Abstract
We studied the identity and function of the 528-bp gene immediately upstream of Legionella pneumophila F2310 ptsP (enzyme INtr). This gene, nudA, encoded for a Nudix hydrolase based on the inferred protein sequence. NudA had hydrolytic activity typical of other Nudix hydrolases, such as Escherichia coli YgdP, in that ApnA’s, in particular diadenosine pentaphosphate (Ap5A), were the preferred substrates. NudA hydrolyzed Ap5A to ATP plus ADP. Both ptsP and nudA were cotranscribed. Bacterial two-hybrid analysis showed no PtsP-NudA interactions. Gene nudA was present in 19 of 20 different L. pneumophila strains tested and in 5 of 10 different Legionella spp. other than L. pneumophila. An in-frame nudA mutation was made in L. pneumophila F2310 to determine the phenotype. The nudA mutant was an auxotroph that grew slowly in liquid and on solid media and had a smaller colony size than its parent. In addition, the mutant was more salt resistant than its parent and grew very poorly at 25°C; all of these characteristics, as well as auxotrophy and slow-growth rate, were reversed by transcomplementation with nudA. The nudA mutant was outcompeted by about fourfold by the parent in competition studies in macrophages; transcomplementation almost completely restored this defect. Competition studies in guinea pigs with L. pneumophila pneumonia showed that the nudA mutant was outcompeted by its parent in both lung and spleen. NudA is of major importance for resisting stress in L. pneumophila and is a virulence factor.
Legionella pneumophila, the most common cause of Legionnaires' disease, is a facultative gram-negative intracellular parasite. Legionnaires' disease is a type of pneumonia that affects mainly adults, especially those who have altered local lung defenses or who have cellular immune system-suppressing diseases (20). A multitude of L. pneumophila virulence factors have been described, the majority of which affect the ability of the bacterium to grow and survive within blood monocytes and alveolar macrophages or within free-living amoebae (14). To attempt to find L. pneumophila virulence factors responsible for growth or survival in vivo, but not necessarily in macrophages, some of us previously performed signature-tagged mutagenesis of L. pneumophila using a guinea pig pneumonia model (21). Several different genes encoding new putative virulence factors were found, including ptsP, mutation of which results in decreased growth in macrophages and loss of virulence for guinea pigs (32). Immediately upstream of ptsP was an open reading frame (ORF) 528 bp in length that was in the same reading frame and appeared to be in the same operon. This reading frame, termed ORF2, shared significant homology with Nudix hydrolases from many different bacteria, including invA, from Rickettsia prowazekii and Pseudomonas aeruginosa and mutT of Escherichia coli. Nudix hydrolases are nucleoside diphosphate pyrophosphatases thought to be important in degrading toxic intracellular compounds and in the virulence of several different bacteria (4, 5, 28, 40). Nudix is an acronym for nucleoside diphosphate linked to another moeity, X, and these enzymes have a characteristic motif, GX5EX7REUXEEXGU (where X is any amino acid and U is I, L, or V) (4). We sought to determine whether ORF2 (renamed nudA) was a L. pneumophila virulence factor and whether it functioned as a Nudix hydrolase.
MATERIALS AND METHODS
Bacteria, plasmids, and growth conditions.
L. pneumophila serogroup1 strain F2310, also known as AA100jm, is a spontaneous streptomycin-resistant mutant of strain 130b (ATCC BAA-74), originally isolated from a patient with combined Legionnaires' disease and pneumococcal and meningococcal pneumonia (21, 43). AA100jm is virulent in guinea pigs, macrophages, and amoebae (21, 45). L. pneumophila bacteria were grown at 35 to 37°C in ambient air on 3-(N-morpholino)-propanesulfonic acid (MOPS)-buffered charcoal yeast extract agar medium supplemented with α-ketoglutarate (BCYEα) (22), in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract broth supplemented with α-ketoglutarate (BYE-α), or to resuscitate frozen bacteria in ACES-buffered charcoal yeast extract broth supplemented with α-ketoglutaric acid (BCYEα broth) (22). BYEα and BCYEα broth-grown bacteria were shaken (150 to 300 rpm) during growth; log-phase broth growth was defined as a bacterial density optical density at 660 nm (OD660) of 0.2, and late-stationary-phase growth was defined as a bacterial density OD660 of >3.0 (measured after 1:10 dilution). Of note, the highly motile coccobacillary form of L. pneumophila is not observed for the late-stationary-phase F2310 strain (unpublished observation). E. coli XL1-Blue (Stratagene) was grown at 37°C either on Luria-Bertani (LB) agar or in LB broth. Selective antimicrobial agents were added to the growth media when appropriate and included kanamycin (Kan), 30 μg/ml; ampicillin, 75 μg/ml; and chloramphenicol, 5 (L. pneumophila) or 30 (E. coli) μg/ml. Plasmid pMMB2002 was a gift from Nicholas Cianciotto (46). Plasmid pUC18 was purchased from Life Technologies, Gaithersburg, MD. A list of bacterial strains and plasmids unique to the present study is shown in Table 1.
TABLE 1.
Unique strains and plasmids
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| Strains | ||
| F2310 | L. pneumophila serogroup 1, strain AA 100jm | 21 |
| F2527 | F2310 ΔnudA::kan (in-frame) | This study |
| F2529 | F2527 containing pMMB2002 | This study |
| F2530 | F2527 containing pBH17 | This study |
| F2531 | F2310 containing pMMB2002 | This study |
| Plasmids | ||
| pMMB2002 | pMMB207 (ΔmobA) | N. Cianciotto (46) |
| pBH3 | pUC18 (nudA+) | This study |
| pBH17 | pMMB2002 (nudA+) | This study |
Nucleic acid manipulation.
All nucleic acid manipulations were accomplished according to standard molecular biology techniques unless otherwise stated (2). PCR was performed by using Taq polymerase (Promega) unless otherwise stated. All reagents used for DNA manipulations were purchased from New England Biolabs unless stated otherwise.
Complete sequencing of nudA gene.
Subcloned DNA from a transposon-mutated and signature-tagged avirulent clone of L. pneumophila, clone mu3h (32) was sequenced in both directions 5′ to the ptsP ORF, by using primer walkout. Whole sequence data were analyzed and aligned by using SeqMan II software, version 5.03 (DNASTAR, Inc., Madison, WI). EditSeq and Protean software (both DNASTAR, version 5.03) were used to translate DNA and to predict protein physical characteristics. GenBank sequence database searching was performed with the BLASTX and BLASTN search algorithms. The TIGR CMR BLAST site was also used for some sequence analyses (http://tigrblast.tigr.org/cmr-BLAST/). Multiple sequence alignments were performed by using Megalign, version 5.03, with the Jotun Hein method (DNASTAR). The Berkeley Drosophila Genome Project promoter prediction program was used to predict promoter sites (http://www.fruitfly.org/seq_tools/promoter.html). Comparison of sequences to published L. pneumophila genome sequences used sequences and search software posted in GenBank, the Pasteur Institute (http://genolist.pasteur.fr/LegioList/), and the Columbia University Genome Center (http://legionella.cu-genome.org/). Detection of Nudix hydrolase orthologs in the published L. pneumophila genomes was performed by searching the PEDANT database (http://pedant.gsf.de/) of each strain with a text search for “MutT.”
Macrophages.
Guinea pig alveolar macrophages were prepared as previously described (21) and cultured in M199 (Invitrogen) supplemented with 10% fetal bovine serum (BioWhittaker, Walkersville, MD).
Guinea pig pneumonia model.
Male 250- to 300-g guinea pigs (Charles River Laboratories) were infected by directly observed injection of mixed F2310 (parent) and F2527 (ΔnudA) L. pneumophila bacteria into the surgically exposed trachea, as detailed previously (18). Three different experiments were conducted, using three different ratios of F2310:2527. The infected animals were euthanized 50 h later by administration of an overdose of intraperitoneal pentobarbital, and the spleens and the right lower lung lobes were removed aseptically. These organs were homogenized, and the bacteria were quantified by duplicate plating of 10-fold dilutions onto Kan-containing BCYEα agar and nonselective BCYEα agar, as described previously (32). Bacterial colonies on the plates were enumerated after 4 to 7 days of incubation at 35°C, depending on colony size. The data from the three different experiments was combined, after normalization of the data, for analysis. The guinea pig experiments were approved by the University of Pennsylvania Institutional Animal Use and Care Committee.
