Abstract
Atherosclerosis-associated disease (ASD) represents a complex pathological condition, characterized by the formation of atherosclerotic plaques within the arterial walls, encompassing cholesterol depositions, which is primarily attributed to elevated levels of low-density lipoprotein-cholesterol (LDL-C). A log-linear association between the absolute magnitude of LDL-C exposure and ASD risk has been widely studied. High levels of LDL-C have been acknowledged as the predominant culprit. The previous research findings have demonstrated that PCSK9 inhibitors (PCSK9i) can remarkably diminish the risk of ASD. The current research has primarily focused on the relevance of PCSK9 to the cardiovascular system and lipid metabolism; however, an increasing body of evidence shows that PCSK9 is pivotal in pathogenic processes in other organ systems. Yet, PCSK9’s impact on the brain is complex and not fully clarified, although several recent studies emphasize a putative role of its impact on various neurodegenerative disorders. Among neurological disorders, not only stroke but neurogenesis, neural cell differentiation, central LDL receptor metabolism, neural cell apoptosis, neuroinflammation, alcohol use disorder (AUD), amyotrophic lateral sclerosis(ALS), and Alzheimer’s Disease (AD) are related to PCSK9. PCSK9 expression in brain is low but greatly upregulated in neurological disorders. Therefore, PCSK9 is a promising pathway for the treatment of central nervous diseases. This review comprehensively describes evidence from the previous research on the effects of PCSK9i on the central nervous system, with a focus on the clinical potential of PCSK9i. We anticipate that this review will generate data that will help biomedical researchers or clinical workers develop treatments for the neurological diseases based on PCSK9i.
Keywords: PCSK9, PCSK9 inhibitors, LDL, Central nervous system diseases, Atherosclerosis
Introduction
Atherosclerosis-related disease (ASD), a lipoprotein-driven disease that leads to plaque formation at specific sites of the arterial tree through intimal inflammation, necrosis, fibrosis, and calcification, causing clinical disease through luminal narrowing or by precipitating thrombin that obstruct blood flow to the heart (coronary heart disease), brain (ischemic stroke), or lower extremities (peripheral vascular disease) [1], has become a global concern. The risk factors of AS gained attention, such as disturbed sleep [2], physical inactivity [3], the microbiome [4], air pollution [5], environmental stress [6], blood pressure [7], smoking [8] and triglyceride-rich lipoproteins [9, 10], particularly high levels of low-density lipoprotein cholesterol (LDL-C) [11]. Currently, anticholesteremic agents have been used to reduce LDL‐C and lower the atherosclerotic risk factors, including statins and nonstatin interventions, such as ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) [12, 13].
Initially, atorvastatin, a well-known lipid-lowering drug, can decrease cholesterol biosynthesis by inhibiting the 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGCR) to lower LDL-C levels [14]. However, < 30% of patients treated with atorvastatin achieve a satisfactory reduction in LDL-C, resulting in a reduction of preventing cardiovascular events [15]. Recently, a growing number of studies have shown consistent evidence that PCSK9 has lately received considerable attention as a target for the decrease of LDL-C levels in patients with inadequately decreased LDL-C [16]. PCSK9, the ninth and last member of a family of serine proteases (also called bacterial subtilisin) [17], is highly expressed in the liver [18]. It controls the levels of plasma LDL-C mainly by influencing the expression levels of the LDL receptor at the surface of hepatic cells [17]. PCSK9 interacts with the LDL receptor (LDLR) on the surface of the hepatocyte cells after secretion into the serum and the complex is subsequently internalized, thereby promoting intracellular retention, and cellular degradation in the lysosomes [19, 20]. PCSK9-induced cellular degradation in the lysosomes was presented in Fig. 1. In addition to the catalytic domain of PCSK9 interacts with the EGF-A domain of the receptor at the neutral pH of the plasma membrane [21]. Thus, the secretion of PCSK9 prevents the receptor from being recycled to the plasma membrane for further clearing of LDL-C [19, 20], leading to a decrease of LDLR on the cell surface of hepatocytes, which decreases the ability of the liver to remove LDL-C from circulation and causes higher levels of circulating LDL-C [20]. Inhibition of PCSK9 therefore offers a novel therapeutic mechanism for the lowering of LDL-C levels. There is a train of factors that affect PCSK9 expression in different ways in humans, including the removal of N-terminal acidic stretch (amino acids 31–53) within the prodomain of PCSK9 [22], the acidic pH within the endosome [21], estrogen [23], growth hormone (GH) [24], glucagon [25], insulin [26, 27], thyroid hormone [28], thyrotropin (TSH) [29], body mass index (BMI) [30], fasting [24] and re-feeding [31, 32], berberine [33, 34], and so on.
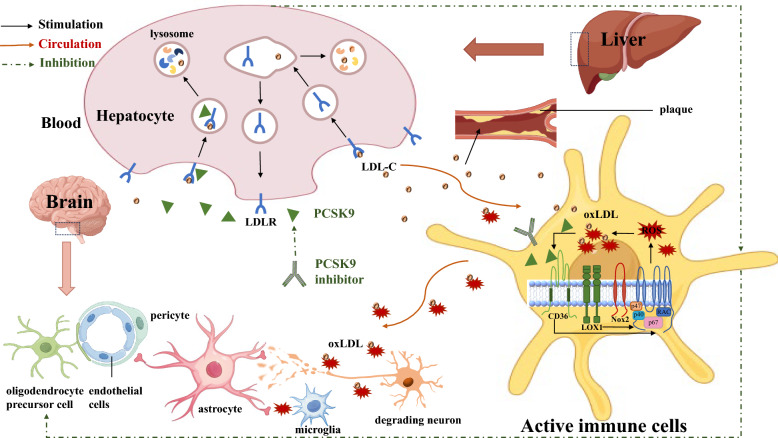
Fig. 1.
PCSK9-induced mechanism of peripheral and central oxidative stress.The role of PCSK9 in the regulation of circulating LDL-C levels is ascribed to binding of circulating PCSK9 to the LDLR and subsequent lysosomal degradation of LDLR. In the liver, PCSK9 binds to the LDL-C and LDLR complex, targeting subsequent lysosomal degradation of LDLR and increasing of plasma LDL-C in the blood vessel. Next, the accumulation and deposition of cholesterol form an atherosclerotic plaque within the artery wall that can narrow the blood vessel, activating and recruiting immune cells such as macrophages. PCSK9 directly binds to CD36 also activates the Nox2 pathway that interact with LDL-C to produce oxLDL in these active immune cells. The latter produces ROS, and oxLDL binds to both CD36 and LOX1, promoting Nox2 activation. In the brain, oxidative stress causing neuronal damage and endothelial cells death is mainly due to oxLDL-mediated excessive ROS production by activated immune cells. However, all these cascades are attenuated when the PCSK9 inhibitor is used. LDL-C low density lipoprotein-cholesterol, LDLR LDL receptor, oxLDL oxidized LDL, ROS reactive oxygen species
PCSK9i were discovered to be a novel kind of lipid-lowering drugs, which blocked the interaction between PCSK9 and LDLR, preventing LDLR degradation in hepatocytes [35]. This increases LDLR availability on cell surfaces, enhancing LDL-C clearance from circulation and reducing plasma LDL-C levels. The possible mechanism of PCSK9i inducing decreased LDLR degradation was presented in Fig. 2. Although the correlation between serum PSK9 levels and risk of ASD has been well established [36], the relation with diseases of central nervous system (CNS) awaits full and accurate data. To describe the influences of PCSK9 in the CNS and discuss clinical perspectives with PCSK9i in the neurological system, we incorporated data from a wide range of scientific disciplines and experimental paradigms in this comprehensive review. Comparisons of common drugs are made in Table 1.
Fig. 2.
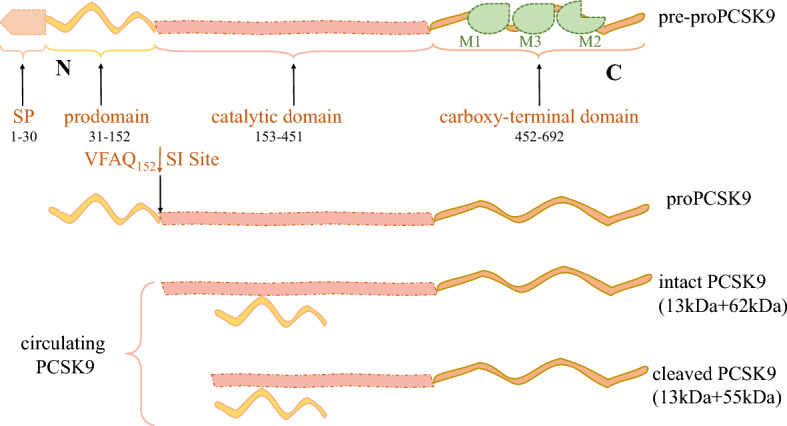
Linear structure, functional domains and key modifications of PCSK9. The structures of proPCSK9 contain the 3 distinct structural domains: the N-terminal domain (prodomain, amino acids 31–152), the catalytic subunit (amino acids 153–451), and the C-terminal domain (CHRD; amino acids 453–692), each playing critical roles in the regulation of PCSK9 and its intracellular traffic. The CHRD is composed of 3 tandem repeats rich in Cys and His residues (Cys/His-rich domain). The LDLR binds the catalytic domain of PCSK9, while the MHC-I complex interacts with the M2 repeat of the CHRD. On the other hand, CAP1 binds the M1 and M3 domains of the CHRD and enhances PCSK9 activity. Prior to the cleavage of the SP (residues 1–30), it was pre-proPCSK9. Following removing of the SP, the generated proPCSK9 is autocatalytically cleaved at its internal VFAQ152SIP site to form an intact PCSK9 (noncovalently bound a 13 kDa prodomain and a 62 kDa mature PCSK9 domain). Furthermore, circulating plasma PCSK9 can be found as another form, a furin-cleaved heterodimer (55 kDa PCSK9 domain + 13 kDa prodomain). CHRD Cys/His-rich domain, SP signal peptide, LDLR low-density lipoprotein receptor
Table 1.
Comparisons of common PCSK9 inhibitors
| Major categories | Drug | Mechanism | Advantages | Primary side effects | Usage | Ref |
|---|---|---|---|---|---|---|
| McAb inhibitors | Alirocumab | It inhibits the circulation of PCSK9, reducing the degradation of LDLR in the liver and reducing LDL-C levels | Safe, efficient, and few adverse reactions | Nasopharyngitis and mild self-limited injection site reactions (e.g., itching, redness, and swelling) | 150 mg every 2 weeks | [144, 145] |
| Evolocumab | 140 mg every 2 weeks or 420 mg every month | [146] | ||||
| Nucleic acid drugs |
ASOs, e.g., AZD8233, MiR-552-3p |
They bind to the mRNA of the target PCSK9 target gene through Watson–Crick base pair interactions and target gene expression is restricted | In clinical trial | In clinical trial | / | [147] |
|
siRNAs, e.g., Inclisiran |
siRNA interferes with mRNA degradation of specific sequences and inhibits PCSK9 gene expression | Safe | Injection site reactions, pain, erythema, rash, and other symptoms during use | / | [148] | |
| CRISPR/Cas9 gene editing systems | The systems can reduce the expression of PCSK9 target genes by inducing host cell DNA double-strand breaks | / | / | / | [149] | |
| Small-molecule drugs | Adnectin BMS-962476 | It blocked the biological activity of PCSK9 by preventing the binding and coassimilation of LDL-R during endocytosis | Highly effective, high affinity for PCSK9 | The contact area of small molecules is limited | / | [150] |
| Vaccine drugs | L-IFPT vaccine | It induced the production of a PCSK9 antibody, which directly targeted and eliminated circulating PCSK9 from the blood | Persistent induced antibodies (> 16 weeks), and high antibody titer | / | / | [151] |
|
CRISPR/ Cas9-targeted knockout drugs |
VT-1001 | They are mainly directed to specific genomic regions by their Cas9 nucleases and cleaved DNA target sequences with a single guide RNA (sgRNA) | / | / | In phase I clinical trials | [152] |
LDLR low-density lipoprotein receptor, LDL-C low-density lipoprotein cholesterol, ASOs antisense oligonucleotides, L-IFPT immunogenic peptide constructs fusing PCSK9-tetanus (IFPT) on the surface of liposome nanoparticles
Search strategy and results
In our literature review, we searched for articles published up to November 2024. We screened several databases, including PubMed, using the following keywords: “proprotein convertase subtilisin/kexin type 9,” “PCSK9,” “proprotein convertase subtilisin/kexin type 9 inhibitor,” “proprotein convertase subtilisin/kexin type 9 inhibitors,” “PCSK9 inhibitor,”and “PCSK9 inhibitors” in combination with “central nervous system diseases,” “central nervous system disorders,” “CNS disease,” “CNS disorders,”“neuroinflammation,” “Alcohol Use Disorder,” “Alzheimer’s Disease,” “Neural Tube Defects,” “Parkinson’s disease,” and “stroke”. Other potentially relevant references of retrieved publications were also selected. We reviewed systematic reviews, viewpoints, meta-analyses, prospective, retrospective, cross-sectional, case–control studies and so on. The CNS diseases, including neuroinflammation, AUD, ALD, ALS, AD, NTDs, PD and stroke, related PCSK9 were included in this review. We comprehensively collected and synthesized articles from previous research on the effects of PCSK9 on the central nervous system, with a focus on the clinical potential of PCSK9i to generate data that will help biomedical researchers or clinical workers develop treatments for the neurological diseases based on PCSK9i.
