Abstract
Background
The genome of a eukaryotic cell is usually organized on a set of chromosomes. Recently, karyotype engineering has been applied to various organisms, but whether and to what extent a naturally evolved genome can resist or tolerate massive artificial manipulations remains unexplored.
Results
Using unicellular yeast models of both Saccharomyces cerevisiae and Schizosaccharomyces pombe, we deliberately construct dozens of single-chromosome strains with different chromosome architectures. Three S. cerevisiae strains have the individual chromosomes fused into a single chromosome, but with the individual chromosomes in different orders. Eighteen S. cerevisiae strains have a single chromosome but with different centromeric sequences. Fifteen S. cerevisiae strains have a single chromosome with the centromere at different distances relative to the telomeres. Two S. pombe strains have a single, circular chromosome, and three strains have a single, linear chromosome with the centromere at different distances relative to the telomeres. All of these single-chromosome strains are viable, but the strains with an acrocentric or a telocentric chromosome have abnormal cell morphologies, and grow more slowly than those with a metacentric or sub-metacentric chromosome, and show increased genome instability with chromosome segregation abnormalities or genome diploidization.
Conclusion
The functional genomes of both the evolutionarily distant yeasts S. cerevisiae and S. pombe are highly tolerant of diversified genome organizations. The phenotypic abnormalities and increased genome instability of the acrocentric/telocentric single-chromosome yeasts suggest that yeasts with metacentric chromosomes have an evolutionary advantage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13059-025-03689-1.
Keywords: Chromosome engineering, Single-chromosome yeast, Genome plasticity, Telocentric chromosome
Background
The genetic information of living organisms on earth is stored in DNA, which is further organized in chromosome(s) [1]. For all eukaryotic chromosomes, centromere and telomere are two essential components. Centromere is the chromosomal region to which kinetochore forms and attaches to spindle microtubules during chromosome segregation in each cell division [2]. A chromosome that contains one centromere is called monocentric chromosome. Monocentric chromosomes are found in many eukaryotes, such as yeasts, plants, and mammals. A chromosome, in which the kinetochore spans most of the chromosome length during mitosis, is called holocentric chromosome. Holocentric chromosomes are common in worms (e.g., Caenorhabditis elegans) and Lepidopteran insects (e.g., Bombyx mori and Spodoptera frugiperda) [3]. The main difference between monocentric and holocentric chromosome is their chromosome configuration during telophase of mitosis, typical monocentric chromatids form the classical V-shaped figures, whereas holocentric chromatids move apart in parallel, which stabilizes chromosome fragments favoring karyotype rearrangements [4].
In species containing monocentric chromosome, the primary sequence in centromeres consists of species-specific kilobase- to megabase-sized arrays of tandem repeats [2]. For example, in fission yeast S. pombe, a centromere region covers ~ 100 kb, including cnt (centromeric central core), dg and dh repeats (pericentromeric repeats), and it is called regional centromere [5]. However, in budding yeast S. cerevisiae, a characteristic sequence of ~ 123-bp, which fully specify centromere identity [6], is called point centromere. Besides the primary sequence, the position of the centromere within a monocentric chromosome also varies widely, resulting in diverse karyotypes across species. Chromosomes containing centromeres next to telomeres, near telomeres, and in the middle of chromosomes are called telocentric, acrocentric, and metacentric chromosomes, respectively [7]. In both yeasts S. cerevisiae and S. pombe, their chromosomes are metacentric or sub-metacentric [8]; in mice Mus musculus, their autosomes are telocentric [9]; while in flies, the chromosomes of different groups are either metacentric or telocentric [10]. Changing centromere position either by chromosomal re-arrangements or by centromere repositioning is likely a driving force for karyotype evolution [10].
A eukaryotic cell usually contains more than one chromosome [11]. There seems to be no tight correlation between the number of chromosomes and the complexity of organisms. In the unicellular baker’s yeast Saccharomyces cerevisiae and fission yeast Schizosaccharomyces pombe, haploid cells have 16 and 3 chromosomes, respectively. In mammals, mouse (Mus musculus) diploid cells have 40 chromosomes; Indian muntjac (Muntiacus muntjak vaginalis) and Chinese muntjac (Muntiacus reevesi), which are two closely related species, have strikingly different chromosome numbers, i.e., 2n = 6/7 and 46, respectively [12]; and human (Homo sapiens) diploid cells have 46 chromosomes. Thus, the chromosomes in a given species have evolved to be relatively stable.
Recently, intentional artificial chromosome engineering has been conducted in both yeast (i.e., S. cerevisiae and S. pombe) and mouse haploid embryonic stem cells (haESCs) [13–18]. Two independent groups performed massive chromosome fusions by deletion of telomeres and centromeres in S. cerevisiae. One group successfully ligated all sixteen chromosomes and obtained both the linear and circular single-chromosome strains SY14 and SY15, respectively [13, 19]. In the single linear chromosome in SY14, the retained CEN15 was roughly positioned in the middle of the giant chromosome [13]. The other group constructed a two-chromosome strain, and their attempts of creating single-chromosome strain were unsuccessful [14]. The authors suspected that overlong chromosome arms might be problematic [14, 20]. The single-chromosome and two-chromosome S. cerevisiae strains displayed drastic changes of global chromosome structures, but showed little changes (~ 0.5%) of gene expression profiles and exhibited no apparent defects in various phenotypes [13, 14]. In S. pombe, the single-chromosome strains were constructed with the fusion orders of Chr3-2–1 and Chr2-1–3, which contained functional cen2 or cen1, respectively. Likewise, the single-chromosome S. pombe strains grew as robustly as the wild-type one, and the expression of more than 90% of genes were unchanged in single-chromosome strains [15]. In mouse haESCs, Wang et al. fused Chr15 and Chr17 together and surprisingly cultivated healthy mice with fused chromosomes [16]. Although Li et al. also successfully fused several pairs of chromosomes, the fusion of Chr1 and Chr2, which are the two largest chromosomes in mouse genome, resulted in the haESCs auto-diploidization, suggesting the existence of a length threshold of the tolerable chromosome arm in mouse cells [17]. Interestingly, naturally evolved giant sex chromosomes have been found in a few mouse species [21], arguing against the hypothesis that chromosome size or chromosome arm length is directly associated with genome instability.
In this work, we employed both S. cerevisiae and S. pombe models to investigate whether and to what extent naturally evolved genomes can tolerate large-scale artificial manipulations with cell viability unscathed. Through successful constructions of more than a dozen of single-chromosome yeast strains, in which either the genome is restructured with different orders of natural chromosomes, or a natural chromosome is fragmented and repositioned at two distal chromosome ends or the centromere is positioned near one of the chromosome ends, we conclude that both yeasts are robust against various genome reconfigurations.
Results
Construction of single-chromosome S. cerevisiae strains FSY1, FSY2, and FSY3
Recently, karyotype engineering has been deliberately attempted in S. cerevisiae. Shao et al. fortunately created the single-chromosome strain SY14 [13], while Luo et al. constructed a strain with two chromosomes, but encountered difficulties in further fusing the two chromosomes into one, suggesting that the inadaptable length of chromosome arms may be a hurdle to overcome [14]. To address whether naturally evolved genome of S. cerevisiae can tolerate a structure different from that of SY14, we used SY13, which has two chromosomes [13], to construct additional single-chromosome strains. We performed chromosome fusions with the left arms of Chr. I (I-L) and Chr. XVI (XVI-L), the left arm of Chr. XVI (XVI-L) and the right arm of Chr. X (X-R), or the right arms of Chr. XI (XI-R) and Chr. X (X-R), which were different from the fusion of the left arm of Chr. I (I-L) and the right arm of Chr. XI (XI-R) of SY13 in SY14 [13]. We obtained three single-chromosome strains FSY1, FSY2, and FSY3 (Fig. 1A; Additional file 1: Fig S1A-C; Additional file 2: Table S1). All of these FSY strains retained CEN15 as the single centromere as SY14, but each displayed a different arrangement of the original sixteen chromosomes (Fig. 1A). To validate the chromosome fusions in FSY strains, we performed pulsed-field gel electrophoresis (PFGE) analysis. The results showed that a larger chromosome band was detected in each FSY strain, similar to the SY14 but different from the SY13 (Fig. 1B), indicating the successful chromosome fusion. However, there still left a slight band of 5.7 Mb (Fig. 1B). We suspected that these chromosomal fragments were originated from mechanical damage during DNA electrophoresis, rather than from spontaneous chromosomal rearrangements. We then examined telomere structures to further validate the proper chromosome fusions. In SY13, there were three telomere signals representing four telomeres from XVI-L (1.3 kb), I-L (1.3 kb), XI-R (2.4 kb), and X-R (5.2 kb) (Additional file 1: Fig S1D). When chromosome fusions occurred in FSY strains, the telomere signals of the corresponding chromosome ends disappeared due to the deletion of the telomere sequences, leaving two telomere signals in each strain as expected (Fig. 1C; Additional file 1: Fig S1D). All of these single-chromosome yeasts, including FSY1, FSY2, and FSY3, showed similar cell morphology but slightly larger cell size compared to SY14 (Fig. 1D). In addition, FSY1 and FSY2 with longer chromosome arm had slightly slower growth but comparable cell doubling time to FSY3 and SY14 (Fig. 1E, F).
Fig. 1.
Creation and characterization of SY13 derived S. cerevisiae strains containing a single chromosome. A Schematic diagram of chromosome fusion. The SY13 and SY14 strains were established in a previous study [13]; FSY1, FSY2, and FSY3 were constructed by fusing two chromosomes in SY13 in different orders/orientations. Each native chromosome is marked with a unique color; the oval dot in red represents the centromere; and the semicircle in dark blue represents the telomere. B PFGE analysis of chromosomes in the SY13, FSY1, FSY2, and FSY3 strains. SY14 is used as a control and wild-type S. pombe chromosomes are used as size markers. Signals indicated with an asterisk are likely to be “damaged chromosome(s)” during DNA electrophoresis. C Southern blotting analysis of telomeres in FSY strains. Genomic DNA extracted from indicated strains was digested with XhoI and hybridized with a telomere-specific probe. Each terminal restriction fragment of the corresponding chromosome(s) is indicated on the right. Asterisks on the right indicate non-specific signals. D Morphology of representative SY13, SY14, and FSY cells in logarithmic phase. Cells were cultured at 30 °C in YPD medium. DIC images are shown. Scale bar, 5 μm. Quantification of cell size (n = 60) for each strain is shown at the right, and the p-value (****p < 0.0001) is shown at the top of the strains. E Growth analysis of FSY cells. Cells were cultured at 30 °C in YPD medium. SY13 and SY14 were used as controls. Error bars represent the standard deviation (s.d.), n = 3. The p-value (****p < 0.0001) at the right of each strain shows the significance of difference in growth rate compared to strain SY14. F Doubling time of FSY cells. For each strain, n = 30, the significance of the difference between the strains is indicated on the right (**p < 0.01). G Serial dilution assays of FSY cells under various genotoxic stress. Cells were grown at 30 °C for 2 days. CPT, camptothecin; HU, hydroxyurea; MMS, methyl methanesulfonate. H Serial dilution assays of single-chromosome yeast cells with replaced centromere sequence under various genotoxic stress. Schematic of centromere replacement in SY14 is shown on the left. SY14CENX represents single-chromosome yeast in which CEN15 was replaced with the conserved ~ 120-bp core centromere sequence of a corresponding chromosome, SY14CENX+ represents single-chromosome yeast in which CEN15 was replaced with the core centromere sequence and its CDEIII-proximal 500-bp pericentromeric sequence. Each centromere was marked with a unique color. Cells were grown at 30 °C for 2 days
To examine genome stability of the newly constructed single-chromosome strains, we challenged them with genotoxic reagents, including Camptothecin (CPT), hydroxyurea (HU), and methyl methanesulfonate (MMS) [22]. CPT acts by trapping topoisomerase I (TOP1) on DNA during replication, and HU generates replication stress by depleting the dNTP pools, while MMS induces nucleotide base methylation. All of them lead to DNA damage during DNA replication. Wild type cells have evolved to deal with DNA damage through DNA repair pathways, assuring the maintenance of genome stability and faithful transmission of genetic information [23]. When these genotoxic drugs were applied to the single-chromosome strains, FSY1 and FSY2, but not FSY3, were more sensitive than SY14 (Fig. 1G), suggesting their lower ability to maintain a stable genome. These results indicate that the S. cerevisiae genome is tolerant to different types of chromosome fusions, although chromosome rearrangements may affect genome stability.
