks in 266 high-risk women [77]. The results showed a lower incidence of PE in women who started the medication before 16 weeks than in those who started after 16 weeks. Progressively, a randomized clinical trial initiated aspirin between 12 and 16 weeks using doses of 75 and 150 mg among 113 high-risk pregnant women. This study noted a higher (though non-significant) reduction in the incidence of PE among those who used a dose of 150 mg compared to those who used 75 mg [68]. Although this study was limited by a small sample size, it was able to detect noticeable reductions with a higher dose of aspirin initiated before 16 weeks just like the aforementioned studies. This suggests a promising trend between higher doses and introduction before 16 weeks. These results are in line with the WHO recommendation, where aspirin should be started at 12 weeks of gestation [49]. This is based on the theory that 12 weeks correspond with the time when the placentation process is completed [59]. However, a more focused study commenced aspirin 100 mg in 397 women with stage 1 hypertension before 16 weeks of gestation. This study found no differences in the incidence of SIP between the aspirin and the control group [33]. Although this study was more focused, it did not report on the women’s adherence levels to the therapy. The major difference in these studies is that the two studies [68, 77] that reported marked reductions included all women at high risk, while the latter study investigated only women with chronic hypertension. Similarly, two other studies [6, 66] initiated aspirin 81 mg and 150 mg before 16 weeks of gestation, respectively. These studies did not report significant effects of aspirin in the high-risk women investigated. Further still, one study meta-analyzed data from eight clinical trials involving 1,426 women at high risk. Aspirin 150 mg was introduced before 11 weeks of gestation. The results showed a non-significant reduction in the incidence of preeclampsia. This study analyzed a large sample of data from RCTs which are considered effective in determining the effectiveness of a drug [65]. However, the analysis was not focused on women with persistent hypertension. Therefore, the results could not be regarded as conclusive. Their investigation was however based on the theory that the first wave of placental invasion occurs between eight and ten weeks of gestation [54]; therefore, introducing aspirin at this time could produce better results.
Timing of aspirin ingestion
Although most clinical guidelines do not specify the time of day aspirin should be ingested, the International Society for the Study of Hypertension in Pregnancy (ISSHP) recommends taking aspirin at bedtime [61]. This is based on the hypothesis that renin and thromboxane production are time-dependent, and their levels decrease tremendously when aspirin is taken at night [4, 61, 69]. Four studies in this review compared different doses of aspirin (75 mg, 100 mg, 150 mg) taken at different times of the day in different populations of women at risk of PE. These included two RCTs, a systematic review of six RCTs, and a prospective chart review. All the four studies reported significant reductions in blood pressure and PE incidence as shown in Table 3. In one of the studies, the researchers randomized 240 women into three different groups and administered 100 mg of aspirin before 16 weeks of gestation. The first group took aspirin when waking up, the second group took it eight hours after waking up, and the third group took it before bedtime. The findings showed a significant decrease in the mean blood pressure readings among those who took aspirin at bedtime compared with other times of the day [30]. Although these studies used varied dosages, initiated the aspirin at different gestational ages and analyzed relatively small samples, they were able to detect significant effects. This suggests that aspirin taken at night can produce better effects, though further research is required to validate these findings.
Adherence to aspirin
The capacity of aspirin to prevent preeclampsia is largely dependent on compliance [76]. A drug cannot work if it is not taken into the body. Two studies in this review reported adherence levels above 90% with subsequent significant reductions in the incidence of PE (see Table 4). One of the studies randomized 600 women at high risk to aspirin 60 mg in a clinical trial [26]. The women complied with 94 per cent, and significant outcomes were achieved. Although the dosage in this study was relatively low, the women prescribed aspirin were followed up at 99% to ensure compliance. Much as the study analyzed a big sample of data, the authors did not report the clinical stage of hypertension in the women studied. Probably most of them were in stage 1, where it is plausible for such a dose to cause an effect [25]. The other study analyzed secondary data of 1,620 high-risk pregnant women [76]. Aspirin 150 mg was used with an adherence level above 90%. The results showed a non-significant reduction in the incidence of PE. This study used data from clinical trials and a higher dose of aspirin. However, the source of the data was not adequately powered to assess adherence appropriately. In addition, pill count was used to assess adherence, which is prone to errors. These studies can be generalizable since they used large sample sizes of data from randomized clinical trials to obtain these results. More so, despite using different dosages, there was agreement that a woman needed to comply more than 90 per cent of the time to achieve the desired effects. However, two studies may be too few to draw a substantive conclusion. Further studies focusing on women with chronic hypertension could improve the validity of these findings. Two studies in this review did not find positive effects. One of the studies analyzed secondary data from a systematic review in which aspirin 150 mg was used with an adherence level above 90% [55]. The other study retrospectively analyzed data from 457 women who used aspirin 81 mg with an adherence of 57% [6]. Although these studies focused on women with hypertension, they relied on self-reports of patients to assess adherence. Self-reports are generally easy to use but they are prone to recall bias and can easily invite socially acceptable responses. Overall, very few studies were found to have evaluated the role of adherence in the effectiveness of aspirin. Therefore, further studies could focus on rigorous follow up of these women and use of combined measures to evaluate adherence. Consideration could be given to measures such as self-reports and electronic adherence monitoring devices or self-reports and aspirin biomarkers which have proven to be more effective [27].
