Abstract
Background
To investigate the diagnostic accuracy of lung ultrasound (LUS) for interstitial lung disease (ILD) in patients with rheumatoid arthritis (RA).
Methods
This retrospective study included patients over 18 years with RA evaluated at the Department of Rheumatology and Immunology of Shantou Central Hospital. All patients underwent chest high-resolution computed tomography (HRCT) and LUS within one month. The LUS was performed in a total of 50 scanning sites (ScS), and the number of B-lines present in each ScS was counted and summed up as B-lines score. A positive judgement was given on LUS when the B-lines score exceeded 10. The presence and patterns of ILD were defined by HRCT findings. ROC curve analysis was used to calculate the accuracy of LUS to detect ILD.
Results
A total of 120 RA patients (86 women, with a median age of 56.0 [50.0–64.0] years) were enrolled. Based on the HRCT, 76 patients were found to have radiographic ILD, with 63 exhibiting nonspecific interstitial pneumonia (NSIP) and 13 showing usual interstitial pneumonia (UIP). Sonographic ILD was detected in 76 patients who underwent LUS examination. The concordance rate between two modalities was 83.33% (Kappa value = 0.64, 95% CI 0.50–0.78). The diagnostic sensitivity and specificity of LUS were 86.84% and 77.27%, respectively. The positive predictive value, negative predictive value, a positive likelihood ratio and a negative likelihood ratio were 86.84%, 77.27%, 3.82, and 0.17, respectively. The number of B-lines in RA with ILD and without ILD on HRCT showed a significant difference (34.0 [15.0–96.5] vs. 6.5 [2.5–10.0], P < 0.001). The presence of 12 B-lines on 50 ScS was the optimal cutoff value for detecting RA-ILD (AUC = 0.89, 95% CI 0.82–0.94, sensitivity of 85.53%, specificity of 81.82%, P < 0.001).
Conclusions
Lung ultrasound is a valuable diagnostic tool for RA-ILD and can be used as a screening method to identify patients who require further evaluation with chest HRCT.
Keywords: Rheumatoid arthritis, Interstitial lung disease, High-resolution computed tomography, Lung ultrasound, B-lines
Background
Rheumatoid arthritis (RA) is a chronic, inflammatory and autoimmune disease characterized by articular synovitis and bone erosion, which affecting 0.5–1% of the worldwide population [1, 2]. Despite significant advances in the treatment of RA, cardiovascular disease and respiratory complications are still two of the leading causes of death among patients [3, 4]. Interstitial lung disease (ILD), as the most prominent manifestation of pulmonary involvement in RA, is linked to a poor prognosis [5, 6]. However, the epidemiological data on ILD prevalence in patients with RA are inconsistent, ranging from 1 to 58%, due to methodological differences in assessment [4]. In addition, although the most majority of RA-ILD are asymptomatic or oligosymptomatic in the early and middle stages, it is still significantly associated with mortality [5–7]. Moreover, even the subclinical RA-ILD or the interstitial lung abnormalities (ILA) are found linked to all-cause mortality [8]. Accordingly, it is essential to screen and identify early RA-ILD in clinical practice.
Currently, chest high-resolution computed tomography (HRCT) is considered as an imaging gold standard for ILD diagnosis, evaluation and follow-up [9]. However, given that RA is more prevalent than systemic sclerosis (SSc) and idiopathic inflammatory myopathy (IIM), and considering that a certain percentage of RA patients are young and children, as well as the high cost of examinations, the use of HRCT for screening purposes can lead to unnecessary radiation exposure, wastage of medical resources, and an increased economic burden [10]. The low sensitivity of traditional chest X-rays and pulmonary function testing (PFT) limits their use in early screening [11, 12]. Chest magnetic resonance imaging (MRI) and Positron Emission Tomography/Computed Tomography (PET/CT) are still in their infancy. High technological access and bottlenecks further restrict their current application [13, 14]. In the past decade, the clinical employment of lung ultrasound (LUS) in connective tissue disease associated ILD (CTD-ILD) has been in the ascendant, although the lung parenchyma is considered the forbidden zone of ultrasound [15]. The severity and extent of ILD, along with the involvement of the pleura, were evaluated by detecting sonographic B-lines and irregularity of the pleural line [16]. Most previous studies confirmed the role of LUS in the whole journey of SSc related ILD (SSc-ILD), especially in early screening and follow-up [17, 18]. However, the clinical value of LUS in RA-ILD is still being evaluated, and most previous retrospective studies were from small sample size and mono-centre [19, 20]. Therefore, the purpose of this study was to further assess the diagnostic accuracy of LUS for RA-ILD in comparison to HRCT and to determine an optimal cutoff value for B-lines in RA-ILD.