Replacement of CEN15 with other CENs in the single chromosome of SY14
During the construction of single-chromosome or two-chromosome strains [13, 14, 19], both groups coincidentally used CEN15 as a residual centromere. There are sixteen point centromeres in the native chromosomes in S. cerevisiae, namely CEN1 to CEN16. They are ~ 120 bp long, and all contain three elements, CDEI, CDEII, and CDEIII [24] (Additional file 1: Fig S2). The short CDEI and CDEIII regions are conserved across sixteen centromeres, whereas the CDEII elements are quite distinct, although they all consist of AT-rich sequences [6]. The CEN15 in the SY14 strain appears to be strong enough to confer segregation of the giant chromosome during cell division [13]. To test whether other centromeres could function as well as CEN15, we replaced CEN15 with other individual CENs (Additional file 1: Fig S2), resulting in a series of strains namely SY14CENX (Fig. 1H, left panel, X refers to the number of native centromeres). The serial dilution assays showed that both SY14CEN8 (CEN15 was replaced by CEN8) and SY14CEN12 (CEN15 was replaced by CEN12) strains grew less robustly than either SY14 or the other derivatives at 30 °C, and were much more sensitive to various genotoxic drug treatments (Fig. 1H, right panel). Previous studies have shown that pericentromeric DNA is also indispensable for full centromere function [25, 26]. We then replaced CEN15 in SY14 with CEN8+ or CEN12+, which contains the corresponding 500-bp pericentromeric sequence proximal to CDEIII. Both SY14CEN8+ and SY14CEN12+ grew more robustly than SY14CEN8 and SY14CEN12 under various conditions (Fig. 1H). These results suggest that all the point centromeres, with conserved DNA sequence and their co-evolved pericentromeric region, are equally functional for chromosome segregation in single-chromosome S. cerevisiae.
Linearization of circular chromosome of SY15 and generation of a series of single-chromosome S. cerevisiae strains
Previous studies have used PCR-mediated or “telomerator”-mediated karyotype engineering in S. cerevisiae [27, 28]. Building on these previous methods and to further test the robustness of S. cerevisiae genome organization, we used the circular chromosome strain SY15 as a starting strain to construct more linear chromosome strains [19]. Our strategy is to insert a telomere cassette into the gene-free region of the circular chromosome (Fig. 2A). The telomere cassette contains two homologous arms for genome integration, two opposing 80-bp telomeric TG1-3/C1-3A repeat sequences for de novo telomere formation, a URA3 marker gene for clone selection, and two identical CRISPR-Cas9 target sites flanking the URA3 gene (Additional file 1: Fig S3A). The induction of Cas9 and gRNA could introduce double-strand breaks at two target sites, resulting in a linear chromosome (Additional file 1: Fig S3A). In order to avoid the potential tragedy of cell death, which could be caused in particular by an inappropriate length of chromosome arms, we arbitrarily inserted telomere sequences at seven different loci on the native Chr. X and Chr. VII, respectively (Fig. 2A; Additional file 2: Table S2). The circular single-chromosome strains with telomere cassettes were named SY15C1 to SY15C7 (Fig. 2A). The Cas9-induced DSBs specifically at the target sites in SY15C cells, exposing the telomeric TG1-3/C1-3A repeat sequences, which served as functional telomeres after telomerase-mediated elongation. We obtained seven additional single linear chromosome yeasts, namely LSY1 to LSY7, in which the roughly balanced chromosome arms are comparable to those in SY14 (Fig. 2A; Additional file 2: Table S2). In contrast to the intact native chromosomes in SY14 and FSY strains (Fig. 1A), the native Chr. X or Chr. VII in these LSY strains was split into two parts and repositioned at two ends of the single chromosome (Fig. 2A; Additional file 1: Fig S3B). PFGE analysis showed that the chromosome in LSY1 to LSY7 strains migrated to the same position as that in the SY14 strain, whereas the circular chromosome in the parental SY15 strain did not enter the gel as expected [19, 28] (Fig. 2B). In both LSY and SY14 cells, in addition to linear single chromosomes, an unexpected 5.7-Mb band could also be detected, which may be resulted from potential mechanical breaks during DNA electrophoresis (Fig. 2B). Further telomere Southern blotting assay confirmed that all the LSY1 to LSY7 cells contained two telomeres, and the size of each telomere terminal restriction fragment (TRF) was elongated by telomerase from 80 bp to approximately 300 bp (Fig. 2C, right panel), fitting well with our original design (Fig. 2C, left panel; Additional file 1: Fig S3C, D). In addition, the cell morphology of LSY1 to LSY7 cells showed no visible abnormality, and their cell size was comparable to that of SY14 cells (Fig. 2D). Although LSY cells had different growth rates, their cell doubling times were statistically comparable (Fig. 2E, F). The successful construction of these strain series reinforced the conclusion that S. cerevisiae chromosomes are highly plastic and can tolerate a wide range of chromosomal shuffling.
Fig. 2.
Generation and characterization of SY15 derived S. cerevisiae strains containing a metacentric single chromosome. A Schematic of circular chromosome structure in SY15 [19] and linear chromosome structures in LSY strains. The telomere cassette (see “Methods” for details) was inserted into individual gene free locus of Chr. VII or Chr. X (indicated on the right of circular chromosome of SY15). Genes flanking the inserted telomere cassette were labeled on top of the lines which indicate linearized single-chromosomes. Specific gRNA expression induced double-strand breaks, leading to chromosome linearization and generation of LSY1 to LSY7 strains with rearranged chromosome structures (shown on the right). B PFGE analysis of chromosomes in the LSY strains. SY14, SY15, and wild-type S. pombe were used as controls. Signals indicated with an asterisk are likely to be “damaged chromosome(s)” during DNA electrophoresis. C Southern blotting assay of telomeres in LSY strains. Genomic DNA extracted from indicated strains were digested with Eco130I (left panel) or XbaI/ClaI (right panel, except that genomic DNA of LSY1 was digested with NotI/ClaI), and hybridized with a telomere-specific probe. Asterisks on the right indicate non-specific signals, triangles marked uncharacterized signals. D Morphology of representative SY15, SY14, and LSY cells in logarithmic phase. Cells were cultured at 30 °C in YPD medium. DIC images are shown. Scale bar, 5 μm. Quantification cell size (n = 60) for each strain is shown at the right, and the p-value (**p < 0.01, ****p < 0.0001) is shown at the top of the strains (compared to the strain SY14). E Growth analysis of LSY cells. Cells were cultured at 30 °C in YPD medium. Error bars represent standard deviation (s.d.), n = 3. The p-value (*p < 0.05, **p < 0.01, ****p < 0.0001) indicated at the right side of strains showed the significance of difference in growth rate compared to strain SY14. F Doubling time of LSY cells. For each strain, n = 30, there are no statistically significant differences between the strains and SY14
It has been proposed that the length limit of chromosome arms is half of the average spindle axis at telophase of cell division [20]. Additionally, FSY1 and FSY2, which had a relatively long chromosome arm (Fig. 1A), were viable despite a reduced growth rate (Fig. 1E) and increased genome instability (Fig. 1G). To further test the correlation between chromosome arm variation and cellular phenotypes, we then inserted the telomere cassette (Additional file 1: Fig S3A) into eight individual gene-free regions of Chr. XII or Chr. XV proximal to the CEN15 and generated a series of strains designated SY15C8 to SY15C15 (Fig. 3A). The induction of Cas9 and gRNA created double-strand breaks to form a linear chromosome, in which the CEN15 was relocated near one end of the chromosome (Fig. 3A). Finally, we generated single-chromosome strains, namely LCSY1 to LCSY8 (Fig. 3A; Additional file 1: Fig S4A; Additional file 2: Table S2), all of which had exceptionally long chromosome arms and could be considered acrocentric. The linear chromosome in the LCSY strains was verified by PFGE (Fig. 3B), and two telomeres of correct size were verified by Southern blotting assay (Fig. 3C; Additional file 1: Fig S4B, C), as previously described. Examination of cell morphology revealed that most LCSY cells had an abnormal cell shape and were larger than both SY14 and SY15 cells (Fig. 3D). The growth rate of LCSY strains was significantly slower than that of SY14 (Fig. 3E). Correspondingly, the cell doubling time of LCSY strains was increased (Fig. 3F). Taken together, these results suggest that the S. cerevisiae genome is robust enough to withstand both massive chromosome reorganization and the centromere position changes without seriously compromising the cell survival.
Fig. 3.
