Abstract
Background
This study aimed to evaluate the impact of anxiety on the pharmacodynamics of remimazolam besylate in patients undergoing gastroscopy.
Methods
Patients undergoing gastroscopy were divided into two groups: an anxiety group (Self -rating Anxiety scale, SAS ≥ 50) and a non-anxiety group (SAS < 50). All patients received intravenous administration of 5 µg/kg alfentanil combined with remimazolam besylate. The biased coin design up-and-down sequential method (BCD-UDM) was used to determine the target doses of remimazolam besylate: the initial dose was 0.1 mg/kg, with a dose gradient of 0.01 mg/kg. If coughing, swallowing, or body movement reactions occurred within the first 2 min from the start of gastroscopy, it was considered a positive reaction, and the dose was increased for the next patient. Otherwise, it was considered a negative reaction, and the dose of remimazolam besylate for the next patient was determined according to the BCD-UDM. Discharge time from the recovery room and adverse reactions were recorded. The ED50, ED90, and their 95% confidence intervals (CI) were calculated.
Results
The ED50 and ED90 of remimazolam besylate in the anxiety group were 0.175 mg/kg (95% CI, 0.140–0.240) and 0.251 mg/kg (95% CI, 0.173–0.250), respectively. In contrast, the ED50 and ED90 of remimazolam besylate in the non-anxiety group were 0.126 mg/kg (95% CI, 0.116–0.150) and 0.148 mg/kg (95% CI, 0.130–0.160), respectively. The ED90 equivalent ratio of the non-anxiety group to the anxiety group was 0.59 (95% CI, 0.468–0.710). The discharge time from the recovery room and the incidence of adverse reactions did not differ significantly between the groups.
Conclusions
When combined with 5 µg/kg alfentanil, the ED50 and ED90 of remimazolam besylate for inhibiting body movement response within two minutes of gastroscopy in anxious patients were 0.175 mg/kg and 0.251 mg/kg, respectively. Anxiety increased the requirement for remimazolam besylate in patients undergoing gastroscopy, but it did not increase the risk of prolonged discharge time or adverse effects.
Trial registration
The study was registered in the Chinese Clinical Trial Registry. (ChiCTR2400086957) on July 15, 2024.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-025-03235-3.
Keywords: Anxiety, Remimazolam besylate, Alfentanil, Gastroscope
Introduction
With advances in comfort medicine, gastroscopy is increasingly performed under general anesthesia, effectively reducing the discomfort associated with the procedure. However, since gastroscopy is an invasive procedure, patients often experience preoperative anxiety due to fear of the procedure, potential anesthesia complications, and concerns about disease outcomes [1, 2]. Preoperative anxiety refers to fear and nervousness about unknown events, representing an unpleasant emotional experience and the most common psychological stress response before surgery [3]. Excessive anxiety can lead to pathophysiological changes that negatively affect the body [4, 5].
Recent studies indicate that preoperative anxiety significantly affects pharmacodynamics [6, 7]. Remimazolam besylate, a novel benzodiazepine, is widely used in painless diagnostic and therapeutic procedures [8]. However, it remains unclear whether anxious patients require the same dosage of remimazolam besylate as non-anxious patients.
Therefore, our study aimed to investigate the impact of preoperative anxiety on the dose-response relationship of remimazolam besylate in inhibiting body movement responses during gastroscopy, focusing on ED50, ED90, and 95% confidence intervals (CI). The goal was to provide a clinical basis for selecting the appropriate dose of remimazolam besylate for anxious patients undergoing gastroscopy.
Materials and methods
Ethics
The study protocol was approved by the Ethics Committees of Shaoxing People’s Hospital (Ethics Approval, 2024-Yanli 048 − 01) and the Affiliated Hospital of Shaoxing University (Ethics Approval, 2023-(Yan)−007−02). The study was registered on the website ClinicalTrials.gov on 15/07/2024(ChiCTR2400086957). Participants were included after obtaining written informed consent.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients undergoing gastroscopy; (2) patients aged 18 to 60 years; (3) body mass index (BMI) ranging from 18 to 28 kg/m²; (4) American Society of Anesthesiologists (ASA) class I–II.
The exclusion criteria included: (1) long-term use of analgesics, psychiatric medications, or alcohol abuse; (2) severe visual or auditory impairments, or other communication difficulties; (3) allergies or contraindications to benzodiazepines, opioids, or any components of the study medications; (4) patients refusing to participate in the study.
