Abstract
Clostridium botulinum neurotoxins (BoNTs) are the most toxic proteins for humans. The current clostridial-derived vaccines against BoNT intoxication have limitations including production and accessibility. Conditions were established to express the soluble receptor binding domain (heavy-chain receptor [HCR]) of BoNT serotypes A and E in Escherichia coli. Sera isolated from mice and rabbits immunized with recombinant HCR/A1 (rHCR/A1) from the classical type A-Hall strain (ATCC 3502) (BoNT/A1) and rHCR/E from BoNT serotype E Beluga (BoNT/EB) neutralized the homologous serotype of BoNT but displayed differences in cross-recognition and cross-protection. Enzyme-linked immunosorbent assay and Western blotting showed that α-rHCR/A1 recognized epitopes within the C terminus of the HCR/A and HCR/E, while α-rHCR/E recognized epitopes within the N terminus or interface between the N and C termini of the HCR proteins. α-rHCR/EB sera possessed detectable neutralizing capacity for BoNT/A1, while α-rHCR/A1 did not neutralize BoNT/E. rHCR/A was an effective immunogen against BoNT/A1 and the Kyoto F infant strain (BoNT/A2), but not BoNT serotype E Alaska (BoNT/EA), while rHCR/EB neutralized BoNT/EA, and under hyperimmunization conditions protected against BoNT/A1 and BoNT/A2. The protection elicited by rHCR/A1 to BoNT/A1 and BoNT/A2 and by rHCR/EB to BoNT/EA indicate that immunization with receptor binding domains elicit protection within sub-serotypes of BoNT. The protection elicited by hyperimmunization with rHCR/E against BoNT/A suggests the presence of common neutralizing epitopes between the serotypes E and A. These results show that a receptor binding domain subunit vaccine protects against serotype variants of BoNTs.
The neurotoxins of Clostridium botulinum (BoNTs) are the most potent protein toxins for humans and are included in the list of Category A Select Agents and Toxins (12). BoNTs comprise seven distinguishable serotypes, A through G, with serotypes A, B, and E responsible for most natural human intoxications (18). Each BoNT serotype is classically defined by the specificity of antibody neutralization. Thus, antibodies that neutralize BoNT serotype A (BoNT/A) do not neutralize the toxicity of BoNT serotypes B through G. Currently available vaccines are composed of chemically inactivated crude isolates of BoNTs. There are two available therapies against botulism, a pentavalent vaccine against serotypes A through E (19) and a heptavalent immunoglobulin against serotypes A through G (27). However, these vaccines are produced from chemically inactivated BoNT that is produced in C. botulinum and is currently in limited supply. There is a need to develop more efficient approaches for vaccine development against botulism.
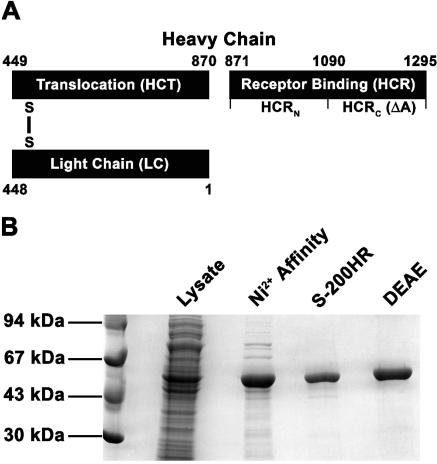
BoNTs are zinc proteases that elicit flaccid paralysis by inhibiting the fusion of neurotransmitter-carrying vesicles to the plasma membrane of peripheral neurons. BoNTs are produced as ∼150-kDa nontoxic single-chain proteins that are activated by proteolytic cleavage to a dichain structure. BoNTs comprise three functional domains, organized as an N-terminal catalytic domain (light chain [LC]), an internal translocation domain (heavy chain translocation [HCT]), and a C-terminal receptor binding domain (heavy chain receptor [HCR]) (Fig. 1A). In addition, HCR can be divided into an N-terminal domain (HCRN) and a C-terminal domain (HCRC). HCRC has been implicated to possess receptor binding capacity for neurons (22). BoNTs enter neurons via receptor-mediated endocytosis. The neurotoxicity of BoNTs is due to the affinity of HCR for protein(s) on the plasma membrane of peripheral neurons (21). The HCR-plasma membrane receptor interaction is enhanced by gangliosides, which are low affinity coreceptors for HCR (11). The translocation capabilities of HCT have been extrapolated from the action of the translocation domain of diphtheria toxin (7). Both native and recombinant HC form channels in artificial lipid bilayers through which the LC can be translocated (16). Upon delivery into the cytosol, LC cleaves neurotransmitter vesicle docking proteins, BoNT/A cleaves SNAP25 between residues 197 and 198 and BoNT/E cleaves SNAP25 between residues 180 and 181, which inactivates SNAP25 (33). In addition to the 7 serotypes of BoNT (A through G) (13, 15), several BoNT variants (subserotypes) have been identified that are immunologically distinguishable within a serotype. The classical type A-Hall strain (ATCC 3502) (BoNT/A1) and the Kyoto F infant strain (BoNT/A2) differ by ∼10% in their primary amino acid sequence (9, 10, 14), while BoNT/EB and BoNT/BA possess ∼92% primary amino acid homology.
FIG. 1.