Discussion
Even though some researchers no longer recommend aspirin to be used in women who are chronically hypertensive [38] for SIP prevention, this review found that aspirin at varying doses had beneficial effects on different populations. For instance, Xiao et al. [77] observed that a dose of 75 mg significantly reduced superimposed preeclampsia among Chinese women. However, most of the studies did not find significant effects when similar doses were used or even when the doses were increased to 150 mg in other populations [6, 16, 55, 58]. This could be attributed to the low obesity levels in the Chinese population [77] compared to the general population. These results may be supported by the hypothesis that the effect of aspirin on an individual is weight-dependent, [16, 22]. Nevertheless, the review observed noticeable reductions in the incidence of SIP in studies that used higher doses of aspirin, [3, 5, 68]. Despite these positive observations, the results were not statistically significant. This suggests that studies that take into account some essential factors such as chronicity of hypertension and weight could find the true effects of aspirin in this population.
Some guidelines from different professional societies agree that starting aspirin prophylaxis before the 16th week is the best course of action [61]. Regarding the gestational timing of aspirin initiation, some studies in this review started as late as 19 weeks, others started before 16 weeks [6, 7, 33, 68], while others were compliant with the 12 weeks as recommended by WHO [3, 66]. Further still, some studies started as early as 11 weeks [14]. However, none of the studies achieved a significant reduction in the incidence of SIP, except one study where a dose of 162 mg was used beginning at 12 weeks [5]. Given this observation, some scholars recommend aspirin to be started before pregnancy in women who are chronically hypertensive [63]. This is thought to stabilize the endometrium before placental implantation takes place. This recommendation is also based on the findings of a study that assessed platelet factor four (a marker for platelet aggregation) in women with chronic hypertension. In this study, increased levels of platelet factor four were present in these women even before pregnancy [14]. This implies that the risk exists even before a woman conceives. In agreement with this idea, a study that initiated aspirin before conception in women who were due for in-vitro fertilization reported reduced cases of preeclampsia [63]. Therefore, further research needs to explore the impact of aspirin when initiated before pregnancy, particularly in women with preexisting hypertension.
According to this review, the majority of the studies did not report the time of day at which aspirin was taken. As a result, there was notable low efficacy reported in these studies. However, the studies that reported consistency in taking aspirin at bedtime produced positive results [30, 62, 68, 77]. This is supported by the theory that thromboxane production is time-dependent and its levels decrease tremendously when aspirin is taken at night [4, 61]. Since very few studies have investigated this phenomenon, further research is needed to validate the time of the day that enhances the effectiveness of aspirin in women with chronic hypertension.
Suboptimal adherence to aspirin is very common and has led to the underestimation of the efficacy of the drug. Like any other medication, aspirin’s ability to prevent preeclampsia depends on proper adherence. This review noted that the outcome of aspirin use in high-risk pregnancies corresponded with the degree of adherence. Respectively, a woman with chronic hypertension had a much lower risk of developing SIP if she took her medication more than 90 percent of the time [26, 76]. However, some studies did not find an impact at > 90% [55]. The discrepancies could be attributed to the differences in the study designs and the populations that were investigated. In this review, most of the researchers who found aspirin less effective in reducing the incidence of SIP did not measure adherence in their studies. For those who did, they used self-reports from patients, which are liable to errors. Although good adherence can prevent up to 90 percent incidence of PE [60], several hindrances surround this aspect. However, some researchers have noted incredible willingness to comply in women who are well empowered with information on the risks/benefits of aspirin [40]. This could be accompanied by continuous reinforcement in the form of regular reminders, peer/family support and addressing their immediate concerns [28, 64, 72]. Further research could consider combining the measures of adherence, such as self-reports and pill count or self-reports and electronic adherence monitoring devices or self-reports and aspirin biomarker monitoring. This could accompany continuous follow-up and support to the women prescribed aspirin.