Methods
Patients
A total of 120 patients with RA from the outpatient and inpatient departments of the Shantou Central Hospital were enrolled from August 2022 to September 2024. The diagnosis of RA was based on the 2010 RA classification criteria of the American College of Rheumatology and European League Against Rheumatism [21]. All patients underwent chest HRCT scans and LUS (independently performed within one month) examination. Patients’ demographic, clinical, and laboratory information was recorded from the electronic hospital database. Patients with a history of pulmonary infection, asthma, chronic obstructive pulmonary disease, occupational lung disease, other types of ILD, radiation lung disease, lung cancer, heart failure, renal failure, children (< 18 years old) and pregnancy were excluded from the study.
Chest HRCT
Chest HRCT was performed in all patients using 128 multi-slice CT (SIEMENS SOMATOM Definition Flash CT, German). All patients were scanned in the supine position from the lung apex to the diaphragm during end-inspiration. The acquisition parameters were as follows: 1 mm collimation and 0.9–1.2 pitch, 120 kV tube voltage and 110 reference mAS. Edge-enhancing B70 kernel was obtained by using filtered back projection for clinical reading with lung window. No intravenous contrast agent was employed. The duration of the CT acquisition was 1–3 s. Matrix was 512*512, and the effective dose was in the range of 1–3 mSv. The presence and pattern of ILD were defined by HRCT findings [22] assessed by an experienced radiologist who was blinded to the clinical, serological, and sonographic information.
Lung ultrasound
Commercially available ultrasound equipment with a 2.5–3.5 MHz cardiac sector transducer providing an imaging depth of 18 cm and a dynamic range of 65 dB was used in this study (Siemens Medical Solutions, Erlangen, Germany). Lung ultrasound was performed by two senior ultrasound physicians who were blinded to clinical, serological and radiographic information related to patients. A B-line was defined as a discrete laser-like vertical artifacts reminiscent of comet tails, originating from the pleural line, and stretching to the bottom of the screen without fading, and moving simultaneously with lung sliding [23] (Fig. 1). The number of B-lines in a total of 50 scanning sites (ScS) was recorded and calculated as B-lines score [24]. When the B-lines were confluent, the semi-quantitative rule was applied for the percentage of white screen divided by 10 on each scanning site. For example, 30% of white screen corresponded to 3 B-lines, 50% to 5 B-lines, and so on [25]. Significant sonographic ILD was according to previous studies with a total B-lines score exceeded 10 [24, 26].
Fig. 1.
Different HRCT patterns corresponded to different LUS patterns. A Normal. B HRCT: Ground-glass opacity (NSIP pattern). C HRCT: Honeycombing (UIP pattern). D Normal pleural line and A-lines visible. E Irregular pleural line and B-lines. F Confluent B-lines (“sonographic white lung”). HRCT, high-resolution computed tomography; LUS, lung ultrasound; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia
Statistical analysis
Continuous variables were expressed as median (interquartile range, IQR), and categorical variables were represented by counts and percentages. Univariate comparisons used the Mann–Whitney U test (two groups), Kruskal–Wallis test (> two groups) or chi-square test (categorical variables), as applicable. Diagnostic accuracy was calculated as (true positives + true negatives)/total cases. Interrater reliability was assessed with Cohen’s Kappa. A receiver operating characteristic (ROC) curve was constructed to assess the performance of B-lines in detecting ILD on HRCT, quantified by the area under curve (AUC). The optimal cutoff value was derived by maximizing the Youden index on the ROC curve. Statistical significance was defined as P < 0.05. All analyses were performed in MedCalc software (version 18.2.1; MedCalc Software Ltd, Ostend, Belgium).
Results
Characteristics of patients with RA
Our study involved 120 patients with RA, of which 86 were female. The median age of patients was 56.0 (50.0–64.0) years and the median duration of the disease was 24.0 (6.5–72.0) months. The demographic, clinical and radiographic data of the patients were described in Table 1. According to the chest HRCT, 76 patients were diagnosed with radiographic ILD, while 44 patients showed no signs of ILD. Respiratory symptoms were present in 38.16% of the 76 patients with ILD through careful clinical history investigation. Of which, cough (19.74%) was the most common symptom, followed by dyspnea (9.21%) and chest distress (9.21%). The radiographic ILD group has a higher prevalence of males, older age, and longer disease duration (P < 0.05). The two major radiographic patterns are nonspecific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP), each accounting for 82.89% and 17.11% respectively. Smoking history, comorbidities, rheumatoid factor (RF), anti-cyclic citrullinated peptide (CCP) antibodies, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) did not differ significantly between two groups (P > 0.05).
Table 1.