Assessment of anxiety
The Self-Rating Anxiety Scale (SAS) was used to assess the degree of anxiety in patients. Developed by Zung, the SAS is a brief and widely applied tool in clinical psychiatry [9]. It sensitively reflects patients’ emotional responses and behavioral changes under stress and is commonly used for preoperative anxiety assessments [10]. The SAS consists of 20 items covering two dimensions: mental and somatic symptoms. Each item is rated on a 4-point scale according to the frequency of anxiety symptoms: 1 = a little of the time, 2 = some of the time, 3 = good part of the time, and 4 = most of the time. The total score was multiplied by 1.25 to obtain a standard score. A standard SAS score of 50 or higher was classified as indicating anxiety. The SAS used in this study is detailed in Supplementary File 1.
Randomization and blinding
Before patient enrollment, the BCD-UDM ordinal sequence was generated using R software (version 4.4.1), and the sequence numbers were sealed in opaque envelopes. After enrollment, the individual responsible for randomization opened the envelopes to retrieve the BCD-UDM allocation results and determine whether adjustment of the remimazolam besylate dose was necessary. Preoperatively, an anesthesiologist not involved in the study assessed patients’ anxiety levels using the SAS to obtain anxiety scores. Based on these scores, another anesthesiologist uninvolved in the study classified patients into anxious or non-anxious groups and preconfigured the drug doses according to the BCD-UDM method. During anesthesia induction, a blinded anesthesiologist administered the drugs, while another blinded anesthesiologist evaluated the sedation effects and body movement responses. Patients, anesthesiologists, gastroscopists, and data analysts were all blinded to the drug doses and randomization process.
Anesthesia procedure
All patients received standardized surgical and anesthetic care. Upon entering the preoperative preparation room, an intravenous line was opened, and 0.2 g of lidocaine hydrochloride gel was orally administered 10 min before the examination. After being transferred to the operating room, routine monitoring was initiated, including electrocardiogram (ECG), oxygen saturation (SpO₂), and non-invasive blood pressure (NIBP). All patients received pure oxygen at a flow rate of 5 L/min via a nasal cannula. Anesthesia induction began with intravenous alfentanil at a dose of 5 µg/kg, followed by remimazolam besylate at a dose determined by the BCD-UDM. The Modified Observer’s Assessment of Alertness and Sedation (MOAA/S) score was assessed every minute after the administration of remimazolam besylate. Gastroscopy was initiated if the MOAA/S score was ≤ 2 at the third minute [11].
MOAA/S monitor
Sedation levels were assessed using the MOAA/S score as follows: a score of 5 indicated the patient is fully awake and responds promptly to their name spoken in a normal tone; a score of 4 indicated a sluggish response to their name spoken in a normal tone; a score of 3 indicated response only after their name is called loudly or repeatedly; a score of 2 indicated response only to mild prodding or shaking; a score of 1 indicated response only to painful stimulus (trapezius squeeze); a score of 0 indicated no response to painful stimulus (trapezius squeeze).
Biased-coin design up-down sequential method
The study design referenced Gao et al. [12]. and utilized the BCD-UDM to determine the target doses of remimazolam besylate. Based on previous studies [13], the initial dose of remimazolam besylate for the first patient in each group was set to 0.1 mg/kg. A positive response was defined as the occurrence of swallowing, coughing, or body movement within the first 2 min from the start of the procedure. In cases of a positive response, the dose of remimazolam besylate for the next patient was increased by 0.01 mg/kg [14]. If a negative response was observed, the next patient was randomized to receive either a lower dose (decreased by 0.01 mg/kg) with a probability of 11% or maintain the same dose with a probability of 89%. The trial continued until the 45th negative response was obtained [15]. In cases where the patient’s MOAA/S score remained above 2 points 3 min after remimazolam besylate administration, the response was considered positive. In cases of a positive response, propofol at a dose of 0.5 mg/kg was administered intravenously as rescue sedation until adequate sedation for the procedure was achieved. The total amount of propofol used for rescue sedation was recorded. Post-procedure, patients were transferred to the post-anesthesia recovery room. Discharge from the recovery room was allowed only when the Aldrete score reached ≥ 9. The time from the completion of the procedure to the patient’s discharge from the recovery room was recorded.
Outcome variables
The primary outcome of the study was ED50 and ED90 of remimazolam besylate for inhibiting body movement response within two minutes of gastroscopy in patients with and without preoperative anxiety. Additionally, the ratio of ED90 between the two groups was calculated.
Secondary outcomes included the dose of propofol used, the number of patients requiring propofol, time to discharge, and the occurrence of respiratory depression, hypotension, nausea and vomiting. Demographic data collected include: gender, age, weight, BMI, ASA classification, education level, history of gastroscopy, gastroscopy duration and SAS score.