PCSK9 structure and regulation
The PCSK9 gene in humans is located at chromosome 1p32, comprising 11 introns and 12 exons [37]. The structures of proPCSK9 contain the 3 distinct structural domains: the N-terminal domain (prodomain, amino acids 31–152), the catalytic subunit (amino acids 153–451), and the C-terminal domain [Cys/His-rich domain (CHRD); amino acids 453–692] (see Fig. 3), each playing critical roles in the regulation of PCSK9 and its intracellular traffic [38]. Prior to the cleavage of the signal peptide (SP) (residues 1–30), it was pre-proPCSK9 [39]. Following removing of the SP, the generated proPCSK9 is autocatalytically cleaved at its internal VFAQ152SIP site to form an intact PCSK9 (noncovalently bound a 13 kDa prodomain and a 62 kDa mature PCSK9 domain) [40]. Furthermore, circulating plasma PCSK9 can be found as another form, a furin-cleaved heterodimer (55 kDa PCSK9 domain + 13 kDa prodomain) [41]. A schematic overview of PCSK9 is shown in Fig. 2.
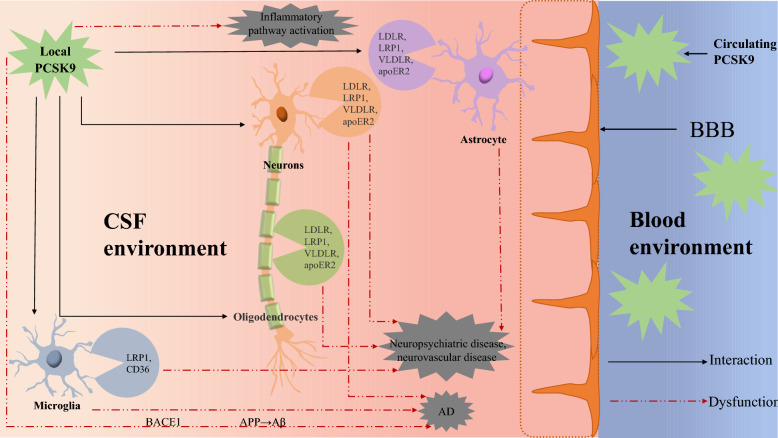
Fig. 3.
Roles for PCSK9 and the underlying mechanisms that link PCSK9 activation and neurological diseases. Under physiological conditions, circulating PCSK9 does not cross into CNS because of the existence of BBB. In the CNS, locally produced PCSK9 may not only act on LDLR, LRP1, VLDLR, and apoER2 that are expressed by neurons, astrocytes, and oligodendrocytes, but also on LRP1 and CD36 that are expressed by microglia. The PCSK9 can modulate these receptors and thereby regulate lipid homeostasis, neurogenesis, apoptosis, and inflammatory processes in the brain. However, central neurological diseases may be accumulated by known molecular pathways when dysfunction of certain receptors occurs. CNS central nerve system, CSF cerebrospinal fluid, BBB blood–brain barrier, LDLR low-density lipoprotein receptor, LRP-1 low-density lipoprotein receptor-related protein-1, VLDLR very LDLR, apoER2 apolipoprotein receptor 2, Aβ amyloid-β, AD Alzheimer’s Disease
Interestingly, a variety of agents or processing and modulation can considerably determine its activation or inactivation via acting in their specific areas to achieve the aim of regulating expression levels of PCSK9. Øystein L. et al. described that the affinity of PCSK9 for the LDLR enhanced by tenfold and the degradation of the LDLR in endosomes or lysosomes of hepatocytes increased by fourfold when removing the prosegment stretch (N-terminal acidic stretch, amino acids 31–53) of PCSK9, resulting in faster endocytosis of the complex into lysosome-like compartments at the neutral pH [22]. Several studies have demonstrated that the PCSK9's affinity for the LDLR increased 150–170-fold at the acidic pH within the endosome than at the neutral pH [21]. Here, several key regulatory hormones that control the expression of the PCSK9 gene have been identified. The serum PCSK9 levels are higher in postmenopausal females than those in premenopausal females in a Han Chinese population, and estrogen levels are inversely correlated to circulating PCSK9 levels in premenopausal females [23]. In pregnant women, PCSK9 levels increase when estrogen levels are at their lowest [42], so this hormone may downregulate PCSK9 expression, serving as a discovered negative regulator of PCSK9 [43]. Subsequent studies revealed that plasma PCSK9 increases with age in girls, but the inverse changes for boys [44], which is independent of the potential roles of estrogen or testosterone but associated with hormonal regulation caused by GH in this process, suggesting that the difference in PCSK9 levels between males and females may be explained by the fact that GH levels at the starting stages of puberty [24]. It is important to mention that these two main types hormones of regulating blood glucose, glucagon and insulin [25–27], also cause significant influences on PCSK9 protein levels. Although the correlation between these hormones and the expression of PCSK9 has been well established, the specific up-regulating or down-regulating remains controversial. There are studies reporting decreases in PCSK9 protein expression in patients with type 2 diabetes [45] and in women with gestational diabetes [46], whereas several studies have reported elevated levels of PCSK9 in type 2 diabetes patients [47]. Furthermore, both increased [48] and decreased [26] expression of PCSK9 have been found with insulin. The above indicates that the expression of PCSK9 is significantly affected by hyperglycemia and hyperinsulinemia via different pathways with cross-interaction when simultaneous hyperglycemia and hyperinsulinemia occur. The finding thyroid hormone reduces the circulating PSCK9 concentration might explain why hyperthyroidism patients with lower plasma LDL levels and hypothyroidism the higher LDL levels [28], while the circulating PCSK9 levels are positively associated with TSH in euthyroid subjects [29]. Interestingly, BMI positively correlated with PCSK9 levels. Indeed, to investigate the effect of obesity and bariatric-induced weight loss on circulating levels of PCSK9 in severely obese patients, in a prospective cohort study, PCSK9 levels were higher in obese patients than in controls (274.6 ± 76.7 ng/mL vs. 201.4 ± 53.3 ng/mL) at baseline, and dropped at 6 months after bariatric surgery (205.5 ± 51.7 ng/mL) along with BMI (from 44.1 ± 5.9 kg/m2 to 33.1 ± 5.6 kg/m2) [30]. In another study, PCSK9 levels were elevated as normal pregnancy progresses and higher in persons who are obese, even after adjustment for insulin resistance [49]. Fasting and re-feeding can affect the expression of PCSK9. Persson et al. monitored circulating PCSK9 in experiments and their results show that serum PCSK9 levels display a diurnal rhythm. Throughout the day, and in response to fasting (> 18 h), circulating PCSK9 displays marked reduction with unchanged serum LDL levels [24]. During fasting in humans, GH is known to be increased reducing circulating PCSK9. This fact could explain the direct relationship between fasting and lower PCSK9 levels and why some reasonable degree of fasting is protective, adding benefits for anti-PCSK9 therapies. Moreover, subjects with a high-fructose diet increased PCSK9 expression [31], whereas rats with cholesterol feeding reduced plasma PCSK9 levels [24], which indicates the species and the diet of re-feeding decide on the expression of PCSK9. Hepatocyte nuclear factor 1α (HNF1α) directly stimulates the transcription of the PCSK9 gene [50], however, berberine can downregulate the HNF1α through the ubiquitin–proteasome degradation pathway to inhibit the PCSK9 transcription [33, 34]. PCSK9i are often prescribed to reduce atherogenic lipid levels and benefit ASD health over time [38].
Relationship between PCSK9 and CNS
Although PCSK9 was first identified in 2003 in neurons of the cerebellum, originally called neural apoptosis-regulated convertase-1 (NARC-1) [18], its function in the CNS is less clear. Under normal conditions, PCSK9 is restricted from entering the brain due to the presence of the blood–brain barrier (BBB) [51]. In observational studies, average serum levels of PCSK9 vary on a diurnal rhythm from 183 ng/ml to 552 ng/ml, with a nadir in the evening and a peak in the early morning. In contrast, average cerebrospinal fluid (CSF) PCSK9 concentration remains at 5 ng/ml for the 24-h observation period [52]. Nevertheless, the permeability of the BBB can be affected when several diseases occur, permitting the diffusion of compounds including PCSK9 otherwise unable to cross the barrier. Alterations in BBB permeability can affect brain PCSK9 concentrations, ordinarily relying exclusively on cerebral own mechanisms for the maintenance of homeostasis in CNS and dynamically regulating PCSK9 expression in the brain. Indeed, the recent systematic reviews showed that PCSK9 expression is highly upregulated during different disease states [53, 54]. Among others, PCSK9 is involved in multiple physical processes such as apoptosis, neurogenesis, neuronal differentiation, cholesterol regulation, and neuroinflammation in the CNS, and thus impacts pathological processes, such as alcohol use disorder (AUD), Alzheimer’s disease (AD), neural tube defects (NTDs), Parkinson’s disease (PD) and ischemic stroke [55]. Therefore, it is a crucial part of determining the specific role of PCSK9 in the brain. Figure 3 implicates major CNS processes in which PCSK9 has an influence, and also shows the known molecular mechanisms of CNS diseases related to PCSK9.