Purification of recombinant HCR/A1. (A) BoNT/A1 is cleaved by Clostridial proteases into a dichain toxin that are linked by a disulfide bond. The N-terminal light chain encodes a zinc protease. The C-terminal heavy chain includes a translocation domain (HCT), and a C-terminal receptor binding domain which can be subdivided into an N-terminal (HCRN) and C-terminal domain (denoted ΔA). (B) rHCR/A1 was purified from E. coli cell paste by a three-column strategy. The clarified extract was purified sequentially using nickel affinity, gel filtration and ion exchange chromatography. rHCR/A (5 μg) was separated by SDS-PAGE under reducing conditions and visualized by staining with silver.
New vaccine strategies for botulism based upon recombinant antigens are currently under development. Native and recombinant HCR purified from C. botulinum and Escherichia coli protect mice against BoNT/A challenge when administered intraparenterally (29, 32). Currently, the HCR domains of the BoNTs are being expressed in the yeast Pichia pastoris (26). While useful as a first generation recombinant BoNT vaccine, this approach has several limitations, including limited genetic manipulation (26). Here, the neutralizing capacities and immunogenic properties of an E. coli-derived HCR against BoNT serotypes A and E are described.
MATERIALS AND METHODS
Materials.
All chemicals were from Sigma-Aldrich unless otherwise stated. Restriction enzymes and DNA polymerases were from Invitrogen. BoNT/A and -E were purified as described previously (8, 30).
Construction of recombinant HCR/A (rHCR/A) and rHCR/E genes.
Total genomic DNA from C. botulinum strain ATCC 3502 (Hall A) was used as a template to amplify full length HC/A (residues 449 through 1295). The PCR product was ligated into the TA cloning vector, pGEM-T (Promega), and the nucleotide sequence of the cloned insert verified. pGEM-HC/A was subsequently used as a template to generate expression constructs. The DNA fragment encoding HCR/A, containing residues 870 through 1295 of BoNT/A, was amplified and subcloned into a modified pET28a (Novagen) expression vector that contained unique KpnI and PstI sites. A similar cloning strategy was used to construct HCR/A2 (residues 871 through 1295) and HCR/E (residues 844 through 1250) using DNA from C. botulinum strains Kyoto F and Beluga, respectively.
rHCR expression in E. coli.
Purification protocols for rHCR/A1, rHCR/A2, rHCR/EB, rHCRC/A1 (ΔA), and HCRC/EB (ΔE) were identical and are described for rHCR/A1. pET28-HCR/A1 was transformed into E. coli strains BL-21 RIL (DE3) (Stratagene). E. coli BL-21 RIL (DE3) (pET28-HCR/A) was grown overnight on Luria-BertaniLB agar with 50 μg/ml kanamycin and 50 μg/ml chloramphenicol. Cells were inoculated into Luria-Bertani medium containing the same antibiotics, grown at 30°C for 2.5 h at 250 rpm to an optical density at 600 nm of ∼0.6, induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and then cultured at 250 rpm overnight at 16°C. Cells (five 0.4-liter cultures) were harvested and lysed with a French Press (2-3 times) in 40 ml ice-cold buffer A (1 mM dithiothreitol, 10 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9) containing EDTA-free protease inhibitor cocktail, 1 mM PMSF, 2.5 μg/ml DNase I and 2.5 μg/ml RNase A. The lysate was clarified by centrifugation at 20,000 × g for 30 min at 4°C and subsequently passed through a 0.45-μm filter. The filtered lysate was loaded onto a column of Ni2+-nitrilotriacetic acid (NTA) resin (5-ml bed volume; Qiagen) that had been equilibrated with 25 ml buffer A containing protease inhibitors. The column was washed with 40 ml buffer A followed by 20 ml buffer B (20 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9) and then eluted with 10 quantities of 1 ml buffer C (250 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9). Peak fractions from the nickel column were pooled, clarified by centrifugation at 12,000 × g for 20 min at 4°C, and subjected to gel filtration using Sephacryl S200 HR (300-ml column equilibrated in buffer D [1 mM EDTA, 20 mM NaCl, 20 mM Tris-HCl {pH 7.9}, 0.1% Triton X-100]). Peak fractions were subjected to anion-exchange chromatography (DEAE-Sephacel, 5 ml). rHCR passed through the column in the void volume, which was pooled, concentrated, and dialyzed overnight into phosphate-buffered saline (PBS)-40% (vol/vol) glycerol (Fig. 1B and Table 1).
TABLE 1.
Purification profile for E. coli-expressed rHCR/Aa
| rHCR/A | Total rHCR/A (mg/liter culture)b | Total protein (mg/liter culture)c | Purification factorc | Yield (%) |
|---|---|---|---|---|
| Extraction | 17 | 525 | 1 | 100 |
| Ni-NTA | 15 | 18 | 14 | 88 |
| Gel filtration | 13 | 15 | 15 | 76 |
| Ion exchange | 12 | 12 | 44 | 71 |
Data are representative of two independent determinations.
Estimated from band intensity on SDS-PAGE gels.
Based on total protein content.
Matrix-assisted laser desorption ionization (MALDI)-time of flight mass spectroscopy (MS) of rHCR/EB.