From the start of remimazolam besylate administration until patients were discharged from the recovery room, perioperative adverse events were recorded. Respiratory depression was defined as a respiratory rate of fewer than 10 breaths per minute persisting for more than one minute and/or a decrease in SpO2 below 90%. In cases where SpO2 dropped below 90%, jaw support or pressure respiration via face mask was administered [16]. Hypotension was defined as a mean arterial pressure (MAP) or systolic blood pressure decreased by more than 20% from baseline. Intravenous ephedrine (6 mg) was administered as needed, with repeated doses if necessary [17]. If a patient experienced nausea or vomiting, ondansetron (4 mg) will be administered.
Sample size
This study uses the BCD-UDM, the sample size required for successful sequential regression must exceed 40 and be a multiple of 9, thus requiring at least 45 negative cases to conclude the trial, typically involving 50–60 participants [15]. Considering an anticipated dropout rate of 20%, we plan to recruit 72 participants in each group. Recruitment will be terminated earlier if the 45th negative case is observed before reaching the planned enrollment target.
Statistical analysis
The statistical analysis was performed by SPSS 25.0. Continuous variables were assessed for normality with the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation and compared using the independent t-tests. Non-normally distributed data were presented as the median and interquartile range (25th to 75th quartile) and analyzed with the Mann–Whitney U test. The Chi-square(χ2) test was used to analyze categorical variables. Two-sided P-values of 0.05 were considered significant.
The ED50 and ED90 of remimazolam besylate for inhibiting body movement response within the first two minutes of gastroscopy were calculated using isotonic regression via the pooled adjacent violators algorithm (PAVA), implemented using the gpava () function from the isotone package in R (version 4.4.1). The 95% CI of the results were calculated by bootstrapping algorithm with 2000 repeated samples, implemented via the boot () function from the boot package (version 1.3–28) [18]. Estimates of the ED90 for remimazolam besylate in each group were obtained, and differences between the groups was quantified by calculating the relative mean potency with a 95% CI, as previously described [19].
Results
General data
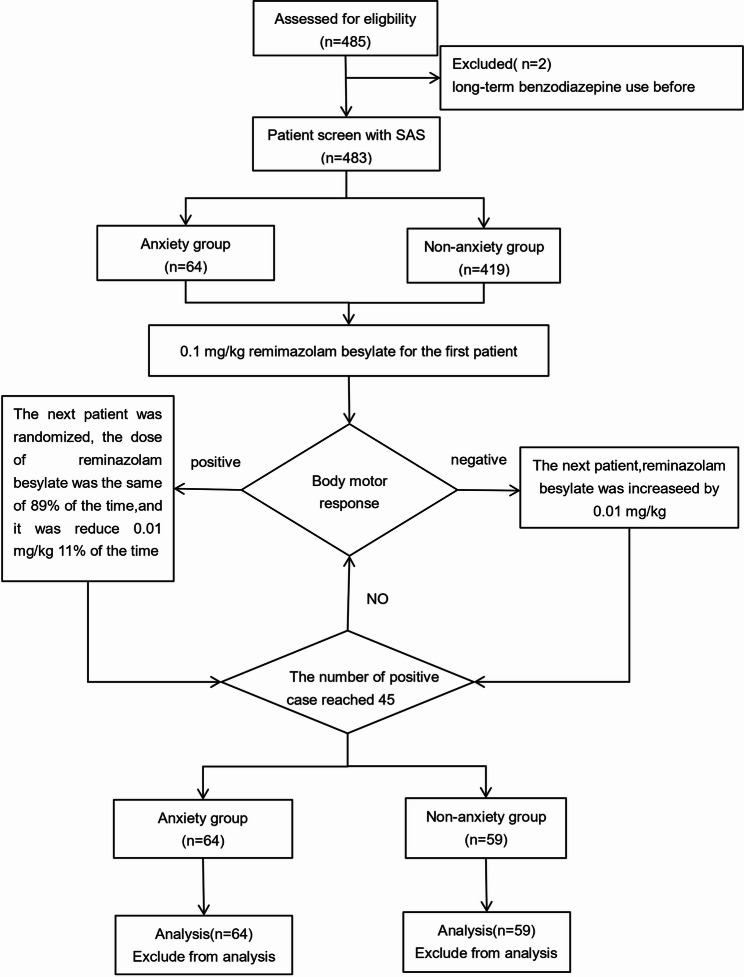
A total of 485 patients were screened in this study, with 66 (13.6%) exhibiting varying degrees of preoperative anxiety: 49 (10.1%) had mild anxiety, 14 (2.9%) had moderate anxiety, and 3 (0.6%) had severe anxiety. Two patients with severe anxiety were excluded because of long-term benzodiazepine use prior to surgery. The 45th negative response was observed in the 64th anxious and 59th non-anxious patient, respectively, triggering early termination as prespecified. A total of 64 anxious and 59 non-anxious patients were included. Detailed information about these participants is presented in Fig. 1.