Strengths and limitations
The strength of this review is that most of the studies evaluated were randomized clinical trials and of recent data. While most of the previous studies assessed one or two important parameters necessary to evaluate drug efficacy, this review was able to appraise four of the most important parameters, that is, dosage, timing of gestation/ingestion and adherence. However, the review was limited by the little evidence assessed on the timing of aspirin ingestion and adherence. This was due to inaccessible articles, which restricted the overall conclusion. Another limitation is that we only considered articles that were published in the English language, which reduced the available evidence for evaluation. We were not able to adequately assess selective reporting to quantify the risk of bias in all the studies, because we were not able to access all the raw data.
Conclusion
This review noted that women with chronic hypertension develop preeclampsia through a variety of pathways. Aspirin addresses only one of them. This suggests that multi-component pharmacological combinations could enhance aspirin’s effectiveness in this population. We also noted that the efficacy of aspirin largely depends on early identification and control of hypertension, weight-adjusted doses, initiation before the 12th week of gestation and taking the drug more than 90% of the time at night/bedtime. However, a substantive conclusion cannot be drawn as very few studies reported on the timing of ingestion and adherence; we think these are also very important aspects in evaluating drug efficacy.
Acknowledgements
We express our gratitude to Mbarara University of Science and Technology for providing us with free internet and space in the library to accomplish this work. We also extend special thanks to the librarian, Mr. Adriko Wilson, who guided us on the process of searching for articles.
Abbreviations
- BMI
Body Mass Index
- PE
Preeclampsia
- RCT
Randomized Controlled Trial
- SIP
Superimposed Preeclampsia
- WHO
World Health Organization
- ACOG
American College of Obstetricians and Gynaecologists
- cOR
Crude Odds Ratio
- CI
Confidence Interval
- DBP
Diastolic Blood Pressure
- HETEs
Hydroxyeicosatetraenoic acids
- L-arginine
Levorotatory Arginine
- OR
Odds Ratio
- PIGF
Placental Growth Factor
- ROBINS
Risk Of Bias In Non-Randomized Studies
- SBP
Systolic Blood Pressure
Authors’ contributions
PA: Searched literature, screened and selected articles, and prepared the manuscript GN: Selected the design, screened articles, edited, and reviewed the manuscript LB: Selected the journal, edited, and reviewed the manuscript JN: Edited and reviewed the manuscript GR: Edited and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
No external funding was received for this review.
Data availability
All the data in this article is included in the paper.
Declarations
Ethics approval and consent to participate
This paper did not require approval by the Ethics Research Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Jameil N, et al. A brief overview of preeclampsia. J Clin Med Res. 2013;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrabi SM, et al. Nitric oxide: physiological functions, delivery, and biomedical applications. Adv Sci. 2023;10(30):2303259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashraf S, et al. To determine efficacy aspirin 150 mg vs 75 mg in prevention of preeclampsia and its associated complications. Pak J Med Dent. 2024;13(4):58–68. [Google Scholar]
- 4.Ayala DE, et al. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30(1–2):260–79. [DOI] [PubMed] [Google Scholar]
- 5.Ayyash M, et al. Efficacy and safety of aspirin 162 mg for preeclampsia prophylaxis in high-risk patients. Am J Perinatol. 2024;41(S 01):e2410–7. [DOI] [PubMed] [Google Scholar]
- 6.Banala C, et al. Impact of the ACOG guideline regarding low-dose aspirin for prevention of superimposed preeclampsia in women with chronic hypertension. Am J Obstet Gynecol. 2020;223(3):419. e411-419. e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bello NA, et al. Prevalence of hypertension among pregnant women when using the 2017 American College of Cardiology/American Heart Association blood pressure guidelines and association with maternal and fetal outcomes. JAMA Netw Open. 2021;4(3):e213808–e213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MA, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. [DOI] [PubMed] [Google Scholar]