Fifteen micrograms rHCR/EB (three independent preparations) was excised from a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and subjected to trypsin digestion (1 μg; Promega) in 50 μl of 100 mM NH4HCO3, pH 8, at 37°C for 24 h. After digestion, gel slices were sonicated twice in 200 μl of 80% acetonitrile and 1% formic acid (in H2O) for 10 min. Eluted material was combined and evaporated, and the pellet was dissolved in 15 μl of 0.1% trifluoroacetic acid (in H2O). Peptide solutions were desalted with C18 Zip Tips (Millipore) that had been equilibrated successively in 15 μl of 100% acetonitrile, 15 μl of 50% acetonitrile (H2O), and 15 μl of 0.1% trifluoroacetic acid in H2O. Resin was washed twice with 0.1% trifluoroacetic acid in H2O. Peptides were eluted in 2 μl of 60% acetonitrile and 0.1% trifluoroacetic acid (H2O saturated with cyano-4-hydroxycinnamic acid) and applied to a sample plate to air dry. Samples were ionized with an N2 UV laser using a PE-Pro mass spectrometer (Applied Biosystems). Two hundred laser shots were conducted at an accelerating voltage of 25,000 V and laser intensity of 2,075 (repetition rate 3 Hz). Scans were processed using Biosystems Voyager 6004 software. Peptide fingerprinting was used to identify the proteins present in the sample, using Protein Prospector (University of California at San Francisco).
Immunization of rabbits and mice with recombinant HCR fragments.
Rabbit antisera against rHCR/A1 and rHCR/E were prepared by Covance, Inc. Briefly, female ELITE NZW rabbits were immunized intradermally with 250 μg rHCRs in Freund's complete adjuvant (day 0), boosted at days 14, 35, 49, and 70 with 125 μg rHCRs in Freund's incomplete adjuvant and terminally bled at day 80.
Female ICR mice (18 to 22 g) were immunized intraperitoneally with 16.7 μg and subcutaneously with 3.3 μg of rHCR/A1 or rHCR/EB mixed with an equal volume of Alhydrogel as adjuvant. Mice were vaccinated at 0, 7, and 14 days. Four days after the final boost mice were challenged with the indicated amount of BoNT/A1, BoNT/A2, or BoNT/EA and monitored for 96 h, at which point survival was scored.
Serum neutralization assay.
Potencies of BoNT were as follows: for A1 Hall BoNT, 30 to 40 pg/50% lethal dose (LD50) or 3.3 × 107 to 2.5 × 107 LD50 per mg toxin; for A2 Kyoto F toxin, 15 to 20 pg/LD50 or 6.67 × 107 to 5 × 107 LD50 per mg toxin; for E Alaska dichain toxin, 15 to 20 pg/LD50 or 6.67 × 107 to 5 × 107 LD50 per mg toxin. Four ng of BoNT/A1 (129 mouse LD50) or 8 ng BoNT/EA (400 mouse LD50) was incubated with serial dilutions of rabbit anti-rHCR/A1 or anti-rHCR/EB serum/ng toxin at the following concentrations: 0.94 μl/ng, 0.75 μl/ng, 0.625 μl/ng, 0.5 μl/ng, 0.375 μl/ng, 0.3125 μl/ng, 0.25 μl/ng, 0.188 μl/ng, 0.125 μl/ng, and 0.0625 μl/ng. After a 2-h incubation at room temperature, samples were injected into three female ICR mice (18 to 22 g), using a volume of 100 μl/mouse. Mice were monitored for 96 h and survival was scored. These experiments were approved by an animal care-and-use committee at the University of Wisconsin at Madison.
ELISA.
rHCRs were diluted to 1 μg/ml in coating buffer (50 mM Na2CO3, pH 9.6) and 100 μl was added to each well of an enhanced binding enzyme-linked immunosorbent assay (ELISA) plate (enzyme immunoassay/RIA high binding plate; Corning) and allowed to adhere overnight at 4°C. Column 1 was incubated with coating buffer alone (no-antigen control). Plates were then washed four times with 400 μl PBS and blocked for 1 h at 37°C with 200 μl per well of 2% (wt/vol) bovine serum albumin in coating buffer. Following a washing step as outlined above, plates were incubated for 1 h at 37°C with serial dilutions of the sera in binding buffer (1% [wt/vol] bovine serum albumin in PBS, 100 μl per well). As controls, column 1 (no antigen) was incubated with the lowest dilution of the serum, while column 2 (no primary antibody) was incubated with binding buffer alone. Following a washing step, plates were incubated for 1 h at 37°C with either donkey anti-mouse or donkey anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (1:12,000) in binding buffer. Plates were washed six times with 400 μl PBS and then incubated with 100 μl per well of tetramethyl benzidine (Pierce) as substrate. The reaction was terminated by addition of 100 μl per well 0.1 M sulfuric acid and absorbance read at 450 nm using an ELISA plate reader (Wallac).
RESULTS
HCR expression in E. coli.
Although the production of recombinant fusion proteins in E. coli is well established, there are several factors which are obstacles for successful production and purification of soluble fusion proteins. While prior expression of botulinum neurotoxin components in E. coli has been reported, low yields and/or poor solubility has limited their use for biochemical analysis and vaccine development (6). We recently developed an expression strategy for the production of large amounts of recombinant BoNT/A LC (1), which prompted a reevaluation of the potential to produce high yields of purified HCR in E. coli.