Fig. 1.
CONSORT diagram describing each stage of the randomized trial
There were no significant differences in the baseline characteristics between the two groups, including age, sex, BMI, ASA classification, education level or history of gastroscopy (P > 0.05, Table 1). However, the anxiety group had significantly higher SAS scores than the non-anxiety group (P < 0.001, Table 1).
Table 1.
Baseline characteristics and intraoperative data
| Characteristics | Anxiety group (n = 64) |
Non-anxiety group (n = 59) |
P |
|---|---|---|---|
| Age, (y) | 48.3 ± 8.3 | 49.2 ± 8.4 | 0.576 |
| Sex, n (%) | |||
| Male | 15(23.4) | 19(32.2) | 0.597 |
| Female | 49(76.6) | 40(67.8) | |
| Weight, (kg) | 61.1 ± 8.2 | 62.5 ± 9.1 | 0.383 |
| Hight, (cm) | 162.5 ± 6.7 | 163.2 ± 7.3 | 0.626 |
| BMI, (kg/m2) | 23.1 ± 2.5 | 23.4 ± 2.4 | 0.490 |
| ASA, n(%) | 0.995 | ||
| I | 54(84.4) | 50(84.7) | |
| II | 10(15.6) | 9(15.3) | |
| Education level, n (%) | |||
| Primary school | 12(18.7) | 8(13.6) | 0.337 |
| Junior high school | 24(37.5) | 31(52.5) | |
| High school | 17(26.6) | 10(16.9) | |
| University or higher | 11(17.2) | 10(16.9) | |
| History of gastroscopy, n (%) | 11(17.2) | 13(22.0) | 0.498 |
| SAS score | 53.4(51.3, 58.8) | 35.0(31.3, 38.8) | < 0.001 |
Notes: Data are expressed as M ± SD, or number (%)
Abbreviations: BMI Body mass index, ASA American Society of Anesthesiologists status, SAS Self-rating anxiety scale
Intraoperative and recovering conditions
No significant differences were observed in gastroscopy examination time, propofol rescue dose, the number of patients requiring propofol or discharge time during the procedure (P > 0.05, Table 2).
Table 2.
Intraoperative and recovering conditions
| Anxiety Group (n = 64) |
Non-anxiety Group (n = 59) |
P | |
|---|---|---|---|
| Gastroscopy examination time, (min) | 6.0 ± 6.9 | 5.4 ± 0.8 | 0.499 |
| Propofol rescue dose, (mg) | 14.5 ± 26.1 | 12.2 ± 25.5 | 0.625 |
| Discharge time, (min) | 21.3 ± 3.9 | 22.3 ± 4.5 | 0.172 |
The ED50 and ED90 of remimazolam besylate for inhibiting body movement response within two minutes of gastroscopy
The dose-response chart illustrates patient responses (positive response or negative response) at each dose level, with the sequence of individuals on the X-axis and the assigned remimazolam besylate dose on the Y-axis. In the anxiety group, 45 patients showed a negative response and 19 a positive response (Fig. 2). In the non-anxiety group, 45 patients exhibit a negative response and 14 a positive response (Fig. 3).
Fig. 2.
Dose-response sequence of remimazolam besylate in the anxiety group for gastroscopy
Fig. 3.
Dose-response sequence of remimazolam besylate in the non-anxiety group for gastroscopy
Utilizing regression and bootstrapping analyses, the ED50 and ED90 of remimazolam besylate in the anxiety group were 0.175 mg/kg (95% CI, 0.140–0.240) and 0.251 mg/kg (95% CI, 0.173–0.250), respectively. In the non-anxiety group, they were lower: 0.126 mg/kg (95% CI, 0.116–0.150) and 0.148 mg/kg (95%CI, 0.130–0.160). The observed and PAVA-adjusted negative response rates for each remimazolam besylate dose are detailed in Table 3 (anxiety group) and Table 4 (non-anxiety group).
Table 3.
