Abstract
Chlamydia pneumoniae is a common respiratory tract pathogen, and persistent infections have been associated with atherosclerosis. We studied the effects of repeated chlamydial inoculations on the inflammatory response and on aortic lipid accumulation in C57BL/6J mice. Mice fed a diet supplemented with 0.2% cholesterol were infected three or six times with C. pneumoniae every fourth week. Sera and lungs were analyzed for inflammatory responses, lung tissues were tested for the presence of C. pneumoniae DNA and RNA, and intimal lipid accumulation in the aortic sinus was quantified. High levels of chlamydial heat shock protein 60 (Hsp60) immunoglobulin G2c subclass antibodies were detected in all of the infected mice, and a positive and statistically significant correlation was found between these antibodies and autoantibodies against mouse Hsp60. Both Hsp60 antibody levels correlated with the severity of lung tissue inflammation. The cholesterol supplement in the diet had no effect on serum cholesterol levels. Significantly larger intimal lipid lesions were seen in the mouse group infected six times (6,542 μm2) than in the control group (1,376 μm2; P = 0.034). In conclusion, repeated inoculations increased aortic sinus lipid accumulation in normocholesterolemic mice. The correlation between the antibodies to mouse and chlamydial Hsp60 proteins and their association with lung inflammation further support the theory of the development of an autoimmune response against heat shock proteins after repeated chlamydial infections.
Chronic sequelae and persistence of the pathogen are well-known phenomena in diseases caused by chlamydia, an obligate intracellular, gram-negative bacterium. Chlamydia trachomatis serovars cause trachoma (i.e., chronic infection and scarring of the eye lids), which is the most common cause of preventable blindness in the world (29), and sexually transmitted diseases connected with pelvic inflammatory disease and tubal factor infertility in women (41). Chlamydia pneumoniae generally causes both upper- and lower-respiratory-tract infections, and persistence of this pathogen has been reported, sometimes despite appropriate treatment (32). An aberrant but viable and persistent form of chlamydia differs from infectious extracellular elementary bodies (EBs) and dividing intracellular reticulate bodies and is induced in vitro by gamma interferon, β-lactam antibiotics, and amino acid starvation (3). Interestingly, tobacco smoke has also been shown to cause the formation of this aberrant chlamydia form in vitro (45). It is not clear yet whether this form of experimental persistence really occurs in vivo in clinical diseases. Restricted growth and a decrease in the development of infectious EB particles have been detected ex vivo in infected human mononuclear cells (2), suggesting that the persistent stage of the bacteria is present in these cells.
Chronic C. pneumoniae infection has been associated with the development of atherosclerosis in several studies (23), and besides chlamydia, other microbes and the pathogen burden have been suggested to be etiological factors as well (46). The role of C. pneumoniae in atherosclerotic diseases is supported by several lines of evidence. C. pneumoniae is able to infect several cell types involved in atherosclerosis in vitro and to induce the production of cytokines and growth factors in these cells (5). Also, the presence of chlamydial particles in atherosclerotic lesions has been demonstrated by isolation, electron microscopy, PCR, and immunohistochemistry (5). It has been proposed that heat shock proteins (Hsp), especially Hsp60 expressed and secreted by pathogens, participate in atherosclerotic development via molecular mimicry and an autoimmune response against self Hsps (26, 44). In agreement with this, serological studies have shown that antibodies to human Hsp60 and chlamydial Hsp60 (cHsp60) are risk factors for atherosclerosis (10), colocalization of cHsp60 with human Hsp60 has been detected in atherosclerotic plaque macrophages (24), and in mice C. pneumoniae inoculations led to the development of mouse Hsp60 (mHsp60) autoantibodies (15, 16). In vivo, intranasal inoculation with C. pneumoniae accelerates atherosclerotic development in chow-fed and cholesterol-fed rabbits (17, 25). Wild-type mice are normocholesterolemic, and most lipids are carried by high-density lipoprotein (12). In these animals, atherosclerotic lesions do not develop spontaneously. In C57BL/6J mice fed a regular diet, chlamydial inoculations have been shown to cause inflammatory changes but not to affect aortic lipid accumulation (8, 33). Susceptibility to intimal lipid accumulation in the aortic sinus of C57BL/6J mice is achieved when a lipid- and cholesterol-rich diet including cholic acid is given to mice (35). An atherogenic effect of C. pneumoniae (21, 33), but not of C. trachomatis (9), has indeed been found in both diet-induced and genetically induced hypercholesterolemic mice.
In our previous study, three C. pneumoniae inoculations were given to increase aortic lipid accumulation in normocholesterolemic C57BL/6J mice fed a diet supplemented with a small amount of cholesterol (15). In the present work, we studied the effect of additional C. pneumoniae inoculations on the development of atherosclerotic changes in the aortic sinus and on the inflammatory response in C57B/6J mice fed a similar cholesterol-enriched diet. In addition, the influence of age at inoculation on the development of chronic, persistent chlamydial infection and on aortic sinus lipid accumulation was tested.
MATERIALS AND METHODS
C. pneumoniae strain and inoculum.
C. pneumoniae isolate Kajaani 7 (K7), which was used in this study, was produced as described previously (43). The purified stock stored in sucrose-phosphate-glutamic acid buffer was found to be free of mycoplasmas by the PCR method, and the number of viable organisms, expressed in inclusion-forming units/ml, was determined by culture in HL cells. The inoculum dose was estimated on this basis, and the infectious dose given to the animals was confirmed by culture in HL cells.
Animal model and samples.
Inbred C57BL/6J female mice purchased from Harlan Netherlands at the age of 6 weeks were randomly divided into five study groups, and 8 to 13 mice were used for each time. The study groups and the numbers of mice at sacrifice when the samples were taken are shown in Table 1. Intranasal inoculations with 2 × 106 inclusion-forming units of C. pneumoniae K7/mouse were given under inhaled methoxyflurane (Metofane; Schering-Plough, United States) anesthesia. To test the effect of several inoculations, the mice in group I were given a total of six inoculations at 4-week intervals. The first inoculation was given at 8 weeks of age, and the last inoculation was given at 28 weeks of age. Group II mice were given three inoculations at ages of 8, 12, and 16 weeks. In order to test the influence of age, mice in groups III and IV received three inoculations at ages of 20, 24, and 28 weeks. With the exception of group IV, the mice were given a slightly cholesterol-enhanced diet (normal chow with 0.2% cholesterol supplement) when they arrived at the facilities. For group IV, cholesterol-supplemented feeding was started at the age of 18 weeks, 2 weeks before the first inoculation. Groups of mice were sacrificed, and all samples from each study group, which included blood samples obtained by heart puncture for serology and lipid analyses, lung samples for PCR, reverse transcription-PCR (RT-PCR), and histopathology, and heart samples for assessment of aortic sinus lipid analysis, were taken from all groups both at 2 weeks after the last inoculation (i.e., at the age of 18 weeks for group II and at the age of 30 weeks for groups I, III, and IV) and again at 32 weeks. In addition, 10 mice from group II were also studied 2 weeks after the primary inoculation, at the age of 10 weeks. At each time, samples from 8 or 10 SPG mock-inoculated mice (control group V) were used as controls. All procedures involving animals were approved by the Animal Care and Use Committee of National Public Health Institute, Helsinki, Finland.