DNA encoding BoNT/A1 residues 870 through 1295 was subcloned into a pET expression vector resulting in an N-terminal His6-HCR/A fusion protein (rHCR/A1). While rHCR/A expression was detected in E. coli BL21(DE3), enhanced expression was achieved in E. coli BL-21(DE3)-RIL, which has been engineered for expression of AT-rich genes. As was reported for the LC of BoNT/A1 (1), induction at 16°C was critical for the stable accumulation of rHCR/A to a concentration of ∼20 mg/liter culture. Expression of recombinant forms of BoNT/A2 and BoNT/E HCR has not been reported. Utilizing the expression conditions established for rHCR/A1; rHCR/A2 and rHCR/EB (Beluga strain, residues 844 through 1250) were expressed at levels comparable to rHCR/A1.
Purification of rHCR from E. coli.
rHCR/A1, rHCR/A2, and rHCR/EB were purified by sequential chromatography on Ni2+-NTA resin, gel filtration, and anion exchange. By gel filtration analysis, the majority of the rHCR/A1 and -A2 migrated as a monomer, while rHCR/E migrated as a dimer. Due to basic isoelectric points, neither rHCR/A1, rHCR/A2, nor rHCR/E bound to DEAE resin at pH 7.9. Passing rHCR/A1, rHCR/A2, or rHCR/E through DEAE resin removed several contaminants, most notably a protease activity. A typical purification from a 1-liter culture yielded ∼15 to 20 mg rHCR/A1, rHCR/A2, or rHCR/EB, which were >95% pure, as determined by SDS-PAGE (Fig. 1 and Table 1). rHCR/A1, rHCR/A2, and rHCR/EB did not degrade upon storage in 10 mM Tris (pH 7.6)-20 mM NaCl at 4°C after >1 week or at −20°C for several months.
Neutralizing capacity of rabbit α-rHCR antibodies.
The neutralizing capacity of polyclonal rHCR/A1 and rHCR/EB serum to the homologous BoNT was determined using a mouse bioassay, where a LD50 corresponds to the quantity of BoNT introduced via intraperitoneal injection that resulted in 50% death after 4 days (29). BoNT/A1 used in this analysis had ∼3.3 × 104 LD50/μg while BoNT/E used in this analysis had ∼1.2 × 104 LD50/μg. Two independent α-rHCR/A1 sera neutralized 5 × 105 and 7 × 105 mouse LD50 of BoNT/A1/ml serum, while α-rHCR/EB neutralized between 1 × 105 and 3 × 105 mouse LD50 of BoNT/EA/ml serum. The Alaska subtype E of BoNT/E was used in challenge experiments, since purification of this subtype from C. botulinum is more efficient than the Beluga subtype of BoNT/E. Controls showed that neither anti-rHCR/A1 nor α-rHCR/EB sera neutralized BoNT/B and that sera from prebleeds did not neutralize BoNT/A1 or BoNT/EA. Using the mouse bioassay, α-rHCR/A1sera did not neutralize BoNT/EA, but α-rHCR/EB sera neutralization of BoNT/A1 could be observed (∼1 × 103 mouse LD50/ml). Although not directly comparable due to different immunization protocols, the neutralizing capacity of the sera was similar to that of humans vaccinated with the pentavalent BoNT toxoid (31).
Immunoreactivity of rabbit anti-rHCR antibodies.
Reactivity of the α-rHCR sera was tested against rHCR/A1, rHCR/A2 and rHCR/E. C-terminal peptides of the HCRs (BoNT/A1 residues 1090 through 1295, termed ΔA) and BoNT/E (residues 1065 through 1250, termed ΔE) were also tested for reactivity to localize antigenic epitopes. Western blot analysis showed that rabbit α-rHCR/A1 sera reacted against rHCR/A1, rHCR/A2 and ΔA with similar reactivity and also cross-reacted with rHCR/EB and ΔE (Fig. 2). ELISA showed that rabbit α-rHCR/A1 sera reacted with rHCE/E and ΔE at a ∼5-fold-lower titer than the rHCR/A antigens. These data indicated that epitope(s) within the C terminus of HCR were immune dominant when rHCR/A1 was used as an immunogen.
FIG. 2.
Immunological characterization of rabbit sera against rHCR/A1 and rHCR/EB. (A) ELISA of rabbit antisera to rHCR/A1 (left panel) or rHCR/EB (right panel), using 100 ng rHCR/A1, 100 ng rHCR/A2, 100 ng rHCR/ΔA, 100 ng rHCR/EB, and 100 ng rHCR/ΔE as capture antigens. (B) Antigens (500 ng) were separated by SDS-PAGE under reducing conditions and visualized by Western blotting with anti-rHCR/A1 sera (left panel) or anti-rHCR/EB (right panel).
Two independent rabbit α-rHCR/EB sera displayed distinctive immune reactive properties relative to the reactivity of the α-rHCR/A1 sera. Western blot analysis showed that rabbit α-rHCR/EB sera reacted against rHCR/EB, but did not react with ΔE or the serotype A antigens. ELISA showed that the rabbit α-rHCR/EB sera titers to ΔE and the type A antigens was detected, but with between 10- and 50-fold lower titers than for rHCR/E. These data indicated that epitopes(s) within the N terminus of rHCR/EB or at the interface of the N-terminal and C-terminal domains were the major epitopes of rHCR/EB. Thus, although HCR/A and HCR/E are 44% identical, the two antigens generated a unique immune response and cross protective antibodies in rabbits.
Immune protection of rHCR against BoNTs.
The neutralizing capacity and unique serum cross-reactivity of the rabbit α-rHCR sera promoted subsequent studies to determine the efficacy of rHCR/A1 and rHCR/EB as vaccine candidates against homologous and heterologous BoNT serotype challenge.