Determination of flagellation.
Plate-grown Legionella bacteria were suspended in sterile distilled water and stained for the presence of flagellae using the Ryu stain (Remel Laboratories, Lenexa, KS) as described previously (19, 38).
Intracellular growth and invasion assays.
Macrophages were prepared as described above and infected with L. pneumophila (multiplicity of infection of 0.1) and then incubated in 5% CO2 in air at 37°C. Culture supernatants were harvested at the indicated times, diluted appropriately with Mueller-Hinton broth, and then plated onto BCYEα agar plates. Bacterial competition studies took advantage of the Kan resistance of L. pneumophila F2527 (ΔnudA) and the Kan susceptibility of its parent. In these competition studies, the concentration of the parent strain was determined by subtracting the total bacterial concentration determined by plating on nonselective BCYEα medium from the bacterial concentration determined by plating on BCYEα medium containing Kan.
The relative abilities of L. pneumophila strains F2310 (parent) and F2527 (ΔnudA) to invade explanted guinea pig alveolar macrophages were determined by using a gentamicin protection assay as previously described (23).
Detection of nudA in other Legionella spp. strains.
The presence of nudA in 20 different strains of L. pneumophila (14 serogroups) was detected by PCR with the nudA primers mu3hU852 (GGCGTTGGCTAAAGTATCGAC) and mu3hL997 (CCGCCAGCTATCGAAT), which gave a 145-bp product. The strains tested included the following ATCC designations:33126, 33152 to 33155, 33215, 33737, 33823, 35096, 35251, 43111, 43130, 43283, 43290, 43703, and 43736. Also tested were L. pneumophila serogroups 1, 2, and 4 (F1381, F1721, and F1948). BCYEα plate-grown bacteria were harvested into 5 mM EDTA to approximate the density of a number 1 McFarland barium sulfate standard. The bacterial suspensions were heated at 99 to 100°C for 20 min. PCR was carried out in a 25-μl volume, using 1 μl of the heated bacterial suspensions as a template.
Southern blotting was used to detect the presence of nudA in 10 different strains of Legionella spp. other than L. pneumophila. A digoxigenin-labeled DNA probe was made from a 343-bp gel-purified EcoRI/EcoRV plasmid restriction fragment internal to nudA, from 23 to 365 bp 3′ to the ORF start. The fragment was labeled as specified by the kit manufacturer (DIG High Prime DNA Labeling and Detection Kit; Roche Applied Science, Indianapolis, IN). Tested were one strain each of L. birminghamensis ATCC 43702), L. dumoffii (ATCC 33343), L. feeleii (ATCC 35072), L. jordanis (ATCC 33623), L. longbeachae serogroup 1 (F850), L. maceachernii (ATCC 35300), L. micdadei (F774), L. quateirensis (ATCC 49507), L. rubrilucens (ATCC 35304), and L. steigerwaltii (ATCC 35302). Genomic DNA from each strain was made by using the CTAB (cetyltrimethylammonium bromide)-NaCl method, and 1 μg of EcoRI-digested DNA was electrophoresed in 0.8% agarose and then transferred to Hybond N+ transfer membranes (Amersham Biosciences). Hybridization was performed at 42°C using the supplied hybridization buffer; the first two washes were carried out at room temperature with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS), and the final two washes were done at 42°C in 0.5× SSC-0.1% SDS. Probe detection was performed according to the kit instructions.
Phenotypic characterization of nudA mutant.
Growth rates of the L. pneumophila bacteria in BYEα broth were determined by monitoring OD660 at the specified times. The starting inoculum for these growth studies was obtained by inoculating BCYEα plates with frozen bacteria and passing the bacteria twice on the plates (35°C). The bacteria were then suspended (Mueller-Hinton broth) to a density equivalent to a 0.5 McFarland barium sulfate standard and inoculated (10 μl) into BYEα broth (10 ml). This broth was then incubated (37°C, 150 rpm) overnight to obtain early log-phase growth, and the bacterial density was adjusted to an OD660 of 0.004; the bacterial suspension was added (10 and 25 μl) to BYEα broths (10 ml). The broths were grown in shaking incubators (25, 37, and 42°C), and the OD was monitored in duplicate over time.
Because salt resistance is a common nonspecific marker of reduced virulence in L. pneumophila (11, 35), we sought to determine whether the nudA mutant was more salt resistant than its parent. Bacterial NaCl resistance was determined by quantitative plating of serial decimal dilutions of late-stationary-phase or early-log-phase broth suspensions of the bacteria onto BYCEα medium made with or without NaCl (100 mM). The broth suspensions were obtained by passing the bacteria twice on BCYEα plates as described above and then inoculating BYEα broths (10 ml) from the plates and growing the bacteria to late stationary phase or early log phase in a shaking incubator.
Colony sizes of bacteria were determined by inoculating BCYEα plates with decimal dilutions of bacterial suspensions made from twice-passed growth on BCYEα plates (35°C). After incubation at 37°C for 4 days, dilution plates with well-isolated colonies were selected for colony size measurement of randomly selected colonies by using a dissecting microscope equipped with a calibrated optical micrometer.
Auxotrophy was characterized by study of growth of L. pneumophila bacteria on a minimal medium as described previously (21) with one exception. The exception was that inoculated minimal medium was incubated for up to 4 weeks to ensure accurate counting of bacterial colonies. Auxotrophs were retested with the same minimal medium supplemented with tryptophan (0.7 to 3.3 mM), ZnCl2 (15 to 74 μM), MgCl2 (150 to 740 μM), or KCl (7.6 to 40 mM) in various combinations; the concentrations of the supplements were based on the differential chemical compositions of Difco yeast extract and the components of the minimal medium (Difco Manual, 11th ed.; Becton Dickinson, Sparks, MD).
Because hypersusceptibility to H2O2 has been described for other Nudix hydrolase mutants, killing of L. pneumophila bacteria by H2O2 (10 mM in sterile distilled water) was tested. This was performed by incubating late-stationary-phase bacteria with the H2O2 for 20 min and then determining viable plate counts of the inoculum and peroxide-exposed bacteria. Late-stationary-phase growth was used for both these studies because pilot studies showed that the log-phase parent was completely killed by peroxide exposure, precluding any comparative studies.
Expression and purification of NudA.
Gene nudA was amplified from pBH3 by standard PCR procedures, subcloned into expression vector pET24a(+) (Novagen, Madison, WI) to form plasmid pETnudA and transformed into the expression host HMS174(DE3) (Novagen). One colony of HMS174(DE3)/pETnudA was inoculated into LB medium (40 ml) containing Kan, incubated at 37°C on a rotary shaker overnight, and inoculated into prewarmed medium (2 liters). When the culture reached an OD600 of ∼0.3, it was transferred to a lower temperature incubator (22°C) and grown to an OD600 of 0.8. IPTG (isopropyl-β-d-thiogalactopyranoside) at 100 mM was added to a final concentration of 0.5 mM, and growth was continued for an additional 12 h. The cells were harvested by centrifugation, washed by suspension in isotonic saline solution, recentrifuged, dispersed in 2.5 volumes of buffer A (50 mM Tris-Cl [pH 7.5], 1 mM EDTA, 0.1 mM dithiothreitol), and disrupted in a French pressure cell at 110 MPa. The insoluble material was removed by centrifugation, and the supernatant containing NudA was adjusted to a protein concentration of 10 mg/ml with buffer A (fraction I). Protein was determined by the method of Bradford (7) with bovine serum albumin as the standard. In the following purification procedure, fractions containing NudA were identified by enzymatic activity, polyacrylamide gel electrophoresis, and spectrophotometry at 260 and 280 nm.
Streptomycin sulfate (10% [wt/vol] in buffer A) was added to fraction I to a final concentration of 1.5%, and the precipitate containing NudA was suspended in buffer A containing 1 M (NH4)2SO4 (fraction II). This was loaded onto a DEAE-Sepharose column, eluted with buffer A, and the fractions containing NudA were pooled. Solid ammonium sulfate was added to a concentration of 70% saturation (0.5g/ml), and the precipitate was dissolved in a minimal volume of buffer A (fraction III), loaded onto a Sephadex G-100 gel-filtration column, and eluted with buffer A containing 200 mM sodium chloride. The fractions containing the purified NudA protein were pooled and dialyzed against buffer A (fraction IV), loaded onto an anion-exchange column (MonoQ; Amersham Pharmacia), and eluted with a gradient of 0.0 to 1.0 M NaCl in buffer A. The fractions containing NudA were pooled (fraction V) and stored at −80°C. This fraction was used for characterization of the enzyme.