TABLE 1.
Study groups
| Group | No. of inoculationsa | 0.2% cholesterol dietb | No. of mice per group at sacrifice
|
|||
|---|---|---|---|---|---|---|
| 10 wk | 18 wk | 30 wk | 32 wk | |||
| I | 6 (8 to 28 wk) | At 6 wk | 8 | 7 | ||
| II | 3 (8 to 16 wk) | At 6 wk | 10 | 9 | 10 | |
| III | 3 (20 to 28 wk) | At 6 wk | 10 | 13 | ||
| IV | 3 (20 to 28 wk) | At 18 wk | 10 | 10 | ||
| V | 6 (SPG) | At 6 wk | 8 | 8 | 8 | 10 |
Three or six inoculations were given once every fourth week starting at the age of either 8 or 20 weeks.
Age at which cholesterol feeding was started.
Antibody measurements.
Mice were sacrificed with CO2, blood was immediately collected by heart puncture, and serum was separated by centrifugation after clotting at room temperature for 30 min. C. pneumoniae total immunoglobulin G (IgG) antibodies were measured with the microimmunofluorescence (MIF) test, using purified, formalin-fixed whole EBs of K7 as the antigen. IgG antibodies were detected using fluorescein isothiocyanate-conjugated anti-mouse IgG (Serotec). In addition, total C. pneumoniae IgG and IgG1 and IgG2c subclass-specific antibodies were measured using EB-coated enzyme immunoassay (EIA) plates (Coated Microstrips; AniLabsystems, Helsinki, Finland). The sample dilutions were 1:100 for total IgG and 1:50 for IgG subtypes. Samples were incubated for 90 min at 37°C, and bound antibodies were detected by alkaline phosphatase-conjugated goat anti-mouse IgG diluted 1:3,000 (Sigma) and goat anti-mouse IgG1 and IgG2c diluted 1:4,000 (Southern Biotech) with phosphatase substrate (Sigma). Mouse Hsp60 antibodies were measured by the EIA method using 96-well plates coated overnight at 37°C with 5 μg/ml recombinant mHsp60 protein (SPP-741; StressGen Biotechnologies Corp.). Serum samples were diluted 1:20 and incubated on the plates for 2 h. Alkaline phosphatase-conjugated anti-mouse IgG and phosphatase substrate, as described above, were used to detect the specific antibodies. C. pneumoniae Hsp60 IgG1 and IgG2c subclass antibodies were measured by the same method. Plates were coated with cHsp60 protein produced at the National Public Health Institute, Helsinki, Finland (1), and 1:40 dilutions from the serum samples were used. For the final results, the photometer absorbance values from each EIA analysis were multiplied by the sample dilution to obtain the EIA units.
Total cholesterol and triglycerides.
Serum total cholesterol levels were measured by enzymatic methods using the Roche Cholesterol CHOD-PAP reagent (kit no. 1489232; Roche, Germany), and triglyceride levels were measured using the Roche GPO-PAP reagent (kit no. 1488872; Roche, Germany) according to the manufacturer's instructions.
Culture of lung tissue.
The right lung was mechanically homogenized in 2 ml of SPG for culture of C. pneumoniae. The remaining lung tissue debris that was separated by centrifugation of the homogenate was stored for DNA detection. The culture of C. pneumoniae from lung tissue was grown as described in detail previously (16). Pathfinder (Sanofi Diagnostics Pasteur) Chlamydia genus-specific monoclonal antibody conjugated to fluorescein isothiocyanate was used to detect the chlamydial inclusions in HL cells.
DNA and RNA detection.
Fifty milligrams of lung tissue debris separated from the lung homogenate was lysed with proteinase K in tissue lysis buffer after homogenization using a FP 120 FastPrep cell disruptor (Savant Instruments, Inc.). After incubation at 56°C overnight, DNA was purified using a commercially available QIAamp tissue kit according to the manufacturer's instructions, excluding the lysis step. The purified DNA was kept frozen at −20°C. A nested PCR assay combining conventional and real-time methods was used to detect the presence of C. pneumoniae DNA in samples. Primers were ordered from TibMolbiol (Germany), and the sequences are shown in the supplemental data online. The first round of nested PCR was conventional, using the outer primers pstfor and pstrev, which amplify a 192-bp product of the C. pneumoniae Pst1 fragment. To prevent carryover contamination, heat-labile uracil-DNA glycosylase enzyme was added, and dTTP was replaced by dUTP in the first step of our PCR. The second round of PCR was performed with a LightCycler real-time-PCR instrument (Roche Applied Science). The real-time PCR was adapted from the PCR described by Ciervo et al. (13). In this protocol, inner primers ln-1 and ln-2 (originally obtained from Maass et al. [28]) amplify a 128-bp product of the Pst1 fragment. A detailed description of the PCR method is given in the supplemental data online.