Low-dose immunization.
Mice were immunized with rHCR/A1 or rHCR/EB in aluminum hydroxide adjuvant (Alhydrogel) and challenged with homologous and heterologous serotypes of BoNT. Immunization with rHCR/A1 or rHCR/E did not elicit distress in mice. Mice immunized with rHCR/A1 were resistant to challenge with up to 100,000 LD50 of either BoNT/A1 or BoNT/A2, but not BoNT/E (Table 2). This is the first demonstration that immunization with the classical type A HCR protects against challenge by a heterologous subserotype of BoNT/A. Similarly, mice immunized with rHCR/EB were resistant to challenge with up to 100,000 LD50 of BoNT/EA but not either BoNT/A1 or BoNT/A2.
TABLE 2.
Protection from BoNT intoxication by rHCRa
| Immunization (2.5 μg antigen) and challenge | Toxin
|
||
|---|---|---|---|
| BoNT/A1 | BoNT/A2 | BoNT/EA | |
| HCR/A1 | |||
| LD50 | |||
| 10 | +,+ | +,+ | −,− |
| 100 | +,+,+,+ | +,+,+,+ | −,− |
| 1,000 | +,+ | +,+ | −,− |
| 100,000 | +,+,+,+ | +,+,+,+ | −,− |
| HCR/E | |||
| 10 | −,− | −,− | +,+ |
| 100 | −,− | −,− | +,+,+,+ |
| 1,000 | −,− | −,− | +,+ |
| 100,000 | −,− | −,− | +,+,+,+ |
Mice were immunized with the indicated serotype of rHCR and then challenged by the indicated amount and serotype of BoNT. Individual mice were inspected for 96 h and scored for survival (+) or death (−).
Hyperimmunization.
In other experiments, mice were immunized with 20 μg of rHCR/A1 or rHCR/EB to determine the effect of hyperimmunization on the protective response. Mice immunized with rHCR/A1 were resistant to challenge with 100,000 mouse LD50 BoNT/A1 and BoNT/A2 but remained sensitive to BoNT/EA. Mice immunized with rHCR/E were resistant to challenge with 100,000 mouse LD50 BoNT/E and were protected against challenge with 10 mouse LD50 of BoNT/A1 or BoNT/A2. In one experiment six of six mice challenged were protected, while in another experiment three of three mice displayed a delay in time to death.
Immunoreactivity of mouse anti-rHCR antibodies.
Pooled sera isolated from mice immunized with either a low or high amount of HCR were analyzed by Western blotting and ELISA. α-rHCR/A1 sera from mice immunized with 20 μg of antigen reacted against rHCR/A1, rHCR/A2, and ΔA by Western blotting and displayed reactivity to rHCR/E and, to a lesser extent, ΔE (Fig. 3). ELISA revealed differences in the relative reactivity of mouse α-rHCR/A1 sera to these antigens where reactivity to HCR/EB and ΔE was ∼10-fold higher than recognition of the type A antigens. Mouse α-rHCR/A1 sera from mice immunized with low doses of antigen (2.5 μg of antigen) displayed similar Western blot and ELISA profiles, although overall titers were two- to threefold lower than observed for the sera from mice immunized with 20 μg of antigen (data not shown).
FIG. 3.
Immunological characterization of mouse sera against rHCR/A1 and rHCR/EB. (A) ELISA of mouse antisera to rHCR/A1 (left panel) or rHCR/EB (right panel), using 100 ng rHCR/A1, 100 ng rHCR/A2, 100 ng rHCR/ΔA, 100 ng rHCR/EB, and 100 ng rHCR/ΔE as capture antigens. (B) Antigens (500 ng) were separated by SDS-PAGE under reducing conditions and visualized by Western blotting with anti-rHCR/A1 sera (left panel) or anti-rHCR/EB (right panel).
Western blot analysis of α-rHCR/E sera from hyperimmunized mice with 20 μg of rHCR/EB showed reactivity to rHCR/EB and to a lesser extent with ΔE and the A serotype antigens. ELISA of mouse α-rHCR/E sera showed that the reactivity to rHCR/E was ∼8-fold higher than ΔE, rHCR/A1 and rHCR/A2, and ∼16-fold higher than ΔA. Sera from animals immunized with lower doses of rHCR/E (2.5 μg of antigen) displayed similar Western blot and ELISA profiles, although reactivity to all antigens was twofold lower than observed for the sera from mice immunized with 20 μg of antigen (data not shown).
Purity of rHCR/E.
The cross serotype protection of serum immunized with rHCR/EB against BoNT/A1 and BoNT/A2 raised the possibility that rHCR/EB was contaminated with rHCR/A1. To address this concern, the purity of the rHCR/EB preparation used for rabbit antibody production and mouse vaccine development was determined. Fifteen μg of rHCR/EB was subjected to SDS-PAGE followed by in-gel tryptic digestion. MALDI MS analysis identified ∼75% of the predicted tryptic peptides of rHCR/EB, but did not identify any tryptic peptides that were unique to rHCR/A1. Moreover, further analysis of two independently prepared preparations of rHCR/EB by both MALDI MS and ELISA produced identical tryptic peptides and immune reactivity, respectively. This indicates that the cross protection elicited by rHCR/EB is intrinsic to the protein and not due to cross contamination among protein preparations.
Structural basis for the cross protection elicited by HCR/A and HCR/E.