Enzyme assays.
Standard reaction mixtures contained (in 50 μl): 50 mM Tris-Cl (pH 8.0), 5 mM Mg2+, 2 mM substrate, 0.1 to 2 mU of NudA, and either 0.5 U of yeast inorganic pyrophosphatase for nucleoside triphosphates and their derivatives or 4 U of alkaline phosphatase for all other substrates. The mixture was incubated at 37°C for 15 min and terminated by adding 250 μl of 4 mM EDTA. The liberated orthophosphate was assayed by the colorimetric procedure of Fiske and SubbaRow (25) as modified by Ames and Dubin (1). One unit of enzyme catalyzes the hydrolysis of 1 μmol of substrate per min under these conditions. The kinetics of Ap5A hydrolysis by NudA were obtained while varying the substrate concentration from 0.05 to 2 mM, and the resulting data were analyzed by using Enzyme Kinetics software (Trinity Software, Ft. Pierce, FL). Organic compounds and enzymes were purchased from Sigma, and inorganic chemicals were analytical grade obtained from common commercial suppliers.
Product determination.
High-pressure liquid chromatography was used to separate the reaction products from the substrates as described previously (15). Briefly, reaction mixtures were increased fivefold, and aliquots were collected at 0, 10, and 40 min. Mixtures were separated by reversed-phase chromatography on a C18 column (YMC, Ltd.), and peaks were identified by comparison to standards (Sigma).
Production of NudA and PtsP antibodies.
Gene ptsP was cloned into pGEX-2T, expressed in E. coli BL21 (vector and host both from Amersham), and purified as previously described (23). Antibodies to the PtsP and NudA expressed proteins were made by rabbit immunization, using a three-injection protocol that included the use of both complete and incomplete Freund adjuvant and the administration of ∼0.3 mg of protein with each injection (Express Line Protocol; Lampire Biological Laboratories, Pipersville, PA). Rabbit serum collected approximately 50 days after the initiating immunization was purified by using a Protein A column (Sigma).
Immunoblot analysis.
To identify expression of NudA and PtsP proteins in F2527 (ΔnudA) and F2310 (parent), immunoblotting was performed as described previously (23). Briefly, plate-grown colonies of bacteria were boiled, lysed in Laemmli sample buffer, and applied to a SDS-polyacrylamide gel electrophoresis gel (12.5 or 15% acrylamide Criterion Precast Gel; Bio-Rad). Separated proteins were transferred electrophoretically to Immobilon-P membranes (Millipore). The membranes were probed with polyclonal rabbit antibodies against NudA (1:100), PtsP (1:100), and donkey horseradish peroxidase-conjugated anti-rabbit secondary antibody (Amersham Biosciences). The proteins were visualized by using chemiluminescence according to the manufacturer's directions (ECL Western Blotting Kit; Amersham Biosciences).
Determination of operon contiguity for nudA and ptsP.
To determine whether nudA and ptsP were on the same operon, the method described by Gay and Stephens was used (27). Briefly, total bacterial RNA was prepared from log-phase growth of L. pneumophila strain F2310. The cDNA was made from the RNA by using primer pTS24U3191 (5′-GGGCATGACGCTTGGCAATTC-3′), which annealed to nudA 441 bp 5′ to its terminus and 442 bp 5′ to the ptsP start site and oriented toward the start of ptsP, using Moloney murine leukemia virus reverse transcriptase and its supplied buffer (Invitrogen). To exclude DNA contamination of the bacterial RNA preparation, a reverse transcription reaction was prepared without adding reverse transcriptase. The cDNA, or total RNA, was then amplified by the PCR using Taq polymerase (Promega) and primers pTS24U3191 and pTS24L4529 (5′-TATAAGCCGCTTCTACCTTGT-3′); pTS24L4529 binds to ptsP 547 bp 3′ of its start. The DNA transcript length was determined by agarose gel electrophoresis.
Interaction between NudA and PtsP.
To determine whether NudA and PtsP interacted, a two-bacteria hybrid system was used (36). This utilized test plasmids pKT25 and pUT18C, positive control plasmids pKT25-zip and pUT18C-zip, and E. coli indicator strains DHM1 and BTH101, all kindly provided by Daniel Ladant. The test genes were cloned in frame into both test plasmids, and the sequence of the insertion junction was determined to confirm that the test genes were inserted correctly. The interaction of PtsP with NudA was determined with ptsP in pKT25 and nudA in pUT18c and also with nudA in pKT25 and ptsP in pUT18c, as previously described (36) and by personal communication (D. Ladant). MacConkey agar containing maltose (1%) was used as the indicator medium, and interactions were tested at both 30 and 37°C.
Cloning and mutation of nudA.
The chromosmal DNA region surrounding nudA was cloned into pUC18 by the use of PCR of L. pneumophila strain F2310 by using Vent DNA polymerase and primers ail-64U (5′-CTCTGGCAATCCTGTTCC-3′) and ail-1583L (5′-CGCCAAGGTAATAAGAAATGA-3′); this yielded pBH3. The sequence of this entire PCR product was confirmed by two-strand sequencing over its entire length. A mutation in nudA was made by using standard methods, which resulted in a truncated nudA, 378 bp in length, containing only the terminal 8 bp of the 69-bp predicted Nudix hydrolase box (4). An in-frame Kan insertion was made in the mutated nudA as previously described, except for the use of EcoRV, rather than SmaI, cloning sites for the Kan insertion (42). The accuracy of the sequence of the 3′ end of the Kan insertion and of most of the mutated nudA ORF 3′ to the Kan insertion was confirmed by single-strand sequencing. The Kan insertion resulted in the termination of the nudA ORF after the first 22 bp of the ORF. The last 356 bp of the interrupted nudA was in the same reading frame as the first 22 bp of nudA, the Kan ORF, and ptsP. The mutated L. pneumophila DNA was cloned into pLAW344 for use in homologous recombination in F2310 (parent), as reported previously (51). The resultant selected clones were shown to contain mutated nudA by PCR and subsequently by immunoblot analysis with NudA antibody (see below). One clone was selected for further studies; this clone was named F2527 (ΔnudA).
A new vector containing just nudA and its putative promoter was constructed to trans-complement nudA by PCR amplification, using PfuTurbo (Stratagene),of plasmid DNA using primers ail3U (5′-AATGGATCCGAGCTCTTGTACTTTGTGGA-3′) and ail3L (5′-AATACTGCAGCCGTTTAAGTATTTTAAGCAT-3′) (the engineered BamHI and PstI sites are underlined). The complementation vector pBH17, made from pMMB2002, included all of the nudA ORF, the putative nudA promoter in 61 bp 5′ to the ORF, and 22 bp 3′ to nudA; the complementation sequence was confirmed by two-strand analysis.
Similar methods were used to mutate orf1 by insertion of a polar Kan gene from pKD368 (21) in a pUC18-based plasmid and homologous recombination of the mutated gene into F2310 (parent), yielding F2482. The presence of the mutated orf1 in strain F2482 was confirmed by methods identical to those used for the nudA mutation in F2310.
Statistical analyses and graphics.
Parametric data were analyzed by the Student t test or one-way analysis of variance (ANOVA), depending on the number of comparisons; Tukey's post hoc comparisons were conducted if ANOVA indicated significant between-group differences. Nonparametric analyses were performed by the method stated in Results. Two-tailed comparisons were conducted. A P value of ≤0.05 was considered significant. Competition data were plotted and analyzed after normalization of the data, so that experiments using different parent/mutant starting ratios could be directly compared; this was done by dividing the actual parent/mutant ratios for each experimental point by the starting parent/mutant ratio. Statistical analyses were performed by using GraphPad InStat (version 3.06) or GraphPad Prism (version 4.02; San Diego, Calif). Graphic presentation of the quantitative data was prepared by using GraphPad Prism.
Nucleotide sequence accession number.
The nudA sequence has been deposited in the GenBank database at the National Center for Biotechnology Information under accession number AY521221.
RESULTS
Sequence and genetic organization of nudA and the adjacent region.