An approximately 10-mg piece of lung tissue from the right lung of each mouse at 32 weeks of age was placed in RNA stabilization solution (RNAlater; QIAGEN) and stored frozen at −20°C for RNA analysis. RNA was extracted using a High Pure RNA tissue kit (Roche). Proteinase K incubation at 56°C for 1 h, which was not included in the kit procedure, was done after homogenization of the tissue, and the extracted RNA was eluted two times with 50 μl of elution buffer. Otherwise, extraction was done as instructed by the manufacturer, and the procedure included DNase incubation to disrupt DNA. PCR primers for nested analysis of the C. pneumoniae 60-kDa heat shock protein GroEL gene were used. Primers were ordered from TibMolbiol (Germany), and the sequences are shown in the supplemental data online. To obtain cDNA, total RNA was transcribed using the Transcriptor reverse transcriptase (Roche) and a specific GroEl-2 reverse primer. The first-round PCR of the nested method was done with conventional PCR, after which all products were diluted 1:100 and amplified with the LightCycler together with C. pneumoniae standards. A detailed description of the RT-PCR method is given in the supplemental data online. The criteria used for a positive result were as follows: (i) a twofold increase in the SYBR Green fluorescence compared with the background fluorescence level; (ii) the crossing point cycle (i.e., the cycle at which fluorescence started to increase from the baseline) appeared earlier than the crossing point of the lowest standard for the LightCycler PCR; and (iii) the melting point from the melting curve analysis was within the range detected for the standards and control (from 81.5°C to 81.9°C)
Histopathology of the lung.
The left lung was removed and fixed in 10% buffered formalin. Formalin-fixed lung specimens were embedded in paraffin, and 4-μm sections were cut and stained with hematoxylin and eosin. Inflammation in the hematoxylin- and eosin-stained sections was evaluated to determine the severity of bronchointerstitial pneumonia on a scale from 0 to 4. In grade 0 sections, no inflammatory reaction was visible. In grade 1 sections, perivascular and peribronchial lymphocyte and plasma cell infiltration with occasional neutrophilic and eosinophilic granulocytes were mild, while in grade 2 sections these effects were moderate, in grade 3 sections they were marked, and in grade 4 sections they were severe. Grade 4 also included patchy consolidation of lung tissue with alveolar macrophages and plasma cells.
Quantitative analysis of lipid accumulation in the aortic sinus.
Lipid lesions in 7 to 13 mice that were 32 weeks old were analyzed in each study group. After the lungs had been dissected, the heart and the adjoining aorta were perfused with 1 ml of phosphate-buffered saline, removed, and fixed in 10% buffered formalin. The upper half of the formalin-fixed heart was embedded in gelatin and frozen. Cross-sections (thickness, 5 μm) were collected from the area of the aortic sinus, where the valve cusps and valves were starting to form until these structures were clearly present and stained with Oil-Red-O. The method used for collecting samples and sections has been described by Paigen et al. (36). Every other section cut by the cryostat microtome was placed on a slide, which gave an average of 24 to 30 sections per mouse, and every third collected section was analyzed. For these sections, the lipid accumulation area from six consecutive sections per mouse from the same area was quantified with computer-assisted image analysis as described previously (15). In some mice, very large lesion stained areas were detected in two or three of the six sections analyzed but in none of the rest of the sections, whereas in some animals, more moderate-sized lesions were present throughout the sections studied. The sum of the lesion areas from six sections was determined for each mouse, and the values are presented below.
Statistics.
The nonparametric Mann-Whitney U test was used for statistical analyses of the lipid accumulation areas and antibody levels for the study groups. Spearman's correlation was used to evaluate the correlation between the different antibodies (SPSS for Windows 10.0.5; SPSS Inc., Chicago, Ill.). The lung histopathology findings were compared to the antibody levels using the nonparametric test for trend across ordered groups (Stata 5.0; Stata Corporation, Inc., College Station, Tex.). Exact P values are reported below as appropriate.
RESULTS
Serum total cholesterol and triglyceride levels.
Total cholesterol and triglyceride levels were not affected by the chlamydial inoculations or 0.2% cholesterol supplement in the diet, as shown in Table 2. In our previous studies on C57BL/6J mice fed either a high-fat diet (21% fat, 0.15% cholesterol, 19.5% casein) or a normal chow diet, the total cholesterol levels measured by the same method when mice were between 8 and 16 weeks of age were around 4 mmol/liter for the high-fat-diet groups and 1.9 mmol/liter for the normal-diet groups (unpublished data).
TABLE 2.
Averages and standard deviations of body weights and total cholesterol and triglyceride levels of the mice for the study groups
| Age of mice (wk) | Group | Body wt (g) (mean ± SD) | Total cholesterol (mmol/liter) (mean ± SD) | Triglycerides (mmol/liter) (mean ± SD) |
|---|---|---|---|---|
| 10a | II | 20.4 ± 0.7 | 2.35 ± 0.53 | 0.63 ± 0.26 |
| V | 21.0 ± 1.3 | 2.04 ± 0.24 | 0.63 ± 0.09 | |
| 18b | II | 21.8 ± 1.4 | 1.80 ± 0.19 | 0.47 ± 0.10 |
| V | 21.9 ± 0.8 | 1.74 ± 0.37 | 0.56 ± 0.03 | |
| 30b | I | 23.0 ± 1.1 | 1.86 ± 0.20 | 0.58 ± 0.18 |
| III | 23.9 ± 1.8 | 1.75 ± 0.14 | 0.59 ± 0.07 | |
| IV | 23.5 ± 1.2 | 1.78 ± 0.20 | 0.65 ± 0.11 | |
| V | 24.1 ± 2.2 | 1.88 ± 0.14 | 0.57 ± 0.06 | |
| 32 | I | 24.9 ± 1.3 | 1.69 ± 0.17 | 0.60 ± 0.07 |
| II | 24.6 ± 2.4 | 1.95 ± 0.41 | 0.56 ± 0.09 | |
| III | 24.7 ± 2.1 | 2.01 ± 0.34 | 0.63 ± 0.18 | |
| IV | 24.5 ± 1.5 | 2.04 ± 0.23 | 0.60 ± 0.09 | |
| V | 24.8 ± 2.2 | 1.89 ± 0.20 | 0.56 ± 0.07 |
Two weeks after the primary inoculation.
Two weeks after the third inoculation.