Construction and characterization of S. cerevisiae strains containing acrocentric chromosome. A Schematic of circular chromosome structure in SY15 and acrocentric chromosome structures in LCSY strains. The telomere cassette was inserted into individual gene free locus of Chr. XII or XV (indicated on the right of circular chromosome of SY15). Genes flanking the inserted telomere cassette were labeled on top of the lines which indicate linearized single-chromosomes. Specific gRNA expression induced double-strand breaks, leading to chromosome linearization and generation of acrocentric chromosomes (shown on the right). B PFGE analysis of chromosomes in the LCSY strains. SY14, SY15, and wild-type S. pombe were used as controls. Signals indicated with an asterisk are likely to be “damaged chromosome(s)” during DNA electrophoresis. C Southern blotting assay of telomeres in LCSY strains. Genomic DNA extracted from indicated strains was digested with Eco130I (left panel) or XbaI/ClaI (right panel) and hybridized with a telomere-specific probe. Asterisks on the right indicate nonspecific signals. D Morphology of representative LCSY cells in logarithmic phase. Shown are DIC images. Scale bar, 5 μm. Quantified cell size of 60 cells from each strain were showed at the right side, and the p-value (****p < 0.0001) indicated at the top of the strains showed the significance of difference in cell size compared to the SY14 strain. E Growth analysis of LCSY cells. Error bars represent standard deviation (s.d.), n = 3. The p-value (***p < 0.001, ****p < 0.0001) indicated at the right side of strains showed the significance of difference in growth rate compared to the SY14 strain. F Doubling time of LCSY cells. For each strain, n = 30, the significance of the difference between the strains is indicated at right (*p < 0.05, ****p < 0.0001)
LCSY strains with acrocentric single chromosomes exhibit severe chromosome mis-segregation and a distinct transcriptome compared to LSY cells
When challenged with genotoxic drugs, the linear single-chromosome LCSY strains with acrocentric chromosome were more sensitive than the LSY strains with metacentric or submetacentric chromosome (Fig. 4A). FACS analysis revealed that compared to the LSY cells, which had a regular DNA content of 1 C and 2 C (Fig. 4B), most of the LCSY cells had 2 C and 4 C DNA content, indicating a genome duplication (Fig. 4B). These results suggest that having acrocentric chromosome in single-chromosome S. cerevisiae leads to genome instability. Further live cell imaging of LCSY8 cells showed that some cells experienced abnormal cell division, including elongated cell shape and failed cytokinesis (Additional file 1: Fig S5). Fluorescence live cell imaging of chromosome segregation during mitosis in LCSY8 cells revealed that although a proportion of the cells underwent normal chromosome segregation, a large number of cells exhibited lagging chromosomes and incomplete sister chromatid separation (Fig. 4C). Correspondingly, the rate of chromosome mis-segregation increased in cells with acrocentric chromosome (Fig. 4D). We conclude that single-chromosome S. cerevisiae strains with an acrocentric chromosome possess a less stable genome, showing as growth defect and genome duplication, which likely due to impaired mitosis.
Fig. 4.
Lopsided chromosome arms in S. cerevisiae LCSY strains leads to chromosome segregation defects. A Serial dilution assays of LSY and LCSY strains under various genotoxic drugs stress. CPT, camptothecin; HU, hydroxyurea; MMS, methyl methanesulfonate. Cells were grown on YPD medium at 30 °C for 2 days. B FACS analysis of DNA content in both LSY and LCSY strains. Two or three independent clones of each strain were tested. SY15 and SY14 were used as controls. C Live-cell fluorescence images of chromosome segregation in LCSY8 strain. (a) shows cells with normal chromosome segregation, (b)–(f) show cells with chromosome segregation errors (highlighted by white arrows). Cells were collected in logarithmic and images were captured during mitosis. Chromosomes were marked by the histone protein Htb1 which was tagged with YFP. Scale bar, 5 μm. D Relative percentage of the cell population which showing impaired chromosome segregation. Grey, cells under normal mitosis; orange, cells with impaired chromosome segregation. The total number of cells counted from three individual image and is given at the top of each column
To address whether the phenotypic alterations observed in the LCSY strains were mainly caused by intentional chromosomal rearrangements or sporadic genetic mutation(s) during genome manipulation, we performed whole-genome sequencing on the representative strains of metacentric LSY1 and LSY4 and acrocentric LCSY7 and LCSY8. The results of the genome sequence assembly showed that the chromosomal translocation had occurred as designed (Figs. 2A, 3A, and 5A). Using the SY14 genome as a reference, dozens of indels and SNVs were detected in all four strains (Fig. 5B). Most of the exonic indels and SNVs were the same in all four strains, consistent with the design that they were derived from SY15 (Fig. 5B; Additional file 3: Table S4). A few strain-specific indels/SNVs were detected in each strain containing the metacentric or telocentric chromosome (Additional file 3: Table S4), suggesting that these indels/SNVs are not the major sources of phenotypic variation in LSY and LCSY strains. However, we could not exclude the possibility that certain indels or SNVs might cause abnormalities (e.g., slow cell growth).
Fig. 5.
Genomic and transcriptomic analysis of LSY and LCSY cells. A Chromosome structure variations of LSY and LCSY cells compared to SY14 cells. SyRI was used to identify structural variations between two whole-genome assemblies. B Venn diagram showing the overlap of all identified SNVs and indels in LSY1, LSY4, LCSY7, and LCSY8 cells. C–F Volcano plots of differentially expressed genes. C SY14 vs LSY1; (D) LSY1 vs LCSY7; (E) LSY1 vs LCSY8; (F) LCSY7 vs LCSY8. Statistical significance of differentially expressed genes (DEGs) were defined using FDR ≤ 0.05 and fold change > 2. Grey dots show genes with no significant differences, significantly upregulated genes are shown on the right side, significant downregulated genes are shown on the left side, significantly differentially expressed genes located on rearranged chromosomes are highlighted with a specific color. G Common GO terms of the DEGs in LSY1 vs LCSY7 and LSY1 vs LCSY8. The color of the bubble represents the significance of enrichment result (FDR) and the size means the statistical significance of the enrichment. H Shared KEGG pathway enrichment analysis of the DEGs in LSY1 vs LCSY7 and LSY1 vs LCSY8. The color of the bubble represents the significance of enrichment result (qvalue) and the size means the statistical significance of the enrichment. (I) Heatmap of gene expressions associated with chromosome segregation GO term (GO:0007059)
Further transcriptome analyses of the representative strains, including metacentric LSY1 and acrocentric LCSY7 and LCSY8, showed that LSY1 had very similar transcriptome profiles to SY14 with only nine genes differentially expressed (FDR ≤ 0.05; fold change > 2) (Fig. 5C; Additional file 1: Fig S6A; Additional file 8). Notably, in LSY1 cells, three genes (i.e., FEX2, YPL278C and YPL277C), which were proximal to the Chr. XVI-L telomere in SY14, were repositioned distal to the telomeres and upregulated due to telomere position effect (TPE) release [29]; while four genes (i.e., NCA3, ASF1, YJL114W and YJL113W) on Chr. X, which were subjected to the position effect of newly formed telomeres, were downregulated (Fig. 5C; Additional file 3: Table S5). Compared to LSY1 cells, 144 and 249 genes were up- and downregulated, respectively, in LCSY7 cells; 311 and 476 genes were up- and downregulated, respectively, in LCSY8 cells (Fig. 5D, E; Additional file 8). In contrast, there were only 20 differentially expressed genes (DEGs) between LCSY7 and LCSY8 cells even though their centromeres were located at opposite ends of the single chromosome (Fig. 5F; Additional file 8). These results suggest that the increased number of DEGs is attributed to the repositioning of the centromere near a chromosome end. Subsequent GO term analysis revealed that the DEGs in both LCSY7 and LCSY8 cells were enriched in various cellular processes compared to LSY1 cells (Additional file 1:Fig S6B, C, left panel), including “extracellular region,” “fungal type cell wall” and “cell surface,” which echo the abnormal cell morphology, as well as “fatty acid beta-oxidation,” “cellular response to oxidative stress,” and “alditol: NADP + 1-oxidoreductase activity,” which may be associated with defects in cell fitness (Fig. 5G). Meanwhile, KEGG enrichment analysis revealed that most of the DEGs in LCSY strains were involved in vegetative metabolism (Fig. 5H; Additional file 1: Fig S6B, C, right panel), coping with the increased cell size (Fig. 3D) and growth defects (Fig. 3E) of LCSY cells. Considering the prolonged cell doubling time (Fig. 3F) and abnormal chromosome segregation of LCSY cells (Fig. 4C), we analyzed genes related to chromosome segregation (GO:0007059) and found that these genes were indeed differentially expressed in LSY and LCSY cells (Fig. 5I). These results support the notion that the S. cerevisiae genome is highly plastic to tolerate different chromosome arrangements, but also suggest that the yeast genome could not be randomly permutated.
Construction of S. pombe strain with single circular chromosome
Both S. pombe and S. cerevisiae are unicellular fungal organisms, and their genome sizes are comparable (~ 13.8 Mb for S. pombe and ~ 12.5 Mb for S. cerevisiae) [30]. However, S. pombe shares more common features with complex eukaryotes including gene structures, chromatin dynamics, and the prevalence of introns, as well as the control of gene expression through pre-mRNA splicing, epigenetic gene silencing, and RNAi pathways [31]. In particular, S. pombe has evolved to have three chromosomes, and each chromosome contains a regional centromere, which is very different from the point centromere in the sixteen chromosomes of S. cerevisiae [30]. Recently, we successfully integrated the entire genome of S. pombe into a single chromosome and obtained two strains with different types of chromosome fusions, namely GXPY666 (Chr3cΔ−2-1cΔ) and GXPY676 (Chr2cΔ−1-3cΔ) [15]. In order to further explore genome plasticity in single-chromosome S. pombe, we first constructed a single-circular chromosome S. pombe strain. By deleting ter1, which encodes the telomerase RNA subunit [32], in strain GXPY666, we obtained several telomerase-null survivors, which have telomeres repaired by homologous recombination or have telomere fused circular chromosome [33, 34] (Fig. 6A). PCR amplification of sub-telomeric DNA revealed that chromosome end fusion had occurred in eight survivors (Fig. 6B, top panel). Further DNA sequencing results confirmed that the chromosome fusion took place at the sub-telomeric H1/H1’ or H3/H3’ homology regions (Fig. 6B, middle and bottom panels) [34]. The survivors containing H1/H1’ and H3/H3’ fusions were designated as type H1 and type H3, respectively.
Fig. 6.
Construction of S. pombe strains with a single circular chromosome. A Schematic of the generation of telomerase-null survivors from single-chromosome fission yeast GXPY666 (Chr3cΔ−2-1cΔ) strain [15]. Deletion of ter1 in GXPY666 results in chromosome circularization and survivor formation. Each native chromosome is marked with a unique color; red dot and grey trapezoid represent centromere; grey dot represents telomere; yellow box shows the location of rDNA. B PCR analysis of chromosome fusion junctions in ter1Δ survivors (top panel). The band amplified from htb1 was used as a positive control. Schematics of circular chromosomes and telomere fusions at H1 and H3 loci in the ter1Δ survivors (middle and bottom panels). The pink line indicates the right end of Chr1; the purple line indicates the left end of Chr3; and grey dot line indicates eroded chromosome ends. H1/H1’ and H3/H3’ are homology regions in each sub-telomeric region [34]. C PFGE-Southern blotting analysis of intact genome and chromosome fragments in type H1 and type H3 survivors. Chromosomes were digested with the restriction enzyme NotI and the fragments were separated by PFGE. A telomere-specific probe was used for Southern blotting hybridization. The single linear chromosome in GXPY666 (Chr3cΔ−2-1cΔ) was used as a control. D Serial dilution assays of type H1 and type H3 survivors. Three independent clones are shown for both type H1 and type H3 strains. Cells were grown under different conditions, including 37 °C and genotoxic drug stress (CPT, HU and MMS)
We then verified the circular chromosome in type H1 and type H3 survivors through PFGE analysis. In the absence of NotI treatment, little intact genomic DNA entered the gel due to its circular nature [35] (Fig. 6C, left panel, lanes of -NotI). After NotI digestion, although the multiple bands could not be well separated under the experimental conditions (Fig. 6C, left panel, lanes of + NotI), the DNA band of ~ 2536 kb, representing the left end of Chr3 (Chr3-L), and the DNA band of 504 kb, representing the right end of Chr1 (Chr1-R) in GXPY666 were not detected with a specific telomeric probe in type H1 and type H3 survivors, consistently indicating that type H1 and type H3 survivors have lost telomeric DNA. Although at this stage, we could not rule out the possibility of a repetitive rDNA break that may lead to a linear chromosome, we carried out a few assays to characterize the type H1 cells and type H3 cells. When grown in 37 °C or treated with various genotoxic drugs, they were more sensitive to these stresses than GXPY666 and wild-type S. pombe (GXPY03) with linear chromosomes (Fig. 6D), supporting the conclusion that type H1 cells and type H3 cells contain a circular chromosome, since survivors with circular chromosomes are much more sensitive to DSB-inducing agents than the survivors with linear chromosomes [36]. Moreover, these strains could be passaged for more than 200 generations (8 re-streaks every 3 days) without detectable changes in the NotI pattern, suggesting a stable genome (Additional file 1: Fig S7A, B). Compared to the wild-type and GXPY666 cells, type H1 and type H3 cells exhibited a slightly larger cell size (Additional file 1: Fig S7C), but a similar cell doubling time (Additional file 1: Fig S7D). Finally, we performed whole-genome sequencing with a type H1 strain (clone 4), and the results confirmed the chromosome fusion at the H1/H1’ homology region, as well as the chromosome rearrangement when it aligned to GXPY666 (Additional file 1: Fig S7E). Genomic variations were observed in type H1, but only two proteins (Cox1-I2b and Cdc10) were mutated to potentially cause functional changes (Additional file 4: Table S6). To this end, we have for the first time created S. pombe strains with a circular single chromosome.