Negative response rates at different remimazolam besylate dose levels and PAVA-adjusted negative response rates in the anxiety group
| Assigned dose | Successful insertion | Trials | Observed response rates | PAVA adjusted response rates |
|---|---|---|---|---|
| 0.1 | 0 | 1 | 0 | 0 |
| 0.11 | 0 | 1 | 0 | 0 |
| 0.12 | 0 | 1 | 0 | 0 |
| 0.13 | 0 | 1 | 0 | 0 |
| 0.14 | 2 | 4 | 0.50 | 0.50 |
| 0.15 | 3 | 4 | 0.75 | 0.75 |
| 0.16 | 2 | 3 | 0.67 | 0.67 |
| 0.17 | 3 | 4 | 0.75 | 0.75 |
| 0.18 | 3 | 4 | 0.75 | 0.75 |
| 0.19 | 4 | 6 | 0.67 | 0.67 |
| 0.20 | 3 | 4 | 0.75 | 0.75 |
| 0.21 | 1 | 2 | 0.50 | 0.50 |
| 0.22 | 4 | 5 | 0.90 | 0.80 |
| 0.23 | 0 | 1 | 0 | 0 |
| 0.24 | 4 | 6 | 0.67 | 0.67 |
| 0.25 | 8 | 9 | 0.89 | 0.89 |
| 0.26 | 8 | 8 | 1.00 | 1.00 |
Table 4.
Negative response rates at different remimazolam besylate dose levels and PAVA-adjusted negative response rates in the non- anxiety group
| Assigned dose | Successful insertion | Trials | Observed response rates | PAVA adjusted response rates |
|---|---|---|---|---|
| 0.1 | 0 | 1 | 0 | 0 |
| 0.11 | 0 | 3 | 0 | 0 |
| 0.12 | 4 | 6 | 0.67 | 0.67 |
| 0.13 | 6 | 9 | 0.67 | 0.60 |
| 0.14 | 13 | 15 | 0.87 | 0.87 |
| 0.15 | 9 | 10 | 0.90 | 0.91 |
| 0.16 | 6 | 7 | 0.86 | 0.86 |
| 0.17 | 6 | 6 | 1.00 | 1.00 |
The ED90 equivalent ratio between the two groups was 0.59 (95% CI, 0.468–0.710), indicating a statistically significant difference, as the 95% CI does not include 1. This confirms a significantly higher ED90 in the anxiety group.
Adverse events
Perioperative adverse events showed no significant differences between groups (all P > 0.05, Table 5). The incidence of respiratory depression was comparable between groups (9.4% vs. 11.9%, P = 0.654), as was dizziness (7.8% vs. 5.1%, P = 0.805). Hypotension was more frequent in the anxiety group (15.6% vs. 8.5%), but not statistically significant (P = 0.226). Notably, no cases of nausea or vomiting were reported in either group.
Table 5.
Adverse events
| Adverse Reactions | Anxiety Group (n = 64) |
Non-anxiety Group (n = 59) | P |
|---|---|---|---|
| Respiratory depression, n (%) | 6(9.4) | 7(11.9) | 0.654 |
| Hypotension, n (%) | 10(15.6) | 5(8.5) | 0.226 |
| Nausea and vomiting, n (%) | 0(0) | 0(0) | > 0.99 |
| Dizziness, n (%) | 5(7.8) | 3(5.1) | 0.805 |
Data are presented as the number of patients, n(%)
Discussion
Our study provides novel evidence that preoperative anxiety significantly increases remimazolam besylate requirements during gastroscopy. When combined with alfentanil 5 µg/kg, the ED50 and ED90 of remimazolam besylate for inhibiting body movement within two minutes of gastroscopy in anxious patients were 0.175 mg/kg and 0.251 mg/kg, respectively, but this increase had no significant effect on prolonged discharge time or the incidence of adverse reactions.
Remimazolam besylate, a novel anesthetic sedative, has been extensively studied for its pharmacodynamics and safety since its market introduction. However, little research has focused on the influence of psychological factors, particularly anxiety [20, 21]. In this study, the ED90 equivalence ratio between the two groups was 0.59, indicating that the ED90 of remimazolam besylate to inhibit body movement responses during gastroscopy in anxious patients increased by approximately 41% compared to non-anxious patients when combined with alfentanil 5 µg/kg. This phenomenon may be associated with heightened arousal and enhanced pain sensitivity in anxious patients. Studies have shown that anxiety induces heightened central nervous system arousal and increases sensitivity to external stimuli, thereby diminishing the sedative effect of anesthetic drugs [22, 23]. Additionally, anxiety is often associated with excessive sympathetic nervous system activation, which heightens the stress response and impairs the ability to achieve sedation [24, 25]. Simultaneously, evidence indicates that anxiety alters spinal pain signaling [26], resulting in heightened pain sensitivity and nociceptive hypersensitivity [5, 27]. The combined effects of heightened arousal and enhanced pain sensitivity may further elevate the requirement for sedative medications.