A convertase implicated in neural apoptosis, neurogenesis, and neuronal differentiation
The reason PCSK9 named NARC-1 is it was initially discovered in an apoptotic cellular model in primary cerebellar neurons [18], and subsequent studies on the effect of PCSK9 showed it had both pro-apoptotic [56, 57] and anti-apoptotic [58, 59] effects in different cell lines. Cerebellar granule neurons (CGNs) were transfected with an enhanced green fluorescent protein (EGFP)–fusion constructs of wild-type NARC 1 and using a laser scanning cytometry-based method to score cell death in transfectants. Wild-type NARC 1 was found to have substantial proapoptotic effects, NH2-terminal deletion constructs of NARC 1 had effects similar to wild type, independently of the potential roles that caspase might have in this process, whereas the expression of COOH-terminal deletion mutants produced a rate of cell death similar to wild-type in the absence of BAF (poly-caspase inhibitor) treatment [56]. Does this mean that the latter is caspase-dependent? When applying BAF, this cell death phenotype was completely insensitive to this rescue, indicating a negative answer [56]. The secreted PCSK9 recognizes the ectodomain of LDLR [60, 61], very LDLR (VLDLR) [62], and apolipoprotein receptor 2 (apoER2) [63], promoting their ubiquitylation and subsequent lysosomal degradation in intracellular acidic compartments [64]. Another study also found that the knockdown of apoER2 but not of others reversed the protection of PCSK9 RNAi. These results identify the important function of apoER2 as a proapoptotic signaling of PCSK9 [57]. However, several studies have reported that the molecular mechanism of PCSK9 siRNA-inhibited oxLDL-induced apoptosis in human umbilical vein endothelial cells (HUVECs) and Bcl/Bax–Cyt C–caspase9–caspase3 pathway may be the involved pathway [58]. Rats were fed with a high‑fat diet (HFD) for 6 weeks and exposed to middle cerebral artery occlusion (MCAO), a lentiviral vector harboring short‑hairpin RNA (shRNA) targeting PCSK9 was injected, and PCSK9 and apoER2 expression levels were assessed by reverse transcription‑quantitative polymerase chain reaction, immunohistochemistry, and western blotting. These results indicated that not only PCSK9 expression increased evidently, but PCSK9 shRNA treatment attenuated cerebral histological injury and neuronal apoptosis in hyperlipidemic mice [59]. Anyway, it is still not certain whether PCSK9 acts to degrade pro-apoptotic-related proteins to initiate apoptosis or anti-apoptotic-related proteins to inhibit the apoptotic cascade. The data about the role of PCSK9 in apoptosis are still very limited and need further investigation to confirm these observations.
The tissue and cell lines of NARC-1 show unique patterns identical to those of other convertases [65] and NARC-1 may be implicated in hepatogenesis, nephrogenesis, neurogenesis, and neuronal differentiation. In mouse embryos, in situ hybridization histochemistry (ISH) revealed NARC-1 expression in the liver at embryonic day En (E9). It was also first detected in the kidney at E15, undetectable in the cortex by postpartum day 6 (P6) and appeared in the medulla at P12 [18]. The mesonephros differentiated into the functional embryonic kidney during the P6 and P12 stages [66], and a possible nephrogenic role of NARC-1 is suggested. Notably, the peaking of transient expression of NARC-1 in the telencephalon was at E12, and NARC-1 expression first appeared in the cerebellum at E15 and was high up to P15 [18]. The one of major brain NARC-1 expression sites was the cerebellum, limited to the external granular layer in adults. The other major brain expression site was the rostral extension of the olfactory peduncle, a location reported to contain multipotential stem cells [67, 68]. In addition, the effects of NARC-1 overexpression in a primary culture of embryonic neural progenitor cells were examined. Immunocytochemical analysis showed that exogenous expression of NARC-1 led to an increase in the number of postmitotic neurons after 4 days in vitro (P < 0.01); in parallel, a decrease in the number of undifferentiated neuroepithelial cells also named mitotic neural progenitor cells (P < 0.005) [18]. These findings show that NARC-1 promotes neurogenesis by driving neuronal differentiation from undifferentiated neural progenitor cells into the neuronal lineage.
A cholesterol regulator of the nervous system
As the most cholesterol-rich tissue/the tissue richest in cholesterol of the human body, the brain’s principal carrier of cholesterol is apoE [69]. Under physiological circumstances, neither plasma lipoproteins nor circulating PCSK9 have the ability to cross the BBB [70], only locally expressed PCSK9 may directly modulate cholesterol homeostasis in the developing brain through potentially interacting with several members of the LDLR family of receptors [LDLR, VLDLR, apoER2, and the LDLR-related protein 1 (LRP1)] in Fig. 3. Like in the liver, PCSK9 promotes lysosomal degradation of LDLR family members that have a high affinity for apoE [64], leading to decreased LDLR levels and a corresponding increase in LDL-C. Evidence suggests that the two separate routes of LDLR degradation induced by PCSK9 primarily include the intracellular and extracellular pathways, leading to hyperlipidemia.
The first critical explanation of the mechanism of how PCSK9 regulates LDL-C levels came from experimental reports of Maxwell and Breslow. In their study, PCSK9 overexpression in wild-type (WT) mice caused a twofold increase in plasma total cholesterol and a fivefold increase in nonhigh-density lipoprotein (HDL) cholesterol primarily due to the increase in LDL cholesterol, with a near absence of LDLR protein [71]. Thus, these results indicate that overexpression of PCSK9 decreases levels of the LDLR and then interferes with LDLR-mediated LDL cholesterol uptake/Pcsk9 plays a major role in the regulation of LDLR-mediated LDL removal from plasma. Maxwell and Breslow also conducted experiment in vitro, transient transfection of PCSK9 into McA-RH7777 rat hepatoma cells induces the degradation of the LDLR by a nonproteasomal mechanism in a postendoplasmic reticulum compartment, resulting in a reduction in LDLR protein and LDL binding [72].
Furthermore, Cohen et al. reported that subjects carried truncating Y142X or C679X heterozygote LOF mutations of PCSK9 showed about 40% reductions in LDL-C levels, confirming the fact that PCSK9 may be acting stoichiometrically on the LDLR [73]. Saavedra et al. demonstrated that LOF mutations displayed a 16–28% increase in the expression levels of cell surface LDLR and a 35% increase in internalization of LDL [74]. However, the three PCSK9 GOF variants F216L [35], R218S [75], and R215H [76] resulted in higher levels of bioactive PCSK9 and enhanced circulating LDL-C. It was reported that GOF mutations of PCSK9 had a 23% decreased expression level of the LDL receptors and a 38% decreased level of internalization of LDL [74]. These results were confirmed by the demonstration that livers of knockout mice lacking PCSK9 manifest about threefold higher LDLR protein levels and a dramatic reduction of plasma LDL-C [61]. In the third year after that, a similar observation was also obtained in complete and hepatocyte-specific PCSK9 knockout mice [77]. As aforementioned, the effect of PCSK9 in promoting LDLR degradation has been obvious, firmly established PCSK9 as an attractive therapeutic target for LDL-C reduction.
Roles of PCSK9 in neuroinflammation
In addition to the effect of PCSK9 on elevating blood LDL-C levels, a multitude of clinical studies clearly demonstrate that PCSK9 plays an important role in neuroinflammation. In the study of Apaijai, the association between PCSK9 inhibition and neuronal responses in a rat model of cardiac ischemic/reperfusion injury (I/R) injury was investigated for the first time. They first used confocal microscopy to observe microglia morphology, and found that cardiac I/R injury could increase astrocytic activation and only pretreatment with PCSK9i did restore the levels in astrocytic activation to the basal levels of the sham group following cardiac I/R. As we all know, amyloid plaque formation can be recognized as one phenomenon of neurodegeneration, which was assessed in these experiments as the expression of Aβ protein levels. And then, in the brains of rats with cardiac I/R injury and vehicle treatment, Aβ was significantly increased by 2.4‐fold (P < 0.001) when compared with that of the sham group, while treatment with PCSK9i at any time point of cardiac I/R injury attenuated the protein levels of Aβ to the basal level of the sham group (P < 0.05), suggesting that cardiac I/R injury induced Aβ aggregation and PCSK9i effectively reduced brain Aβ levels in rats with cardiac I/R injury. Furthermore, to determine the direct effects of PCSK9i on the brain with and without cardiac I/R injury, they also evaluated the levels of PCSK9 in the brain using an immunoblot. Brain PCSK9 protein levels did not decrease with PCSK9i treatment in rats without cardiac I/R, and the same not decreased results also in mice with PCSK9i administration before cardiac I/R, during cardiac ischemia, or at the onset of reperfusion [78]. Those findings suggest that PCK9i attenuates brain damage caused by cardiac I/R via decreasing microglial and astrocytic hyperactivation, and β‐amyloid aggregation without reducing PCSK9 levels in the brain.
ApoE has emerged as a pivotal anti-inflammatory agent in a number of neurodegenerative disorders (NDD), including AD, ischemic stroke [79], PD, and multiple sclerosis (MS) [80]. An increasing body of evidence suggests that apoE and apoE-mimetic peptides exert prominent anti-inflammatory effects [81]. In addition, Listeria monocytogenes (LM) induced more hepatic damage in apoE-/- mice as judged by increased serum concentrations of alanine aminotransferase at day 1 (apoE-/- 301 ± 45 U/ml, apoE +/+ 101 ± 9 U/ml, P = 0.01) and more intense inflammatory cascade as significantly increased serum concentrations of TNF-alpha (TNFα) at day 3 (127 ± 43 pg/ml versus 20 ± 17, P = 0.003) compared to apoE +/+ mice, which suggested apoE deficient mice have impaired innate immune responses to Listeria monocytogenes [82]. Thus, targeting apoE may be a potential anti-inflammatory approach for the prevention and treatment of various neurodegenerative and peripheral diseases in humans [83]. In order to confirm these findings in vivo, Wang et al. treated htau mice with binding domain of apoE (aa 133–152, ApoEp) with 6 lysines (6KApoEp) by daily intranasal (i.n.) administration for 28 weeks and found that mice treated with 6KApoEp showed reduced concanavalin A (ConA)-induced splenocyte IFNγ release compared with treatment with 6 K alone [84]. As a corollary, the modulation of systemic inflammation is an initiative factor of PCSK9i and triggers beneficial cascade responses. Vilella et al. conducted in vitro studies, U373 human astrocytoma cells were treated with Aβ fibrils and human recombinant PCSK9, and found that PCSK9 significantly increased IL6, IL1B, and TNFΑ mRNA levels in Aβ fibrils-treated U373 cells [85]. The GPNMB/CD44 pathway was shown to limit the release of two classic pro-inflammatory factors, IL-6 and iNOS. In the study of Zheng et al., western blotting and immunofluorescence results showed that mRNA levels of IL-6 and iNOS increased significantly, while PCSK9i effectively reduced temporary MACO (tMCAO)-induced overexpression of IL-6 and iNOS [79]. These findings indicated that PCSK9i treatment attenuated the inflammatory response in the tMCAO model. The recent study has shown that PCSK9i may dampen neuroinflammation via the GPNMB/CD44 signaling pathway, further exerting their protective effects [86], while the exact mechanisms are unknown. The data about the details of PCSK9 in neuroinflammation are still very limited and require further investigation to confirm these mechanisms.
PCSK9i as a potential therapeutic target for AUD and ALD
Characterized by an impaired ability to control or stop alcohol intake, AUD is a chronic relapsing condition [87] and is associated with the dysfunction of several organ systems including alcohol-associated liver disease (ALD) and progressive neurodegeneration in the brain [88]. Generally, the tissue damage precipitated by excessive alcohol consumption leads to increased translocation of bacterial products into circulation (translocation of bacterial products from the intestinal lumen to the systemic circulation), an increase in production of pro-inflammatory cytokines, and persistent activation of immune cells, which manifests as ALD in the liver [89] or presents with symptoms of neurotoxicity including cognitive deficits, numbness and pain in the hands and feet, disordered thinking, dementia, and short-term memory loss in the brain [90, 91].