Construction of S. pombe with telocentric single chromosome
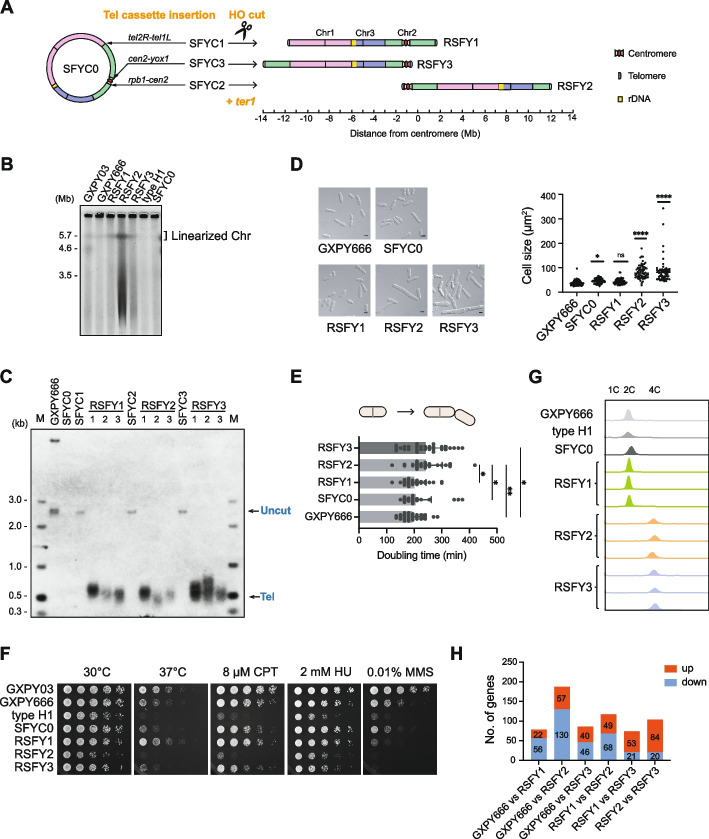
With the circular single-chromosome S. pombe strains in hand, we could literally integrate a telomere cassette into any gene-free locus on the circular chromosome as we had done in S. cerevisiae (Figs. 2A and 3A). The telomere cassette contained two homologous arms for genome integration (DR1 and DR2), two opposite 237-bp telomeric TTAC(A)G2-6/C2-6(T)GTAA repeat sequences for de novo telomere formation, an ura4 marker gene for clone selection, and two HO digestion sites flanking the ura4 gene (Additional file 1: Fig S8A). The ura4 gene, which was introduced into the type H1 genome during ter1 deletion, was first removed, resulting in a strain hereafter referred to as SFYC0. The telomere cassette was then inserted into the genomic locus between the fusion sites of Chr2 and Chr1, or into two gene-free loci on either side of the cen2 in close proximity to the centromere region (Fig. 7A; Additional file 2: Table S3). Induction of the nmt1-driven expression of the HO endonuclease by thiamine removal resulted in DSBs at the HO recognition sites, while ter1 was placed back at its original locus. The artificial telomeric sequences positioned at both chromosomal ends were exposed and then elongated by functional telomerase (Additional file 1: Fig S8A). We successfully generated three S. pombe strains containing a linear chromosome, they were designated as RSFY1, RSFY2, and RSFY3 (Fig. 7A; Additional file 1: Fig S8B; Additional file 2: Table S3). The PFGE assay was used to examine the chromosomal structure, and a chromosomal band with a size comparable to that of strain GXPY666 was detected in RSFY1, RSFY2, and RSFY3 strains but not in type H1 and SFYC0 strains with circular chromosome (Fig. 7B). Telomere Southern blotting assay showed that the bands representing the uncut telomere cassette were present in SFYC cells, but disappeared in RSFY cells after chromosome linearization (Fig. 7C). Instead, there was a new telomere signal of approximately 500 bp appeared in RSFY cells, extended from the initial 237-bp telomeric sequence, suggesting that the reactivated telomerase in these strains functions at the de novo telomeres (Fig. 7C).
Fig. 7.
Construction and characterization of S. pombe strains with sub-metacentric and telocentric single chromosomes. A Schematic of construction of single linear chromosome in SFYC0 strain. The telomere cassette (see “Methods” for details) was inserted into individual gene-free locus of Chr2 (indicated on the right of the circular chromosome of SFYC0). Genes flanking the inserted telomere cassette were labeled above the lines. HO expression induces double-strand breaks, leading to chromosome linearization, while ter1 was simultaneously reinserted into its original locus, generating stable strains with rearranged chromosome structures, designated RSFY1, RSFY2, and RSFY3 (shown on the right). Each native chromosome is marked with a unique color; red dot and grey trapezoid represent centromere; grey dot represents telomere; yellow box shows the location of rDNA. B PFGE analysis of chromosomes in RSFY1, RSFY2, and RSFY3. SFYC0 cells and type H1 survivor with circular chromosomes were used as control. The wild-type S. pombe (GXPY03) chromosome were used as size markers. C Southern blotting analysis of telomeres in RSFY1, RSFY2, and RSFY3 strains. GXPY666 (single-chromosome Chr3cΔ−2-1cΔ), SFYC0 (circular chromosome), SFYC1, SFYC2, and SFYC3 (circular chromosome with inserted telomere cassette) were used as controls. “Uncut” indicates bands containing the entire telomere cassette, “Tel” marks newly formed telomeres in a single linear chromosome. D Cell morphology of RSFY1, RSFY2, and RSFY3 strains in logarithmic phase. Cells were cultured in YES medium. DIC images are shown. Scale bar, 5 μm. Quantification cell size (n = 60) for each strain is shown at the right, and the p-value (*p < 0.05, ****p < 0.0001) is shown at the top of the strains (compared to the strain GXPY666). E Doubling time of RSFY1, RSFY2, and RSFY3 cells. For each strain, n = 30, the significance of the difference between the strains is indicated at right (*p < 0.05, **p < 0.01). F Serial dilution assays of RSFY1, RSFY2, and RSFY3 under different conditions. Cells were grown with different treatments, including 37 °C and genotoxic drug stress (CPT, HU, and MMS). G FACS analysis of DNA content in RSFY1, RSFY2, and RSFY3 strains. Three independent clones from each strain were tested. GXPY666, type H1 and SFYC0 were used as controls. H Numbers of DEGs between single-chromosome fission yeast strains. Red, upregulated genes; blue, downregulated genes
RSFY1 contained a single chromosome in which the three native chromosomes are arranged in the sequence of Chr1-3–2, which differs from the previously reported GXPY666 (Chr3-2–1) and GXPY676 (Chr2-1–3) [15]. Notably, the rDNA arrays in RSFY1 are located in the middle of the single chromosome (Fig. 7A), whereas in both GXPY666 (Chr3-2–1) and GXPY676 (Chr2-1–3) strains, the rDNA arrays are located at one end of the single chromosome [15]. Additionally, RSFY1 had a sub-metacentric chromosome, whereas RSFY2 and RSFY3 had a telocentric chromosome. Compared to GXPY666, significant cell length elongation and larger cell size were observed in RSFY2 and RSFY3 cells, but not in RSFY1 cells (Fig. 7D). Similarly, RSFY2 and RSFY3 had a prolonged cell doubling time compared to GXPY666 and RSFY1 (Fig. 7E). Furthermore, RSFY2 and RSFY3 showed slower growth at 37 °C culture, or on YES medium containing different genotoxic drugs, whereas RSFY1 grew as robustly as GXPY666 (Fig. 7F). Accordingly, FACS analysis revealed that individual clones of RSFY1 strains had a regular DNA content with a majority of 2 C, but RSFY2 and RSFY3 strains had a DNA content of 4 C as a result of genome duplication (Fig. 7G). These results suggest that telocentric chromosome imposes a significant burden on single-chromosome S. pombe cells, causing cell cycle prolongation and genome duplication, as we have seen in single-chromosome S. cerevisiae.
We then performed whole-genome sequencing on RSFY1 and RSFY2 cells. Alignment of RSFY1 and RSFY2 to GXPY666 confirmed that chromosome reorganizations were successful in single-chromosome S. pombe (Fig. 7A; Additional file 1: Fig S9A). A number of variations were detected in the genomes of both RSFY1 and RSFY2, most of which were inherited from the circular chromosome of type H1, while only a few strain-specific variations could cause mutations in proteins (e.g., Rpp21-K46N in RSFY2) (Additional file 4: Table S6 and S7). Additional transcriptome analysis revealed that gene expression in single-chromosome S. pombe was perturbed by chromosome reorganization, as all the RSFY cells containing either a sub-metacentric or a telocentric chromosome, had slightly different transcriptome levels (no more than 4%) compared to each other (Fig. 7H; Additional file 9). The loci of DEGs between GXPY666 and RSFY1 cells were distributed among all the three native chromosomes, but most of the significantly upregulated genes (e.g., SPAC750.03c, SPAC750.01 and pdc102) and downregulated genes (e.g., nhe1, dark2, gal1 and gal10) were concentrated at the loci where telomeres were removed (the right end of Chr1) and new telomeres were formed (the right end of Chr2 and the left end of Chr1), respectively, consistent with loss and gain of telomere position effect (TPE) (Additional file 1: Fig S9B; Additional file 4: Table S8) [37, 38]. Similarly, gain and loss of TPE was also observed in RSFY2 and RSFY3 when compared to RSFY1 (Additional file 1: Fig S9C, D). However, in both RSFY2 and RSFY3 cells with telocentric chromosome, genes near the heterochromatic centromere were less affected by new telomeres, probably due to the pre-existing silencing effect of the centromere (Additional file 1: Fig S9C-E; Additional file 4: Table S8) [37]. Apart from those affected by TPE, 99 DEGs in RSFY2 and 55 DEGs in RSFY3 were enriched in several GO terms, including “plasma membrane” for RSFY2, as well as “cytoplasm” and “extracellular region” for RSFY3 (Additional file 1: Fig S10A), corresponding with their observed abnormal cell morphologies (Fig. 7D), and alterations in various metabolic pathways (Additional file 1: Fig S10B). Interestingly, the DEGs of these two strains could be enriched for the GO terms of “nucleus” and “DNA recombination,” which may correlate with chromosome reorganization (Additional file 1: Fig S10A). In summary, we found that the S. pombe genome is highly plastic to tolerate multiple chromosome reorganization. Although the telocentric chromosome can support vegetative cell growth, it is not conducive to maintaining a stable genome in single-chromosome S. pombe.