Despite the relatively low primary amino acid homology among the BoNTs (30 to 50% identity), the crystal structures of HCRs of BoNT/A1, BoNT/B, and tetanus toxin share overall structural similarity (15). Using Swiss Model, the predicted structures of HCR/A2 (Kyoto F, 90% identity to HCR/A1) and HCR/EB (Beluga, 44% identity to HCR/A1) were determined. While HCR/A1, HCR/A2, and HCR/EB showed similar overall topology to their templates BoNT/A1, BoNT/B, and tetanus HCR (Fig. 4A, upper panel), four regions (1-3, 24) between HCR/A1 and HCR/E showed low structural homology. These loops were located at interface of the sub-domains of HCR (loops 1, 2, and 4) or towards the C terminus of the molecule. Since these loop regions represent the only major structural differences between HCR/A and HCR/E, these loops may represent epitope(s) for serological distinction.
FIG. 4.
Protein modeling of HCR/A2 (Kyoto F) and HCR/E (Beluga). (A) Using the structures of BoNT/A (pdb:3bta), BoNT/B (pdb:1epw), and tetanus HCR (pdb:1doh) as templates, the three-dimensional structures of HCR/A2 and HCR/EB were generated using Swiss-Model. Ribbon diagrams of HCR/A1 (blue), HCR/A2 (red), and HCR/EB (black) are displayed in the upper panels. Molecular surface electrostatic potentials of each protein were computed using the Coulomb method and are displayed in the lower panels (blue, positive charge; red, negative charge; white, neutral). The regions of lowest structural homology between HCR/A1, HCRA2, and HCR/E are circled and labeled 1 through 4. (B) Enlarged view of region 5 highlighting the primary residues contributing to the electrostatic surface of the molecule (left panel). Sequence alignment of the peptides forming this region are displayed on the right with conserved charge residues highlighted.
The surface electrostatic potential of HCR/A1, HCR/A2, and HCR/E was also calculated (Fig. 4A, lower panel). The charge distribution of HCR/A1 and HCR/A2 were similar, with an acidic C-terminal domain surface and a basic/neutral N-terminal domain surface. HCR/EB showed a different distribution of surface electrostatic potential relative to HCR/A. The C-terminal domain surface was highly basic, while the surface of the N-terminal domain was primarily neutral. However, one region of charge conservation was identified in the N-terminal domain (position 5). The acidic surface potential within this region results from both structural and primary amino acid conservation. region (Fig. 4B). Thus, this region could represent a common conformational epitope among the A and E serotypes of BoNT.
DISCUSSION
The botulinum neurotoxins can be beneficially employed for the treatment of several involuntary muscle disorders, but have also been given high priority for the development of vaccines and therapies to prevent intoxication (24). Botulism can be prevented by administration of neutralizing antibodies or vaccination. The licensed trivalent antitoxin contains neutralizing antibodies against botulinum toxin types A, B, and E, the serotypes that most commonly cause of human botulism. Passive immunity is currently provided through administration of equine antitoxin distributed by the CDC. While only limited data is available on the safety of current BoNT vaccines, studies of recipients of equine botulinum antitoxin in the United States demonstrate various acute reactions (3). The current vaccine is a pentavalent botulinum toxoid (A through E), which is effective but has several limitations including cost, efficacy and accessibility. Exposure to other serotypes of BoNT can be addressed with an investigational heptavalent (ABCDEFG) antitoxin (14).
Previous studies have indicated that major protective epitopes of BoNT/A are located in the receptor binding domain (HCR) (6, 25). Thus, the use of HCR/A has been included in strategies for botulinum antibody therapy and vaccine development. The HCR component of BoNTs has several potential advantages over currently available C. botulinum-derived antigens. Production of HCR in a heterologous system facilitates large scale production and removes the possibility of contamination with other neurotoxins and clostridial components. This strategy was originally applied to BoNT/A, using an E. coli-based expression system (6). HCR/A expressed and purified from E. coli protected mice against challenged with active toxin. Moreover, purified HCR/A was as efficacious in protecting against challenge with BoNT/A as the pentavalent toxoid vaccine. Thus, HCR/A had the properties required for use as a vaccine candidate. However, in these early studies HCR/A was not expressed at levels sufficient for vaccine development and so was not pursued further. The limited utility of HCR/A expressed in E. coli prompted the development of the methylotrophic yeast Pichia pastoris as a heterologous host for expression of HCR fragments (4, 5). rHCR/A expressed in P. pastoris is highly immunogenic and induces protective immunity in mice and represent a useful first generation for vaccine development, but expression of HCRs in P. pastoris can be a challenge with respect to genetic manipulation and ease of purification (26). Popoff and coworkers have recently expressed HCR/A in E. coli and mapped the major protective epitopes of the BoNT to HCR (32).
The C. botulinum A Hall-hyper (28) has been used widely for the production of BoNT/A vaccines, studies on neurotoxin biochemistry, pharmacology, and crystallography (17, 23) and in the manufacture of therapeutic BoNT. Comparison of the BoNT/A amino acid sequences from C. botulinum type A-Hall-hyper strain with other BoNT/A sequences revealed subtypes within serotype A (9, 10, 24). BoNT produced by the Kyoto F strain shares ∼90% identity with the Hall-A strain and has been designated as BoNT/A2. These findings have raised the question of whether an antigen based upon a single strain can protect against all strain variants. The current study addresses this concern by showing that vaccination with rHCR/A1 protected against challenge by both BoNT/A1 and BoNT/A2. While several serotypes of BoNT HCR have been used in vaccine development, HCR/E derived vaccines are currently lacking. Here we report for the first time that rHCR/EB elicits protective immunity to BoNT/EA. In these experiments, HCR/E engineered from the Beluga subtype protected from challenge with the BoNT/E from the Alaska strain of C. botulinum (20). This shows efficient protection from immunization with heterologous HCR/E sub-types.