AUD comorbid with liver disease is extremely common [89], and ALD is one of the most common causes of liver-related diseases, with cirrhosis in approximately 10–20% of patients [92]. The exact mechanisms currently known are as follows. Firstly, reactive-oxygen species (ROS) generated during alcohol metabolism cause cellular damage and then changes in the gut microbiota (dysbiosis) and the immune cascade reaction to such changes persistently aggravates the development and progression of ALD [93]. Secondly, the dysregulation of homeostatic mechanisms regulating injury and repair in the liver also is the key mechanism in the occurrence and progression of ALD, including disruption of pathways regulating cell death and survival, exorbitant activated immune responses, and organ–organ interactions [94]. Meanwhile, the reason neurotoxic effect on the brain of alcohol is long-term alcohol consumption can damage BBB integrity. Wei et al. used the mouse models of cognitive deficit induced by d-galactose or lipopolysaccharide (the important risk conditions in humans on cognitive function) to evaluate the effects of long-term alcohol consumption on BBB integrity. The BBB integrity was significantly destroyed with remarkably increased permeability and the expression of protein most closely related with the structure and function of BBB integrity down-regulated, such as zonula occludens-1, VE-cadherin, occludin, LRP1, receptor for advanced glycation end products, major facilitator superfamily domain-containing protein-2a and aquaporin-4, after alcohol administration for 30 days in these models [95]. Lee and colleagues conducted a study between controls and AUD subjects, and reported that PCSK9 in CSF was significantly increased in the AUD group at day 5 and day 21 compared to the controls (P < 0.0001) and plasma PCSK9 levels were parallel to CSF PCSK9 levels in AUD individuals (P = 0.0493) [54]. AUD results in an estimated 88,000 deaths each year annually and an annual cost of over 250 billion per year in the United States [96, 97]. Chronic alcohol consumption resulted in hypermethylation of the PCSK9 gene and increased expression of PCSK9 in the liver and CNS [98], which may be responsible for the pathogenesis of AUD. Given the observed increase in PCSK9 with chronic alcohol consumption and the role of PCSK9 in cholesterol regulation and inflammation, inhibition of PCSK9 may be a potential therapeutic option for AUD and ALD. To test this assumed possibility, one study administered alirocumab [one of Food and Drug Administration (FDA)-approved PCSK9i] to a rat model receiving a chronic alcohol liquid diet, showing improved alanine transaminase (ALT) and aspartate transaminase (AST) levels, and ameliorated hepatic inflammation by reducing pro-inflammatory cytokines (e.g., TNFα and IL-1β) [99]. Compared with control groups, patients with liver fibrosis and a fibrosis mouse model both had heightened PCSK9 expression. In the diseased mice, genetic deletion of PCSK9 improved liver inflammation and fibrosis with reduced hepatocyte necrosis markers ALT and AST, suggesting that PCSK9 inhibition can attenuate liver inflammation and hepatocyte injury [100]. More clinical studies are required to clarify the benefits of PCSK9 gene-targeted antimethylation for the treatment of AUD.
The significant protective effect of PCSK9i against amyotrophic lateral sclerosis (ALS)
ALS is a fatal NDD that, by definition, is characterized by selective and progressive involvement of upper and lower motor neurons [101]. Certainly, locomotor system dysfunction is the most prominent clinical manifestations of ALS, ultimately leading to respiratory failure approximately 3–5 years after symptom onset [102]. Little investigations about the impacts of PCSK9 in ALS have been studied, despite the recognized association between lipid metabolism and the severity of ALS [103]. In June 2024, Huang et al. collected single nucleotide polymorphisms (SNPs) of PCSK9 from published statistics of genome-wide association studies and performed drug target MR analyses to detect a causal relationship between PCSK9i and the risk of NDD, ALS included [104]. The drug target Mendelian Randomization (MR) analysis revealed that PCSK9i significantly decreased the risk of ALS (OR [95%] = 0.89 [0.77–1.00], P = 0.048). Furthermore, to explore whether PCSK9i play a role in the pathogenesis of ALS through lipid lowering mechanisms, they also utilized the effects of conventional lowering lipid drugs HMGCR inhibitors (HMGCRi, statin targets) for comparison with PCSK9i [104], and the analysis of lacking HMGCRi exhibited a similar effect. PCSK9 interferes with the antiapoptotic signaling pathway by modulating the apoER2 [18, 105], thereby promoting neuronal apoptosis [57, 106], suggesting that PCSK9i reduced the risk of developing ALS through pathways beyond lipid lowering. This may be a potential mechanism of action for PCSK9i to protect against ALS. However, PCSK9 exhibited antiapoptotic properties in U251 human glioma cells [107]. Given conflicting roles of PCSK9 in apoptosis in previous studies, the role of PCSK9i in different NDD may be inconsistent and requires further exploration. Hence, the precise mechanisms underlying PCSK9 involvement in ALS onset and progression remain unknown, the potential neuroprotective role against ALS of PCSK9i requires further exploration.
PCSK9 acts as a key regulator: potential implications in AD and pathogenesis
To the best of our knowledge, a successive accumulation of cerebral amyloid-β (Aβ) is one peculiar hallmark of AD pathogenesis, which correlates with the progressive cognitive decline in AD. The clearance of cerebral Aβ exceeds its production rate in physiological conditions [108], however, only Aβ clearance decreased accompanied by an unaffected production rate when patients suffering from AD [109]. During the process of cerebral Aβ clearance, as a key mediator, LRP1 mediates the rapid transport of Aβ across the BBB into the periphery [110, 111], and this approach can remove a significant percentage of Aβ. Although PCSK9 exists, it binds to members of the low-density LRP family at the cell surface and targets them for lysosomal degradation, reducing the number of functional receptors. Meanwhile, there is evidence that elevated PCSK9 levels in the CSF are associated with AD patients [112]. In reality, Zimetti and others previously observed that the CSF of patients affected by AD displays higher PCSK9 concentrations than non-AD subjects (P = 0.0049) [112], especially the frontal cortex, the most involved region with AD, showed elevated PCSK9 expression [113]. These data suggest an involvement of PCSK9 in the disease but their link deserves further investigations, however, the study of Courtemanche et al. shows a trend for increased CSF PCSK9 levels also in non-AD NDD [114], confirming a link to the neurodegenerative process but not specifically to AD.
In the experiments of Mazura et al., FDA-approved monoclonal anti-PCSK9 antibodies were injected intraperitoneally into the AD mouse model 5xFAD to inhibit endogenous PCSK9 activity to achieve the aim of evaluating whether therapeutic targeting of peripheral PCSK9 would increase Aβ brain clearance in vivo. Finishing the above operations, they observed that treating 5 × FAD mice with low-concentrated Alirocumab for 10 weeks resulted in significantly reduced Aβ pathology compared to control-treated 5xFAD mice accompanied by unaffected general behavior [115]. This study strongly supports the idea that peripheral PCSK9 inhibition could be a novel and easily implementable tool to increase cerebral Aβ clearance rate, thus targeting Aβ brain accumulation in AD patients even in moderate cognitive impairment (MCI) stage with already available FDA-approved PCSK9 inhibitors. In vivo, PCSK9 ablation in 5XFAD mice significantly improved the performance at the Morris water maze test; accompanied by a reduced corticohippocampal Aβ burden [85]. Consistently, in vivo studies showed a protective effect of PCSK9 ablation against cognitive impairments, which is associated with improved Aβ pathology and attenuated neuroinflammation in mouse models of AD. In other word, PCSK9i may reduce neuroinflammation and Aβ aggregation in AD by preserving LDLR/LRP1-mediated Aβ clearance. Nevertheless, Vilella et al. conducted in vitro studies, U373 human astrocytoma cells were treated with Aβ fibrils and human recombinant PCSK9, and using q-PCR to evaluate mRNA expression of the proinflammatory cytokines and inflammasome-related genes. They found PCSK9 significantly increased IL6, IL1B, and TNFΑ mRNA levels in Aβ fibrils-treated U373 cells [85]. Thus, in vitro data indicated a pro-inflammatory role of PCSK9, whose results were opposite to in vivo evidence. However, the relationship between PCSK9 and AD is still largely unknown, as genetic studies apparently did not find an association between PCSK9 variants and AD risk or prevalence [116, 117]. International Genomics of Alzheimer’s Project used Egger Mendelian randomization analysis to summarize data, giving a risk ratio for AD of 0.24 (0.02–2.79) for 26 PCSK9 and HMGCR variants. In other words, low LDL cholesterol levels due to PCSK9 and HMGCR variants had no causal effect on the high risk of AD [116]. These studies are not entirely conclusive and future studies may need to look at other compound heterozygotes to capture variability.
PCSK9 and relevance for NTDs
NTDs are severe congenital malformations affecting approximately 0.6–6 per 1000 pregnancies [118], which are correlated with CSF PCSK9 concentrations in human patients. An et al. observed the space–time expression changes of PCSK9 at different stages of fetal development and noticed a marked decrease in the sera of NTDs pregnancies and a gradual increase in the sera of normal pregnancies with embryonic development. Leveraging the known effects of changes in PCSK9 to identify novel phenotypes in which this protein and its inhibitors may have an impact. PCSK9 is known to be expressed mainly in primary embryonic telencephalon cells between embryonic days 13–15 [18, 119], a key time point in the disease pathway for spina bifida. The potential pathway, by which decreased PCSK9 and further lead to development of NTDs, is spina bifida code (P = 2.7*10–4) [120]. They not only identified PCSK9 as diagnostic efficacy of potential NTDs serum molecule biomarkers (area under the curve in the receiver: 0.763, 95% CI 065–0.88), but found markedly decreased PCSK9 expression in the spinal cords and placentas of spina bifida aperta (SBA) rat fetuses [121]. These studies have shown that PCSK9 could serve as a novel molecular biomarker for the noninvasive prenatal screening of NTDs. Jerome et al. analyzed 29,722 patients’ genotype and clinical data using phenome-wide association study (PheWAS) methods revealing a potential novel association between PCSK9 loss of function variant (R46L) and an increased risk of spina bifida (OR 5.90, P = 0.00027) [120]. Only one of the case carriers had LDL data available in the electronic health record (EHR), this study was underpowered for further covariate analysis of LDL values. Additional covariate analyses suggest that alterations in glucose homeostasis may be a risk of PCSK9 loss of function [122, 123]. These potential confounding factors may have enabled further insights into potential pathogenesis as well as will be necessary to inform a more complete evaluation of any relative contribution of PCSK9 inhibition to NTDs. This article provides a methodology to assess the connections between inhibition of PCSK9 and NTDs. Further investigation into pathogenesis as well as surveillance may be warranted.
Association between plasma PCSK9 levels and lipid profile in patients with PD
In clinical practice, PCSK9i are mainly used in the cardiovascular field. More recently, an involvement of PCSK9 in PD has been suggested. Jahed et al. found that LDL, HDL, and total cholesterol levels were significantly lower in PD patients [124], indicating higher cholesterol can have a protective effect on PD occurrence. Consistent with this finding, Ikeda et al. concluded that total cholesterol and LDL level were lower in 119 patients with PD compared with 120 healthy individuals. Jahed et al. also investigated the role of lipid profile and PCSK9 levels between 31 individuals diagnosed with PD and 31 healthy people. Compared with the control group, the PD group exhibited significantly lower LDL (84.2 ± 24.9 mg/dl vs. 105.5 ± 16.8 mg/dl, P < 0.001), HDL (45.5 ± 8.7 mg/dl vs. 51.1 ± 9.5 mg/dl, P < 0.001), and total cholesterol levels (155.3 ± 31.2 mg/dl vs. 192.8 ± 32.5 mg/dl, P < 0.001). The PCSK9 levels of participants in the PD group and control group were 141.6 ± 70.0 and 129.7 ± 51.0 ng/ml, but no significant difference was found (P = 0.500) [124]. Based on this foundation of PCSK9 combined and HMGCR variants associated with a 9.3% lower LDL cholesterol level, Benn et al. conducted observational analyses and concluded that the risk ratio for a lifelong 1 mmol/L lower LDL cholesterol level was 1.02 (0.26–4.00) for PD after adjusting for age, sex, and year of birth [116]. They did not observe any statistically significant data between the loss function of PCSK9 and increased risk of PD in 111,194 subjects, suggesting that Low LDL cholesterol levels due to PCSK9 and HMGCR variants had no causal effect on the high risk of PD. Rotigotine (Neupro), a nonergoline dopamine agonist licensed for the treatment of PD [125], suppresses the expressions of LDLR as well as PCSK9. In 2021, Kang’s findings showed that oxLDL alone increased the mRNA expressions of PCSK9 and LDLR, as compared to the control. However, the introduction of Rotigotine reduced these levels, demonstrating that treatment with Rotigotine decreased the protein expression levels of PCSK9 and LDLR [126]. Given this inhibition of Rotigotine to PCSK9, focusing on the inhibition of the PCSK9 pathway may act as an essential part of highlighting the potential use of PCSK9i in the treatment of PD. The current evidence regarding the prognostic value of lipid profiles and PCSK9 in PD remains limited and inconsistent. One of the limitations of these studies exploring the link between plasma PCSK9 levels and lipid profile in PD patients was small scale which might affect the results. Whether PCSK9i can bring more clinical benefits to treat PD needs to be confirmed by further studies.