Demographic, clinical and radiographic data of patients
| Baselines characteristics | All patients (N = 120) |
Patients with radiographic ILD (n = 76) | Patients without radiographic ILD (n = 44) | P value |
|---|---|---|---|---|
| Gender, n (%) | 0.022 | |||
| Female | 86 (71.67) | 49 (64.47) | 37 (84.09) | |
| Male | 34 (28.33) | 27 (35.53) | 7 (15.91) | |
| Age, years, median (IQR) | 56.0 (50.0–64.0) | 59.5 (53.0–66.0) | 52.0 (42.0–57.0) | < 0.001 |
| Disease duration in months, median (IQR) | 24.0 (6.5–72.0) | 36.0 (12.0–90.0) | 12.0 (6.0–42.0) | 0.026 |
| Smoking history, n (%) | 0.145 | |||
| Yes | 28 (23.33) | 21 (27.63) | 7 (15.91) | |
| No | 92 (76.67) | 55 (72.37) | 37 (84.09) | |
| Comorbidities, n (%) | ||||
| Hypertension | 29 (24.17) | 21 (27.63) | 8 (18.18) | 0.105 |
| Diabetes mellitus | 9 (7.50) | 8 (10.53) | 1 (2.27) | 0.149 |
| Cardiovascular disease | 5 (4.17) | 5 (6.58) | 0 (0) | 0.156 |
| RF, n (%) | 0.087 | |||
| Negative | 23 (19.17) | 11 (14.47) | 12 (27.27) | |
| Positive | 97 (80.83) | 65 (85.53) | 32 (72.73) | |
| Anti-CCP autoantibody, n (%) | 0.081 | |||
| Negative | 15 (12.50) | 9 (11.84) | 6 (13.64) | |
| Positive | 105 (87.50) | 67 (88.16) | 38 (86.36) | |
| ESR, mm/h, median (IQR) | 43.0 (27.5–64.3) | 45.0 (28.0–64.8) | 42.5 (24.0–62.0) | 0.710 |
| CRP, mg/L, median (IQR) | 20.3 (9.7–48.9) | 20.3 (10.6–43.6) | 21.2 (8.1–53.3) | 0.667 |
| Total B-lines, median (IQR) | 18.0 (7.0–55.5) | 34.0 (15.0–96.5) | 6.5 (2.5–10.0) | < 0.001 |
| Respiratory symptoms, n (%) | 0.401 | |||
| Dyspnea | 9 (7.50) | 7 (9.21) | 2 (4.55) | |
| Cough | 17 (14.17) | 15 (19.74) | 2 (4.55) | |
| Chest distress | 7 (5.83) | 7 (9.21) | 0 (0) | |
| HRCT patterns, n (%) | NA | |||
| UIP | 13 (10.83) | 13 (17.11) | 0 (0) | |
| NSIP | 63 (52.50) | 63 (82.89) | 0 (0) | |
| Current treatment of RA | ||||
| GC, n (%) | 112 (93.33) | 73 (96.05) | 39 (88.64) | 0.118 |
| NSAIDs, n (%) | 80 (66.67) | 49 (40.83) | 31 (70.45) | 0.505 |
| MTX, n (%) | 53 (44.17) | 23 (19.17) | 30 (68.18) | 0.000 |
| LEF, n (%) | 4 (3.33) | 1 (1.31) | 3 (6.82) | 0.107 |
| SSZ, n (%) | 53 (44.17) | 29 (38.16) | 24 (54.55) | 0.083 |
| HCQ, n (%) | 47 (39.17) | 25 (32.89) | 22 (50.00) | 0.066 |
| TNFi, n (%) | 2 (1.67) | 0 (0) | 2 (4.55) | 0.062 |
| JAKi, n (%) | 54 (45.00) | 27 (35.53) | 27 (61.36) | 0.006 |
| MMF, n (%) | 4 (3.33) | 4 (5.26) | 0 (0) | 0.130 |
| CTX, n (%) | 0 (0) | 0 (0) | 0 (0) | 0.004 |
| Anti-fibrosis drugs, n (%) | 0 (0) | 5 (4.17) | 0 (0) | 0.084 |
CCP cyclic citrullinated peptide, CRP C-reactive protein, CTX cyclophosphamide, ESR erythrocyte sedimentation rate, GC glucocorticoids, HCQ hydroxychloroquine, HRCT high-resolution computed tomography, IQR interquartile range, JAKi janus kinase inhibitors, LEF leflunomide, MTX methotrexate, MMF mycophenolate mofetil, NA not applicable, NSAIDs nonsteroidal anti-Inflammatory drugs, NSIP nonspecific interstitial pneumonia, RA rheumatoid arthritis, RF rheumatoid factor, SSZ sulfasalazine, TNFi tumor necrosis factor inhibitor, UIP usual interstitial pneumonia
Lung ultrasound findings
The B-lines score was significantly higher in the radiographic ILD group than the non-ILD group (34.0[15.0–96.5] vs. 6.5[2.5–10.0], P < 0.001). Subgroup analysis further revealed that the B-lines score was much higher in the UIP group than in the NSIP group (161.0 [117.8–212.0] vs. 28.0 [14.0–57.7], P < 0.001) (Fig. 2).
Fig. 2.