However, the results of the present study differ from those of previous reports [28]. Chuang et al. [28] reported no significant correlation between the propofol dose required to induce loss of consciousness and preoperative anxiety scores in colonoscopy patients when combined with 10 µg/kg alfentanil. This difference may be attributed to variations in examination techniques, alfentanil dosage and anxiety measurement tools. First, regarding the procedure, gastroscopy induces pharyngeal reflexes and intense vagus nerve stimulation, leading to significantly higher cortical arousal compared to colonoscopy. A Phase IIa study demonstrated that even with a high dose of remimazolam (0.2 mg/kg), the success rate of gastroscopy was only 64%, and frequent additional sedation was needed to suppress the body movement reactions [29]. In contrast, the phase III clinical trial for colonoscopy revealed that 7.5 mg of remimazolam achieved a procedural success rate of 91.3%, indicating lower stimulation intensity during colonoscopy and correspondingly reduced sedation requirements [30]. Second, regarding pharmacological action, alfentanil exhibits a dose-dependent sedative effect that potentiates the efficacy of other sedative agents [31], thereby reducing the intraoperative requirement for sedative medications [32]. The literature indicates that 10 µg/kg alfentanil alone suffices to achieve moderate sedation in patients undergoing colonoscopy [33]. At this dose, alfentanil likely provides adequate analgesia and sedation to suppress nociceptive stimuli without requiring additional sedative medication. The lower alfentanil dose used in this study (5 µg/kg) may be insufficient to fully suppress stimuli, necessitating an increased dose of remimazolam besylate as compensation. Third, the assessment tools differed between studies: we used the SAS, whereas Chuang et al. had used the Beck Anxiety Inventory (BAI). Although both are validated instruments, they assess different dimensions of anxiety. The BAI primarily targets somatic symptoms [34], while the SAS covers both psychological and physiological manifestations. These differences may explain the variability in anxiety classification and its observed effects on sedation.
Although the SAS is a widely accepted self-reported measure of anxiety in both clinical and research settings, the State-Trait Anxiety Inventory (STAI) is often regarded as the research gold standard due to its comprehensive assessment of both trait and state anxiety [35]. The STAI consists of 40 items and typically requires about 30 min to complete, which limits its use in routine clinical practice. Moreover, STAI scores are inconsistently interpreted across studies. Deivasigamani defined anxiety levels as mild (20–37), moderate (38–44), and severe (≥ 45) [36], whereas Shen used a single cut-off of 40 to distinguish between anxious and non-anxious patients in surgical populations [37]. Variability in population characteristics and threshold selection has led to inconsistent anxiety grading systems. In contrast, the SAS is particularly well-suited for clinical settings that demand rapid assessment and decision-making.
Although anxiety significantly increased the induction dose requirement for remimazolam besylate, it did not prolong discharge time. No severe adverse effects (e.g., respiratory depression, hypotension, dizziness) or statistically significant differences in adverse event incidence were observed between groups. These findings may be attributed to the rapid onset, fast metabolism, and mild respiratory and circulatory effects of remimazolam besylate [38]. Additionally, the study population was relatively healthy (ASA I-II), the procedure was non-surgical (gastroscopy), and the examination duration was short with minimal nociceptive stimuli.
Limitation
This study has several limitations. First, patients over 60 years old were excluded, leaving the characteristics of elderly and super-elderly populations unexamined. Second, the absence of gender-stratified analysis limited the identification of potential differences in drug responses between male and female patients, potentially affecting the generalizability of the findings. Third, observations were limited to body movement responses during the first 2 min of gastroscopic insertion, without body movements throughout the procedure. Fourth, anxiety levels were not stratified, future studies should explore how different anxiety levels influence the pharmacodynamics of remimazolam besylate. Finally, relying solely on the SAS may introduce misclassification due to subjective underreporting. Future studies should incorporate objective measures such as heart rate variability or cortisol to validate anxiety levels.
Conclusion
When combined with 5 µg/kg alfentanil, the ED50 and ED90 of remimazolam besylate for inhibiting body movement responses within 2 min of gastroscopy in anxious patients were 0.175 mg/kg and 0.251 mg/kg, respectively. Anxiety increased the requirement for remimazolam besylate in patients undergoing gastroscopy, but it did not increase the risk of prolonged discharge time or adverse effects.
Supplementary Information
Supplementary Material 1. Supplementary File 1. Self-Rating Anxiety Scale (SAS).
Acknowledgements
Not applicable.
CONSORT statement
This study adheres to the CONSORT 2010 guidelines for reporting randomized trials.