C. pneumoniae and Hsp60 antibodies.
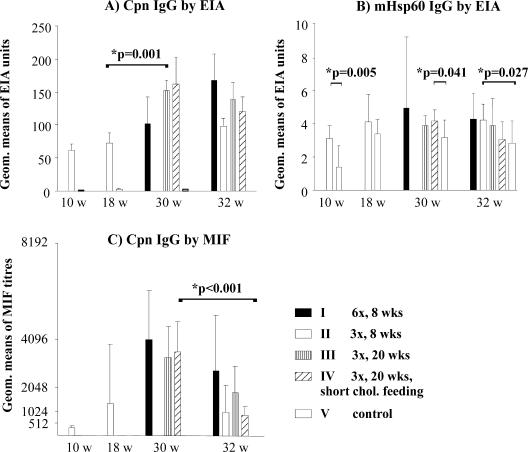
The total C. pneumoniae IgG antibodies measured by MIF and EIA are shown in Fig. 1 to compare the methods. Significant decreases in the C. pneumoniae IgG antibody titers between 30 and 32 weeks were detected for the MIF titers (group III, P = 0.061; group IV, P < 0.001) (Fig. 1C), whereas the antibody levels measured by EIA seemed to be more stable. Both methods detected the increase in antibody levels 2 weeks after the primary inoculation (at 10 weeks of age), and no C. pneumoniae antibodies were detected in SPG mock-inoculated mice. Regardless of the time of cholesterol supplement addition to the diet, the C. pneumoniae IgG antibody levels were equally high as determined by the two methods 2 weeks after the third inoculation for the groups inoculated at 20, 24, and 28 weeks of age (groups III and IV). However, the infection age affected the antibody levels. At an age of 18 weeks, 2 weeks after the third inoculation, the titers in group II mice inoculated at 8, 12, and 16 weeks were clearly lower than the titers in group III and IV mice at an age of 30 weeks (as described above), and the difference was significant when it was measured by the EIA method (for group II versus groups III and IV, P = 0.001) (Fig. 1A).
FIG. 1.
Total C. pneumoniae IgG antibody levels measured by the MIF and EIA methods and levels of mouse Hsp60 IgG antibodies measured by EIA. The study group, the number of inoculations given (e.g., six inoculations [6x]), and the age at which the first inoculation was given (e.g., 8 weeks) are indicated. Cpn, C. pneumoniae; chol., cholesterol; w, weeks; Geom., geometric.
Levels of mouse Hsp60 IgG antibodies measured by the EIA are shown in Fig. 1B. Although the measured mHsp60 IgG antibody levels were low (the absorbance values at the later times for the infected groups generally varied between 0.15 and 0.3), statistically significant differences between the groups could still be detected. An increase in the mHsp60 IgG levels was seen in the uninfected control mice between 10 and 18 weeks of age, but C. pneumoniae inoculations clearly further raised the levels. A statistically significant increase compared to the uninfected control group (group V) was observed for group II after primary inoculation at 10 weeks (P = 0.005), for group IV (inoculated three times at older ages) at 30 weeks (P = 0.041), and for group II (inoculated three times at younger ages) at 32 weeks (P = 0.027) (Fig. 1B).
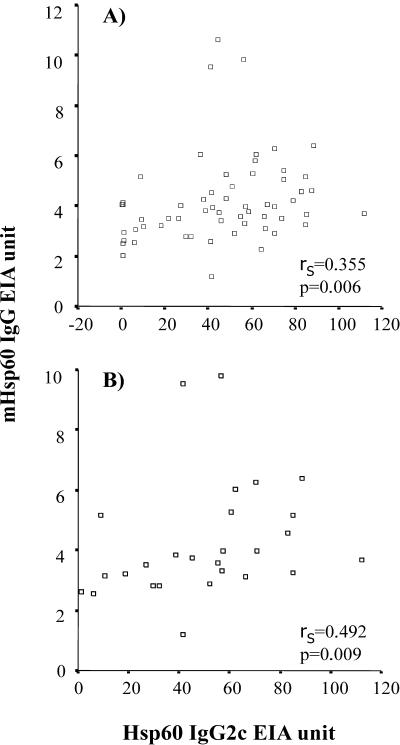
IgG1 and IgG2c subclass antibody levels in serum for whole chlamydial EB particles and for chlamydial Hsp60 protein were measured to roughly evaluate the type of immunological response in the mice. As Table 3 shows, the IgG2c levels in each of the infected groups were clearly higher than the IgG1 levels, especially in the case of the cHsp60 antibodies. No significant differences in any of the subclass levels were detected between the study groups. Since hardly any IgG1-type antibodies were detected, further analyses were done with IgG2c subclass antibodies. A positive correlation (rS = 0.355, P = 0.006) was seen between the levels of IgG2c antibodies to cHsp60 and mHsp60 when all the infected groups from each time were combined (n = 59) and tested for Spearman's correlation (Fig. 2A). In mice at the age of 32 weeks (n = 27) this correlation was more prominent (rS = 0.492, P = 0.009) (Fig. 2B).
TABLE 3.
EIA units of IgG subtypes
| Age of mice (wk) | Group | EIA units
|
|||
|---|---|---|---|---|---|
| cHsp60 IgG1 | cHsp60 IgG2c | C. pneumoniae IgG1 | C. pneumoniae IgG2c | ||
| 10a | II | 1.7 (1.4-2.0)c | 1.1 (0.6-2.1) | 0.4 (0.3-0.6) | 2.8 (2.2-3.6) |
| V | 1.4 (1.1-1.8) | 0.6 (0.3-0.9) | 0.0 (0.0-0.3) | 0.0 (0.0-0.0) | |
| 18b | II | 1.8 (1.2-2.5) | 37.2 (17.5-79.0) | 0.7 (0.3-1.6) | 8.1 (6.0-10.9) |
| V | 1.4 (1.1-1.7) | 0.7 (0.6-0.8) | 0.0 (0.0-0.3) | 0.2 (0.1-0.4) | |
| 30b | I | 2.4 (1.7-3.5) | 67.6 (52.0-87.8) | 0.8 (0.3-2.1) | 7.0 (5.0-9.9) |
| III | 1.9 (1.2-2.9) | 53.4 (36.5-78.2) | 0.4 (0.2-0.8) | 5.5 (4.3-7.2) | |
| IV | 3.0 (0.9-9.5) | 48.4 (33.3-70.3) | 0.5 (0.3-0.8) | 4.9 (3.7-6.3) | |
| V | 1.4 (1.0-2.2) | 1.0 (0.7-1.3) | 0.0 (0.0-0.2) | 0.4 (0.3-0.5) | |
| 32 | I | 4.3 (1.9-9.8) | 51.9 (21.6-124.4) | 0.8 (0.4-1.5) | 6.6 (5.0-8.6) |
| II | 1.8 (1.5-2.2) | 42.1 (19.7-90.2) | 0.8 (0.5-1.2) | 6.4 (4.7-8.3) | |
| III | 2.1 (1.2-3.8) | 33.2 (19.3-57.0) | 0.7 (0.4-1.4) | 4.1 (3.5-4.8) | |
| IV | 1.9 (1.3-2.7) | 27.7 (7.1-107.7) | 1.0 (0.6-1.7) | 4.3 (3.5-5.4) | |
| V | 1.1 (0.8-1.5) | 0.7 (0.4-1.1) | 0.0 (0.0-0.2) | 0.2 (0.1-0.9) | |
Two weeks after the primary inoculation;
Two weeks after the third inoculation.