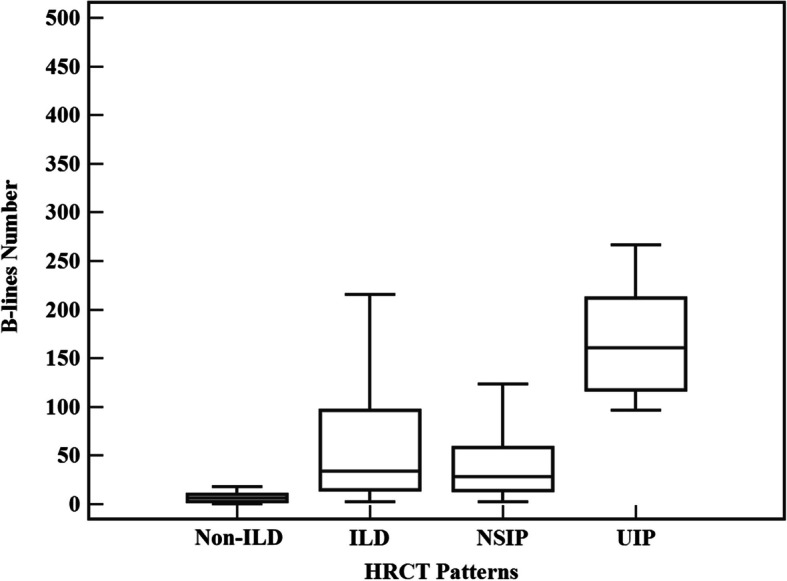
Significant difference of in the number of B-lines among different HRCT patterns. HRCT, high-resolution computed tomography; ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia
The diagnostic concordance between LUS and HRCT
Sonographic ILD, which is measured by the total B-lines score exceeding 10, was detected in 76 patients (Table 2). The concordance rate between LUS and HRCT is 83.33% (Kappa value = 0.64, 95% CI 0.50–0.78). The sensitivity and specificity of LUS were 86.84% (95% CI 77.1%−93.5%) and 77.27% (95% CI 62.2%−88.5%), respectively. The positive predictive value (PPV), negative predictive value (NPV), a positive likelihood ratio (LR +) and a negative likelihood ratio (LR-) were 86.84% (95% CI 79.2%−92.0%), 77.27% (95% CI 65.1%−86.1%), 3.82(95% CI 2.2–6.6), and 0.17 (95% CI 0.09–0.3), respectively.
Table 2.
The concordance analysis of LUS and HRCT for RA-ILD
| LUS | HRCT | Sensitivity | Specificity | PPV | NPV | LR + | LR- | Kappa Value | Overall Accuracy | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ILD | Non-ILD | ||||||||||
| B-lines > 10 | 66 | 10 |
86.84% (77.1%−93.5%) |
77.27% (62.2%−88.5%) |
86.84% (79.2%−92.0%) |
77.27% (65.1%−86.1%) |
3.82 (2.2–6.6) |
0.17 (0.09–0.3) |
0.64 (0.05–0.78) |
83.33% | < 0.001 |
| B-lines ≤ 10 | 10 | 34 | |||||||||
| B-lines > 12 | 65 | 8 | 85.53% (75.6%−92.5%) |
81.82% (67.3%−91.8%) |
89.00% (81.2%−93.9%) |
76.60% (65.1%−85.2%) |
4.7 (2.5–8.9) |
0.18 (0.1–0.3) |
0.66 (0.53–0.80) |
84.17% | < 0.001 |
| B-lines ≤ 12 | 11 | 36 | |||||||||
CI confidence interval, HRCT high-resolution computed tomography, ILD interstitial lung disease, LR + positive likelihood ratio, LR- negative likelihood ratio, LUS lung ultrasound, NPV negative predictive value, PPV positive predictive value, RA-ILD rheumatoid arthritis related interstitial lung disease, Values in parentheses denote 95% CI
The best cutoff value of B-lines score to discriminate RA-ILD
Receiver operating characteristic (ROC) curve analysis showed a total of B-lines exceeding12 (AUC = 0.89, 95% CI 0.82–0.94, P < 0.001) on 50 ScS was the best cutoff value for detecting RA-ILD with an overall accuracy rate of 84.17% (Fig. 3). The sensitivity, specificity, PPV, NPV, LR + and LR- were 85.53% (95%CI 75.6%−92.5%), 81.82% (95%CI 67.3%−91.8%), 89.0% (95%CI 81.2%−93.9%), 76.60% (95%CI 65.1%−85.2%), 4.7(95%CI 2.5–8.9), and 0.18(95%CI 0.1–0.3), respectively.
Fig. 3.

ROC curve showing accuracy of LUS in identifying the presence of radiographic ILD. AUC, area under curve; ILD, interstitial lung disease; ROC, receiver operating characteristic
Discussion
Interstitial lung disease is a common extra-articular manifestation of RA, which is linked to morbidity and mortality. Although several risk factors for the development of ILD in RA patients have been identified, ILD can still occur even in the absence of these risk factors [27]. According to previous epidemiological researches, UIP and fibrotic NSIP are the primary radiographic manifestations in individuals with symptomatic RA-ILD. Therefore, in order to improve outcomes, it is crucial to detect RA-ILD early, allowing for timely implementation of appropriate treatment strategies. However, ideal screening tools for RA-ILD have not been available until now. Apart from traditional imaging tools, LUS is playing an increasingly active role in this field.