The effects of PCSK9i on brain stroke prevention
PCSK9i are a nonstatin preparation that can reduce LDL-C levels and lowering LDL-C levels can reduce ischemic complications. Therefore, there are some relevant reports in the field of neurology. In the studies of Oyama and colleagues, the second FDA-approved PCSK9 inhibitor evolocumab reduced first acute arterial events [including acute coronary, cerebrovascular (ischemic stroke, transient ischemic attack, or urgent cerebral revascularization), or peripheral vascular] by 19% [hazard ratio (HR): 0.81, 95% CI 0.74–0.88; P < 0.001] in comparison with the placebo group, with significant individual reductions in cerebrovascular events [HR 0.77, 95% CI 0.65–0.92)]. There were 3437 total events in 27 564 patients with stable atherosclerotic cardiovascular disease (ASCVD), with the evolocumab therapy group reducing total events by 24% [incidence rate ratio (RR): 0.76, 95% CI 0.69–0.85]. The patients receiving evolocumab therapy were noted to have a reduction in acute arterial events over time, with a 16% reduction [HR 0.84, 95% CI 0.75–0.95)] in the first year followed by a 24% reduction [HR 0.76, 95% CI 0.67–0.85)] thereafter [127]. Qin et al. analyzed clinical data from 57,440 participants in seven randomized controlled trials (RCTs), including 29,850 patients treated with PCSK9i and 27,590 control participants. They observed an association between PCSK9i and significant reductions in total brain stroke risk (RR: 0.77, 95% CI 0.67–0.88, P < 0.001) and such relationship with ischemic brain stroke risk (RR; 0.76, 95% CI 0.66–0.89, P < 0.001) when compared with the control group. There was no significant difference in the risk of hemorrhagic brain stroke (RR: 1.00, 95% CI 0.66–1.51, P = 0.999) between patients treated with PCSK9i and controls [128]. Similarly, Schlunk’s results did not find an independent effect of PCSK9 inhibition on intracerebral hemorrhage (ICH) growth [129]. In the study of Guo et al., neuroprotective effects against cerebral IRI were attributed to LDLR upregulation and nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome inactivation (LDLR/NLRP3 axis) induced by MARCH1, which is achieved by the downregulation of PCSK9 expression [130]. These findings are consistent with a recent study indicating that PCSK9 expression was obviously elevated in the brain tissues of MCAO-induced ischemic stroke model and that siRNA-mediated knockdown of PCSK9 improved neurological function, and reduced brain infarct size [131]. These results confirm that PCSK9i have protective effects on preventing again ischemic stroke, and pave the way for larger prospective studies and ultimately guidelines for secondary prophylactic treatment of patients with stroke with lipid-lowering agents.
PCSK9’s role in other severe disorders
PCSK9i are a group of drugs that controls LDL receptor expression at the plasma membrane, and PCSK9’s receptors are widely distributed in the brain, heart, immune systems, kidneys, liver, small intestine, vascular structure, and other organs. PCSK9 plays essential roles in hyperlipidemia and ASD and is also related to other diseases, including viral infections, sepsis, tumors, and immune checkpoint regulation in cancer, highlighting the potential therapeutic effects of PCSK9i in treating these diseases. This section will introduce their application in more detail.
The PCSK9 has a vital effect on the activation of a number of enveloped viruses [132], including dengue virus (DENV). Gan et al. showed that DENV infection enhances the mRNA expression of PCSK9 in hepatocytes in cell cultures, which was supported by the detection of elevated plasma PCSK9 levels in DENV-infected patients with high levels of viremia [133]. After observing this unexpected role of PCSK9 in dengue pathogenesis, Seidah used the inhibitory PCSK9-mAb alirocumab to achieve the effect of blocking PCSK9 function, resulting in lower viremia [134]. PCSK9 promotes viral cellular entry through LDLR degradation, while its pharmacological inhibition significantly reduces viral load in circulation. Furthermore, the novel outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, named coronavirus disease 2019 (COVID-19), seems to be associated with the potential influence on vascular inflammation of PCSK9. To investigate the impact of PCSK9 inhibition vs placebo on clinical and laboratory outcomes in patients with severe COVID-19, Navarese et al. randomly grouped 60 patients hospitalized for severe COVID-19 to 1:1, receiving a single 140-mg subcutaneous injection of evolocumab or placebo, and recorded the outcome at 30 days and circulating IL-6 levels at 7 and 30 days from baseline. They found that patients with PCSK9 inhibition had lower rates of death or need for intubation within 30 days (23.3% vs 53.3%), and had lower IL-6 levels (30-day decline: − 56% vs − 21%) compared to the placebo group, respectively [135]. In COVID-19, PCSK9i lowers IL-6 and mortality by modulating vascular inflammation. The pro-inflammatory effects of PCSK9 contribute to sepsis pathogenesis, and emerging evidence suggests PCSK9 inhibitors could be therapeutic by modulating cytokine release syndrome. PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for antivirus. The relevant findings of this trial justify a larger study in patients with COVID-19 and other virus-infected conditions, given the potential implications.
Although PCSK9 is the best known for regulating cholesterol metabolism, growing research indicates its involvement in cellular aging and the development of age-related diseases. Since cancer incidence increases with age, PCSK9 has recently emerged as a potential modulator of tumor progression and patient outcomes, warranting further investigation into its oncogenic role. As highly demanding anabolic steps, tumor growth and metastasis are especially relevant to PCSK9 [136]. In 2012, Sun et al. reported that PCSK9 deficiency reduces melanoma metastasis in the liver because it can reduce cholesterol levels and possibly elevate TNFα-mediated apoptosis [137]. In 2021, Suh et al. illustrated that PCSK9 expression suppressed TNFα-mediated apoptosis of melanoma cells [138]. PCSK9 enhances TNFα-mediated apoptosis in melanoma cells by modulating cholesterol metabolism and LDLR recycling, which subsequently affects TNF receptor (TNFR) cell surface expression and downstream apoptotic signaling. Specifically, PCSK9 inhibition increases LDLR availability, promoting intracellular cholesterol accumulation that alters membrane lipid composition and TNFR clustering, thereby sensitizing melanoma cells to TNFα-induced apoptosis. These findings suggest that targeting PCSK9 could potentiate TNFα-based therapies in melanoma by overcoming apoptotic resistance, offering a promising combinatorial strategy for cancer treatment. The dual impact of PCSK9 on both cholesterol homeostasis and death receptor signaling highlights its potential as a therapeutic target to enhance treatment efficacy in apoptosis-resistant cancers. Moreover, in a large-scale two-sample Mendelian randomization analysis involving over 400,000 participants, Christoph Nowak et al. demonstrate that genetically elevated LDL-C levels are significantly associated with increased breast cancer risk (P = 0.020). That is to say, the PCSK9 LOF variant was associated with a lower incidence of breast cancer, and the GOF variant with higher incidence [139]. Zoltan Ungvari et al. systematically evaluated PCSK9 expression and its prognostic value across multiple malignancies using transcriptomic data from TCGA and GEO databases. Through Cox regression and Kaplan–Meier analyses, Zoltan Ungvari et al. identified tumor-specific survival associations: elevated PCSK9 expression predicted improved overall survival (OS) in breast and ovarian cancers, but poorer OS in bladder cancer, renal clear cell carcinoma, melanoma, and pancreatic cancer. No significant OS associations were found for colon, liver, gastric, lung, prostate, head and neck cancers, or low-grade gliomas. These findings establish PCSK9 as a context-dependent prognostic biomarker, demonstrating paradoxical survival effects across different tumor types. The observed dual roles suggest PCSK9 may influence cancer progression through tissue-specific mechanisms. Future investigations should focus on elucidating these differential molecular pathways and evaluating PCSK9's potential as a therapeutic target in oncology [140]. In addition, injection of a PCSK9 mAb enhanced the levels of MHC-I receptors and led to enhanced T-cell response to several tumors [141]. Immune checkpoint therapy, particularly PD-1/PD-L1 blockade, has shown remarkable success in treating various cancers. However, many patients exhibit poor responses, underscoring the need for strategies to enhance its efficacy. Cholesterol metabolism, which influences tumor progression through bioactive metabolites and membrane dynamics, has emerged as a potential modulator of immunotherapy. The recent studies suggest that inhibiting PCSK9, a key regulator of cholesterol metabolism, can amplify the antitumor effects of PD-1/PD-L1 blockade. Mechanistically, PCSK9 inhibition promotes MHC-I recycling, enhances LDL receptor-mediated T-cell receptor signaling, and remodels the tumor microenvironment (TME) by optimizing immune cell infiltration. These effects bolster cytotoxic T lymphocyte (CTL) activity, the primary effector cells in anti-PD-1/PD-L1 therapy. Thus, combining PCSK9 inhibition with immune checkpoint blockade represents a promising therapeutic approach [142]. Accordingly, PCSK9 expression could be a valuable biomarker for the clinical prognostic outcome of certain types of malignancies.
Limitations
We discussed the potential of PCSK9i beyond the treatment of hypercholesterolemia, highlighting their benefits in various neurological conditions and their potential as a therapeutic target. However, there are several disadvantages or limitations in the information presented. This article mentions a wide range of neurological conditions (AD, AUD, ALS, NTDs, and PD) with lack of understanding the precise mechanisms of action. More and more clinical trials or studies are needed to draw strong conclusions to support the observed connection, which implies that current evidence may be insufficient or preliminary. The primary limitations of PCSK9 inhibitors in broader therapeutic applications include (1) incomplete understanding of their tissue-specific effects beyond hepatic cholesterol metabolism, (2) potential off-target impacts on immune cell function due to PCSK9’s pleiotropic roles, and (3) limited clinical data on long-term safety in noncardiovascular contexts. Future research should prioritize multi-omics approaches to elucidate PCSK9’s extra-hepatic mechanisms, develop targeted delivery systems to minimize systemic effects, and conduct well-designed clinical trials evaluating combination therapies with immune checkpoint inhibitors or conventional chemotherapeutics. In addition, investigating isoform-specific modulation rather than global PCSK9 inhibition may yield more precise therapeutic strategies. In addition, we thought that findings from PCSK9i could be translated into therapeutic or preventive approaches for CNS disorders, but it does not address the challenges in translating preclinical or clinical trial results into approved therapies. The key challenges in translating preclinical PCSK9 inhibitor findings into clinical neurology therapies include (1) the blood–brain barrier (BBB) limiting CNS bioavailability of current monoclonal antibody inhibitors, (2) insufficient understanding of PCSK9’s cell-type-specific roles in neurons versus glia, (3) potential disruption of physiological cholesterol homeostasis required for synaptic plasticity, and (4) lack of validated biomarkers to assess target engagement in the human CNS. Future research should focus on developing BBB-penetrant small molecule inhibitors or nanoparticle delivery systems [143], conducting cell-specific knockout studies to elucidate neural mechanisms, establishing noninvasive CNS cholesterol imaging techniques, and designing adaptive clinical trials that account for the slow progression of many neurological disorders while monitoring both neurological and systemic metabolic effects. In summary, while it lacks depth in providing evidence, discussing risks, and considering the practical aspects of translating research into clinical practice, we present an optimistic view of PCSK9i’s potential in treating or neurological conditions in the future.