The values are geometric means. The values in parentheses are 95% confidence intervals.
FIG. 2.
Spearman's correlation between chlamydial Hsp60 IgG2c and mouse Hsp60 IgG levels in all mice (A) and in mice at 32 weeks of age (B).
Chlamydia culture.
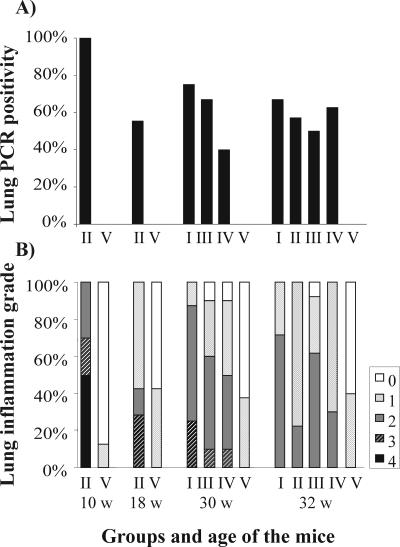
Every mouse lung was culture positive 2 weeks after the primary inoculation; the inclusion counts for group II mice which were inoculated at a young age were very high, whereas for the same group at 18 weeks (i.e., 2 weeks after the third inoculation) only 1 of 10 mice was culture positive. At 30 and 32 weeks of age, no culture-positive samples were detected in any of the study groups.
DNA and RNA detection.
The percentages of lung tissue samples that were positive for chlamydial DNA in the study groups are shown in Fig. 3A. Samples from all mice in group II taken 2 weeks after the primary inoculation were positive as determined by PCR. Fifty-five percent of the mice inoculated at 8, 12, and 16 weeks of age were PCR positive 2 weeks after the third inoculation, although the isolation of chlamydia from lung tissue was successful in only one case at this time. At the ages of 30 and 32 weeks, 40% to 75% of the mice in the infected groups were still PCR positive, and the positivity rate was 57% for group II mice that were inoculated three times at younger ages.
FIG. 3.
(A) C. pneumoniae DNA positivity in lung tissue samples measured by nested PCR. (B) Lung inflammatory reactions measuring perivascular and peribronchial mononuclear cell infiltrates on a scale from 0 (no inflammation) to 4 (severe infiltration with occasional alveolar macrophages). Group I, six inoculations, starting at the age of 8 weeks; group II, three inoculations, starting at the age of 8 weeks; group III, three inoculations, starting at the age of 20 weeks; group IV, three inoculations, starting at the age of 20 weeks, and cholesterol feeding started at 18 weeks; group V, uninfected control. w, weeks.
None of the mouse lungs at 32 weeks of age were positive for chlamydial RNA as determined by the method used in this study. To confirm this, all samples from the conventional PCR run were diluted 1:10 and analyzed again with the LightCycler with the inner primers. One positive control sample collected after the acute infection was highly positive in all analyses.
Histopathology of lungs.
The percentages of mice with different grades of lung inflammation in the study groups are shown in Fig. 3B. The most severe inflammation (grade 4) was only detected 2 weeks after the primary inoculation at 10 weeks of age for group II mice, and 2 weeks after the third inoculation, the histopathological changes were already milder. Similarly, at 30 weeks of age for the other infected groups, no severe inflammation was seen in the mice 2 weeks after the last inoculation. However, moderate inflammation persisted in 10% to 70% of the infected mice at 32 weeks of age, and this finding suggests that an inflammatory agent was present in the lung tissue since moderate (grade 2) inflammation was not detected in the uninfected mice. In the uninfected control groups, only mild inflammation (grade 1), possibly due to the SPG mock inoculation, was seen in less than 50% of the mice at each time.
To analyze the possible connection between the inflammatory reaction in lung tissue and the antibody findings, we tested the histopathological grades against mHsp60 IgG, cHsp60 IgG2c, chlamydial IgG2c, and chlamydial total IgG antibody levels measured by both MIF and EIA. A significant trend was found when the histopathology grades were compared to the mHsp60 IgG and cHsp60 IgG2c antibody levels in the sera taken 2 weeks after the last inoculation, but not at 32 weeks; higher antibody levels were associated with more severe inflammation. Medians and interquartile ranges for mHsp60 IgG and cHsp60 IgG2c for the different histopathology grades are shown in Table 4.
TABLE 4.
Medians and interquartile ranges for mHsp60 IgG and cHsp60 IgG2c levels in mice with different lung inflammation grades
| IgG | Inflammation grade in histopathology | Median of EIA (interquartile range) | P valuea |
|---|---|---|---|
| mHsp60 IgG | 1 | 3.64 (3.43-4.80) | |
| 2 | 4.17 (3.50-4.76) | ||
| 3 | 4.61 (4.41-5.02) | 0.01 | |
| cHsp60 IgG2c | 1 | 45.76 (41.21-65.22) | |
| 2 | 49.68 (37.92-67.52) | ||
| 3 | 79.32 (77.20-83.56) | 0.02 |
Test for trend across ordered groups.
Quantitative analysis of lipid accumulation in the aortic sinus.
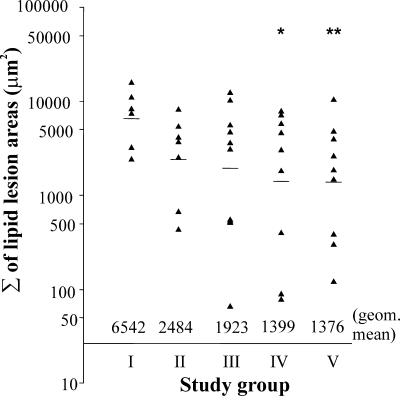
Lipid lesion areas from six Oil-Red-O-stained sections, as described in Materials and Methods, were quantified for each mouse. The stained area of a single lesion in the cross-sections varied from 0 to 6,288 μm2, and the sum of lesion areas from the six sections for each mouse are shown in Fig. 4. The largest stained areas of the lesions in the aortic sinus were usually present in the area where the valve cusps and valves just started to appear. In the case of samples from a few mice, cutting of the sinus sections was started too late, and the area of interest was not on the sections collected. These mice were excluded from the final analysis. As shown in Fig. 4, six repeated C. pneumoniae inoculations of group I mice increased the sum of the lesion areas significantly compared to the uninfected mice. Relatively large lesions were detected in the cross-sections collected for individual mice in every study group, including the uninfected controls, but there were no mice with small lesion areas in group I. The difference between group I mice inoculated six times (geometric mean, 6,542 μm2) and the uninfected control mice in group V (1,376 μm2) was statistically significant (P = 0.034). The lipid lesion areas for group IV mice inoculated three times at an older age with the shortest period of cholesterol feeding were comparable to those for the control mice and significantly less than those for group I mice (1,399 μm2; P = 0.045).