In our cohort, we found that 63.33% of patients with RA have radiographic ILD, and up to 61.84% of them have no respiratory symptoms. Along with published studies [7, 28], our data further confirms that asymptomatic ILD is more prevalent in early RA. Compared to some previous findings [29–32], we found that NSIP was the most common radiographic pattern, rather than UIP. One explanation for this phenomenon is owing to our proactive screening strategy, which had led to discovery more early-stage ILDs.
Compared to the non-ILD group, patients with radiographic ILD had significantly higher B-lines score. In addition, B-lines score of UIP subgroup was significantly higher than the NSIP subgroup. These results are in accordance with previous studies on SSc-ILD and others CTD-ILD [33–36]. Mena-Vazquez et al. also observed that the trend of B-lines number increased in the UIP pattern compared to the NSIP pattern (100.7 ± 88.2 vs. 83.7 ± 36.6, P > 0.05) in RA-ILD [32]. These findings indicate that a higher B-lines score could reflect more severe lung fibrosis.
Although the crude agreement rate between LUS and HRCT for diagnosing ILD reached 83.88%, the Kappa statistic (κ = 0.64) indicates only moderate to substantial agreement. This discrepancy arises because the Kappa statistic corrects for chance agreement and tends to yield more conservative estimates in conditions with low disease prevalence. Notably, the κ value of 0.64 remains significantly higher than the level expected by chance alone (P < 0.001), demonstrating a substantial diagnostic concordance between LUS and HRCT. Future efforts should focus on standardizing LUS operating procedures to enhance its reproducibility. Thus, our data suggest that LUS could be an alternative imaging tool for screening and diagnosing ILD in patients with RA. However, these findings require confirmation and validation through more prospective studies with larger sample size.
Since the lack of a standardized examining protocol for LUS in RA-ILD diagnosis, various scanning methods with different numbers of ScS (including 72, 65, 50, 14, and 10) have been used across different centers. Recently, a meta-analysis that included a total of 487 patients with CTD demonstrated that the simplified method, which consists of 14 ScS, is the most effective scanning approach compared to others, with a sensitivity of 98.2% and a specificity of 87.5%. However, the analysis noted a small number of patients in each subgroup and significant heterogeneity among the studies involved [37]. The 50 ScS method provides comprehensive coverage of the anterior, lateral, and posterior thorax with reasonable time efficiency, while avoiding interference from diaphragmatic motion, abdominal organs, and cardiac activity during LUS assessment. Therefore, we recommend the 50 ScS protocol for RA-ILD screening.
Table 3 summarized most of the previous studies that have explored the potential application of LUS as a screening and diagnostic tool for RA-ILD [28, 32, 38–42]. Although diagnostic efficacy of LUS for RA-ILD varies, with sensitivity ranging from 62.2% to 98.3% and specificity ranging from 14.7% to 97.62%, it shows a good correlation with the HRCT score. Up to now, the optimal cutoff value of B-lines for RA-ILD remains uncertain. According to the ROC curve analysis, we determined that using a cutoff value of more than 12 B-lines on 50 ScS was the most accurate for detecting RA-ILD, offering an optimal balance between sensitivity and specificity.
Table 3.
Main studies on LUS in RA-ILD
| Study | Sample Size | Radiographic ILD, n (%) | Scanning Sites | Probe | Reference Image | Correlation Coefficient, (r) | Concordance Rate | Kappa Value | Cutoff value (B-Lines) | AUC | Sensitivity | Specificity | PPV | NPV | LR + | LR- | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cogliati et al., 2014, [38] | 39 | 13 (33.3%) | 72 ScS | Convex probe 5–2 MHz | HRCT | 0.806 | NA | NA | > 17 according to ROC curve | NA | 92% (78%−100%) | 72% (54%−90%) | NA | NA | NA | NA | NA |
| Moazedi-Fuerst et al. 2014, [39] | 64 | 17 (26.6%) | 18 ScS | Convex probe 3.5 MHz | HRCT | NA | 88% | 0.92 | NA | NA | 97.1% | 97.3% | 94.3% | 98.6% | NA | NA | < 0.001 |
| Mena-Vázquez et al. 2021, [32] | 71 | 35 (49.2%) | 8 ScS | Linear probe 2.5–3.5 MHz | HRCT | NA | NA | NA | ≥ 5.5 according to ROC curve | 0.91 (0.83–0.94) | 62.2% | 91.3% | 88.4% | 69.5% | NA | NA | < 0.0001 |
| Gutierrez et al. 2022, [40]. | 74 | 27 (36.5%) | 14 ScS | Linear probe 4–12 MHz | HRCT | 0.837 | 90% | NA | ≥ 6 according to previous study | NA | 92% | 89% | 83% | 95% | 8.36 | 0.09 | < 0.001 |