The region surrounding ptsP was sequenced on both strands 753 bp 5′ and 664 bp 3′; an additional 149 bp of single-strand sequence was obtained in the 3′ region, making a total of 813 bp of single and double-strand sequence 3′ to ptsP. A 528-bp ORF was 5′ to ptsP, and in the same reading frame; initially termed ORF2, this was finally named nudA. The termination of nudA was immediately upstream of the ATG start of ptsP, with no intervening bases. A predicted promoter (ACTTTGTGGAAAAATCAAGTTATTAACGGTTTATTCTAAGCAGTGACATCATG) (score 1.0) was identified starting 50 bp 5′ of the nudA start site, with a predicted transcriptional start (underlined) 10 bp 5′ to the ATG start (italic). A second ORF (orf1) was identified 3′ of ptsP, 807 bp in length; orf1 was in a different reading frame and began 1 bp 5′ to the TGA termination of ptsP. A promoter was predicted to begin 91 bp 5′ of the start of orf1, with a transcriptional start 50 bp upstream of the start site (score 0.99). A schematic of the genetic organization of this region is shown in Fig. 1.
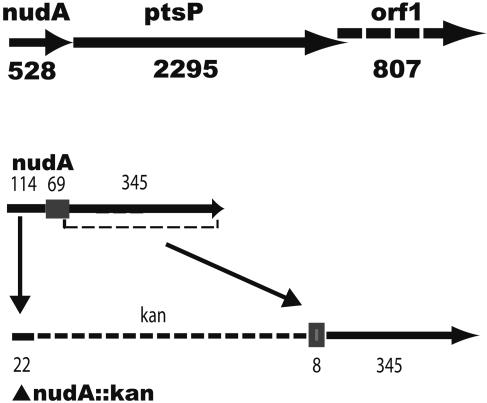
FIG. 1.
Schematic drawings of the genetic organization of nudA (top) and of the nudA mutation used to study its function (bottom). Both drawings are drawn to scale. The top drawing shows the relationship of nudA to ptsP and orf1. Genes nudA and ptsP are in the same reading frame, which is different from the reading frame of orf1. The bottom drawing shows the mutation and insertion used to make the in-frame mutation nudA; the gray box (69 bp in length) indicates the region coding for the active Nudix site (Nudix box). The mutation was made by deleting 143 bp of nudA, including most of the Nudix box, and inserting an in-frame Kan cassette. The numbers above or below the drawings indicate the size in base pairs.
The GC content of nudA was 43.4%, compared to a total genome GC content of 38% (12, 13). For comparison, the GC contents of ptsP and orf1 were 42.8 and 38.5%, respectively. NudA was predicted to have a mass of 20,577 Da and an isoelectric point of 9.8 for this 174-amino-acid protein. Immunoblot analysis of purified expressed NudA and NudA present in boiled bacterial cells showed both bands to have an approximate mass of 20,600 Da.
Sequence similarity searching for the nudA sequence of the GenBank database, using the blastx program, revealed that nudA was homologous to a large number of Nudix hydrolases, with the closest matches being Pseudomonas putida or P. fluorescens putative (di)nucleoside polyphosphate hydrolase (60% identities [89 of 147], 77% positives [114 of 147], expect 7e − 49), P. syringae MutT (59% identities [87 of 147], 77% positives [114 of 147], 4e − 48), and Vibrio parahaemolyticus MutT (49% identities [85 of 173], 67% positives [116 of 173], 8e − 46). Other matches of note included P. aeruginosa YgdP (8e − 46), V. cholerae MutT (2e−45), Coxiella burnetii MutT-nudix family (5e − 40), Rickettsia conorii InvA (3e − 21), and R. prowazekii InvA (5e − 20).
The predicted sequence of NudA contained a 23-amino-acid sequence, GGLAPGETAMQAMYRELHEEVGL, which conforms to the conserved motif of the putative Nudix active site, GX5EX7REUXEEXGU (where X is any amino acid and U is I, L, or V) (4). The Nudix conserved domain was found in the predicted sequence of NudA when it was searched in GenBank using a conserved domain search, with high scoring matches for the pfam00293, COG0494, COG1051, and COG2816 conserved domains with respective scores and E values of 69.1, 3e-13; 52.7, 2e-8; 45.8, 3e-6; and 39.9, 2e-4. These conserved domains correspond to Nudix, MutT, ADP-ribose pyrophosphatase, and NPY1 domains, respectively.
Text searches of the PEDANT database for three published L. pneumophila genomes showed that the Philadelphia 1, Paris, and Lens strains contained five, six, and five different Nudix homologs, respectively (http://pedant.gsf.de). All of these contained a stereotypical Nudix motif, had widely varying ionization constants ranging from 5.2 to 9.7, and widely varying GC contents. One Nudix hydrolase from each strain was nearly identical to the nudA of strain F2310. No genome appeared to have more than one copy of each different Nudix hydrolase.
There was only a one-base difference between the nudA sequence of strain F2310 (parent) and that of Philadelphia 1, Paris, and Lens. These differences were at position 138 (G to A) for Philadelphia 1, 181 (T to C) for Paris, and 238 (A to G) for Lens, all of which resulted in silent mutations, with 100% identity of the predicted protein sequences (12, 13). The genetic organization of nudA, ptsP, and orf1 was identical in F2310, Philadelphia 1, Paris, and Lens (12, 13).
Examination of the genetic organization of nudA and ptsP homologs in other bacteria was determined by using the TIGR CMR BLAST search and region view module. This database search showed that homologs of nudA immediately preceded ptsP homologs in P. aeruginosa PA01, Y. pestis C092, E. coli MG1655 and EDL933, C. burnetii RSA493, and V. cholerae N16961. However, a nudA homolog was not found to be adjacent to a ptsP homolog in Rickettsia conorii Malish7, Salmonella enterica serovar Typhimurium SGSC1412, Haemophilus influenzae KW20, or Neisseria meningitidis 2492. A smaller survey of the relation of orf1 to ptsP found the same organization in V. vulnificus CMCP6 and V. cholerae N16961 as was seen in F2310 but not in P. aeruginosa PA01, E. coli MG1655, Y. pestis C092, P. multocida PM70, H. influenzae KW20, C. burnetii RSA493, or N. meningitidis MC58.
Multiple homologs of ORF1 were also found in the GenBank or TIGR databases, all of which were predicted or hypothetical permeases, with the closest matches being from Desulfovibrio desulfuricans (28% identities [64 of 222], 50% positives [111 of 222], 2e−25) and Geobacter metallireducens (28% identities [62 of 218], 50% positives [109 of 218], 7e−23) but also including P. aeruginosa (2e−19), V. cholerae (2e−19), and P. multocida (3e−17). All three sequenced L. pneumophila strains, Philadelphia 1, Paris, and Lens, had 98 to 99% nucleotide identity to orf1 over the entire 807-bp region.
Presence of nudA in other Legionella strains.
The presence of nudA in other L. pneumophila strains, including strains from serogroups 1 to 14, was studied by PCR. A PCR product of the correct size was found in 19 of 20 strains tested. The one negative strain was one of two serogroup 5 strains tested. Southern blotting for nudA gave positive bands in one strain each of L. dumoffii, L. jordanis, L. longbeachae serogroup 1, L. micdadei, and L. quateirensis; no bands were detected in single strains of L. birminghamensis, L. feeleii, L. maceachernii, L. rubrilucens, or L. steigerwaltii. All of the positive Southern blots for the non-L.pneumophila species revealed bands that were different from one another by at least several thousand base pairs and from the reference band of strain F2310 (parent).
Determination of nudA operon.
The reverse transcriptase reaction product of total bacterial RNA, using a primer binding to nudA, and directed 3′, was ∼1,000 bp in length, as measured by gel electrophoresis, and the same size as the PCR product of this reaction when bacterial DNA was used as the template. If ptsP is transcribed on an operon independent of nudA, no PCR product would be expected, since the PCR primers used included one that bound to nudA and one to ptsP. The negative control, F2310 (parent) total RNA used in the reverse transcriptase reaction without reverse transcriptase, had no product in the PCR, indicating the lack of contamination by bacterial DNA. These results indicate that nudA and ptsP are cotranscribed.
Interaction between NudA and PtsP.
To determine whether the adjacent and highly conserved relationship of nudA to ptsP on the same operon had a correlate with interactions with their respective encoded proteins, the physical interactions of these two proteins were studied using two bacterial hybrid interaction studies. These studies showed no interaction of NudA and PtsP, regardless of which expression vector was used to express either protein, at both 30 and 37°C, using host strain DHM1. Positive control plasmids pKT25-zip and pUT18c-zip showed interaction, as expected, and a variety of negative controls showed no interaction, all with host strain DHM1. Use of host strain BTH101 gave unreliable results with the control plasmids and no evidence of interaction of NudA and PtsP.