Future prospects of PCSK9 inhibitors: expanding applications and overcoming challenges
The FDA’s approval of PCSK9 inhibitors for indications beyond hypercholesterolemia will depend on robust clinical evidence demonstrating therapeutic efficacy, a favorable risk–benefit profile, validated biomarkers, and mechanistic insights linking PCSK9 to disease pathology. Pharmaceutical companies face challenges, such as high development costs, complex trial designs (especially for neurological disorders), market competition, and reimbursement uncertainties. In addition, optimizing drug formulations—particularly for blood–brain barrier penetration in CNS diseases—remains a key hurdle.
PCSK9 inhibitors demonstrate superior LDL-C reduction efficacy (50–60% decrease) as compared to statins (30–50%) and ezetimibe (15–20%), with particularly strong benefits in statin–intolerant patients, while maintaining a favorable safety profile with mostly mild injection-site reactions. Unlike statins which exhibit pleiotropic anti-inflammatory effects or ezetimibe’s intestinal cholesterol absorption blockade, PCSK9 inhibitors uniquely modulate LDL receptor recycling, offering potential advantages in broader applications including neuroprotection, autoimmune diseases, and cancer immunotherapy through their immunomodulatory effects on T-cells and tumor microenvironment. However, their current injectable administration and higher cost limit accessibility as compared to oral statins and ezetimibe, although emerging oral PCSK9-targeting drugs may bridge this gap in the future.
The most promising noncardiovascular research directions for PCSK9 inhibitors include: (1) neurodegenerative disorders, AD and PD, where modulating brain cholesterol metabolism may reduce amyloid/tau pathology and neuroinflammation; (2) oncology, particularly in enhancing immune checkpoint inhibitor efficacy by optimizing T-cell function through LDLR-mediated mechanisms; (3) autoimmune diseases like rheumatoid arthritis, leveraging PCSK9’s immunomodulatory effects on macrophage polarization; and (4) sepsis management, targeting PCSK9’s role in inflammatory cytokine regulation. Emerging evidence also suggests potential applications in nonalcoholic fatty liver disease (NAFLD) through hepatic lipid metabolism modulation and in diabetic complications via vascular protection mechanisms. The current research is actively exploring blood–brain barrier–penetrant formulations and combination therapies to maximize therapeutic potential across these diverse conditions.
However, the cost and accessibility barriers limit widespread adoption. High pricing and prior authorization requirements restrict patient access, particularly in low- and middle-income countries. Future strategies to improve affordability may include biosimilar development, value-based pricing models, and expanded insurance coverage. In addition, advancements in oral small-molecule PCSK9 inhibitors could enhance convenience and reduce costs as compared to injectable monoclonal antibodies. Looking ahead, interdisciplinary research, public–private partnerships, and innovative trial designs will be critical to fully realize the therapeutic potential of PCSK9 inhibition across diverse diseases while ensuring equitable patient access.
Conclusion
It is becoming clear that the reach of PCSK9i extends beyond the treatment of hypercholesterolemia and its associated vascular complications. The function of PCSK9 inhibition in clinical trials has shown added benefits of AD, AUD, ALS, NTDs, and PD, leading to the design of efficacious strategies to reduce PCSK9 expression. Nevertheless, more clinical trials aimed at PCSK9 inhibition are expected to conclude effective and strong conclusions. PCSK9 inhibition is considered an attractive therapeutic target for therapy, especially in light of the fact that a large proportion of high-risk neurological diseases are associated with PCSK9. It remains to be determined whether FDA-approved PCSK9i and more findings can be translated into newly approved therapeutic approaches for targeted CNS disorders. It would be fascinating if such drugs could be designed, produced in large quantities, and become prescriptions for clinical practice application in the future. Thus, we expect more PCSK9i for the treatment to be approved by more countries’ FDA in the future. In this review, we mainly focus on introducing the roles of PCSK9 and its trials, and briefly describe the latest research progress of target drugs. We anticipate that this study will help biomedical researchers or clinical workers treat PCSK9-related CNS diseases by providing ideas for developing novel drug strategies or scientific research ideas.
Acknowledgements
This work was supported by the project of Beijing Tsinghua Changgung Hospital.
Abbreviations
- ASD
Atherosclerosis-related disease
- LDL-C
Low-density lipoprotein cholesterol
- PCSK9i
Proprotein convertase subtilisin/kexin type 9 inhibitors
- HMGCR
3-Hydroxy -3- methylglutaryl-assisted enzyme A reductase
- LDLR
LDL receptor
- GH
Growth hormone
- TSH
Thyrotropin
- BMI
Body mass index
- CNS
Central nervous system
- CHRD
Cys/His-rich domain
- SP
Signal peptide
- HNF1α
Hepatocyte nuclear factor 1α
- NARC-1
Neural apoptosis-regulated convertase-1
- BBB
Blood–brain barrier
- CSF
Cerebrospinal fluid
- AUD
Alcohol use disorder
- AD
Alzheimer’s disease
- NTDs
Neural tube defects
- PD
Parkinson’s disease
- CGNs
Cerebellar granule neurons
- EGFP
Enhanced green fluorescent protein
- VLDLR
Very LDLR
- ApoER2
Apolipoprotein receptor 2
- HUVECs
Human umbilical vein endothelial cells
- HFD
High‑fat diet
- MCAO
Middle cerebral artery occlusion
- shRNA
Short‑hairpin RNA
- ISH
In situ hybridization histochemistry
- LRP1
LDLR-related protein 1
- WT
Wild-type
- HDL
High-density lipoprotein
- I/R
Ischemic/reperfusion injury
- NDD
Neurodegenerative disorders
- FDA MS
Multiple sclerosis
- LM
Listeria monocytogenes
- TNFα
TNF-alpha
- i.n
Intranasal
- tMCAO
Temporary MACO
- ALD
Alcohol-associated liver disease
- ROS
Reactive oxygen species
- FDA
Food and drug administration
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALS
Amyotrophic lateral sclerosis
- NDD
Neurodegenerative disorders
- SNPs
Single nucleotide polymorphisms
- MR
Mendelian randomization
- HMGCRi
HMGCR inhibitors
- Aβ
Amyloid-β
- MCI
Moderate cognitive impairment
- SBA
Spina bifida aperta
- PheWAS
Phenome-wide association study
- HER
Electronic health record
- HR
Hazard ratio
- ASCVD
Atherosclerotic cardiovascular disease
- RR
Rate ratio
- RCTs
Randomized controlled trials
- ICH
Intracerebral hemorrhage
- NLRP3
Nucleotide-binding oligomerization domain-like receptor protein 3
- DENV
Dengue virus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- TNFR
TNF receptor
- OS
Overall survival
- TME
Tumor microenvironment
- CTL
Cytotoxic T lymphocyte
- BBB
Blood–brain barrier
- NAFLD
Non-alcoholic fatty liver disease
Author contributions
All authors contributed to the study conception and design. Xiaoxiao Zheng, Wei Yuan, Ling Li, Hongyue Ma, and Mingxia Zhu conceived the idea, revised all the literature, and wrote the manuscript. Xiuli Li and Xinhong Feng contributed to the revision of the manuscript. All authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Funding
This work was not supported by any funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66. [DOI] [PubMed] [Google Scholar]
- 2.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallardo-Alfaro L, Bibiloni MDM, Mascaró CM, Montemayor S, Ruiz-Canela M, Salas-Salvadó J, et al. Leisure-time physical activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-Plus study. Nutrients. 2020. 10.3390/nu12041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrington WT, Lusis AJ. Atherosclerosis: association between the gut microbiome and atherosclerosis. Nat Rev Cardiol. 2017;14(12):699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Newby DE, Rajagopalan S. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens. 2017;31(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Münzel T. Up in the air: links between the environment and cardiovascular disease. Cardiovasc Res. 2019;115(13):e144–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Y, Tang O, Brady TM, Miller ER 3rd, Heiss G, Appel LJ, et al. Simplified blood pressure measurement approaches and implications for hypertension screening: the atherosclerosis risk in communities study. J Hypertens. 2021;39(3):447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotlyarov S. The role of smoking in the mechanisms of development of chronic obstructive pulmonary disease and atherosclerosis. Int J Mol Sci. 2023. 10.3390/ijms24108725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35. [DOI] [PubMed] [Google Scholar]
- 10.Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60(8):716–21. [DOI] [PubMed] [Google Scholar]
- 11.Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cholesterol Treatment Trialists C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarese EP, Kołodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia. Ann Intern Med. 2015;163(1):40–51. [DOI] [PubMed] [Google Scholar]
- 14.Carbonell T, Freire E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry. 2005;44(35):11741–8. [DOI] [PubMed] [Google Scholar]
- 15.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–90. [DOI] [PubMed] [Google Scholar]
- 16.Grześk G, Dorota B, Wołowiec Ł, Wołowiec A, Osiak J, Kozakiewicz M, et al. Safety of PCSK9 inhibitors. Biomed Pharmacother. 2022. 10.1016/j.biopha.2022.113957. [DOI] [PubMed] [Google Scholar]
- 17.Siezen RJ, Leunissen JA. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6(3):501–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100(3):928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marais AD, Kim JB, Wasserman SM, Lambert G. PCSK9 inhibition in LDL cholesterol reduction: genetics and therapeutic implications of very low plasma lipoprotein levels. Pharmacol Ther. 2015;145:58–66. [DOI] [PubMed] [Google Scholar]
- 20.Lambert G, Sjouke B, Choque B, Kastelein JJP, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53(12):2515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surdo PL, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S, et al. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 2011;12(12):1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holla ØL, Laerdahl JK, Strøm TB, Tveten K, Cameron J, Berge KE, et al. Removal of acidic residues of the prodomain of PCSK9 increases its activity towards the LDL receptor. Biochem Biophys Res Commun. 2011;406(2):234–8. [DOI] [PubMed] [Google Scholar]
- 23.Guo W, Fu J, Chen X, Gao B, Fu Z, Fan H, et al. The effects of estrogen on serum level and hepatocyte expression of PCSK9. Metabolism. 2015;64(4):554–60. [DOI] [PubMed] [Google Scholar]
- 24.Persson L, Cao G, Ståhle L, Sjöberg BG, Troutt JS, Konrad RJ, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. 2010;30(12):2666–72. [DOI] [PubMed] [Google Scholar]
- 25.Spolitu S, Okamoto H, Dai W, Zadroga JA, Wittchen ES, Gromada J, et al. Hepatic glucagon signaling regulates PCSK9 and low-density lipoprotein cholesterol. Circ Res. 2019;124(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awan Z, Dubuc G, Faraj M, Dufour R, Seidah NG, Davignon J, et al. The effect of insulin on circulating PCSK9 in postmenopausal obese women. Clin Biochem. 2014;47(12):1033–9. [DOI] [PubMed] [Google Scholar]
- 27.Veilleux A, Grenier É, Marceau P, Carpentier AC, Richard D, Levy E. Intestinal lipid handling. Arterioscler Thromb Vasc Biol. 2014;34(3):644–53. [DOI] [PubMed] [Google Scholar]
- 28.Bonde Y, Breuer O, Lütjohann D, Sjöberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55(11):2408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong Y, Ma Y, Ye Z, Fu Z, Yang P, Gao B, et al. Thyroid stimulating hormone exhibits the impact on LDLR/LDL-c via up-regulating hepatic PCSK9 expression. Metabolism. 2017;76:32–41. [DOI] [PubMed] [Google Scholar]