| Carlo et al. 2022, [41]. | 72 | 25 (34.7%) | 14 ScS | linear probe 4–13 MHz | HRCT | 0.559 | NA | NA | > 9 according to ROC curve | 0.838 | 70.0% | 97.62% | NA | NA | 29.4 | NA | < 0.0001 |
| Otaola et al. 2024, [42]. | 106 | 32 (30.2%) | 14 ScS | Convex probe 1–8 MHz | HRCT | NA | NA | NA | ≥ 5 according to previous study | 0.82 (0.75–0.89) | 90.6% (75.0%−98.0%) | 73% (61.4%–82.6%) | 59.2% | 94.7% | NA | NA | NA |
| Santos-Moreno et al. 2024, [28]. | 192 | 117 (60.9%) | 8 ScS | Convex probe 2–5 MHz | HRCT | NA | NA | NA | > 11.5 according to ROC curve | 0.63 (0.55–0.71) | 93% | NA | NA | NA | NA | NA | < 0.003 |
| Our study, 2025 | 120 | 76 (63.3%) | 50 ScS | Cardiac probe 2.5–3.5 MHz | HRCT | 0.649 | 84.17% | 0.66 | > 12 according to ROC curve | 0.89 (0.82–0.94) | 85.53% (75.6%−92.5%) | 81.82% (67.3%−91.8%) | 89.00% | 76.60% | 4.7 | 0.18 | < 0.001 |
AUC area under curve, CI confidence interval, HRCT high-resolution computed tomography, LR + positive likelihood ratio, LR- negative likelihood ratio, LUS lung ultrasound, MHz Mega Hertz, NA not applicable, NPV negative predictive value, PPV positive predictive value, RA-ILD rheumatoid arthritis related interstitial lung disease, ScS scanning sites, Values of AUC, sensitivity and specificity in parentheses denote 95%CI
In this study, LUS detected 61.84% of patients without respiratory symptoms. There were 10 false positives and 10 false negatives cases identified by LUS. Among these, 8 cases were incorrectly diagnosed with ILD due to scars at the lung bases, but there was no radiographic evidence of ILD. Two cases may have been over-evaluated in LUS interpretation. Additionally, 10 cases were false negatives for ILD due to the blind spots in the scanning area.
There were several limitations in our study that should be considered. Firstly, this was a single-center, retrospective study, which may have introduced bias in patient selection. Secondly, not describing abnormalities in the pleural lines could lead to missed diagnosis, as some previous studies have indicated that irregularities of the pleural line are the significant sonographic manifestation of ILD [43]. Thirdly, PFTs were not included in this study due to some missing data, as approximately a quarter of patients failed to complete PFTs test. Finally, lung sonographic B-lines is not a unique sign of ILD and cannot discriminate between the inflammatory and fibrotic patterns of ILD. To enhance diagnostic accuracy and reproducibility for detecting RA-ILD, the standardization and validation of LUS examination should be fully established. Furthermore, incorporating artificial intelligence along with serum biomarkers such as Krebs von den Lungen-6 (KL-6), surfactant protein-D (SP-D), matrix metalloproteinase-9 (MMP-9), as well as clinical information like velcro crackles, could help develop a more comprehensive methodology.
Conclusions
In summary, our study demonstrates LUS can be a valid diagnostic tool for RA-ILD. The presence of 12 B-lines on 50 ScS was the optimal threshold for detecting ILD in patients with RA. LUS can be utilized as a screening method to identify patients with RA who require more advanced chest HRCT. Our results need to be confirmed by prospective, multicentric and large-sample size studies.
Acknowledgements
We thank all the patients who participated in our study and all the professional staff at our hospital.
Abbreviations
- LUS
Lung ultrasound
- ILD
Interstitial lung disease
- RA
Rheumatoid arthritis
- HRCT
High resolution computed tomography
- ScS
Scanning sites
- NSIP
Nonspecific interstitial pneumonia
- UIP
Usual interstitial pneumonia
- CI
Confidence interval
- AUC
Area under curve
- ILA
Interstitial lung abnormalities
- IIM
Idiopathic inflammatory myopathy
- PFT
Pulmonary function testing
- MRI
Magnetic resonance imaging
- CTD
Connective tissue disease
- SSc
Systemic sclerosis
- IQR
Interquartile range
- ROC
Receiver operating characteristic
- RF
Rheumatoid factor
- CCP
Cyclic citrullinated peptide
- ESR
Erythrocyte sedimentation rate
- CRP
C-reactive protein
- PPV
Positive predictive value
- NPV
Negative predictive value
- LR +
Positive likelihood ratio
- LR-
Negative likelihood ratio
- KL-6
Krebs von den Lungen-6
- SP-D
Surfactant protein-D
- MMP-9
Matrix metalloproteinase-9
Authors’ contributions
All authors contributed to the study conception and design. SYZ, ZXZ, JHZ, and MGH collected clinical, laboratory, radiographic and sonographic data. GZD analyzed the chest HRCT data. QZC and SQC performed LUS. JQL, WJZ, and YKW analyzed and interpreted the data. SJH and KDZ prepared the ethical materials. SYZ and YKW wrote the manuscript. BR, CB, AMHV, MMC and DEF reviewed and polished the article. All authors read and approved the final manuscript.