- 9.Burton GJ, et al. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366. [DOI] [PubMed]
- 10.Camarena Pulido E, et al. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: a double-blind, randomized, clinical trial. Hypertens Pregnancy. 2016;35(2):217–25. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier C, et al. Aspirin responsiveness at a dose of 80 mg and its impact on birth weight when used in twin pregnancies: the GAP pilot randomized trial. Am J Perinatol. 2022;39(13):1396–400. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright N, Munro E. The limitations of randomized controlled trials in predicting effectiveness. J Eval Clin Pract. 2010;16(2):260–6. [DOI] [PubMed] [Google Scholar]
- 13.Catov JM, et al. Risk of early or severe preeclampsia related to pre-existing conditions. Int J Epidemiol. 2007;36(2):412–9. [DOI] [PubMed] [Google Scholar]
- 14.Chaemsaithong P, et al. Does low-dose aspirin initiated before 11 weeks’ gestation reduce the rate of preeclampsia? Am J Obstet Gynecol. 2020;222(5):437–50. [DOI] [PubMed] [Google Scholar]
- 15.Chappell LC, et al. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension. 2008;51(4):1002–9. [DOI] [PubMed] [Google Scholar]
- 16.Demuth B, et al. Aspirin at 75 to 81 mg daily for the prevention of preterm pre-eclampsia: systematic review and meta-analysis. J Clin Med. 2024;13(4):1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorniak-Wall T, et al. The role of L-arginine in the prevention and treatment of pre-eclampsia: a systematic review of randomised trials. J Hum Hypertens. 2014;28(4):230–5. [DOI] [PubMed] [Google Scholar]
- 18.Dumont A, et al. Effect of aspirin in pregnant women is dependent on increase in bleeding time. Am J Obstet Gynecol. 1999;180(1):135–40. [DOI] [PubMed] [Google Scholar]
- 19.Elhalag RH, et al. Efficacy of aspirin for prevention of preeclampsia in twin pregnancy: a meta-analysis. Reprod Female Child Health. 2024;3(3): e97. [Google Scholar]
- 20.English FA, et al. Risk factors and effective management of preeclampsia. Integ Blood Pressure Control. 2015;7–12. [DOI] [PMC free article] [PubMed]
- 21.Finnegan C, Breathnach FM. The role of aspirin for preeclampsia prevention in women with diabetes. Curr DiabRep. 2020;20:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Ghesquiere L, et al. Optimal dose of aspirin for the prevention of preterm preeclampsia. Am J Obstet Gynecol. 2023;229(5):574–5. [DOI] [PubMed] [Google Scholar]
- 23.Guerrier G, et al. (2013). Factors associated with severe preeclampsia and eclampsia in Jahun, Nigeria. Int J Womens Health: 509–513. [DOI] [PMC free article] [PubMed]
- 24.Gui S, et al. Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review. J Renin Angiotensin Aldosterone Syst. 2014;15(1):88–96. [DOI] [PubMed] [Google Scholar]
- 25.Hauspurg A, et al. Aspirin effect on adverse pregnancy outcomes associated with stage 1 hypertension in a high-risk cohort. Hypertension. 2018;72(1):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauth J, et al. Low-dose aspirin therapy to prevent preeclampsia. Int J Gynecol Obstet. 1994;44(1):97–97. [DOI] [PubMed] [Google Scholar]
- 27.Hawkshead J, Krousel-Wood MA. Techniques for measuring medication adherence in hypertensive patients in outpatient settings: advantages and limitations. Dis Manag Health Out. 2007;15:109–18. [Google Scholar]
- 28.Helou A, et al. ‘I wish my body was stronger’: A qualitative study of attitudes and behaviours regarding treatment of hypertensive disorders of pregnancy. SAGE Open Medicine. 2021;9:20503121211032480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henrique AJ, et al. Resultado perinatal em mulheres portadoras de hipertensão arterial crônica: revisão integrativa da literatura. Rev Bras Enferm. 2012;65:1000–10. [DOI] [PubMed] [Google Scholar]
- 30.Hermida RC, et al. Administration time–dependent effects of aspirin in women at differing risk for preeclampsia. Hypertension. 1999;34(4):1016–23. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, et al. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions. 2019;205–28.