- 30.Zenti MG, Lupo MG, De Martin S, Altomari A, Galvan S, Aventaggiato M, et al. Impact of bariatric surgery-induced weight loss on circulating PCSK9 levels in obese patients. Nutr Metab Cardiovasc Dis. 2020;30(12):2372–8. [DOI] [PubMed] [Google Scholar]
- 31.Cariou B, Langhi C, Le Bras M, Bortolotti M, Lê KA, Theytaz F, et al. Plasma PCSK9 concentrations during an oral fat load and after short term high-fat, high-fat high-protein and high-fructose diets. Nutr Metab Lond. 2013;10(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudling M, Angelin B, Gälman C, Persson L. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology. 2009;150(3):1140–6. [DOI] [PubMed] [Google Scholar]
- 33.Ataei S, Kesharwani P, Sahebkar A. Berberine: Ins and outs of a nature-made PCSK9 inhibitor. Excli j. 2022;21:1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong B, Li H, Singh AB, Cao A, Liu J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1α protein expression through the ubiquitin-proteasome degradation pathway. J Biol Chem. 2015;290(7):4047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383(9911):60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barale C, Melchionda E, Morotti A, Russo I. PCSK9 biology and its role in atherothrombosis. Int J Mol Sci. 2021. 10.3390/ijms22115880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidah NG, Prat A. The multifaceted biology of PCSK9. Endocr Rev. 2022;43(3):558–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279(47):48865–75. [DOI] [PubMed] [Google Scholar]
- 40.Piper DE, Jackson S, Liu Q, Romanow WG, Shetterly S, Thibault ST, et al. The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure. 2007;15(5):545–52. [DOI] [PubMed] [Google Scholar]
- 41.Lipari MT, Li W, Moran P, Kong-Beltran M, Sai T, Lai J, et al. Furin-cleaved proprotein convertase subtilisin/kexin type 9 (PCSK9) is active and modulates low density lipoprotein receptor and serum cholesterol levels. J Biol Chem. 2012;287(52):43482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ooi TC, Raymond A, Cousins M, Favreau C, Taljaard M, Gavin C, et al. Relationship between testosterone, estradiol and circulating PCSK9: cross-sectional and interventional studies in humans. Clin Chim Acta. 2015;446:97–104. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh M, Gälman C, Rudling M, Angelin B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res. 2015;56(2):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94(7):2537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziv E, Rabasa-Lhoret Rm, Spahis S, Seidah NG, Précourt LP, Elchebly M, et al. Intestinal and hepatic cholesterol carriers in diabetic Psammomys obesus. Endocrinology. 2010;151(3):958–70. [DOI] [PubMed] [Google Scholar]
- 46.Dubé E, Ethier-Chiasson M, Lafond J. Modulation of cholesterol transport by insulin-treated gestational diabetes mellitus in human full-term placenta1. Biol Reprod. 2013. 10.1095/biolreprod.112.105619. [DOI] [PubMed] [Google Scholar]
- 47.Momtazi AA, Banach M, Pirro M, Stein EA, Sahebkar A. PCSK9 and diabetes: is there a link? Drug Discov Today. 2017;22(6):883–95. [DOI] [PubMed] [Google Scholar]
- 48.Miao J, Manthena PV, Haas ME, Ling AV, Shin D-J, Graham MJ, et al. Role of insulin in the regulation of proprotein convertase subtilisin/kexin type 9. Arterioscler Thromb Vasc Biol. 2015;35(7):1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wild RA, Weedin E, Cox K, Zhao YD, Wrenn DS, Lopez D, et al. Proprotein convertase subtilisin kexin 9 (PCSK9) and nonHDL particles rise during normal pregnancy and differ by BMI. J Clin Lipidol. 2022;16(4):483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong B, Wu M, Li H, Kraemer FB, Adeli K, Seidah NG, et al. Strong induction of PCSK9 gene expression through HNF1α and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res. 2010;51(6):1486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt RJ, Beyer TP, Bensch WR, Qian Y-W, Lin A, Kowala M, et al. Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem Biophys Res Commun. 2008;370(4):634–40. [DOI] [PubMed] [Google Scholar]
- 52.Chen YQ, Troutt JS, Konrad RJ. PCSK9 is present in human cerebrospinal fluid and is maintained at remarkably constant concentrations throughout the course of the day. Lipids. 2014;49(5):445–55. [DOI] [PubMed] [Google Scholar]
- 53.Rousselet E, Marcinkiewicz J, Kriz J, Zhou A, Hatten ME, Prat A, et al. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J Lipid Res. 2011;52(7):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JS, Rosoff D, Luo A, Longley M, Phillips M, Charlet K, et al. PCSK9 is increased in cerebrospinal fluid of individuals with alcohol use disorder. Alcohol Clin Exp Res. 2019;43(6):1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell AS, Wagner J, Rosoff DB, Lohoff FW. Proprotein convertase subtilisin/kexin type 9 (PCSK9) in the central nervous system. Neurosci Biobehav Rev. 2023. 10.1016/j.neubiorev.2023.105155. [DOI] [PubMed] [Google Scholar]
- 56.Bingham B, Shen R, Kotnis S, Lo CF, Ozenberger BA, Ghosh N, et al. Proapoptotic effects of NARC 1 (= PCSK9), the gene encoding a novel serine proteinase. Cytometry A. 2006;69A(11):1123–31. [DOI] [PubMed] [Google Scholar]
- 57.Kysenius K, Muggalla P, Mätlik K, Arumäe U, Huttunen HJ. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell Mol Life Sci. 2012;69(11):1903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C-Y, Tang Z-H, Jiang L, Li X-F, Jiang Z-S, Liu L-S. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax–caspase9–caspase3 pathway. Mol Cell Biochem. 2011;359(1–2):347–58. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Wang Z, Shi J, Jiang Q, Wang H, Li X, et al. Inhibition of proprotein convertase subtilisin/kexin type 9 attenuates neuronal apoptosis following focal cerebral ischemia via apolipoprotein E receptor 2 downregulation in hyperlipidemic mice. Int J Mol Med. 2018;42(4):2098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu H-m, Adijiang A, Mah M, Zhang D-w. Characterization of the role of EGF-A of low density lipoprotein receptor in PCSK9 binding. J Lipid Res. 2013;54(12):3345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102(15):5374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sparks RP, Arango AS, Jenkins JL, Guida WC, Tajkhorshid E, Sparks CE, et al. An allosteric binding site on Sortilin regulates the trafficking of VLDL, PCSK9, and LDLR in hepatocytes. Biochemistry. 2020;59(45):4321–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai X-q, Peng J, Wang M-m, Xiao J, Xiang Q, Ren Z, et al. PCSK9: a potential regulator of apoE/apoER2 against inflammation in atherosclerosis? Clin Chim Acta. 2018;483:192–6. [DOI] [PubMed] [Google Scholar]
- 64.Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. Pcsk9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Commun. 2008;375(1):69–73. [DOI] [PubMed] [Google Scholar]
- 65.Seidah NG, Mowla SJ, Hamelin J, Mamarbachi AM, Benjannet S, Touré BB, et al. Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci USA. 1999;96(4):1321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morizane R, Bonventre JV. Kidney organoids: a translational journey. Trends Mol Med. 2017;23(3):246–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, et al. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22(2):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Insausti R, Córcoles-Parada M, Ubero MM, Rodado A, Insausti AM, Muñoz-López M. Cytoarchitectonic areas of the gyrus ambiens in the human brain. Front Neuroanat. 2019. 10.3389/fnana.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45(8):1375–97. [DOI] [PubMed] [Google Scholar]
- 70.Mazura AD, Pietrzik CU. Endocrine regulation of microvascular receptor—mediated transcytosis and its therapeutic opportunities: insights by PCSK9—mediated regulation. Pharmaceutics. 2023. 10.3390/pharmaceutics15041268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA. 2004;101(18):7100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci USA. 2005;102(6):2069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37(2):161–5. [DOI] [PubMed] [Google Scholar]
- 74.Luna Saavedra YG, Day R, Seidah NG. The M2 module of the Cys-His-rich domain (CHRD) of PCSK9 protein is needed for the extracellular low-density lipoprotein receptor (LDLR) degradation pathway. J Biol Chem. 2012;287(52):43492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, et al. Novel mutations of thePCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat. 2005;26(5):497. [DOI] [PubMed] [Google Scholar]
- 76.Cameron J, Holla ØL, Ranheim T, Kulseth MA, Berge KE, Leren TP. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum Mol Genet. 2006;15(9):1551–8. [DOI] [PubMed] [Google Scholar]
- 77.Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48(2):646–54. [DOI] [PubMed] [Google Scholar]
- 78.Apaijai N, Moisescu DM, Palee S, McSweeney CM, Saiyasit N, Maneechote C, et al. Pretreatment with PCSK9 inhibitor protects the brain against cardiac ischemia/reperfusion injury through a reduction of neuronal inflammation and amyloid beta aggregation. J Am Heart Assoc. 2019. 10.1161/JAHA.118.010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng Y, Zhu T, Li G, Xu L, Zhang Y. PCSK9 inhibitor protects against ischemic cerebral injury by attenuating inflammation via the GPNMB/CD44 pathway. Int Immunopharmacol. 2024;126: 111195. [DOI] [PubMed] [Google Scholar]
- 80.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Datta G, White CR, Dashti N, Chaddha M, Palgunachari MN, Gupta H, et al. Anti-inflammatory and recycling properties of an apolipoprotein mimetic peptide, Ac-hE18A-NH(2). Atherosclerosis. 2010;208(1):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39(9):1740–3. [PubMed] [Google Scholar]
- 83.Giau VV, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015;11:1723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Hou H, Zi D, Habib A, Tan J, Sawmiller D. Novel apoE receptor mimetics reduce LPS-induced microglial inflammation. Am J Transl Res. 2019;11(8):5076–85. [PMC free article] [PubMed] [Google Scholar]
- 85.Vilella A, Bodria M, Papotti B, Zanotti I, Zimetti F, Remaggi G, et al. PCSK9 ablation attenuates Aβ pathology, neuroinflammation and cognitive dysfunctions in 5XFAD mice. Brain Behav Immun. 2024;115:517–34. [DOI] [PubMed] [Google Scholar]
- 86.Zheng Y, Zhu T, Li G, Xu L, Zhang Y. PCSK9 inhibitor protects against ischemic cerebral injury by attenuating inflammation via the GPNMB/CD44 pathway. Int Immunopharmacol. 2024. 10.1016/j.intimp.2023.111195. [DOI] [PubMed] [Google Scholar]
- 87.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiat. 2017;74(9):911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee JS, O’Connell EM, Pacher P, Lohoff FW. PCSK9 and the gut-liver-brain axis: a novel therapeutic target for immune regulation in alcohol use disorder. J Clin Med. 2021. 10.3390/jcm10081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alavi M, Janjua NZ, Chong M, Grebely J, Aspinall EJ, Innes H, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: an international study. J Hepatol. 2018;68(3):393–401. [DOI] [PubMed] [Google Scholar]
- 90.McIntosh C. Alcohol and the nervous system. J Neurol Neurosurg Psychiatry. 2004;75(suppl_3):iii16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Egervari G, Siciliano CA, Whiteley EL, Ron D. Alcohol and the brain: from genes to circuits. Trends Neurosci. 2021;44(12):1004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326(2):165–76. [DOI] [PubMed] [Google Scholar]
- 93.Nagy LE, Ding W-X, Cresci G, Saikia P, Shah VH. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology. 2016;150(8):1756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu X, Fan X, Miyata T, Kim A, Cajigas-Du Ross CK, Ray S, et al. Recent advances in understanding of pathogenesis of alcohol-associated liver disease. Annu Rev Pathol Mech Dis. 2023;18(1):411–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei J, Dai Y, Wen W, Li J, Ye LL, Xu S, et al. Blood-brain barrier integrity is the primary target of alcohol abuse. Chem Biol Interact. 2021. 10.1016/j.cbi.2021.109400. [DOI] [PubMed] [Google Scholar]