Funding
This work is supported by grants from the National Natural Science Foundation of China (No. 82271853) and the ZHONGNANSHAN MEDICAL FOUNDATION OF GUANGDONG RPOVINCE (ZNSXS-20240011).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by the Shantou Central Hospital Ethics Committee (2024-KY-018). It was conducted by the principles of the Declaration of Helsinki. All patients signed the informed consents.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaoyu Zheng, Zexuan Zhou, and Guangzhou Du contributed equally to this work.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis Nat Rev Dis Primers. 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 3.Lee EE, Shin A, Lee J, Lee JH, Ha YJ, Lee YJ, et al. All-cause and cause-specific mortality of patients with rheumatoid arthritis in Korea: A nation-wide population-based study. Joint Bone Spine. 2022;89(1): 105269. [DOI] [PubMed] [Google Scholar]
- 4.Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V. The Lung in Rheumatoid Arthritis: Focus on Interstitial Lung Disease. Arthritis Rheumatol. 2018;70(10):1544–54. [DOI] [PubMed] [Google Scholar]
- 5.Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology. 2007;46(2):350–357. [DOI] [PubMed] [Google Scholar]
- 6.Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Lokke A, Bendstrup E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017;76(10):1700–6. [DOI] [PubMed] [Google Scholar]
- 7.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168(2):159–66. [DOI] [PubMed] [Google Scholar]
- 8.McDermott GC, Hayashi K, Yoshida K, Moll M, Cho MH, Doyle TJ, et al. Prevalence and mortality associations of interstitial lung abnormalities in rheumatoid arthritis within a multicentre prospective cohort of smokers. Rheumatology. 2023;62(SI3):SI286-SI295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swensen SJ, Aughenbaugh GL, Myers JL. Diffuse lung disease: diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiology. 1997;205(1):229–34. [DOI] [PubMed] [Google Scholar]
- 10.Picano E, Matucci-Cerinic M. Unnecessary radiation exposure from medical imaging in the rheumatology patient. Rheumatology. 2011;50(9):1537–9. [DOI] [PubMed] [Google Scholar]
- 11.Suliman YA, Dobrota R, Huscher D, Nguyen-Kim TD, Maurer B, Jordan S, et al. Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease. Arthritis Rheumatol. 2015;67(12):3256–61. [DOI] [PubMed] [Google Scholar]
- 12.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56(8):622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang P, Luo Q, Wang X, Fang Q, Fu Z, Li J, et al. Comprehensive Analysis of Fibroblast Activation Protein Expression in Interstitial Lung Diseases. Am J Resp Crit Care. 2023;207(2):160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landini N, Orlandi M, Calistri L, Nardi C, Ciet P, Bellando-Randone S, et al. Advanced and traditional chest MRI sequence for the clinical assessment of systemic sclerosis related interstitial lung disease, compared to CT: disease extent analysis and correlations with pulmonary function tests. Eur J Radiol. 2024;170: 111239. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther. 2017;19(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Chen S, Zheng S, Zhou Z, Zhang W, Du G, et al. A versatile role for lung ultrasound in systemic autoimmune rheumatic diseases related pulmonary involvement: a narrative review. Arthritis Res Ther. 2024;26(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappelli S, Bellando RS, Camiciottoli G, De Paulis A, Guiducci S, Matucci-Cerinic M. Interstitial lung disease in systemic sclerosis: where do we stand? Eur Respir Rev. 2015;24(137):411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barskova T, Gargani L, Guiducci S, Randone SB, Bruni C, Carnesecchi G, et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann Rheum Dis. 2013;72(3):390–5. [DOI] [PubMed] [Google Scholar]
- 19.Lepri G, Markovic M, Bellando-Randone S, Sebastiani M, Guiducci S. The burden of interstitial lung involvement in rheumatoid arthritis: could lung ultrasound have a role in its detection? a literature review. Diagnostics. 2024;14(13). [DOI] [PMC free article] [PubMed]
- 20.Wang Y, Chen S, Zheng S, Lin J, Hu S, Zhuang J, et al. The role of lung ultrasound B-lines and serum KL-6 in the screening and follow-up of rheumatoid arthritis patients for an identification of interstitial lung disease: review of the literature, proposal for a preliminary algorithm, and clinical application to cases. Arthritis Res Ther. 2021;23(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CR, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. [DOI] [PubMed] [Google Scholar]