- 32.Hu X, et al. The optimal dosage of aspirin for preventing preeclampsia in high-risk pregnant women: a network meta-analysis of 23 randomized controlled trials. J Clin Hypertens. 2024;26(5):455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huai J, et al. Preventive effect of aspirin on preeclampsia in high-risk pregnant women with stage 1 hypertension. J Clin Hypertens. 2021;23(5):1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L-T, et al. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int J Mol Sci. 2012;13(11):14606–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jüni P, et al. Risk of bias in non-randomized studies of interventions (ROBINS-I): detailed guidance. Br Med J. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kametas NA, et al. Chronic hypertension and superimposed preeclampsia: screening and diagnosis. Am J Obstet Gynecol. 2022;226(2):S1182–95. [DOI] [PubMed] [Google Scholar]
- 37.Khansari S, et al. The effect of L-arginine in the prevention of preeclampsia and intrauterine growth of the fetus in primigravid women: a randomized clinical trial. Biomed Res Ther. 2024;11(4):6357–62. [Google Scholar]
- 38.Lecarpentier E, Haddad B. Aspirin for the prevention of placenta-mediated complications in pregnant women with chronic hypertension. J Gynecol Obstet Hum Reprod. 2020;49(9):101845. [DOI] [PubMed] [Google Scholar]
- 39.Lecarpentier E, et al. Risk factors of superimposed preeclampsia in women with essential chronic hypertension treated before pregnancy. PLoS ONE. 2013;8(5):e62140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorreia ADA, et al. 281 Aspirin acceptance rate and perceived preeclampsia risk after the implementation of universal screening for preeclampsia. Am J Obstet Gynecol. 2021;224(2):S184. [Google Scholar]
- 41.Magee LA, et al. Toward personalized management of chronic hypertension in pregnancy. Am J Obstet Gynecol. 2022;226(2):S1196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meher S, et al. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 1996;2010(2). [DOI] [PMC free article] [PubMed]
- 43.Menichini D, et al. L-Arginine supplementation in pregnancy: a systematic review of maternal and fetal outcomes. J Matern Fetal Neonatal Med. 2023;36(1):2217465. [DOI] [PubMed] [Google Scholar]
- 44.Mol BW, et al. Pre-eclampsia. The Lancet. 2016;387(10022):999–1011. [DOI] [PubMed] [Google Scholar]
- 45.Muldoon KA, et al. Persisting risk factors for preeclampsia among high-risk pregnancies already using prophylactic aspirin: a multi-country retrospective investigation. J Matern Fetal Neonatal Med. 2023;36(1):2200879. [DOI] [PubMed] [Google Scholar]
- 46.Naderipour F, et al. Efficacy of L-arginine for preventing preeclampsia and improving maternal and neonatal outcomes in high-risk pregnancies: a systematic review and meta-analysis. Int J Fertil Steril. 2024;18(4):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngene NC, Moodley J. (2024). Preventing maternal morbidity and mortality from preeclampsia and eclampsia particularly in low-and middle-income countries. Best Pract Res Clin Obstet Gynaecol 102473. [DOI] [PubMed]
- 48.Noris M, et al. Mechanisms of disease: pre-eclampsia. Nat Clin Pract Nephrol. 2005;1(2):98–114. [DOI] [PubMed] [Google Scholar]
- 49.Organization WH. WHO recommendations on antiplatelet agents for the prevention of pre-eclampsia, World Health Organization. 2021. [PubMed]
- 50.Oyekan A, McGiff J. Cytochrome P-450-derived eicosanoids participate in the renal functional effects of ET-1 in the anesthetized rat. Am J Physiol. 1998;274(1):R52–61. [DOI] [PubMed] [Google Scholar]
- 51.Panagodage S, et al. Low-dose acetylsalicylic acid treatment modulates the production of cytokines and improves trophoblast function in an in vitro model of early-onset preeclampsia. Am J Pathol. 2016;186(12):3217–24. [DOI] [PubMed] [Google Scholar]
- 52.Pascale JV, et al. 20-Hydroxyeicosatetraenoic acid (20-HETE): bioactions, receptors, vascular function, cardiometabolic disease and beyond. Adv Pharmacology, Elsevier. 2023;97:229–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phoswa WN, Khaliq OP. The role of oxidative stress in hypertensive disorders of pregnancy (preeclampsia, gestational hypertension) and metabolic disorder of pregnancy (gestational diabetes mellitus). Oxid Med Cell Longev. 2021;2021(1):5581570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pijnenborg R, et al. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4(4):397–413. [DOI] [PubMed] [Google Scholar]