- 96.Goldfine CE, Tom JJ, Im DD, Yudkoff B, Anand A, Taylor JJ, et al. The therapeutic use and efficacy of ketamine in alcohol use disorder and alcohol withdrawal syndrome: a scoping review. Front Psychiatry. 2023. 10.3389/fpsyt.2023.1141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516–24. [DOI] [PubMed] [Google Scholar]
- 98.Lohoff FW, Sorcher JL, Rosen AD, Mauro KL, Fanelli RR, Momenan R, et al. Methylomic profiling and replication implicates deregulation of PCSK9 in alcohol use disorder. Mol Psychiatry. 2018;23(9):1900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JS, Mukhopadhyay P, Matyas C, Trojnar E, Paloczi J, Yang YR, et al. Pcsk9 inhibition as a novel therapeutic target for alcoholic liver disease. Sci Rep. 2019;9(1):17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zou Y, Li S, Xu B, Guo H, Zhang S, Cai Y. Inhibition of proprotein convertase subtilisin/kexin type 9 ameliorates liver fibrosis via mitigation of intestinal endotoxemia. Inflammation. 2020;43(1):251–63. [DOI] [PubMed] [Google Scholar]
- 101.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tzeplaeff L, Wilfling S, Requardt MV, Herdick M. Current state and future directions in the therapy of ALS. Cells. 2023. 10.3390/cells12111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van JanseMantgem MR, van Rheenen W, Hackeng AV, van Es MA, Veldink JH, van den Berg LH, et al. Association between serum lipids and survival in patients with amyotrophic lateral sclerosis: a meta-analysis and population-based study. Neurology. 2023;100(10):e1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Q, Zhang Q, Cao B. Causal relationship between PCSK9 inhibitor and common neurodegenerative diseases: a drug target Mendelian randomization study. Brain Behav. 2024;14(6): e3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chiang LW, Grenier JM, Ettwiller L, Jenkins LP, Ficenec D, Martin J, et al. An orchestrated gene expression component of neuronal programmed cell death revealed by cDNA array analysis. Proc Natl Acad Sci USA. 2001;98(5):2814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bingham B, Shen R, Kotnis S, Lo CF, Ozenberger BA, Ghosh N, et al. Proapoptotic effects of NARC 1 (= PCSK9), the gene encoding a novel serine proteinase. Cytometry A. 2006;69(11):1123–31. [DOI] [PubMed] [Google Scholar]
- 107.Piao MX, Bai JW, Zhang PF, Zhang YZ. PCSK9 regulates apoptosis in human neuroglioma u251 cells via mitochondrial signaling pathways. Int J Clin Exp Pathol. 2015;8(3):2787–94. [PMC free article] [PubMed] [Google Scholar]
- 108.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12(7):856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem. 2010;115(5):1077–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Storck SE, Meister S, Nahrath J, Meißner JN, Schubert N, Di Spiezio A, et al. Endothelial LRP1 transports amyloid-β1–42 across the blood-brain barrier. J Clin Invest. 2015;126(1):123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zimetti F, Caffarra P, Ronda N, Favari E, Adorni MP, Zanotti I, et al. Increased PCSK9 cerebrospinal fluid concentrations in Alzheimer’s disease. J Alzheimers Dis. 2017;55(1):315–20. [DOI] [PubMed] [Google Scholar]
- 113.Lakshmana MK, Picard C, Poirier A, Bélanger S, Labonté A, Auld D, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) in Alzheimer’s disease: a genetic and proteomic multi-cohort study. PLoS ONE. 2019. 10.1371/journal.pone.0220254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Courtemanche H, Bigot E, Pichelin M, Guyomarch B, Boutoleau-Bretonnière C, Le May C, et al. PCSK9 concentrations in cerebrospinal fluid are not specifically increased in Alzheimer’s disease. J Alzheimers Dis. 2018;62(4):1519–25. [DOI] [PubMed] [Google Scholar]
- 115.Mazura AD, Ohler A, Storck SE, Kurtyka M, Scharfenberg F, Weggen S, et al. PCSK9 acts as a key regulator of Aβ clearance across the blood–brain barrier. Cell Mol Life Sci. 2022. 10.1007/s00018-022-04237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjærg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCRgenetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: mendelian randomisation study. BMJ. 2017. 10.1136/bmj.j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosoff DB, Bell AS, Jung J, Wagner J, Mavromatis LA, Lohoff FW. Mendelian randomization study of PCSK9 and HMG-CoA reductase inhibition and cognitive function. J Am Coll Cardiol. 2022;80(7):653–62. [DOI] [PubMed] [Google Scholar]
- 118.Molloy AM, Kappen C. Papers from the 7th international neural tube defects conference. Birth Defects Res A Clin Mol Teratol. 2012;94(10):747–8. [DOI] [PubMed] [Google Scholar]
- 119.Poirier S, Prat A, Marcinkiewicz E, Paquin J, Chitramuthu BP, Baranowski D, et al. Implication of the proprotein convertase NARC-1/PCSK9 in the development of the nervous system. J Neurochem. 2006;98(3):838–50. [DOI] [PubMed] [Google Scholar]
- 120.Jerome RN, Pulley JM, Roden DM, Shirey-Rice JK, Bastarache LA, Bernard GR, et al. Using human “experiments of nature” to predict drug safety issues: an example with PCSK9 inhibitors. Drug Saf. 2018;41(3):303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.An D, Wei X, Li H, Gu H, Huang T, Zhao G, et al. Identification of PCSK9 as a novel serum biomarker for the prenatal diagnosis of neural tube defects using iTRAQ quantitative proteomics. Sci Rep. 2015. 10.1038/srep17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst-Hunter Z, Lyall DM, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2017;5(2):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144–53. [DOI] [PubMed] [Google Scholar]
- 124.Jahed MR, Habibi SAH, Vaseghi G, Amiri H, Montazeri H, Eshraghi A. Association between plasma PCSK9 levels and lipid profile in patients with Parkinson’s disease and comparison with healthy subjects. Curr J Neurol. 2022;21(4):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woitalla D, Dunac A, Safavi A, Ceravolo MG, Gomez Esteban JC, Pavese N, et al. A noninterventional study evaluating the effectiveness of rotigotine and levodopa combination therapy in younger versus older patients with Parkinson’s disease. Expert Opin Pharmacother. 2018;19(9):937–45. [DOI] [PubMed] [Google Scholar]
- 126.Kang H, Yu H, Fan J, Cao G. Rotigotine protects against oxidized low-density lipoprotein(ox-LDL)-induced damages in human umbilical vein endothelial cells(HUVECs). Bioengineered. 2021;12(2):10568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oyama K, Giugliano RP, Tang M, Bonaca MP, Saver JL, Murphy SA, et al. Effect of evolocumab on acute arterial events across all vascular territories : results from the FOURIER trial. Eur Heart J. 2021;42(47):4821–9. [DOI] [PubMed] [Google Scholar]
- 128.Qin J, Liu L, Su XD, Wang BB, Fu BS, Cui JZ, et al. The effect of PCSK9 inhibitors on brain stroke prevention: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31(8):2234–43. [DOI] [PubMed] [Google Scholar]
- 129.Schlunk F, Fischer P, Princen HMG, Rex A, Prinz V, Foddis M, et al. Effects of inhibition or deletion of PCSK9 (proprotein convertase subtilisin/kexin type 9) on intracerebral hemorrhage volumes in mice. Stroke. 2020;51(11):e297–8. [DOI] [PubMed] [Google Scholar]
- 130.Guo H, Li W, Yang Z, Xing X. E3 ubiquitin ligase MARCH1 reduces inflammation and pyroptosis in cerebral ischemia-reperfusion injury via PCSK9 downregulation. Mamm Genome. 2024;35(3):346–61. [DOI] [PubMed] [Google Scholar]
- 131.Pu S, Jia C, Li Z, Zang Y. Protective mechanism of proprotein convertase subtilisin-like kexin type 9 inhibitor on rats with middle cerebral artery occlusion-induced cerebral ischemic infarction. Comput Intell Neurosci. 2022;2022:4964262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seidah NG, Prat A, Pirillo A, Catapano AL, Norata GD. Novel strategies to target proprotein convertase subtilisin kexin 9: beyond monoclonal antibodies. Cardiovasc Res. 2019;115(3):510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gan ES, Tan HC, Le DHT, Huynh TT, Wills B, Seidah NG, et al. Dengue virus induces PCSK9 expression to alter antiviral responses and disease outcomes. J Clin Invest. 2020;130(10):5223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seidah NG. The PCSK9 discovery, an inactive protease with varied functions in hypercholesterolemia, viral infections, and cancer. J Lipid Res. 2021. 10.1016/j.jlr.2021.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Navarese EP, Podhajski P, Gurbel PA, Grzelakowska K, Ruscio E, Tantry U, et al. PCSK9 inhibition during the inflammatory stage of SARS-CoV-2 infection. J Am Coll Cardiol. 2023;81(3):224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol. 2013;4: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012;14(12):1122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suh JM, Son Y, Yoo J-Y, Goh Y, Seidah NG, Lee S, et al. Proprotein convertase subtilisin/kexin type 9 is required for Ahnak-mediated metastasis of melanoma into lung epithelial cells. Neoplasia. 2021;23(9):993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nowak C, Ärnlöv J. A mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. 2018. 10.1038/s41467-018-06467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ungvari Z, Menyhart O, Lehoczki A, Fekete M, Bianchini G, Győrffy B. PCSK9 expression and cancer survival: a prognostic biomarker at the intersection of oncology and geroscience. Geroscience. 2025. 10.1007/s11357-025-01733-3. [DOI] [PubMed] [Google Scholar]
- 141.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020. 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun S, Ma J, Zuo T, Shi J, Sun L, Meng C, et al. Inhibition of PCSK9: a promising enhancer for anti-PD-1/PD-L1 immunotherapy. Research (Wash D C). 2024;7: 0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Inamdar A, Gurupadayya B, Halagali P, Tippavajhala VK, Khan F, Pathak R, et al. Unraveling neurological drug delivery: polymeric nanocarriers for enhanced blood-brain barrier penetration. Curr Drug Targets. 2025;26(4):243–66. [DOI] [PubMed] [Google Scholar]
- 144.Doggrell SA, Lynch KA. Is there enough evidence with evolocumab and alirocumab (antibodies to proprotein convertase substilisin-kexin type, PCSK9) on cardiovascular outcomes to use them widely? Evaluation of Sabatine MS, Giugliano RP, Wiviott SD et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500-1509, and Robinson JG, Farnier M, Krempf M et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1488-99. Expert Opin Biol Ther. 2015;15(12):1671–5. [DOI] [PubMed] [Google Scholar]
- 145.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. [DOI] [PubMed] [Google Scholar]
- 146.Safaeian L, Mirian M, Bahrizadeh S. Evolocumab, a PCSK9 inhibitor, protects human endothelial cells against H2O2-induced oxidative stress. Arch Physiol Biochem. 2022;128(6):1681–6. [DOI] [PubMed] [Google Scholar]
- 147.Ma N, Fan L, Dong Y, Xu X, Yu C, Chen J, et al. New PCSK9 inhibitor miR-552-3p reduces LDL-C via enhancing LDLR in high fat diet-fed mice. Pharmacol Res. 2021;167: 105562. [DOI] [PubMed] [Google Scholar]
- 148.Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30. [DOI] [PubMed] [Google Scholar]
- 149.Wang X, Raghavan A, Chen T, Qiao L, Zhang Y, Ding Q, et al. CRISPR-Cas9 targeting of PCSK9 in human hepatocytes in vivo-brief report. Arterioscler Thromb Vasc Biol. 2016;36(5):783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mitchell T, Chao G, Sitkoff D, Lo F, Monshizadegan H, Meyers D, et al. Pharmacologic profile of the Adnectin BMS-962476, a small protein biologic alternative to PCSK9 antibodies for low-density lipoprotein lowering. J Pharmacol Exp Ther. 2014;350(2):412–24. [DOI] [PubMed] [Google Scholar]
- 151.Momtazi-Borojeni AA, Jaafari MR, Banach M, Gorabi AM, Sahraei H, Sahebkar A. Pre-clinical evaluation of the nanoliposomal antiPCSK9 vaccine in healthy non-human primates. Vaccines. 2021. 10.3390/vaccines9070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chadwick AC, Musunuru K. Treatment of dyslipidemia using CRISPR/Cas9 genome editing. Curr Atheroscler Rep. 2017;19(7):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.