NudA growth-phase dependence.
Immunoblot analysis of F2310 (parent) fractions reacted with NudA antibody showed that NudA production appeared to be independent of bacterial growth phase, since approximately equal amounts of NudA were observed in immunoblots of log-phase and late-stationary-phase cultures.
Expression and purification of NudA.
NudA expressed well from pETnudA in HMS174(DE3), and the purification procedure yielded approximately 10 mg, with an estimated purity by gel electrophoresis of >95% (data not shown). It is noteworthy that NudA precipitated with the nucleic acid fraction upon addition of streptomycin at the first step in the purification scheme.
Substrate specificity of NudA.
The Nudix hydrolase superfamily of enzymes, catalyzing the hydrolysis of a variety of nucleoside diphosphate derivatives, may be divided into subfamilies depending on their preferred substrates. Since the “Nudix box” containing the amino acid signature sequence characteristic of the superfamily is highly conserved among all its members, the specificities for different substrates must be determined in regions of the protein outside of the Nudix motif. By comparing amino acid sequences of different members of the superfamily with the same or similar specificities, certain landmark amino acids outside of the Nudix box have been identified that are predictive of likely substrates for newly discovered proteins (7). The following is part of the sequence of NudA containing amino acids 30 to 80 (from the N terminus): 30AWQFPQGGLAPGETAMQAMYRELHEEVGLDKGDVEILGSTRRWLKYRLPK80. The amino acids indicated in boldface italics represent the highly conserved Nudix signature sequence. Seventeen amino acids downstream of this Nudix box is a tyrosine (boldface, underlined) that is predictive of a subfamily of Nudix hydrolases active on diadenosine polyphosphates (17). Accordingly, we tested a number of these compounds with purified NudA and found that Ap5A was the preferred substrate. In Table 2, the relative rates of hydrolysis of the related compounds from Ap3A to Ap6A are compared. A chain length of five phosphates appears to be optimal with Ap6A hydrolyzed at 60% of the rate of Ap5A. The tetra- and tripolyphosphate derivatives were practically not hydrolyzed. Also listed in the table are known substrates of other Nudix hydrolases. The inactivity toward these compounds indicates that NudA is specific for the higher diadenosine polyphosphates and may therefore be considered a member of the diadenosine polyphosphate subfamily.
TABLE 2.
NudA substrate specificity
| Substrate | Amt hydrolyzed (nmol) | Relative activity (%) |
|---|---|---|
| Ap5A | 6.1 | 100 |
| Ap6A | 3.7 | 60 |
| Ap4A | 0.1 | 2 |
| Ap3A | <0.05 | |
| Othersa | <0.05 |
ADP-ribose, GDP-mannose, UDP-mannose, FAD, NADH, (d)ATP, (d)CTP, (d)GTP, and (d)TTP.
Products of the reaction.
The course of hydrolysis of Ap5A, analyzed by high-pressure liquid chromatography at various time points revealed a decrease in Ap5A and a commensurate increase in ATP and ADP. No other peaks appeared, and no Pi was formed during the incubation. Thus, the hydrolysis of Ap5A may be described by the following equation: 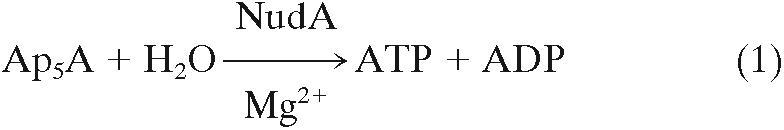
Other properties of the enzyme.
As with all of the orthologous invasion-associated Nudix hydrolases studied thus far (5, 10, 15, 28), NudA had an alkaline pH optimum. The maximum rate of Ap5A hydrolysis was observed at pH 8.0, with activities falling off to 35 and 50% at pH 7 and 9.5, respectively. A divalent cation was absolutely required and was best satisfied by Mg2+. Mn2+ was ca. 15% as effective, whereas Zn2+ and Ca2+ were inactive. Analysis of the kinetic parameters of the hydrolysis of Ap5A by NudA showed the following: Vmax, 4.3 ± 0.3 U/mg; Kcat, 1.49 s−1; Km, 0.3 ± 0.06 mM; and Kcat/Km, 4.96 × 103 s−1 M−1.
Production of NudA and PtsP in the nudA mutant and its complement.
Immunoblot analysis of the boiled nudA mutant (F2527) with either NudA or PtsP antibody showed that the mutant produced no detectable NudA but that PtsP was present in normal amounts and of expected size. A transposon-insertion mutant of ptsP, F2345 (32), originating from parent strain F2310, produced normal amounts of NudA and no detectable PtsP. In contrast, the parent strain (F2310) was shown to contain both NudA and PtsP under the same conditions. In addition, the transcomplemented nudA mutant (F2530) was shown to contain NudA, although the density of the NudA band was slightly less than that of the parent strain when equivalent amounts of total cells were used in the immunoblot study (data not shown). These data showed that the nudA mutant was defective in the production of NudA and that it had no polar effect on the production of PtsP. Also, the normal production of NudA by the ptsP mutant demonstrated that ptsP has no role in the regulation of nudA.
Phenotype of nudA mutant and effect of transcomplementation.
L. pneumophila strain F2527 (ΔnudA) grew more slowly than did F2310 (parent) on both solid media and in broth and with a smaller colony size (Fig. 2). When both the parent and mutant were inoculated in equal amounts and grown (37°C) in the same tube of BYEα broth, the parent outcompeted the mutant 4-, 6-, and 9-fold after 18, 22, and 25 h, respectively. The mutant took about 1 day longer to grow on solid media than did F2310. F2527 was 400 to 580 times more salt tolerant than its parent. For F2310, only 0.02 and 0.005% of bacteria grown to log phase and stationary phase, respectively, grew on salt supplemented BCYEα medium compared to growth on non-salt-containing medium. In contrast, 9.6 and 2.9% of log-phase and stationary-phase F2527 bacteria grew on the same salt-supplemented medium. Like the parent, F2527 possessed one to two polar flagellae.
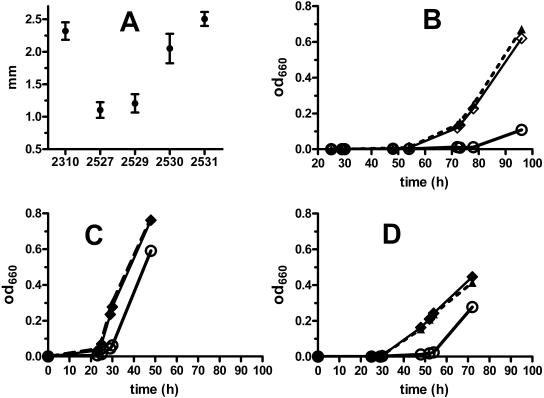
FIG. 2.
Colony diameters (A) and growth rates in broth (B to D) of L. pneumophila F2310 (parent) and various nudA mutants. (A) Colony diameters (±95% confidence interval) of L. pneumophila strains grown on BCYEα medium (37°C for 4 days; n = 28 for each datum point). P < 0.0001 for group comparison (one-way ANOVA); P < 0.001 for F2310 versus F2527 (ΔnudA) or F2529 (ΔnudA + pMMB2002), P < 0.001 for F2529 versus F2530 (ΔnudA + nudA in pMMB2002) or F2531 (parent + pMMB2002), and P < 0.001 for F2530 versus F2531; all other comparisons were not significant. (B) Growth of F2529, F2530, or F2531 in BYEα broth at 25°C as measured by spectroscopy in duplicate. The growth curves of F2310 and F2527 were indistinguishable from those of F2531 and F2529, respectively, and are not shown for graphical clarity. Symbols: ○, F2529; ▴ (dotted line), F2530; ⋄, F2531. (C and D) Same as panel B, including symbols, but at 37 and 42°C, respectively. A second study of broth growth gave results nearly identical to those shown in panels B to D.
There was no clear susceptibility or resistance of F2527 to H2O2 compared to that of F2310 in the two experiments performed (data not shown).
The nudA mutant was an auxotroph in that only 2 × 10−4 % of its inoculum grew on minimal medium, in contrast to ca. 40% of the parental inoculum. Prototrophy was not restored to the auxotrophic nudA mutant bacteria by the supplementation of the minimal medium by tryptophan or the mineral salts tested.