Authors’ contributions
B.Z.Cha: Conducted research, acquired and analyzed data, interpreted the results, and wrote and revised the manuscript.S.Y.Hu: Contributed to study conception, design, and manuscript revision; N.P.Chen: Managed anesthesia and contributed to patient recruitment; J.F.Cao, C.X, and K.Q: Conducted the study and acquired data; F.Q.Luo: Conceived and designed the study, conducted the study, planned, analyzed, and interpreted the data, and prepared and revised the manuscript.All authors reviewed the final manuscript.
Funding
None.
Data availability
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committees of Shaoxing People’s Hospital (2024-Yanli 048 − 01) and the Affiliated Hospital of Shaoxing University(2023-(Yan)-007-02). Trial registration was conducted in the Chinese Clinical Trial Registry on July 15, 2024 (ChiCTR2400086957). All patients provided written informed consent before participating in the study and methods.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang M, Lu LL, Zhao M, et al. Associations of anxiety with discomfort and tolerance in Chinese patients undergoing Esophagogastroduodenoscopy. PLoS ONE. 2019;14(2):e0212180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan AA, Ali A, Khan AS, et al. Effects of visual aid on state anxiety, fear and stress level in patients undergoing endoscopy: a randomized controlled trial. Ann Med. 2023;55(1):1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhart L, Aust H, Schuster M, et al. Preoperative anxiety in adults - across-section study on specific fears and risk factors. BMC Psychiatry. 2020;20(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang HJ, Koh E, Kang Y. Susceptibility of women to cardiovascular disease and the prevention potential of mind-body intervention by changes in neural circuits and cardiovascular physiology. Biomolecules. 2021;11(5):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmaz IF, Yilmaz CY, Daskaya H, et al. The effect of preoperative anxiety and pain sensitivity on preoperative hemodynamics, Propofol consumption, and postoperative recovery and pain in endoscopic ultrasonography. Pain Ther. 2021;10(2):1283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gürbulak B, Üçüncü MZ, Yardımcı E, et al. Impact of anxiety on sedative medication dosage in patients undergoing Esophagogastroduodenoscopy. Wideochir Inne Tech Maloinwazyjne. 2018;13(2):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larach DB, Sahara MJ, As-Sanie S, et al. Patient factors associated with opioid consumption in the month following major surgery. Ann Surg. 2021;273(3):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa EC, Espírito Santo PA, Baraldo S, et al. Remimazolam versus Propofol for sedation in Gastrointestinal endoscopic procedures: a systematic review and meta-analysis. Br J Anaesth. 2024;132(6):1219–29. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Wang S, Niu X, et al. The effects of transcranial magnetic stimulation on patients with chronic insomnia: a prospective functional near-infrared spectroscopy study. Sleep Med. 2025;131:106517. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Yang W, Pan T, et al. Comparative study of rhBMP-2 assisted femoral neck system and cannulated screws in femoral neck fractures: clinical efficacy and psychological status. Sci Rep. 2025;15(1):12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui X, Cheng Z, Li H, et al. Efficacy and safety of different doses of remimazolam tosilate applied in upper Gastrointestinal endoscopy: a prospective randomized controlled double-blind trial. Drug Des Devel Ther. 2023;17:2889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Chen Y, Wang W, et al. The 90% minimum effective volume and concentration of ropivacaine for ultrasound-guided median nerve block in children aged 1–3 years: A biased-coin design up-and-down sequential allocation trial. J Clin Anesth. 2022;79:110754. [DOI] [PubMed] [Google Scholar]
- 13.Zuo RH, Zhuang JW, Chen QM, et al. Median effective dose of remimazolam combined with alfentanil for painless gastroscopy. Int J Anesthesiology Resusc. 2022;43(08):813–7. [Google Scholar]
- 14.Wang P, Xue S, Zhang L, et al. Determination of ED50 and ED95 of remimazolam besylate combined with alfentanil for adult gastroscopy: a prospective dose-finding study. Braz J Anesthesiol. 2024;74(4):844518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose Estimation in anesthesia research. Anesthesiology. 2007;107(1):144–52. [DOI] [PubMed] [Google Scholar]
- 16.Park SJ, Min SK, Choi G, et al. The degree of respiratory depression according to the effect-site concentration in remimazolam target-controlled infusion: a randomised controlled trial. Eur J Anaesthesiol. 2024;41(10):728–37. [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Zhang J, Hu Z, et al. The efficacy and safety of remimazolam in painless colonoscopy: a prospective, randomized clinical trial. Front Med (Lausanne). 2024;11:1434767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stylianou M, Proschan M, Flournoy N. Estimating the probability of toxicity at the target dose following an upanddown design[J]. Stat Med. 2003;22(4):535–43. [DOI] [PubMed] [Google Scholar]