Discussion
The speciation of every organism on earth appears to be linked to the origin and evolution of both genes and their organization into chromosomes. Different species are likely to have their own gene content, chromosome structure, and number. Thus, chromosomal rearrangements have a substantial impact on speciation [39]. It remains a mystery why the genome and chromosomes of a given species have evolved to their present state, and thus to what extent the genome and chromosomes of a species can tolerate natural changes or artificial manipulation. Reproductive isolation of living organisms is a key indicator of species identity [40, 41]. Recently, single-chromosome strains of S. cerevisiae and S. pombe have been successfully constructed [13, 15]. Karyotype engineering in both yeasts through chromosome fusion leads to reproductive isolation [14, 15]. In this study, we further constructed a variety of new single-chromosome yeast strains in which either the genome is restructured with different orders of natural chromosomes (Figs. 1A and 7A), or a natural chromosome is arbitrarily divided into two segments and positioned proximal to two chromosome ends, or the centromere is positioned in the middle of the chromosome (metacentric) (Fig. 2A), or proximal to one of the chromosome ends (acrocentric and telocentric) (Figs. 3A and 7A). All of these single-chromosome strains are viable, indicating that the functional genomes of the two yeast models are highly robust to support cell survival despite massive reconfiguration.
In addition to metacentric chromosomes, naturally evolved telocentric chromosomes are prevalent in higher eukaryotic organisms such as flies and mice [9, 10]. However, in the unicellular organisms S. cerevisiae and S. pombe, their naturally evolved chromosomes are metacentric [8]. The S. cerevisiae and S. pombe strains with acrocentric or telocentric chromosomes artificially generated in this work (Fig. 3A, 7A) are competent in vegetative growth, but at the cellular level, they showed less growth fitness (Figs. 3E, F and 7E) and increased genome instability (Figs. 4A, B and 7F, G), and experienced impaired mitosis as previously found in plants and mouse cell lines [17, 20, 42]. Thus, the naturally evolved metacentric chromosomes in both S. cerevisiae and S. pombe strains seem to have more advantages than the artificial acrocentric or telocentric ones. At the molecular level, the transcriptomes of both acrocentric and telocentric chromosome strains showed significant differences from those of strains with metacentric chromosome (Figs. 6C–F and 7H; Additional file 1: Fig S9B-E), and DEGs are associated with GO terms of “membrane” and “extracellular region” (Additional file 1: Fig S6B, C, S10A) and the KEGG enrichment in “Metabolic” pathway (Additional file 1: Fig S6B, C, S10B).
Centromere repositioning proximal to a telomere in single-chromosome yeast likely results in an over-long chromosome arm (i.e., unbalanced chromosome arms), leading to more frequent chromosome segregation errors (Fig. 4C, D). These results support the hypothesis that excessively long chromosome arms can lead to impaired cell mitosis [20, 42]. A recent study by Kunchala et al. showed that S. cerevisiae cells with fewer but longer chromosomes have smaller spindle pole bodies, fewer microtubules, and longer spindles [43]. In addition, fewer chromosomes would face a stronger outward force, suggesting that there should be a balance between inward and outward force in a metaphase spindle during mitosis, and that the outward force may be determined by kinetochore or microtubule function [44]. It is possible that centromeres located near telomeres could affect kinetochore assembly or alter chromosome conformation in the centromere region, thereby exacerbating the imbalance between inward and outward forces and leading to impaired chromosome segregation.
Regional centromere is adopted by most eukaryotes, including fission yeast, metazoans and plant cells [2], suggesting that from the revolutionary point of view the regional centromere is superior to the point centromere as seen in budding yeast. In flies and mice, regional centromeres are often found proximal to telomeres and appear to be robust enough to fulfil their functions during cell division [9, 10]. In contrast, the regional centromere in S. pombe (i.e., in RSFY strains), which artificially positioned proximal to a telomere, does not function as perfect as those in mouse cells (Fig. 7D–F). There are some differences between centromeres in fission yeast and mouse. For example, in fission yeast, centromere regions cover 40–100 kb and consist of two inverted repeats flanking a central core [45]. The centromeric DNA in mouse is usually megabase-sized, having both centromeric minor satellites (miSats) and pericentromeric major satellites (maSats) [46]. Thus, centromeres in the mouse genome are much larger than those in fission yeast which allows for more nucleosome formation. Additionally, the variable maSats and miSats arrays in the mouse centromeric region facilitate the organization of a more elaborate centromere structure, which has a higher plasticity to respond to mechanical forces stemming from kinetochore-associated microtubules.
Although the construction of diverse single-chromosome yeast strains in this study provides useful research models and references for further studies of chromosome structure and function, speciation and centromere evolution, a number of questions remain to be addressed in the future. For example, (1) the reorganization of the yeast genome was purposeful (i.e., acrocentric or telocentric chromosome) rather than systematic. Abundant single-chromosome strains could be generated from systematic linearization every “x” kb in circular chromosome of S. cerevisiae and S. pombe [19], which would be much more informative. (2) Whole-genome sequencing of both S. cerevisiae and S. pombe strains confirmed the chromosome rearrangements in our study, but the potential synthetic effects of SNVs/indels on cellular phenotypes could not be completely excluded. (3) The acrocentric and telocentric strains showed growth defects, impaired mitosis, genome duplication, and altered gene expression profiles. It was tempting to suggest that acrocentric or telocentric chromosomes have a higher probability of experiencing unequal chromosome segregation, leading to impaired mitosis, genome duplication, and slower cell growth, but we were unable to establish a causal relationship between cellular phenotypes and transcriptome changes. (4) The focus of this work was on chromosome rearrangement in yeast, emphasizing the positioning of the centromere within a chromosome and its effects on cell growth and genome stability. Such a simple view of limited DNA sequence differences in a chromosome does not touch on the hierarchical structure of a chromosome and seems inadequate to explain the complex phenotypes seen at either the cellular or population level. It is worth considering whether centromere repositioning would cause variation in centromere structure, given that both centromere and pericentromeric regions can be organized into a bottlebrush-like structure in both budding yeast and mammals [47–50].
Conclusions
Here, we demonstrate that the genome of both budding yeast S. cerevisiae and fission yeast S. pombe are robust and can tolerate massive chromosome shuffling. However, single-chromosome yeast strains with acrocentric or telocentric chromosomes display decreased cell viability and increased genome instability than strains with metacentric chromosome, suggesting the evolutionary advantage of existing metacentric chromosome in these two yeast models.
Methods
Yeast strains and plasmids
Yeast strains constructed in this study are listed in Additional file 5: Table S9 and Table S10. Plasmids used in this study are listed in Additional file 5: Table S11 and their sequence were provided as GenBank files in Additional file 6. For single-chromosome S. cerevisiae strains, they were derived from SY13 (Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) with two chromosomes [13] or SY15 (Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) with a single circular chromosome by CRISPR-Cas9 editing [19]. The plasmids used for co-expressing of Cas9 and site-specific guide RNAs were constructed based on pRS series in previous study [51, 52]. As for single-chromosome S. pombe strains, they were all constructed from GXPY666 (Chr3cΔ−2-1cΔ, h+his3-D1 leu1-32 ura4-D18 ade6-M210 ars1::[4JR-41XH-HO](his+)) [15].
Chromosome fusion in S. cerevisiae
The method we used to fuse chromosomes has been described in previous study [52] (Additional file 1: Fig S1A). In brief, we prepared two DNA targeting cassettes, one for centromere deletion, the other for chromosome ends fusion, and a gRNA plasmid containing three gRNA expression cassettes. All of them were co-transformed into SY13 cells harboring pCas9. The transformation products were plated on the synthetic drop-out medium SC-Ura-His-Leu (omitting uracil, histidine, and leucine) and cultured at 30 °C for several days. The positive colonies were verified by PCR analysis and sequencing, and then inoculated into SC-Leu liquid medium and grown to stationary phase at 30 °C. Then, cells were transferred to SC-Leu medium containing 2% galactose with an initial optical density of OD600 = 0.1, grown overnight and plated on SC medium containing 1 mg/ml 5’-FOA. The loss of pgRNA and pCas9 were verified by colony re-streak on synthetic drop-out plate SC-His and SC-Leu.
Chromosome linearization in S. cerevisiae
The procedure of CRISPR-Cas9 mediated circular chromosome linearization (Additional file 1: Fig S3A) is as follows. Different parts of the telomere cassette were cloned into pRS303 plasmids through enzyme digestion and ligation. These cassettes contained seven modules: (1) 200 to 400-bp homology arm 1 (DR1); (2) an 80-bp telomeric seed fragment with TG1-3/C1-3A repeat sequence; (3) a 20-bp gRNA target sequence; (4) a URA3 marker gene; (5) another 20-bp gRNA target sequence; (6) a second 80-bp telomeric seed fragment with C1-3A/TG1-3 repeat sequence; (7) 200 to 400-bp homology arm 2 (DR2) (Additional file 6). The guide RNA expression plasmid (pgRNA4) was constructed from pRS426. The telomere cassette digested from constructed plasmid was transformed into SY15 cells harboring pCas9, and the transformation products were plated on the synthetic drop-out medium SC-Ura-Leu. The positive colonies which had successfully integrated the DNA targeting cassette were verified by PCR analysis and sequencing. Then pgRNA was introduced into these cells for linearization, and positive clones were selected on SC-Leu-His plate with 1 mg/ml 5’-FOA, verified by PCR analysis, and then re-streaked on SC-Leu plate containing 2% galactose. The loss of pgRNA was tested by colony re-streak on SC-His plate.