Transcomplementation of the nudA mutant (F2530) completely restored the growth rate of the bacterium in broth at 25, 37, and 42°C (Fig. 2B to D). The colony size of the complemented mutant was also almost completely restored to that of the parent (Fig. 2A).
Salt resistance of the nudA mutant was lost with transcomplementation with nudA. In a comparison of log phase grown bacteria, an average of 0.6 ± 0.2% (standard error of the mean, n = 4, with pooled data from two independent experiments) of the nudA mutant bacteria complemented with an empty plasmid (F2529) were salt resistant. Transcomplementation of the mutant with nudA (F2530) resulted in 0.07 ± 0.03% (n = 4, pooled data) salt-resistant bacteria versus 0.02 ± 0.007% (n = 4, pooled data) salt resistance of the parent with an empty plasmid (F2531). ANOVA showed a significant between-group salt resistance difference (P = 0.01), and post hoc analysis showed no significant difference between the salt resistance of F2530 and F2531, whereas the salt resistance of F2529 was significantly greater than that of F2530 or F2531 (P < 0.05).
Transcomplementation with nudA of the nudA mutant almost completely restored prototrophy. Only 5 × 10−5 % of an inoculum of F2529 (ΔnudA + pMMB2002) grew on minimal medium. In contrast, 0.5 and 21% of the inocula of F2530 (ΔnudA + nudA in pMMB2002) and F2531 (parent + pMMB2002), respectively, grew on the same media. Transcomplementation thus increased the prototrophy of the mutant by ca. 10,000-fold.
Virulence of nudA mutant in guinea pig alveolar macrophages and guinea pigs.
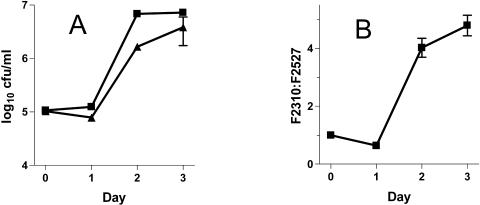
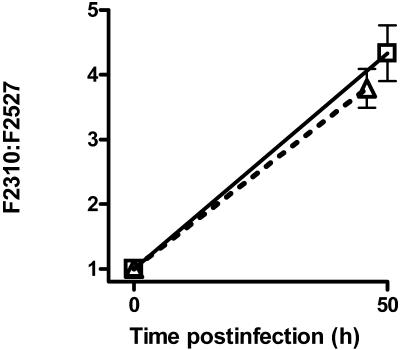
The nudA mutant (F2527) grew slightly less well in guinea pig alveolar macrophages than did its parent (F2310) (Fig. 3A). Only on day 2 postinfection was there a significant difference between the mean bacterial concentrations, with the ratio of F2310 to F2527 on that day being equal to 4.1 (P = 0.001 [nonpaired t test]). To help determine whether F2527 was truly less virulent than F2310 in macrophages, bacterial competition studies were undertaken; this method can demonstrate significant L. pneumophila virulence defects when single bacterial studies show none (23).When F2310 and F2527 were mixed together in similar amounts before infecting macrophages, the parent outcompeted the mutant by 4.0-fold and 4.8-fold after 2 and 3 days, respectively (P < 0.001 for day 0 or 1 versus day 2 or 3 [unpaired t tests with Welch's correction for unequal variances]) (Fig. 3B). The parent bacterium also outcompeted the nudA mutant when guinea pigs were infected with a mixture of both strains by intratracheal inoculation. At 50 h after infection F2310 outcompeted F2527 by 4.3- and 3.8-fold in the lung and spleen, respectively (Fig. 4).
FIG. 3.
Growth of F2527 (ΔnudA) and F2310 (parent) in guinea pig alveolar macrophages. (A) Comparison of growth of F2310 and F2527 when each was independently used to infect macrophages. Error bars show the standard error of the mean (n = 3). Two other independent experiments gave the same results. Symbols:▪, F2310; ▴, F2527. (B) Competition between F2310 and F2527, when the bacteria were mixed together before infection of the macrophages; data are normalized and pooled from three independent experiments and are presented as the mean ratios of F2310 to F2527. Error bars show standard error of the mean (n = 9). Three other independent experiments gave the same results.
FIG. 4.
Multiplication of a mixture of F2310 (parent) and F2527 (ΔnudA) in guinea pigs infected by the intratracheal route. The datum points represent mean values of the ratio of F2310 to F2527; error bars represent the standard errors of the mean of three independent experiments (total n = 17 animals). The 2310/2527 ratios at 50 h postinfection for both lung and spleen differ significantly from 1.0 (ratio at time zero) by one-sample t tests (P < 0.0001). Symbols: □, lung; ▵ (dotted line), spleen.
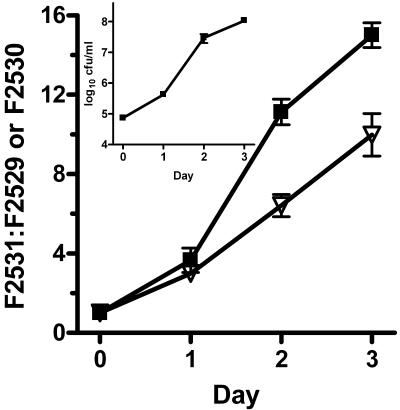
Transcomplementation of the nudA mutant partially restored its decreased virulence for guinea pig alveolar macrophages (Fig. 5). In competition studies with the parent, the transcomplemented mutant was significantly more virulent than the mutant containing an empty plasmid (P ≤ 0.002 [nonpaired t test]) but was still significantly outcompeted by the parent (P = 0.002 [one-sample t test with comparison with hypothetical mean F2531/F2530 ratio of 1.0]).
FIG. 5.
Effect of nudA transcomplementation on growth within guinea pig alveolar macrophages. Bacterial strains F2531 (parent + pMMB2002), and F2529 (ΔnudA + pMMB2002) or F2530 (ΔnudA + nudA in pMMB2002) were mixed together before the bacteria were added to the macrophages. The data were pooled from two independent experiments, were normalized, and are represented as the mean ratios of either F2531 to F2529 or F2531 to F2530. Error bars represent standard error of the mean (n = 6). P ≤ 0.002 for F2531/F2530 versus F2531/F2529 ratios on days 2 and 3 (P = 0.3 for day 1, all by nonpaired t tests). The inset shows the growth of F2531 alone in macrophages. Note that a lower ratio indicates greater virulence of the strain in reference to that of F2531 and that a ratio of 1 would mean virulence equivalent to F2531. One other set of experiments gave similar results. Symbols: ▪, F2531:F2529; ▿, F2531:F2530.
A polar mutation in orf1 had no effect on the ability of the mutant L. pneumophila strain to grow in guinea pig alveolar macrophages. The growth curves of the parent strain and the orf1 mutant strain in the macrophages were identical (data not shown).
Gentamicin protection assays showed that F2527 (nudA mutant) was not less invasive of guinea pig alveolar macrophages than was its parent (F2310). The percentage of extracellular bacteria that were internalized after a two hour incubation was 0.017% (n = 5, 95% confidence interval = 0.012 to 0.022%) and 0.014% (n = 6, 95% confidence interval = 0.010 to 0.019%) for the mutant and parent, respectively.
DISCUSSION
Nudix hydrolases are a family of ubiquitous enzymes found in viruses, bacteria, and eukaryotes, including humans, with pleiotropic functions and multiple substrate specificities (4, 41). Although originally described solely as a mutation prevention mechanism, as MutT in E. coli, it is now apparent that Nudix hydrolases have different functions in multiple hosts, including bacterial pathogenesis (3, 5, 15, 44), mRNA decapping in mammals and vertebrates (29, 50), and viral pathogenesis (10, 49). Most bacteria studied contain one or more different Nudix hydrolases, unrelated to genome size (52). For example, E. coli K-12 contains 12, Neisseria meningitidis contains 1 to 2, Mycobacterium tuberculosis H37RV contains 7, Bacillus anthracis contains 30, and Mycoplasma pneumoniae M129 contains no Nudix hydrolases (52). Two bacteria, Bartonella bacilliformis, and E. coli K1, have been shown to have Nudix hydrolases, ialA and ygdP, respectively, that contribute to erythrocyte and human brain microvascular endothelial cell invasion, respectively (3, 5, 15, 44). In addition, Rickettsia prowazekii has been shown to have a Nudix hydrolase invA homolog that is of unknown function but could be a virulence gene for this organism (28).