- 22.Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intens Care Med. 2012;38(4):577–91. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez M, Salaffi F, Carotti M, Tardella M, Pineda C, Bertolazzi C, et al. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders–preliminary results. Arthritis Res Ther. 2011;13(4):R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasoun. 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tardella M, Gutierrez M, Salaffi F, Carotti M, Ariani A, Bertolazzi C, et al. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: correlation with high-resolution computed tomography. J Rheumatol. 2012;39(8):1641–7. [DOI] [PubMed] [Google Scholar]
- 27.Koduri G, Solomon JJ. Identification, Monitoring, and Management of Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2023;75(12):2067–77. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Moreno P, Linares-Contreras MF, Rodriguez-Vargas GS, Rodriguez-Linares P, Mata-Hurtado A, Ibata L, et al. Usefulness of Lung Ultrasound as a Method for Early Diagnosis of Interstitial Lung Disease in Patients with Rheumatoid Arthritis. Open Access Rheumato. 2024;16:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EJ, Collard HR, King TJ. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136(5):1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yunt ZX, Chung JH, Hobbs S, Fernandez-Perez ER, Olson AL, Huie TJ, et al. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: Relationship to survival. Resp Med. 2017;126:100–4. [DOI] [PubMed] [Google Scholar]
- 31.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology. 2014;53(9):1676–82. [DOI] [PubMed] [Google Scholar]
- 32.Mena-Vazquez N, Jimenez-Nunez FG, Godoy-Navarrete FJ, Manrique-Arija S, Aguilar-Hurtado MC, Romero-Barco CM, et al. Utility of pulmonary ultrasound to identify interstitial lung disease in patients with rheumatoid arthritis. Clin Rheumatol. 2021;40(6):2377–85. [DOI] [PubMed] [Google Scholar]
- 33.Gargani L, Romei C, Bruni C, Lepri G, El-Aoufy K, Orlandi M, et al. Lung ultrasound B-lines in systemic sclerosis: cut-off values and methodological indications for interstitial lung disease screening. Rheumatology. 2022; 61(SI):SI56-SI64. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe S, Yomono K, Yamamoto S, Suzuki M, Gono T, Kuwana M. Lung ultrasound in the assessment of interstitial lung disease in patients with connective tissue disease: Performance in comparison with high-resolution computed tomography. Mod Rheumatol. 2024;35(1):79–87. [DOI] [PubMed] [Google Scholar]
- 35.Bruni C, Mattolini L, Tofani L, Gargani L, Landini N, Roma N, et al. Lung ultrasound b-lines in the evaluation of the extent of interstitial lung disease in systemic sclerosis. Diagnostics. 2022;12(7). [DOI] [PMC free article] [PubMed]
- 36.Tardella M, Di Carlo M, Carotti M, Filippucci E, Grassi W, Salaffi F. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: Cut-off point definition for the presence of significant pulmonary fibrosis. Medicine. 2018;97(18): e0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie HQ, Zhang WW, Sun S, Chen XM, Yuan SF, Gong ZH, et al. A simplified lung ultrasound for the diagnosis of interstitial lung disease in connective tissue disease: a meta-analysis. Arthritis Res Ther. 2019;21(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cogliati C, Antivalle M, Torzillo D, Birocchi S, Norsa A, Bianco R, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology. 2014;53(8):1497–503. [DOI] [PubMed] [Google Scholar]
- 39.Moazedi-Fuerst FC, Kielhauser SM, Scheidl S, Tripolt NJ, Lutfi A, Yazdani-Biuki B, et al. Ultrasound screening for interstitial lung disease in rheumatoid arthritis. Clin Exp Rheumatol. 2014;32(2):199–203. [PubMed] [Google Scholar]
- 40.Gutierrez M, Ruta S, Clavijo-Cornejo D, Fuentes-Moreno G, Reyes-Long S, Bertolazzi C. The emerging role of ultrasound in detecting interstitial lung disease in patients with rheumatoid arthritis. Joint Bone Spine. 2022;89(6): 105407. [DOI] [PubMed] [Google Scholar]
- 41.Di Carlo M, Tardella M, Filippucci E, Carotti M, Salaffi F. Lung ultrasound in patients with rheumatoid arthritis: definition of significant interstitial lung disease. Clin Exp Rheumatol. 2022;40(3):495–500. [DOI] [PubMed] [Google Scholar]
- 42.Otaola M, Paulin F, Rosemffet M, Balcazar J, Perandones M, Orausclio P, et al. Lung ultrasound is a promising screening tool to rule out interstitial lung disease in patients with rheumatoid arthritis. Respirology. 2024;29(7):588–95. [DOI] [PubMed] [Google Scholar]
- 43.Buda N, Piskunowicz M, Porzezinska M, Kosiak W, Zdrojewski Z. Lung Ultrasonography in the Evaluation of Interstitial Lung Disease in Systemic Connective Tissue Diseases: Criteria and Severity of Pulmonary Fibrosis - Analysis of 52 Patients. Ultraschall Med. 2016;37(4):379–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.