FIG. 4.
Lipid lesion areas in the aortic sinuses of the mice (and geometric means for the groups) inoculated with C. pneumoniae. Samples were taken at the age of 32 weeks. Group I, six inoculations, starting at the age of 8 weeks; group II, three inoculations, starting at the age of 8 weeks; group III, three inoculations, starting at the age of 20 weeks; group IV, three inoculations, starting at the age of 20 weeks, and cholesterol feeding started at 18 weeks; group V, uninfected control. One asterisk and two asterisks indicate significant differences compared with group I (P = 0.034 and P = 0.045, respectively). geom., geometric.
DISCUSSION
C. pneumoniae inoculations led to a self-restricting lung infection and histopathology in the mice in the present study, and generally, no signs of clinical illness were detected, except in one animal in group I that developed severe pneumonia after repeated inoculations and was euthanized during the study. No statistically significant differences were found in body weight, total cholesterol, or triglyceride levels between groups, and as described previously by Erkkilä et al. (15), the mice remained normocholesterolemic despite the small cholesterol supplement (0.2%) in the diet. Levels of high-density lipoprotein, low-density lipoprotein (LDL), or oxidized LDL were not measured here, but alterations in these levels without changes in the total cholesterol or triglyceride levels may have occurred. LDL oxidation has been shown to be induced at least in vitro by C. pneumoniae (22) and in vivo by infection and inflammation in general (31). However, in a previous study with LDL receptor-deficient mice, infection with C. pneumoniae had no effect on serum LDL levels (21). Grade 2 inflammation with moderate perivascular and peribronchial infiltration of mononuclear cells in lung tissue, which was not seen in mock-inoculated control mice, persisted at 32 weeks of age in the mice. This, together with the positive PCR findings for the lung tissue of the mice, especially the findings for group II, suggests the persistent presence of chlamydia in lung tissue, as described in previous studies (15, 43) with this mouse model.
However, we were not able to find chlamydial mRNA in the lung tissue with nested reverse transcriptase PCR combining the conventional and real-time PCR methods and targeting the C. pneumoniae groEL gene. If chlamydial mRNA is present in chronically infected tissues, the levels must be very low. We attempted to purify the extracted total RNA by removing eukaryotic 18S rRNA, 28S rRNA, and polyadenylated RNAs with a MICROBEnrich kit (Ambion) but failed to obtain positive findings. The tissue sample taken for RNA extraction (15 to 30 μg) was only a small fraction of the right lung, and the samples were stored in RNA stabilization solution (RNAlater; QIAGEN) for 2 years at −20°C before extraction. All these factors may have affected the chlamydial RNA levels in the tissue, although samples collected 4 days after an acute infection and stored similarly for 1 year had high levels of chlamydial RNA when they were measured with the same RT-PCR method (results not shown). We also tested for the presence of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase RNA, in two of the extracted samples from each study group by a method described previously (42). Glyceraldehyde-3-phosphate dehydrogenase was found to be expressed in all of the samples from the study groups and in the positive control sample, thus showing the adequacy of the samples. The optimal target for measuring mRNA expression in the chronic state of infection in vivo can be discussed as well. The groEL gene encoding the C. pneumoniae Hsp60 protein has been shown to be up-regulated in murine alveolar macrophages (20) and in some (14, 34) but not all (4, 30) previous in vitro studies after gamma interferon-induced persistence of infection. Whether this reflects correctly the state of persistence in vivo is not clear; however, Gerard et al. previously detected the presence of transcripts from chlamydial Hsp60 in both C. trachomatis (18) and C. pneumoniae (19) DNA-positive synovial samples.
In serological analyses in the present study, two different methods, EIA and MIF, were compared for measuring the total IgG levels in mouse serum. The two methods detected antibodies in infected mice equally well, and no antibodies were found in uninfected mice. The MIF method was more efficient for detecting very high antibody levels, but when measured by EIA, the antibody levels seemed to be more stable between 30 and 32 weeks and there was less variation within the groups. As Fig. 1 shows, the results of the two analyses are roughly parallel, and both methods thus seem to be reliable for measuring the presence of C. pneumoniae antibodies in mice. We also determined the levels of the IgG1 and IgG2c subclass antibodies against both whole C. pneumoniae EB antigen and chlamydial Hsp60 protein. These analyses showed that IgG1 antibodies were present only at trace levels, whereas the IgG2c antibody levels were high, especially those against the cHsp60 protein. The dominance of subtype IgG2c was expected, since the predominance of the TH1 response in chlamydial murine infection models has been shown previously (37, 40). Only in group I, with six chlamydial inoculations, did the cHsp60 IgG1 antibody levels increase above the baseline at 32 weeks of age, 4 weeks after the last inoculation, suggesting that there was a weak TH2-type response as well. Chlamydial Hsp60 is known to be abundant during the whole developmental cycle and to be highly immunogenic (38). In the present study, high cHsp60 antibody levels correlated with severe lung inflammatory reactions. Importantly, a similar correlation was also found between mHsp60 IgG antibody levels and lung inflammation grades, and there was a further positive and statistically significant correlation between the mHsp60 IgG and cHsp60 IgG2c antibody levels in these mice. In agreement with the previous results (15), the mHsp60 IgG levels in chlamydia-infected groups were also higher than those in SPG mock-inoculated mice at every time. These results strongly support the theory that during infection, when both bacterial and host Hsp60 are overexpressed due to the stressful conditions for both the bacteria and the host, bacterial Hsp60 may elicit the production of autoantibodies against self heat shock proteins and thereby worsen the pathological consequences in a target tissue.