Our studies show that L. pneumophila nudA is a Nudix hydrolase that is a virulence factor for intracellular and guinea pig infection. A polar effect of the nudA mutation on a cotranscribed gene that would account for either the virulence defect or stress-related defects is very unlikely. The mutation insertion was by design nonpolar, and functional evidence for readthrough of the mutation into the downstream ptsP gene was shown by the expression of normal amounts of PtsP in the nudA mutant. In addition, transcomplementation of the nudA mutant restored significant, albeit not complete, virulence for macrophage multiplication. We attribute incomplete complementation to the complexity of the mixed infection assay and to the reduced expression of NudA in the transcomplemented mutant, as shown by immunoblot. It is probable that sufficient levels of NudA were produced by the transcomplemented strain to reverse the mutation-associated changes in metabolism and growth but that they were insufficient for complete restoration of virulence.
The markedly reduced ability of F2527(ΔnudA) to grow at 25°C is similar to the reduced ability of E. coli ΔygdP to grow at 18°C (31). Nudix hydrolase substrates such as Ap4A are hypothesized to be “alarmones” that are synthesized in high concentrations in the presence of heat shock or oxidative stress (6, 34, 37, 39). In E. coli these alarmones bind to heat shock and oxidative stress proteins (26, 34), and it is thought that alarmone synthesis may modulate the heat stress response (34). E. coli apaH is a non-Nudix dinucleoside hydrolase that normally hydrolyzes Ap4A to ADP and Ap5A to ADP + ATP (24, 33). E coli apaH mutants accumulate Ap4A, have increased susceptibility to heat and UV light, are unable to grow at 43°C, and are nonmotile; the mechanism of these phenotypes is thought to be due to the modulation of RpoF by excess Ap4A and to alteration in cyclic AMP and by binding to heat shock and oxidative stress proteins (24, 34). Helicobacter pylori nudA, which preferentially hydrolyzes Ap4A, is important for survival to oxidative stress caused by H2O2 but not heat shock (40). We show that the L. pneumophila nudA mutation is responsible for delayed growth at 25, 37, and 42°C, reduced colony size, auxotrophy, and salt resistance but not increased susceptibility to H2O2 or decreased motility as has been reported for other Nudix hydrolase mutants. Salt resistance has not been previously studied in Nudix hydrolase mutants, and its presence in the L. pneumophila nudA mutant is difficult to explain in terms of the expected increased susceptibility to stress observed in Nudix hydrolase mutants of other bacteria (4). It is noteworthy that salt resistance can be a nonspecific marker of reduced virulence of L. pneumophila by unknown mechanisms (11, 35).
Another alarmone, ppGpp, has been shown to trigger the entry of the Lp02 strain of L. pneumophila into the late stationary phase under limited nutrient conditions via a RpoS-dependent mechanism and to enhance the virulence of the bacterium for macrophages (30). Late-stationary-phase growth of this strain also greatly enhances NaCl sensitivity and heat resistance (8). The L. pneumophila nudA mutant is reduced in virulence and is salt resistant and heat sensitive, which superficially suggests that nudA could play a role in ppGpp modulation. However, ppGpp is not a substrate of NudA, NudA expression is independent of bacterial growth phase, and the virulence defect in the nudA mutant is much less severe than that seen in log phase Lp02 bacteria in comparison to late-stationary-phase bacteria. It is more likely that the growth-phase-related characteristics of Lp02 and the effect of NudA on F2310 share similar phenotypes but not mechanisms. A caveat, however, is that L. pneumophila strain F2310 (parent) is unlike strain Lp02 in several ways, including its overall greater virulence and the lack of virulence enhancement in the postexponential phase (48).
The enzymatic characteristics of L. pneumophila NudA closely resemble the homologous Nudix hydrolase invasion-associated proteins InvA from R. prowazekii (9) and YgdP from E. coli (2). IalA, the homologue from B. bacilliformis, also belongs in this group but prefers Ap4A instead of Ap5A (9, 16).
Our studies do not support a role for L. pneumophila NudA as an invasin, based on the similar invasion abilities of the nudA mutant and its parent, as well as on the lack of differences between these strains for macrophage multiplication on day 1 postinfection. This is in contrast to the invasin-like nature of the E. coli K-12 and B. bacilliformis nudA orthologs (3, 44).
Our findings on nudA can probably be generalized to other L. pneumophila strains based on the highly conserved NudA sequence in the three L. pneumophila strains that have been completely sequenced and on the results presented by us on the presence of nudA in 95% of L. pneumophila strains tested by us. The nudA gene also appears to be common in other Legionella spp. based on the identification of nudA-homologous sequences in 5 of 10 other Legionella spp. tested by Southern hybridization. It is possible, but unproven, that nudA is a virulence factor for these other species.
The genetic organization of nudA in relation to ptsP was conserved in the four L. pneumophila strains of known sequence and a number of other bacteria. The highly conserved nature of this genetic organization and cotranscription in the F2310 (parent) L. pneumophila strain suggests some interaction between the two genes. In addition, the GC contents of both genes are significantly higher than that of the total genome and different from the immediate downstream gene, orf1, suggesting that both nudA and ptsP originated from the same non-L. pneumophila organism. We were unable to find evidence of protein-protein interaction with a bacterial two-hybrid system. In addition, mutations in each gene had no apparent effect on expression of the other. These results do not exclude trans interactions between nudA and ptsP, especially ones related to production of energetic phosphate compounds by NudA hydrolysis of Ap5A or degradation of NpnN by the enzyme. In fact, Ismail et al. speculate that in Salmonella enterica serovar Typhimurium, NudA hydrolysis of NpnN has a role in the regulation of PtsP by binding to the regulatory domain of PtsP (33).
The mechanism by which NudA exerts its pleiotropic phenotype in L. pneumophila is unknown. One of us has speculated that Nudix hydrolases perform a “housecleaning” role by hydrolyzing potentially toxic nucleotides and by preventing the unbalanced accumulation of normal metabolites (4). In the case of L. pneumophila, NudA appears to prevent inimical responses to external stress. The presence of this enzyme confers a growth advantage in macrophages and therefore appears to have a role in both extracellular and intracellular survival of the bacterium. Whether the slow-growth of the nudA mutant in macrophages is solely related to its slower growth in vitro, or to factors exclusive to the intracellular environment is unknown but possible. The intracellular environment of L. pneumophila is complex and dynamic, making extrapolation of extracellular growth characteristics to intracellular growth characteristics tenuous. Based on the profound effect of a nudA mutation on 25°C growth, it is reasonable to hypothesize that NudA plays a role in the survival of the intra-amebal bacterium at the same or lower temperature in the environment. Future studies are needed to determine the role of NudA in the environmental persistence of L. pneumophila, its interactions with other bacterial genes, and its effect on bacterial persistence and relapse in mammalians infected with L. pneumophila.
Acknowledgments
Edward Schenck and Elizabeth McAvoy provided excellent technical assistance. Nicholas Cianciotto and Daniel Ladant kindly donated plasmids and host strains as specified in the text.