Chromosome circularization and linearization in S. pombe strains
In fission yeast S. pombe, we knocked out the telomerase RNA gene ter1 in GXPY666 (Chr3cΔ−2-1cΔ) [15] to generate survivors with circular chromosomes (namely type H1 and type H3). The chromosome end fusions were verified by PCR analysis and sequencing as previously reported [34]. Next, ura4 marker gene which introduced through ter1 knock out was removed from genome through homologous recombination, resulting a strain named SFYC0. The strategies to insert telomere cassettes and to linearize circular chromosome were similar to the method we used in budding yeast (Additional file 1: Fig S8A). First, the telomere cassettes were cloned into pRS303 plasmids and were digested from plasmids before transformation. The telomere cassette includes nine modules: (1) 200 to 400-bp homology arm 1 (DR1); (2) a leu2 marker gene; (3) a 237-bp telomeric seed fragment with TTAC(A)G2-6/C2-6(T)GTAA repeat sequence; (4) a HO enzyme digestion sequence; (5) a ura4 marker gene; (6) a second HO enzyme digestion sequence; (7) another 237-bp telomeric seed fragment with C2-6 (T)GTAA/TTAC(A)G2-6 repeat sequence; (8) a natMX6 marker gene; (9) 200 to 400-bp homology arm 2 (DR2) (Additional file 6). The constructed telomere cassettes were transformed into SFYC0 cells, and then plated on the synthetic drop-out medium PMG-Ura + Thiamine [53]. The positive clones verified by PCR-sequencing were inoculated into YES liquid medium and grown to log phase at 30 °C. Then, ter1 rescue cassettes (ter1::bsdMX6) were transformed into these cells and plated on PMG-Thiamine medium with 1 mg/ml 5’-FOA and 0.03 mg/ml Blasticidin. The positive clones were identified by PCR analysis.
Telomere Southern blotting
Telomere Southern blotting was performed as previously described [54]. The extracted genomic DNA was digested with restriction enzyme(s) as indicated in the figures (Additional file 1: Fig S1C, S2C, D, S3B, C, S8C), and then separated on a 1.0% agarose gel. The DNA transferred to a Hybond-N+ Nylon membrane (GE Healthcare) was hybridized with a telomeric probe labeled with α-32P dCTP (255-bp C1-3A repeats for S. cerevisiae genome) or 3′-biotin (5′-TGTAACCCCTGTAACCCCTGTAACCCC-3′ for S. pombe genome).
Pulsed-field gel electrophoresis
DNA plugs for pulse-field gel electrophoresis (PFGE) were prepared according to the manufacturer’s instructions (Bio-Rad) and previous reports [13, 15, 55] with the following modifications. About 1 × 109 cells were collected and washed twice with 5 ml of chilled 50 mM EDTA. The cells were resuspended in 400 μl of cell suspension buffer (2 × CSB, 10 mM Tris pH 7.2, 20 mM NaCl, 100 mM EDTA, 4 mg/ml lyticase and 10 mg/ml lysing) and then mixed with 400 μl of 2% low melt agarose (Bio-Rad). 100 μl of the cell-agarose mixture was transferred into plug molds (Bio-Rad). The plugs were placed at 4 °C for 20 min to allow the gelling of the agarose. The solidified plugs were then immersed in 1.3 ml lyticase buffer (10 mM Tris pH 7.2, 100 mM EDTA and 2 mg/ml lyticase), and incubated at 37 °C for 1 h (for S. cerevisiae cells) or 3 h (for S. pombe cells); in 1.3 ml PK buffer (10 mM Tris pH 7.2, 0.5 M EDTA, 1% lauroyl sarcosine, 0.2% sodium deoxycholate and 1 mg/ml proteinase K) at 50 °C for 4.5 h, and washed four times in 5 ml wash buffer (20 mM Tris, pH 8.0, 50 mM EDTA) at room temperature with gentle agitation for 30 min every time, and then subjected to electrophoresis.
For S. cerevisiae samples (Fig. 1B, 2B, 3B), the prepared DNA plugs were run in 0.7% Pulsed-Filed Certified Agarose in 1 × TAE at 6 °C, and the electrophoresis program was as follows: first run: initial switch time 1800 s; final switch time 1800 s; run time 27 h; voltage gradient 1.5 V/cm; angle 106°; second run: initial switch time 1500 s; final switch time 1500 s; run time 27 h; voltage gradient 2 V/cm; angle 100°; third run: initial switch time 1200 s; final switch time 1200 s; run time 27 h; voltage gradient 2.5 V/cm; angle 96°. For S. pombe samples (Fig. 7B), the prepared DNA plugs were run in 0.8% Pulsed-Filed Certified Agarose in 1 × TAE at 14 °C, and the electrophoresis program was as follows: first run: initial switch time 1200 s; final switch time 1200 s; run time 24 h; voltage gradient 2 V/cm; angle 96°; second run: initial switch time 1500 s; final switch time 1500 s; run time 24 h; voltage gradient 2 V/cm; angle 100°; third run: initial switch time 1800 s; final switch time 1800 s; run time 24 h; voltage gradient 2 V/cm; angle 106°. NotI-digested chromosomal DNA of S. pombe (Fig. 6C; Additional file 1: Fig S7B) were separated in 1% Pulsed-Filed Certified Agarose in 0.5 × TBE at 14 °C with the setting: first run: initial switch time 60 s; final switch time 60 s; run time 15 h; voltage gradient 6 V/cm; angle 120°; Second run: initial switch time 90 s; final switch time 90 s; run time 9 h; voltage gradient 6 V/cm; angle 120°. The gel was stained with GelstainRed nucleic acid dye (US EVERBRIGHT) and was imaged under UV light for 200–300 ms by Tanon Chemi Dog 5200 T, and the image were then processed using Photoshop with the invert function.
Cell morphology and cell size analysis
Strains were freshly streaked on YPD (for S. cerevisiae) or YES (for S. pombe) plates. For each strain, a single clone was picked and inoculated into liquid medium, cells were cultured at 30 °C until log phase (OD600 = 1.0). Then, 1 ml of fresh cells were harvested and washed twice with sterile H2O. Samples were resuspended with 100 μl of liquid medium and sonicated for 10 s at 95% power (Q800R2 Shearing Sonicator, QSONICA) before imaging. Live cell imaging was performed using a Zeiss Axioplan2 microscope with a × 100 oil objective. For each strain, n = 60 cells were counted, and their cell size were measured using ImageJ. The significance of difference in cell size between these strains was analyzed using Kruskal–Wallis H Test.
Growth curve measurement
Three individual fresh colonies of yeast strains were inoculated into 3 ml of liquid medium and incubated overnight at 30 °C. Then the cell cultures were diluted to OD600 = 0.1 in 30 ml of liquid medium and cultured to stationary phase at 30 °C. OD600 was measured hourly by V-5600 Visible Spectrophotometer. The growth curve was plotted using GraphPad Prism 9. The significance of the differences growth rate between these strains and SY14 was calculated by two-way ANOVA test.
Doubling time measurement using microfluidic assay
The microfluidic devices and experimental setup were described previously [56–59]. Strains were freshly streaked onto YPD (for S. cerevisiae) or YES (for S. pombe) plates. A single clone was picked and inoculated into 2 ml of liquid medium, cells were cultured overnight at 30 °C to stationary phase. From this culture, 2 μl of the cell culture was re-inoculated into 20 ml of liquid medium and cultured to OD600 = 0.6–0.8. Cells were collected and loaded into PDMS microfluidic devices for imaging with a Nikon Inverted microscope ECLIPSE Ti2-E equipped with a C14440-20UP digital CMOS camera and a × 60 oil objective. Images were acquired every 10 min for 6 h (for S. cerevisiae) or every 15 min for 21.5–38 h (for S. pombe). Doubling time of strains were calculated as the intervals between the completion (i.e., formation of two daughter cells) of the previous and the current cell divisions. For each strain, n = 30 cells were analyzed, and the significance of the differences in cell doubling time between these strains were analyzed through Kruskal–Wallis H Test.
Serial dilution assay
Yeast strains were inoculated and cultured in liquid medium at 30 °C for overnight. The cultures were then harvested and diluted to OD600≈0.5 in sterile water. Five-fold serially diluted cells were spotted on medium containing different genotoxic drugs (e.g., HU, MMS, and CPT) [22]. Plates were incubated at 30 °C for 2–3 days.
Fluorescence-activated cell sorting (FACS) assay
A single fresh colony was inoculated into liquid medium and cultured overnight at 30°C. One milliliter cultured cells were collected and washed twice with cold ddH2O, and fixed with 70% ethanol overnight at 4 °C. The cells were then washed with sodium citrate buffer (50 mM, pH 7.2) and treated with RNase A (10 mg/ml, Thermo Scientific) at 37 °C for 2 to 3 h and then with proteinase K (0.2 mg/ml, sigma) at 50 °C for 1 h. The cells were further washed with sodium citrate buffer, resuspended in 500 μl sodium citrate buffer, and sonicated for 30 s at 95% power (Q800R2 Shearing Sonicator, QSONICA). Finally, the DNA of the cells was stained with 20 μg/ml propidium iodide (PI) at 4 °C overnight or room temperature for 1 h. FACS analysis was performed on a BD LSRFortessa instrument.
Fluorescence live cell imaging
Cells were freshly inoculated and grown into liquid medium at 30 °C until the OD600 = 0.8. Chromosomes were labeled with YFP-fused Htb1, and the sequence of the HTB1-YFP fusion is provided in Additional file 6. Live cell imaging was performed with a Zeiss LSM880 Ariyscan microscope equipped with a 63 × oil objective lens with a YFP filter (514 nm) and bright channel at room temperature (25 °C), images were analyzed and Airyscan processed with Zeiss ZEN 2.3 SP1 FP3 (black, 64 bit). For each strain, hundreds of cells were counted from three individual images. The proportion of different cell populations was analyzed and visualized by GraphPad Prism 9.
Genome sequencing and assembly
Total DNA was extracted using the QIAGEN Genomic-tips 20/G with Genomic DNA Buffer Set according to the manufacturer’s instruction. DNA quality was assessed by NanoDrop, gel electrophoresis, and Qubit. Whole-genome sequencing was performed on the lllumina PE150 platform and the PacBio Sequel system from AZENTA Life Sciences. Next generation sequencing library preparations were done according to the manufacturer’s protocol. For each sample, 200 ng of genomic DNA was randomly fragmented by Covaris to an average size of 300–350 bp. The fragments were treated with End Prep Enzyme Mix for end repairing, 5’ phosphorylation and 3’ adenylation to add adaptors to both ends. Size selection of adaptor-ligated DNA was then performed using DNA Cleanup beads. Each sample was then amplified by PCR for 8 cycles using P5 and P7 primers, with both primers carrying sequences which can anneal with flowcell to perform bridge PCR and the P7 primer carries a six base index to allow for multiplexing. The PCR products were purified and validated using an Agilent 2100 Bioanalyzer. Fastp (V0.23.0) was used to remove the adaptors, polymerase chain reaction (PCR) primers, N base more than 14, and Q20 lower than 40%. The qualified libraries were sequenced pair end PE150 on the lllumina Novaseq System. Sentieon pipeline (V202112.02) was used to map clean data to the reference genome, remove duplications, and call SNV/indel [60]. Annotation for SNV/indel was performed by Annovar (V21 Apr 2018) [61].
For the PacBio sequencing library, 5–10 μg genomic DNA was sheared into 10–15 kb fragments using a g-TUBE device. Then library was then constructed using the SMRTbell® Express Template Preparation Kit 2.0 according to the manufacture’s instruction. The library was sequenced on the PacBio Sequel platform. The PacBio reads were assembled using Hifiasm/Canu [62]. The genome was then recorrected with software Pilon using previous Illumina data. Alignments between contigs and reference genomes (SY14 or GXPY666) were generated by MUMmer-nucmer (4.6.2beta2) [63]. SYRI was utilized to identify genomic rearrangements between assembly contigs and the reference genome using default parameters [64].