Classically, botulinum serotypes are defined by the lack of cross-protection between neutralizing anti-sera, i.e., anti-type A sera does not neutralize BoNT from other serotypes. The cross protection elicited by hyper immunization with HCR/E to BoNT/A1 intoxication suggests the presence of cross protective epitope(s) within the BoNTs. The enhanced cross protection elicited by HCR/EB relative to HCR/A may be due to a polyclonal epitope response to the HCRs, where antibodies that recognize multiple epitopes are required for neutralization (2) or may represent the expansion of a minor common epitope that is stimulated upon immunization with large amounts of antigen. While it is not practical to envision that this level of cross protection will yield a common protective immunogen using HCR subunit vaccination, identification of the mechanism responsible for this cross-protection may lead to the development of reagents with cross-neutralizing capabilities. Earlier studies by Middlebrook and coworkers reported some cross-protection of mice against BoNT/E when immunized by BoNT/A (6, 19).
Molecular modeling predicts the structures of HCR/A2 and HCR/EB (Fig. 4). HCR/A2 has ∼90% homology with HCR/A1 and is predicted to have similar structures and overall electrostatic potential. This is consistent with the cross-protection observed with immunization with rHCR/A1. HCR/EB has 44% homology with HCR/A1. While the overall predicted structures are similar, HCR/A1 differs from HCR/EB in four loop regions (Loops 1 through 4), which are candidate epitopes for the differential protection elicited by HCR/EB relative to HCR/A1. HCR comprises two domains, the N-terminal domain (residues 870 through 1095) and the C-terminal domain (residues 1096 through 1295). The C-terminal domain has been proposed to include the receptor binding domain (20). Popoff and coworkers implicated a role for epitopes within the interface of these two domains of HCR for effective immunization (32). Thus loops 1, 2, and 4, which lie within the interface (Fig. 4), may define serotype specific neutralization epitopes. Alternatively, while predicted electrostatic properties of HCR/A1 and HCR/A2 are similar, HCR/A electrostatic properties are different from HCR/E and common regions of electrostatic potential may contribute to the common epitopes among the HCRs of the BoNTs. Current studies address the nature potential common neutralizing epitopes of BoNT/A and BoNT/E.
The immunogenic potency of E. coli-derived rHCRs represent tools that allow genetic manipulation to develop the next generation of vaccines and therapies against botulism, as well as reagents to elucidate the cell biology of BoNT intoxication of neurons. The subtype protection elicited by HCR/A1 and HCREB predicts that a well designed heavy chain subunit vaccine can protect against variant subtypes of the BoNTs.
Acknowledgments
This work was supported by a grant from the Great Lakes Regional Center of Excellence (GLRCE) from NIH-NIAID U54 AI057153.
The assistance of Na Li at the Medical College of Wisconsin in the preparation the rHCR and the Protein-Nucleic Acid Facility at the Medical College of Wisconsin for the MALDI-MS analysis of HCR/E is acknowledged.
Editor: D. L. Burns
REFERENCES
- 1.Baldwin, M. R., M. Bradshaw, E. A. Johnson, and J. T. Barbieri. 2004. The C-terminus of botulinum neurotoxin type A light chain contributes to solubility, catalysis, and stability. Protein Expr. Purif. 37:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky, J. A., I. J. Berkower, and S. L. Epstein. 1999. Antigen-antibody interactions and monoclonal antibodies, p. 91-94. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 3.Black, R. E., and R. A. Gunn. 1980. Hypersensitivity reactions associated with botulinal antitoxin. Am. J. Med. 69:567-570. [DOI] [PubMed] [Google Scholar]
- 4.Byrne, M. P., and L. A. Smith. 2000. Development of vaccines for prevention of botulism. Biochimie 82:955-966. [DOI] [PubMed] [Google Scholar]
- 5.Byrne, M. P., T. J. Smith, V. A. Montgomery, and L. A. Smith. 1998. Purification, potency, and efficacy of the botulinum neurotoxin type A binding domain from Pichia pastoris as a recombinant vaccine candidate. Infect. Immun. 66:4817-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton, M. A., J. M. Clayton, D. R. Brown, and J. L. Middlebrook. 1995. Protective vaccination with a recombinant fragment of Clostridium botulinum neurotoxin serotype A expressed from a synthetic gene in Escherichia coli. Infect. Immun. 63:2738-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier, R. J. 2001. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39:1793-1803. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta, B. R., L. J. Berry, and D. A. Boroff. 1970. Purification of Clostridium botulinum type A toxin. Biochim. Biophys. Acta 214:343-349. [DOI] [PubMed] [Google Scholar]
- 9.Dineen, S. S., M. Bradshaw, and E. A. Johnson. 2003. Neurotoxin gene clusters in Clostridium botulinum type A strains: sequence comparison and evolutionary implications. Curr. Microbiol. 46:345-352. [DOI] [PubMed] [Google Scholar]
- 10.Dineen, S. S., M. Bradshaw, C. E. Karasek, and E. A. Johnson. 2004. Nucleotide sequence and transcriptional analysis of the type A2 neurotoxin gene cluster in Clostridium botulinum. FEMS Microbiol. Lett. 235:9-16. [DOI] [PubMed] [Google Scholar]
- 11.Dong, M., D. A. Richards, M. C. Goodnough, W. H. Tepp, E. A. Johnson, and E. R. Chapman. 2003. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 162:1293-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federal Register. 2002. Agricultural bioterrorism protecton act of 2002; possession, use, and transfer of select agents and toxins, interim final rule. 67:76908-76938.