Part of this study was supported by U.S. Public Health Service grant GM18649 from the National Institute of General Medical Sciences to M.J.B.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ames, B. N., and D. T. Dubin. 1960. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J. Biol. Chem. 235:769-775. [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Badger, J. L., C. A. Wass, and K. S. Kim. 2000. Identification of Escherichia coli K1 genes contributing to human brain microvascular endothelial cell invasion by differential fluorescence induction. Mol. Microbiol. 36:174-182. [DOI] [PubMed] [Google Scholar]
- 4.Bessman, M. J., D. N. Frick, and S. F. O'Handley. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 271:25059-25062. [DOI] [PubMed] [Google Scholar]
- 5.Bessman, M. J., J. D. Walsh, C. A. Dunn, J. Swaminathan, J. E. Weldon, and J. Shen. 2001. The gene ygdP, associated with the invasiveness of Escherichia coli K1, designates a Nudix hydrolase, Orf176, active on adenosine (5′)-pentaphospho-(5′)-adenosine (Ap5A). J. Biol. Chem. 276:37834-37838. [DOI] [PubMed] [Google Scholar]
- 6.Bochner, B. R., P. C. Lee, S. W. Wilson, C. W. Cutler, and B. N. Ames. 1984. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell 37:225-232. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartwright, J. L., P. Britton, M. F. Minnick, and A. G. McLennan. 1999. The IalA invasion gene of Bartonella bacilliformis encodes a (de)nucleoside polyphosphate hydrolase of the MutT motif family and has homologs in other invasive bacteria. Biochem. Biophys. Res. Commun. 256:474-479. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright, J. L., S. T. Safrany, L. K. Dixon, E. Darzynkiewicz, J. Stepinski, R. Burke, and A. G. McLennan. 2002. The g5R (D250) gene of African swine fever virus encodes a Nudix hydrolase that preferentially degrades diphosphoinositol polyphosphates. J. Virol. 76:1415-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catrenich, C. E., and W. Johnson. 1989. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect. Immun. 57:1862-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 13.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 14.Cianciotto, N. P. 2001. Pathogenicity of Legionella pneumophila. Int. J. Med. Microbiol. 291:331-343. [DOI] [PubMed] [Google Scholar]
- 15.Conyers, G. B., and M. J. Bessman. 1999. The gene, ialA, associated with the invasion of human erythrocytes by Bartonella bacilliformis, designates a Nudix hydrolase active on dinucleoside 5′-polyphosphates. J. Biol. Chem. 274:1203-1206. [DOI] [PubMed] [Google Scholar]
- 16.Conyers, G. B., G. Wu, M. J. Bessman, and A. S. Mildvan. 2000. Metal requirements of a diadenosine pyrophosphatase from Bartonella bacilliformis: magnetic resonance and kinetic studies of the role of Mn2+. Biochemistry 39:2347-2354. [DOI] [PubMed] [Google Scholar]
- 17.Dunn, C. A., S. F. O'Handley, D. N. Frick, and M. J. Bessman. 1999. Studies on the ADP-ribose pyrophosphatase subfamily of the Nudix hydrolases and tentative identification of trgB, a gene associated with tellurite resistance. J. Biol. Chem. 274:32318-32324. [DOI] [PubMed] [Google Scholar]
- 18.Edelstein, P. H. 1999. The guinea pig model of Legionnaires' disease, p. 303-314. In O. Zak and M. A. Sande (ed.), Handbook of animal models of infection. Academic Press, Ltd., London, England.
- 19.Edelstein, P. H. 1985. Legionnaires' disease laboratory manual. National Technical Information Service, Springfield, Va.
- 20.Edelstein, P. H., and N. P. Cianciotto. 2001. Legionella species and Legionnaires' disease. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York, N.Y. [Online.]
- 21.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelstein, P. H., and M. A. C. Edelstein. 1993. Comparison of three buffers used in the formulation of buffered charcoal yeast extract medium. J. Clin. Microbiol. 31:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelstein, P. H., B. Hu, F. Higa, and M. A. Edelstein. 2003. lvgA, a novel Legionella pneumophila virulence factor. Infect. Immun. 71:2394-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr, S. B., D. N. Arnosti, M. J. Chamberlin, and B. N. Ames. 1989. An apaH mutation causes AppppA to accumulate and affects motility and catabolite repression in Escherichia coli. Proc. Natl. Acad. Sci. USA 86:5010-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiske, C. H., and Y. SubbaRow. 1925. The colorimetric determination of phosphorous. J. Biol. Chem. 66:375-400. [Google Scholar]
- 26.Fuge, E. K., and S. B. Farr. 1993. AppppA-binding protein E89 is the Escherichia coli heat shock protein ClpB. J. Bacteriol. 175:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay, K., and D. S. Stephens. 2001. Structure and dissemination of a chromosomal insertion element encoding macrolide efflux in Streptococcus pneumoniae. J. Infect. Dis. 184:56-65. [DOI] [PubMed] [Google Scholar]
- 28.Gaywee, J., W. Xu, S. Radulovic, M. J. Bessman, and A. F. Azad. 2002. The Rickettsia prowazekii invasion gene homolog (invA) encodes a Nudix hydrolase active on adenosine (5′)-pentaphospho-(5′)-adenosine. Mol. Cell Proteomics 1:179-185. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh, T., B. Peterson, N. Tomasevic, and B. A. Peculis. 2004. Xenopus U8 snoRNA binding protein is a conserved nuclear decapping enzyme. Mol. Cell 13:817-828. [DOI] [PubMed] [Google Scholar]
- 30.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 31.Hand, N. J., and T. J. Silhavy. 2003. Null mutations in a Nudix gene, ygdP, implicate an alarmone response in a novel suppression of hybrid jamming. J. Bacteriol. 185:6530-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higa, F., and P. H. Edelstein. 2001. Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect. Immun. 69:4782-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ismail, T. M., C. A. Hart, and A. G. McLennan. 2003. Regulation of dinucleoside polyphosphate pools by the YgdP and ApaH hydrolases is essential for the ability of Salmonella enterica serovar Typhimurium to invade cultured mammalian cells. J. Biol. Chem. 278:32602-32607. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone, D. B., and S. B. Farr. 1991. AppppA binds to several proteins in Escherichia coli, including the heat shock and oxidative stress proteins DnaK, GroEL, E89, C45, and C40. EMBO J. 10:3897-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi, A. D., and M. S. Swanson. 1999. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect. Immun. 67:4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 95:5752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisselev, L. L., J. Justesen, A. D. Wolfson, and L. Y. Frolova. 1998. Diadenosine oligophosphates (Ap(n)A), a novel class of signalling molecules? FEBS Lett. 427:157-163. [DOI] [PubMed] [Google Scholar]
- 38.Kodaka, H., A. Y. Armfield, G. L. Lombard, and V. R. Dowell, Jr. 1982. Practical procedure for demonstrating bacterial flagella. J. Clin. Microbiol. 16:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, P. C., B. R. Bochner, and B. N. Ames. 1983. Diadenosine 5′,5"-P1,P4-tetraphosphate and related adenylylated nucleotides in Salmonella typhimurium. J. Biol. Chem. 258:6827-6834. [PubMed] [Google Scholar]
- 40.Lundin, A., C. Nilsson, M. Gerhard, D. I. Andersson, M. Krabbe, and L. Engstrand. 2003. The NudA protein in the gastric pathogen Helicobacter pylori is an ubiquitous and constitutively expressed dinucleoside polyphosphate hydrolase. J. Biol. Chem. 278:12574-12578. [DOI] [PubMed] [Google Scholar]
- 41.McLennan, A. G. 1999. The MutT motif family of nucleotide phosphohydrolases in man and human pathogens. Int. J. Mol. Med. 4:79-89. [DOI] [PubMed] [Google Scholar]
- 42.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer, R. D., P. H. Edelstein, B. D. Kirby, M. H. Louie, M. E. Mulligan, A. A. Morgenstein, and S. M. Finegold. 1980. Legionnaires' disease: unusual clinical and laboratory features. Ann. Intern. Med. 93:240-243. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell, S. J., and M. F. Minnick. 1995. Characterization of a two-gene locus from Bartonella bacilliformis associated with the ability to invade human erythrocytes. Infect. Immun. 63:1552-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffat, J. F., P. H. Edelstein, D. P. Regula, Jr., J. D. Cirillo, and L. S. Tompkins. 1994. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol. Microbiol. 12:693-705. [DOI] [PubMed] [Google Scholar]
- 46.Rossier, O., S. R. Starkenburg, and N. P. Cianciotto. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72:310-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy, C. R., and R. R. Isberg. 1997. Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect. Immun. 65:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samrakandi, M. M., S. L. Cirillo, D. A. Ridenour, L. E. Bermudez, and J. D. Cirillo. 2002. Genetic and phenotypic differences between Legionella pneumophila strains. J. Clin. Microbiol. 40:1352-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shors, T., J. G. Keck, and B. Moss. 1999. Down regulation of gene expression by the vaccinia virus D10 protein. J. Virol. 73:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, Z., X. Jiao, A. Carr-Schmid, and M. Kiledjian. 2002. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc. Natl. Acad. Sci. USA 99:12663-12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiater, L. A., A. B. Sadosky, and H. A. Shuman. 1994. Mutagenesis of Legionella pneumophila using Tn903 dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11:641-653. [DOI] [PubMed] [Google Scholar]
- 52.Xu, W., C. A. Dunn, C. R. Jones, G. D'Souza, and M. J. Bessman. 2004. The 26 Nudix hydrolases of Bacillus cereus, a close relative of Bacillus anthracis. J. Biol. Chem. 279:24861-24865. [DOI] [PubMed] [Google Scholar]