- 55.Poon LC, et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. 2017;217(5):585. e581. [DOI] [PubMed] [Google Scholar]
- 56.Rana S, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–112. [DOI] [PubMed] [Google Scholar]
- 57.Rani N, et al. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J Obstet Gynaecol Res. 2010;36(6):1189–94. [DOI] [PubMed] [Google Scholar]
- 58.Richards EM, et al. Low-dose aspirin for the prevention of superimposed preeclampsia in women with chronic hypertension: a systematic review and meta-analysis. Am J Obstet Gynecol. 2023;228(4):395–408. [DOI] [PubMed] [Google Scholar]
- 59.Roberge S, et al. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287-293. e281. [DOI] [PubMed] [Google Scholar]
- 60.Rolnik DL, et al. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2022;226(2):S1108–19. [DOI] [PubMed] [Google Scholar]
- 61.Rottenstreich A. Controversies and clarifications regarding the role of aspirin in preeclampsia prevention: a focused review. J Clin Med. 2024;13(15):4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruan Y. Effect of administration of low-dose aspirin before bedtime on blood pressure in hypertensive patients: a meta-analysis. Int J Cardiol. 2011;152:S85–6. [Google Scholar]
- 63.Rubinstein M, et al. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertil Steril. 1999;71(5):825–9. [DOI] [PubMed] [Google Scholar]
- 64.Shanmugalingam R, et al. Factors that influence adherence to aspirin therapy in the prevention of preeclampsia amongst high-risk pregnant women: a mixed method analysis. PLoS ONE. 2020;15(2):e0229622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma N, et al. Randomized clinical trial: gold standard of experimental designs-importance, advantages, disadvantages and prejudices. Revista Pesquisa em Fisioterapia. 2020;10(3):512–9. [Google Scholar]
- 66.Shen L, et al. ASPRE trial: risk factors for development of preterm pre-eclampsia despite aspirin prophylaxis. Ultrasound Obstet Gynecol. 2021;58(4):546–52. [DOI] [PubMed] [Google Scholar]
- 67.Sibai BM, et al. Low-dose aspirin in pregnancy. Obstet Gynecol. 1989;74(4):551–7. [PubMed] [Google Scholar]
- 68.Sinha N, et al. A randomized controlled study comparing the efficacy of 75mg versus 150mg aspirin for the prevention of preeclampsia in high-risk pregnant women. Cureus. 2023;15(5). [DOI] [PMC free article] [PubMed]
- 69.Snoep JD, et al. Time-dependent effects of low-dose aspirin on plasma renin activity, aldosterone, cortisol, and catecholamines. Hypertension. 2009;54(5):1136–42. [DOI] [PubMed] [Google Scholar]
- 70.Staff AC. The two-stage placental model of preeclampsia: An update. J Reprod Immunol. 2019;134:1–10. [DOI] [PubMed] [Google Scholar]
- 71.Tesfa E, et al. Prevalence and determinants of hypertensive disorders of pregnancy in Ethiopia: a systematic review and meta-analysis. PLoS ONE. 2020;15(9):e0239048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vinogradov R, et al. Aspirin non-adherence in pregnant women at risk of preeclampsia (ANA): a qualitative study. Health Psychol Behav Med. 2021;9(1):681–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wandabwa J, et al. Risk factors for severe pre-eclampsia and eclamsia in Mulago Hospital, Kampala, Uganda. East Afr Med J. 2010;87(10). [PubMed]
- 74.Whiting P, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whittemore R. Rigour in integrative reviews. Rev Res Evid Nurs Pract Syst Rev. 2007;149–56.
- 76.Wright D, et al. Aspirin for Evidence-based preeclampsia prevention trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol. 2017;217(6):685. e681-685. e685. [DOI] [PubMed] [Google Scholar]
- 77.Xiao Y, et al. Aspirin 75 mg to prevent preeclampsia in high-risk pregnancies: a retrospective real-world study in China. Eur J Med Res. 2023;28(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou A, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468(7320):108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in this article is included in the paper.