RNA-seq analysis
Cells cultured at log phase (OD600 = 1.0) were collected, and their RNA was isolated using Trizol (Invitrogen) method [65]. Libraries were prepared from 1 μg total RNA using VAHTS® Universal V8 RNA-seq Library Prep Kit for Illumina. The libraries with different indexes were multiplexed and loaded onto the Illumina HiSeq platform by AZENTA Life Sciences using the 2 × 150-bp pair-end configuration according to the manufacturer’s instructions. Clean data were obtained using Cutadapt (V1.9.1, phred cutoff: 20, error rate: 0.1, adapter overlap: 1 bp, minimum length: 75, proportion of N:0.1) and aligned to the reference genome (S288C: GCA_000146045.2 or 972 h-: GCA_000002945.2) via software Hisat2 (v0.6.1) [66]. HTSeq (v0.6.1) estimated gene and isoform expression levels from the pair-end clean data according to the reference gene file. Significant differentially expressed genes (DEGs) were identified by DEseq2 [67] with a definition of fold change more than 2 and FDR ≤ 0.05. GOSeq (v1.34.1) was used to identify Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms with a significant padj ≤ 0.05 in S. cerevisiae samples. DEGs in S. pombe samples were enriched using KOBAS (kobas.cbi.pku.edu.cn/in). Volcano plots and scatter plots were generated and visualized using ggplot2 in R [68].
Successive passages of single circular chromosome S. pombe
For each strain, a single clone was picked from three individual survivors of type H1 and type H3 and re-streaked on YES medium and cultured at 30 °C for every 3 days. Clones were re-streaked for over 8 times.
Statistics and reproducibility
The methods used to compare different groups are described in the appropriate sections. Statistical significance was considered at a p-value < 0.05 threshold. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. WGS experiments were independent sample, and RNA-seq analysis included three biological replicates.
Supplementary Information
Additional file 1: Supplementary figures in this study. Fig S1. Schematics of chromosome fusion in SY13. Fig S2. Comparison of the core DNA sequences of CEN1 to CEN16 in Saccharomyces cerevisiae. Fig S3. Circular chromosome linearization in SY15. Fig S4. Validation of acrocentric chromosomes in LCSY1-LCSY8 cells. Fig S5. Single-chromosome cells with acrocentric chromosome show abnormal cell division. Fig S6. Functional enrichment analysis based on DEGs of LCSY7 and LCSY8 compared to LSY1. Fig S7. Characteristics of circular single-chromosome S. pombe. Fig S8. Circular chromosome linearization in SFYC0 and validation of linear single-chromosome S. pombe. Fig S9. Genomic and transcriptomic analysis of RSFY cells. Fig S10. Functional enrichment analysis based on DEGs of RSFY2 and RSFY3 compared to RSFY1.
Additional file 2: Details of the strains creation. Table S1. Details of the creation of FSY strains. Table S2. Details of the creation of LSY and LCSY strains. Table S3. Details of the creation of RSFY strains.
Additional file 3: Supporting information of genome and transcriptome in single-chromosome S. cerevisiae. Table S4. SNVs and indels in LSY1, LSY4, LCSY7 and LCSY8 compared to SY14. Table S5. Genes affected by telomere position effectin linear single-chromosome S. cerevisiae.
Additional file 4: Supporting information of genome and transcriptome in single-chromosome S. pombe. Table S6. SNVs and indels in type H1 compared to GXPY666. Table S7. SNVs and indels in RSFY1 and RSFY2 compared to GXPY666. Table S8. DEGs affected by telomere position effectin linear single-chromosome S. pombe.
Additional file 5: Strains and plasmids used in this study. Table S9. S. cerevisiae strains used in this study. Table S10. S. pombe strains used in this study. Table S11. Plasmids used in this study.
Additional file 6. GenBank files of plasmids and modified DNA sequences in this study.
Additional file 7. Primers used to verify chromosome fusions and chromosome linearization in this study.
Additional file 8. Differentially expressed genes between S. cerevisiae strains.
Additional file 9. Differentially expressed genes between S. pombe strains.
Additional file 10. Detail information of sequenced strains.
Acknowledgements
We thank lab members for helpful discussion.
Peer review information
Andrew Cosgrove was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Review history
The review history is available as Additional file 11.
Authors’ contributions
J.-Q.Z. and Z.-J.W. conceived the study, J.-Q.Z. and X.Z. analyzed the data and wrote the manuscript with input from Z.-J.W. and X.G.. X.Z. performed most of the experiments. T.Y. and X.G. constructed circular-chromosome S. pombe. X.Z. and S.L. analyzed sequencing data. F.P. and Z.Z. performed doubling time experiment. J.-Q.Z. supervised the project. All authors read and approved the final manuscript.
Funding
This work was support by grants from National Key Research and Development Program of China (2023YFA0913400); National Natural Science Foundation of China (No. 32150004) to J.-Q.Z.; and National Natural Science Foundation of China (No. 32200419) to Z.-J.W..
Data availability
All of the data for this study have been made publicly available. Raw sequencing data of whole-genome sequencing and RNA-seq have been deposited at Sequence Read Archive (SRA) with BioProject accession number PRJNA1187269 [69] for S. cerevisiae and PRJNA1198638 [70] for S. pombe. The details of sequenced strains within the BioProject have been listed in Additional file 10. Previously published raw sequencing data for whole-genome sequencing are available in PRJNA429985 [71] (SY14) and PRJNA792625 [72] (GXPY666). All data are available in the manuscript or the supplementary information. The sequence of plasmids and modified DNA sequences in this study have been provided in Additional file 6. DEGs between S. cerevisiae strains (SY14, LSY1, LCSY7 and LCSY8) can be found in Additional file 8, and DEGs between S. pombe strains (GXPY666, RSFY1, RSFY2 and RSFY3) can be found in Additional file 9. The strains in this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Ethical approval is not applicable for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uhler C, Shivashankar GV. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol. 2017;18:717–27. [DOI] [PubMed] [Google Scholar]
- 2.Talbert PB, Henikoff S. What makes a centromere? Exp Cell Res. 2020;389:111895. [DOI] [PubMed] [Google Scholar]
- 3.Senaratne AP, Cortes-Silva N, Drinnenberg IA. Evolution of holocentric chromosomes: drivers, diversity, and deterrents. Semin Cell Dev Biol. 2022;127:90–9. [DOI] [PubMed] [Google Scholar]
- 4.Mandrioli M, Manicardi GC. Holocentric chromosomes. PLoS Genet. 2020;16:e1008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–80. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald-Hayes M, Clarke L, Carbon J. Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell. 1982;29:235–44. [DOI] [PubMed] [Google Scholar]
- 7.Pierce BA. Genetics : a conceptual approach. 7th ed. New York: W. H. Freeman & Company; 2019. [Google Scholar]
- 8.Loidl J. Chromosomes of the budding yeast Saccharomyces cerevisiae. Int Rev Cytol. 2003;222:141–96. [DOI] [PubMed] [Google Scholar]
- 9.Kipling D, Ackford HE, Taylor BA, Cooke HJ. Mouse minor satellite DNA genetically maps to the centromere and is physically linked to the proximal telomere. Genomics. 1991;11:235–41. [DOI] [PubMed] [Google Scholar]
- 10.Bracewell R, Chatla K, Nalley MJ, Bachtrog D. Dynamic turnover of centromeres drives karyotype evolution in Drosophila. Elife. 2019;8:e49002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendich AJ, Drlica K. Prokaryotic and eukaryotic chromosomes: what’s the difference? BioEssays. 2000;22:481–6. [DOI] [PubMed] [Google Scholar]
- 12.Mudd AB, Bredeson JV, Baum R, Hockemeyer D, Rokhsar DS. Analysis of muntjac deer genome and chromatin architecture reveals rapid karyotype evolution. Commun Biol. 2020;3:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao Y, Lu N, Wu Z, Cai C, Wang S, Zhang LL, Zhou F, Xiao S, Liu L, Zeng X, et al. Creating a functional single-chromosome yeast. Nature. 2018;560:331–5. [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Sun X, Cormack BP, Boeke JD. Karyotype engineering by chromosome fusion leads to reproductive isolation in yeast. Nature. 2018;560:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu X, Ye T, Zhang XR, Nie L, Wang H, Li W, Lu R, Fu C, Du LL, Zhou JQ. Single-chromosome fission yeast models reveal the configuration robustness of a functional genome. Cell Rep. 2022;40:111237. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Qu Z, Fang Y, Chen Y, Peng J, Song J, Li J, Shi J, Zhou JQ, Zhao Y. Chromosome territory reorganization through artificial chromosome fusion is compatible with cell fate determination and mouse development. Cell Discov. 2023;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LB, Li ZK, Wang LY, Xu K, Ji TT, Mao YH, Ma SN, Liu T, Tu CF, Zhao Q, et al. A sustainable mouse karyotype created by programmed chromosome fusion. Science. 2022;377:967–75. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XM, Yan M, Yang Z, Xiang H, Tang W, Cai X, Wu Q, Liu X, Pei G, Li J. Creation of artificial karyotypes in mice reveals robustness of genome organization. Cell Res. 2022;32:1026–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao Y, Lu N, Cai C, Zhou F, Wang S, Zhao Z, Zhao G, Zhou JQ, Xue X, Qin Z. A single circular chromosome yeast. Cell Res. 2019;29:87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schubert I, Oud JL. There is an upper limit of chromosome size for normal development of an organism. Cell. 1997;88:515–20. [DOI] [PubMed] [Google Scholar]
- 21.Lamelas L, Arroyo M, Fernandez FJ, Marchal JA, Sanchez A. Structural and evolutionary relationships in the giant sex chromosomes of three microtus species. Genes (Basel). 2018;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travesa A, Wittenberg C. Turned on by genotoxic stress. Cell Cycle. 2012;11:3145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017;58:235–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke L. Centromeres: proteins, protein complexes, and repeated domains at centromeres of simple eukaryotes. Curr Opin Genet Dev. 1998;8:212–8. [DOI] [PubMed] [Google Scholar]
- 25.Lauer S, Luo J, Lazar-Stefanita L, Zhang W, McCulloch LH, Fanfani V, Lobzaev E, Haase MAB, Easo N, Zhao Y, et al. Context-dependent neocentromere activity in synthetic yeast chromosome VIII. Cell Genom. 2023;3:100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan R, Lian T, Zhou BR, He E, Wu C, Singleton M, Bai Y. Structural and dynamic mechanisms of CBF3-guided centromeric nucleosome formation. Nat Commun. 2021;12:1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiyama M, Ikushima S, Nakazawa T, Kaneko Y, Harashima S. PCR-mediated repeated chromosome splitting in Saccharomyces cerevisiae. Biotechniques. 2005;38:909–14. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell LA, Boeke JD. Circular permutation of a synthetic eukaryotic chromosome with the telomerator. Proc Natl Acad Sci U S A. 2014;111:17003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–62. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman CS, Wood V, Fantes PA. An ancient yeast for young geneticists: a primer on the schizosaccharomyces pombe model system. Genetics. 2015;201:403–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyas A, Freitas AV, Ralston ZA, Tang Z. Fission yeast schizosaccharomyces pombe: a unicellular, “micromammal” model organism. Curr Protoc. 2021;1:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonardi J, Box JA, Bunch JT, Baumann P. TER1, the RNA subunit of fission yeast telomerase. Nat Struct Mol Biol. 2008;15:26–33. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura TM, Cooper JP, Cech TR. Two modes of survival of fission yeast without telomerase. Science. 1998;282:493–6. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Baumann P. Chromosome fusions following telomere loss are mediated by single-strand annealing. Mol Cell. 2008;31:463–73. [DOI] [PubMed] [Google Scholar]