Repeated C. pneumoniae inoculations significantly increased the area of subendothelial lipid accumulation at the age of 32 weeks in the mice inoculated six times (group I) in the present study. As Fig. 4 shows, relatively large lipid lesion areas were also detected in some of the SPG mock-inoculated mice (group V), but the significant difference between the uninfected and repeatedly infected groups was evidently due to the lack of smaller lesions in the infected group. C. pneumoniae infections thus contributed to the development of intimal lipid accumulation in the aortic sinuses of the normocholesterolemic mice fed a slightly cholesterol-supplemented diet, confirming our previous results (15). In the previous study, the compositions of lipid lesions in the aortas collected from mice at the age of 31 weeks were similar, but the lesion areas were smaller than those seen in the present study. This was due to the fact that cross-sections were collected from slightly different areas of the aortic sinus, as well as calculated differently, in these two studies.
The duration of cholesterol feeding had no significant effect on the lesion areas when groups III and IV were compared in this study. However, in group IV mice, which had a short period of cholesterol feeding that started just before the inoculations, the lesion areas were comparable to those in the SPG mock-inoculated mice and also significantly smaller than those in the mice infected six times. As shown in the previous murine models (6, 7), fat and cholesterol are needed for the development of atherosclerosis in C57BL mice, but our previous results and the results of the present study suggest that overt hypercholesterolemia is not necessary for C. pneumoniae infections to affect the vascular system. The theory of pathogen burden (46) suggests that the existence of active pathogen-induced inflammatory responses in general has an important role in the development of atherosclerosis as well. The fact that several repeated inoculations led to areas with higher lipid accumulation in every mouse in the present study also supports this theory, although only C. pneumoniae was used in our study. In humans, infections have been associated with changes in the coronary and carotid arteries; Pesonen et al. (39) reported intimal thickening in coronary samples from autopsied children associated with different infections, and Liuba et al. (27) showed that in vivo acute infections may lead to thickening of the carotid intima media in children.
The effects of infection age on lipid accumulation and on inflammatory reactions were studied as well. Previous studies with apolipoprotein E-deficient (apoE) mice suggested that the persistence of chlamydia in infected mice was greater and concomitantly detected when the mice were infected at an older age (33). It has also been suggested that chlamydia is more prone to infect aortas with preexisting lipid lesions (21). In this study, we tested whether three repeated inoculations given at the ages of 20, 24, and 28 weeks (group III) led to increased lipid lesion areas. The mice were compared to mice inoculated three times at the ages of 8, 12, and 16 weeks (group II), but no difference in the lesion areas was seen between the groups. We were not able to test the presence of chlamydia in the aortic tissue, but the persistence of chlamydial DNA in lung tissue was not increased in the group inoculated at an older age. Serological data based on total chlamydia IgG antibodies and cHsp60 IgG2c antibodies suggest that the antibody response to repeated inoculations is greater when infections are started at an older age (group III, results at 30 weeks) than when mice are inoculated at a younger age (group II, results at 18 weeks). Previously, B-cell-mediated adaptive immunity has been shown to be atheroprotective in mice (11), and the stronger antibody responses in the older mice might actually prevent atherosclerotic development.
In the present study, we detected intimal lipid accumulation by Oil-red-O staining in the aortic sinuses of C57BL/6J mice fed a slightly cholesterol-supplemented diet. All mice inoculated six times with chlamydia developed large lipid lesion areas, which differed significantly from the lesion areas in the uninfected control group. High IgG subtype 2c antibody levels, especially against chlamydial Hsp60, as markers of a TH1-type immune response were found, and a significant correlation between the IgG antibodies to chlamydial and mouse Hsp60s was demonstrated. In addition, both these antibody levels also correlated with the severity of the lung inflammatory response, further supporting the theory of development of an autoimmune response against heat shock proteins after chlamydial infection. The age at which the infections were started did not affect the intimal lipid accumulation in the present model, but further studies are needed to confirm this finding.
Supplementary Material
Acknowledgments
We thank Elise Saario for interpreting the histopathology results.
We thank the Sigrid Juselius Foundation, the Academy of Finland, and the Finnish Technology Foundation for financial support.
Editor: J. N. Weiser
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Airaksinen, U., T. Penttilä, E. Wahlström, J. M. Vuola, M. Puolakkainen, and M. Sarvas. 2003. Production of Chlamydia pneumoniae proteins in Bacillus subtilis and their use in characterizing immune responses in the experimental infection model. Clin. Diagn. Lab Immunol. 10:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Airenne, S., H. M. Surcel, H. Alakärppä, K. Laitinen, J. Paavonen, P. Saikku, and A. Laurila. 1999. Chlamydia pneumoniae infection in human monocytes. Infect. Immun. 67:1445-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belland, R. J., D. E. Nelson, D. Virok, D. D. Crane, D. Hogan, D. Sturdevant, W. L. Beatty, and H. D. Caldwell. 2003. Transcriptome analysis of chlamydial growth during IFN-{gamma}-mediated persistence and reactivation. Proc. Natl. Acad. Sci. USA 100:15971-15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belland, R. J., S. P. Ouellette, J. Gieffers, and G. I. Byrne. 2004. Chlamydia pneumoniae and atherosclerosis. Cell Microbiol. 6:117-127. [DOI] [PubMed] [Google Scholar]
- 6.Blessing, E., L. A. Campbell, M. E. Rosenfeld, N. Chough, and C. C. Kuo. 2001. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis 158:13-17. [DOI] [PubMed] [Google Scholar]