- 13.Gimenez, D. F., and J. A. Gimenez. 1993. Serological subtypes of botulinal neurotoxins, p. 421-431. In B. R. DasGupta (ed.), Botulinum and tetanus neurotoxins: neurotransmission and biomedical aspects. Plenum Press, New York, N.Y.
- 14.Hibbs, R. G., J. T. Weber, A. Corwin, B. M. Allos, M. S. Abd el Rehim, S. E. Sharkawy, J. E. Sarn, and K. T. McKee, Jr. 1996. Experience with the use of an investigational F(ab′)2 heptavalent botulism immune globulin of equine origin during an outbreak of type E botulism in Egypt. Clin. Infect. Dis. 23:337-340. [DOI] [PubMed] [Google Scholar]
- 15.Izumi, N., H. Kondo, I. Ohishi, and G. Sakaguchi. 1983. Purification and characterization of alpha-toxin of Clostridium oedematiens type A. Jpn. J. Med. Sci. Biol. 36:135-146. [PubMed] [Google Scholar]
- 16.Koriazova, L. K., and M. Montal. 2003. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 10:13-18. [DOI] [PubMed] [Google Scholar]
- 17.Lacy, D. B., W. Tepp, A. C. Cohen, B. R. DasGupta, and R. C. Stevens. 1998. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat. Struct. Biol 5:898-902. [DOI] [PubMed] [Google Scholar]
- 18.Marvaud, J. C., S. Raffestin, and M. R. Popoff. 2002. Botulism: the agent, mode of action of the botulinum neurotoxins, forms of acquisition, treatment and prevention. C R Biol. 325:863-878, 879-883. [In French.] [DOI] [PubMed] [Google Scholar]
- 19.Middlebrook, J. L. 1995. Protection strategies against botulinum toxin. Adv. Exp. Med. Biol. 383:93-98. [DOI] [PubMed] [Google Scholar]
- 20.Montecucco, C., O. Rossetto, and G. Schiavo. 2004. Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 12:442-446. [DOI] [PubMed] [Google Scholar]
- 21.Montecucco, C., and G. Schiavo. 1994. Mechanism of action of tetanus and botulinum neurotoxins. Mol. Microbiol. 13:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Montecucco, C., and G. Schiavo. 1995. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 28:423-472. [DOI] [PubMed] [Google Scholar]
- 23.Montecucco, C., G. Schiavo, V. Tugnoli, and D. de Grandis. 1996. Botulinum neurotoxins: mechanism of action and therapeutic applications. Mol. Med. Today 2:418-424. [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Allergy and Infectious Diseases. 2002. Summary of the NIAID expert panel on botulinum toxins. National Institute of Allergy and Infectious Diseases, Bethesda, Md.
- 25.Nowakowski, A., C. Wang, D. B. Powers, P. Amersdorfer, T. J. Smith, V. A. Montgomery, R. Sheridan, R. Blake, L. A. Smith, and J. D. Marks. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 99:11346-11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter, K. J., W. Zhang, L. A. Smith, and M. M. Meagher. 2000. Production and purification of the heavy chain fragment C of botulinum neurotoxin, serotype A, expressed in the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 19:393-402. [DOI] [PubMed] [Google Scholar]
- 27.Robinson, R. F., and M. C. Nahata. 2003. Management of botulism. Ann. Pharmacother. 37:127-131. [DOI] [PubMed] [Google Scholar]
- 28.Schantz, E. J., and E. A. Johnson. 1997. Botulinum toxin: the story of its development for the treatment of human disease. Perspect. Biol. Med. 40:317-327. [DOI] [PubMed] [Google Scholar]
- 29.Schantz, E. J., and E. A. Johnson. 1990. Dose standardisation of botulinum toxin. Lancet 335:421. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt, J. J., and L. S. Siegel. 1986. Purification of type E botulinum neurotoxin by high-performance ion exchange chromatography. Anal. Biochem. 156:213-219. [DOI] [PubMed] [Google Scholar]
- 31.Siegel, L. S. 1988. Human immune response to botulinum pentavalent (ABCDE) toxoid determined by a neutralization test and by an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 26:2351-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavallaie, M., A. Chenal, D. Gillet, Y. Pereira, M. Manich, M. Gibert, S. Raffestin, M. R. Popoff, and J. C. Marvaud. 2004. Interaction between the two subdomains of the C-terminal part of the botulinum neurotoxin A is essential for the generation of protective antibodies. FEBS Lett. 572:299-306. [DOI] [PubMed] [Google Scholar]
- 33.Tonello, F., S. Morante, O. Rossetto, G. Schiavo, and C. Montecucco. 1996. Tetanus and botulism neurotoxins: a novel group of zinc-endopeptidases. Adv. Exp. Med. Biol. 389:251-260. [PubMed] [Google Scholar]