- 35.Naito T, Matsuura A, Ishikawa F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat Genet. 1998;20:203–6. [DOI] [PubMed] [Google Scholar]
- 36.Jain D, Hebden AK, Nakamura TM, Miller KM, Cooper JP. HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature. 2010;467:223–7. [DOI] [PubMed] [Google Scholar]
- 37.Allshire RC, Ekwall K. Epigenetic Regulation of Chromatin States in Schizosaccharomyces pombe. Cold Spring Harb Perspect Biol. 2015;7:a018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oizumi Y, Kaji T, Tashiro S, Takeshita Y, Date Y, Kanoh J. Complete sequences of Schizosaccharomyces pombe subtelomeres reveal multiple patterns of genome variation. Nat Commun. 2021;12:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucek K, Gimenez MD, Joron M, Rafajlovic M, Searle JB, Walden N, Westram AM, Faria R. The impact of chromosomal rearrangements in speciation: from micro- to macroevolution. Cold Spring Harb Perspect Biol. 2023;15:a041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozdag GO, Ono J. Evolution and molecular bases of reproductive isolation. Curr Opin Genet Dev. 2022;76:101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang Y, Zhang Q. The molecular and evolutionary basis of reproductive isolation in plants. J Genet Genomics. 2018;45:613–20. [DOI] [PubMed] [Google Scholar]
- 42.Hudakova S, Künzel G, Endo TR, Schubert I. Barley chromosome arms longer than half of the spindle axis interfere with nuclear divisions. Cytogenet Genome Res. 2002;98:101–7. [DOI] [PubMed] [Google Scholar]
- 43.Kunchala P, Varberg JM, O’Toole E, Gardner J, Smith SE, McClain M, Jaspersen SL, Hawley RS, Gerton JL. Plasticity of the mitotic spindle in response to karyotype variation. Curr Biol. 2024;34(3416–3428):e3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helsen J, Reza H, Carvalho R, Sherlock G, Dey G: Spindle architecture constrains karyotype in budding yeast. bioRxiv 2024. [DOI] [PMC free article] [PubMed]
- 45.Allshire R. Dissecting Fission Yeast Centromeres via Silencing. Paris: In Mapping Protein/DNA Interactions by Cross-Linking; 2001. [PubMed] [Google Scholar]
- 46.Liu J, Li Q, Hu Y, Yu Y, Zheng K, Li D, Qin L, Yu X. The complete telomere-to-telomere sequence of a mouse genome. Science. 2024;386:1141–6. [DOI] [PubMed] [Google Scholar]
- 47.Bloom K. Chromosome segregation: Brushing up on centromeres. Curr Biol. 2024;34:R565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrimore J, Bloom K. Shaping centromeres to resist mitotic spindle forces. J Cell Sci. 2022;135:jcs259532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacristan C, Samejima K, Ruiz LA, Deb M, Lambers MLA, Buckle A, Brackley CA, Robertson D, Hori T, Webb S, et al. Vertebrate centromeres in mitosis are functionally bipartite structures stabilized by cohesin. Cell. 2024;187(3006–3023):e3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen Gupta A, Seidel C, Tsuchiya D, McKinney S, Yu Z, Smith SE, Unruh JR, Gerton JL. Defining a core configuration for human centromeres during mitosis. Nat Commun. 2023;14:7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao Y, Lu N, Xue X, Qin Z. Creating functional chromosome fusions in yeast with CRISPR-Cas9. Nat Protoc. 2019;14:2521–45. [DOI] [PubMed] [Google Scholar]
- 53.Ormond KE, Hallquist MLG, Buchanan AH, Dondanville D, Cho MK, Smith M, Roche M, Brothers KB, Coughlin CR 2nd, Hercher L, et al. Developing a conceptual, reproducible, rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background. Genet Med. 2019;21:727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Y, Tang HB, Liu NN, Tong XJ, Dang W, Duan YM, Fu XH, Zhang Y, Peng J, Meng FL, Zhou JQ. Telomerase-null survivor screening identifies novel telomere recombination regulators. PLoS Genet. 2013;9:e1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hage AE, Houseley J. Resolution of budding yeast chromosomes using pulsed-field gel electrophoresis. Methods Mol Biol. 2013;1054:195–207. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Jin M, O’Laughlin R, Bittihn P, Tsimring LS, Pillus L, Hasty J, Hao N. Multigenerational silencing dynamics control cell aging. Proc Natl Acad Sci U S A. 2017;114:11253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Jiang Y, Paxman J, O’Laughlin R, Klepin S, Zhu Y, Pillus L, Tsimring LS, Hasty J, Hao N. A programmable fate decision landscape underlies single-cell aging in yeast. Science. 2020;369:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Z, Liu Y, Feng Y, Klepin S, Tsimring LS, Pillus L, Hasty J, Hao N. Engineering longevity-design of a synthetic gene oscillator to slow cellular aging. Science. 2023;380:376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paxman J, Zhou Z, O’Laughlin R, Liu Y, Li Y, Tian W, Su H, Jiang Y, Holness SE, Stasiowski E, et al. Age-dependent aggregation of ribosomal RNA-binding proteins links deterioration in chromatin stability with challenges to proteostasis. Elife. 2022;11:e75978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nurk S, Walenz BP, Rhie A, Vollger MR, Logsdon GA, Grothe R, Miga KH, Eichler EE, Phillippy AM, Koren S. HiCanu: accurate assembly of segmental duplications, satellites, and allelic variants from high-fidelity long reads. Genome Res. 2020;30:1291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcais G, Delcher AL, Phillippy AM, Coston R, Salzberg SL, Zimin A. MUMmer4: A fast and versatile genome alignment system. PLoS Comput Biol. 2018;14:e1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goel M, Sun H, Jiao WB, Schneeberger K. SyRI: finding genomic rearrangements and local sequence differences from whole-genome assemblies. Genome Biol. 2019;20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gayral P, Weinert L, Chiari Y, Tsagkogeorga G, Ballenghien M, Galtier N. Next-generation sequencing of transcriptomes: a guide to RNA isolation in nonmodel animals. Mol Ecol Resour. 2011;11:650–61. [DOI] [PubMed] [Google Scholar]
- 66.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ginestet C. ggplot2: Elegant Graphics for Data Analysis. J R Stat Soc Ser A Stat Soc. 2011;174:245–6. [Google Scholar]
- 69.Zhu X, Liu S, Ye T, Gu X, Pu F, Zhou Z, Wu ZJ, Zhou JQ. Sequencing data of the genome and transcriptome of single-chromosome budding yeast. Sequence Read Archive. 2024. https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1187269.
- 70.Zhu X, Liu S, Ye T, Gu X, Pu F, Zhou Z, Wu ZJ, Zhou JQ. Sequencing data of the genome and transcriptome of single-chromosome fission yeast Sequence Read Archive. 2024. https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1198638.
- 71.Shao Y, Lu N, Cai C, Zhou F, Wang S, Zhao Z, Zhao G, Zhou JQ, Xue X, Qin Z. Creating a functional single-chromosome yeast. Sequence Read Archive. 2018. https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA429985. [DOI] [PubMed]
- 72.Gu X, Ye T, Zhang XR, Nie L, Wang H, Li W, Lu R, Fu C, Du LL, Zhou JQ. Single-chromosome fission yeast models reveal the configuration robustness of a functional genome. Sequence Read Archive. 2021. https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA792625. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary figures in this study. Fig S1. Schematics of chromosome fusion in SY13. Fig S2. Comparison of the core DNA sequences of CEN1 to CEN16 in Saccharomyces cerevisiae. Fig S3. Circular chromosome linearization in SY15. Fig S4. Validation of acrocentric chromosomes in LCSY1-LCSY8 cells. Fig S5. Single-chromosome cells with acrocentric chromosome show abnormal cell division. Fig S6. Functional enrichment analysis based on DEGs of LCSY7 and LCSY8 compared to LSY1. Fig S7. Characteristics of circular single-chromosome S. pombe. Fig S8. Circular chromosome linearization in SFYC0 and validation of linear single-chromosome S. pombe. Fig S9. Genomic and transcriptomic analysis of RSFY cells. Fig S10. Functional enrichment analysis based on DEGs of RSFY2 and RSFY3 compared to RSFY1.
Additional file 2: Details of the strains creation. Table S1. Details of the creation of FSY strains. Table S2. Details of the creation of LSY and LCSY strains. Table S3. Details of the creation of RSFY strains.
Additional file 3: Supporting information of genome and transcriptome in single-chromosome S. cerevisiae. Table S4. SNVs and indels in LSY1, LSY4, LCSY7 and LCSY8 compared to SY14. Table S5. Genes affected by telomere position effectin linear single-chromosome S. cerevisiae.
Additional file 4: Supporting information of genome and transcriptome in single-chromosome S. pombe. Table S6. SNVs and indels in type H1 compared to GXPY666. Table S7. SNVs and indels in RSFY1 and RSFY2 compared to GXPY666. Table S8. DEGs affected by telomere position effectin linear single-chromosome S. pombe.
Additional file 5: Strains and plasmids used in this study. Table S9. S. cerevisiae strains used in this study. Table S10. S. pombe strains used in this study. Table S11. Plasmids used in this study.
Additional file 6. GenBank files of plasmids and modified DNA sequences in this study.
Additional file 7. Primers used to verify chromosome fusions and chromosome linearization in this study.
Additional file 8. Differentially expressed genes between S. cerevisiae strains.
Additional file 9. Differentially expressed genes between S. pombe strains.
Additional file 10. Detail information of sequenced strains.
Data Availability Statement
All of the data for this study have been made publicly available. Raw sequencing data of whole-genome sequencing and RNA-seq have been deposited at Sequence Read Archive (SRA) with BioProject accession number PRJNA1187269 [69] for S. cerevisiae and PRJNA1198638 [70] for S. pombe. The details of sequenced strains within the BioProject have been listed in Additional file 10. Previously published raw sequencing data for whole-genome sequencing are available in PRJNA429985 [71] (SY14) and PRJNA792625 [72] (GXPY666). All data are available in the manuscript or the supplementary information. The sequence of plasmids and modified DNA sequences in this study have been provided in Additional file 6. DEGs between S. cerevisiae strains (SY14, LSY1, LCSY7 and LCSY8) can be found in Additional file 8, and DEGs between S. pombe strains (GXPY666, RSFY1, RSFY2 and RSFY3) can be found in Additional file 9. The strains in this study are available from the corresponding author upon request.