- 19.Huang XD, Chen JB, Dong XY, et al. The impact of Fentanyl on the effective dose of remimazolam-induced sedation in elderly female patients: an up-and-down sequential allocation trial. Drug Des Devel Ther. 2024;18:3729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng D, Yang ZH, Li YN, et al. Effect of gender factor on efficacy of remimazolam combined with alfentanil in patients undergoing Gastrointestinal endoscopy. Chin J Anesthesiology. 2023;43(01):76–9. [Google Scholar]
- 21.Chae D, Kim HC, Song Y, et al. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129(1):49–57. [DOI] [PubMed] [Google Scholar]
- 22.Suwaluk A, Chutabhakdikul N. Altered development of prefrontal GABAergic functions and Anxiety-like behavior in adolescent offspring induced by prenatal stress. Brain Sci. 2022;12(8):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma GQ, Hu YT, Wei F, et al. Effect of preoperative anxiety on consciousness and autonomic nervous activity during Propofol anesthesia. Chin J Anesthesiology. 2025;45(01):42–8. [Google Scholar]
- 24.Uysal Aİ, Altıparmak B, Korkmaz TM, et al. The effect of preoperative anxiety level on mean platelet volume and Propofol consumption. BMC Anesthesiol. 2020;20(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gras S, Servin F, Bedairia E, et al. The effect of preoperative heart rate and anxiety on the Propofol dose required for loss of consciousness. Anesth Analg. 2010;110(1):89–93. [DOI] [PubMed] [Google Scholar]
- 26.Guan L, Qiu M, Li N, et al. Inhibitory gamma-aminobutyric acidergic neurons in the anterior cingulate cortex participate in the comorbidity of pain and emotion. Neural Regen Res. 2024;20:2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kil HK, Kim WO, Chung WY, et al. Preoperative anxiety and pain sensitivity are independent predictors of Propofol and Sevoflurane requirements in general anaesthesia. Br J Anaesth. 2012;108(1):119–25. [DOI] [PubMed] [Google Scholar]
- 28.Chung KC, Juang SE, Lee KC, et al. The effect of pre-procedure anxiety on sedative requirements for sedation during colonoscopy. Anaesthesia. 2013;68(3):253–9. [DOI] [PubMed] [Google Scholar]
- 29.Borkett KM, Riff DS, Schwartz HI, et al. A phase iia, randomized, double-blind study of remimazolam (CNS 7056) versus Midazolam for sedation in upper Gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–80. [DOI] [PubMed] [Google Scholar]
- 30.Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and Midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–37. [DOI] [PubMed] [Google Scholar]
- 31.Yang LJ, Li YH, Liang YP, et al. Comparison of anesthetic effects of different target plasma concentrations of alfentanil combined with remimazolam for hysteroscopic surgery. Chin J Anesthesiology. 2023;43(06):746–8. [Google Scholar]
- 32.Deng S, Huang X, Lei X. Effects of different doses of alfentanil combined with target-controlled infusion (TCI) of Propofol for daytime hysteroscopy. Heliyon. 2024;10(14):e34161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Chen X, Zheng X, et al. Effects of single-use alfentanil versus Propofol on cognitive functions after colonoscopy: a randomized controlled trial. Heliyon. 2023;9(6):e17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lykke J, Hesse M, Austin SF. Validity of the BPRS, the BDI and the BAI in dual diagnosis patients. Addict Behav. 2008;33(2):292–300. [DOI] [PubMed] [Google Scholar]
- 35.Yue QQ, Feng GH, Peng T. What is the current state of anxiety and its related factors in Chinese patients undergoing colonoscopy? A cross-sectional study. BMC Psychol. 2025;13(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deivasigamani S, Adams ES, Kotamarti S, et al. Comparison of procedural anxiety and pain associated with conventional transrectal ultrasound prostate biopsy to magnetic resonance imaging-ultrasound fusion-guided biopsy: a prospective cohort trial. Prostate Cancer Prostatic Dis. 2024;27(2):294–9. [DOI] [PubMed] [Google Scholar]
- 37.Shen Y, Yin L, Hu B, et al. Preoperative anxiety’s impact on the median effective dose of Esketamine for alleviating Propofol injection pain in patients undergoing painless abortion: A randomized, double-blind, controlled trial. Drug Des Devel Ther. 2024;18:5863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao MJ, Hu HF, Li XL, et al. The safety and efficacy between remimazolam and Propofol in intravenous anesthesia of endoscopy operation: a systematic review and meta-analysis. Int J Surg. 2023;109(11):3566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Supplementary File 1. Self-Rating Anxiety Scale (SAS).
Data Availability Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.