- 7.Blessing, E., L. A. Campbell, M. E. Rosenfeld, and C. C. Kuo. 2002. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis: infection prior to induction of hyperlipidemia does not accelerate development of atherosclerotic lesions in C57BL/6J mice. Infect. Immun. 70:5332-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blessing, E., T. M. Lin, L. A. Campbell, M. E. Rosenfeld, D. Lloyd, and C. Kuo. 2000. Chlamydia pneumoniae induces inflammatory changes in the heart and aorta of normocholesterolemic C57BL/6J mice. Infect. Immun. 68:4765-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blessing, E., S. Nagano, L. A. Campbell, M. E. Rosenfeld, and C. C. Kuo. 2000. Effect of Chlamydia trachomatis infection on atherosclerosis in apolipoprotein E-deficient mice. Infect. Immun. 68:7195-7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burian, K., Z. Kis, D. Virok, V. Endresz, Z. Prohaszka, J. Duba, K. Berencsi, K. Boda, L. Horvath, L. Romics, G. Fust, and E. Gonczol. 2001. Independent and joint effects of antibodies to human heat-shock protein 60 and Chlamydia pneumoniae infection in the development of coronary atherosclerosis. Circulation 103:1503-1508. [DOI] [PubMed] [Google Scholar]
- 11.Caligiuri, G., A. Nicoletti, B. Poirier, and G. K. Hansson. 2002. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Investig. 109:745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman, M. J. 1986. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 128:70-143. [DOI] [PubMed] [Google Scholar]
- 13.Ciervo, A., A. Petrucca, and A. Cassone. 2003. Identification and quantification of Chlamydia pneumoniae in human atherosclerotic plaques by LightCycler real-time-PCR. Mol. Cell. Probes 17:107-111. [DOI] [PubMed] [Google Scholar]
- 14.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69:2442-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erkkilä, L., K. Laitinen, K. Haasio, T. Tiirola, M. Jauhiainen, H. A. Lehr, K. Aalto-Setälä, P. Saikku, and M. Leinonen. 2004. Heat shock protein 60 autoimmunity and early lipid lesions in cholesterol-fed C57BL/6JBom mice during Chlamydia pneumoniae infection. Atherosclerosis 177:321-328. [DOI] [PubMed] [Google Scholar]
- 16.Erkkilä, L., K. Laitinen, A. Laurila, P. Saikku, and M. Leinonen. 2002. Experimental Chlamydia pneumoniae infection in NIH/S mice: effect of reinoculation with chlamydial or cell preparation on culture, PCR and histological findings of lung tissue. Vaccine 20:2318-2324. [DOI] [PubMed] [Google Scholar]
- 17.Fong, I. W., B. Chiu, E. Viira, D. Jang, and J. B. Mahony. 1999. De novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect. Immun. 67:6048-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerard, H. C., P. J. Branigan, H. R. Schumacher, Jr., and A. P. Hudson. 1998. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J. Rheumatol. 25:734-742. [PubMed] [Google Scholar]
- 19.Gerard, H. C., H. R. Schumacher, H. El-Gabalawy, R. Goldbach-Mansky, and A. P. Hudson. 2000. Chlamydia pneumoniae present in the human synovium are viable and metabolically active. Microb. Pathog. 29:17-24. [DOI] [PubMed] [Google Scholar]
- 20.Haranaga, S., H. Yamaguchi, H. Ikejima, H. Friedman, and Y. Yamamoto. 2003. Chlamydia pneumoniae infection of alveolar macrophages: a model. J. Infect. Dis. 187:1107-1115. [DOI] [PubMed] [Google Scholar]
- 21.Hu, H., G. N. Pierce, and G. Zhong. 1999. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J. Clin. Investig. 103:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalayoglu, M. V., B. Hoerneman, D. LaVerda, S. G. Morrison, R. P. Morrison, and G. I. Byrne. 1999. Cellular oxidation of low-density lipoprotein by Chlamydia pneumoniae. J. Infect. Dis. 180:780-790. [DOI] [PubMed] [Google Scholar]
- 23.Kalayoglu, M. V., P. Libby, and G. I. Byrne. 2002. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA 288:2724-2731. [DOI] [PubMed] [Google Scholar]
- 24.Kol, A., G. K. Sukhova, A. H. Lichtman, and P. Libby. 1998. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98:300-307. [DOI] [PubMed] [Google Scholar]
- 25.Laitinen, K., A. Laurila, L. Pyhälä, M. Leinonen, and P. Saikku. 1997. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect. Immun. 65:4832-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb, D. J., W. El-Sankary, and G. A. Ferns. 2003. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis 167:177-185. [DOI] [PubMed] [Google Scholar]
- 27.Liuba, P., J. Persson, J. Luoma, S. Ylä-Herttuala, and E. Pesonen. 2003. Acute infections in children are accompanied by oxidative modification of LDL and decrease of HDL cholesterol, and are followed by thickening of carotid intima-media. Eur. Heart J. 24:515-521. [DOI] [PubMed] [Google Scholar]
- 28.Maass, M., E. Krause, P. M. Engel, and S. Kruger. 1997. Endovascular presence of Chlamydia pneumoniae in patients with hemodynamically effective carotid artery stenosis. Angiology 48:699-706. [DOI] [PubMed] [Google Scholar]
- 29.Mabey, D. C., A. W. Solomon, and A. Foster. 2003. Trachoma. Lancet 362:223-229. [DOI] [PubMed] [Google Scholar]
- 30.Mathews, S., C. George, C. Flegg, D. Stenzel, and P. Timms. 2001. Differential expression of ompA, ompB, pyk, nlpD and Cpn0585 genes between normal and interferon-gamma treated cultures of Chlamydia pneumoniae. Microb. Pathog. 30:337-345. [DOI] [PubMed] [Google Scholar]
- 31.Memon, R. A., I. Staprans, M. Noor, W. M. Holleran, Y. Uchida, A. H. Moser, K. R. Feingold, and C. Grunfeld. 2000. Infection and inflammation induce LDL oxidation in vivo. Arterioscler. Thromb. Vasc. Biol. 20:1536-1542. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita, N., H. Fukano, K. Yoshida, Y. Niki, and T. Matsushima. 2003. Chlamydia pneumoniae infection in adult patients with persistent cough. J. Med. Microbiol. 52:265-269. [DOI] [PubMed] [Google Scholar]
- 33.Moazed, T. C., C. Kuo, J. T. Grayston, and L. A. Campbell. 1997. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J. Infect. Dis. 175:883-890. [DOI] [PubMed] [Google Scholar]
- 34.Molestina, R. E., J. B. Klein, R. D. Miller, W. H. Pierce, J. A. Ramirez, and J. T. Summersgill. 2002. Proteomic analysis of differentially expressed Chlamydia pneumoniae genes during persistent infection of HEp-2 cells. Infect. Immun. 70:2976-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paigen, B., A. Morrow, C. Brandon, D. Mitchell, and P. Holmes. 1985. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 57:65-73. [DOI] [PubMed] [Google Scholar]
- 36.Paigen, B., A. Morrow, P. A. Holmes, D. Mitchell, and R. A. Williams. 1987. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68:231-240. [DOI] [PubMed] [Google Scholar]
- 37.Pal, S., E. M. Peterson, and L. M. de la Maza. 2003. Induction of protective immunity against a Chlamydia trachomatis genital infection in three genetically distinct strains of mice. Immunology 110:368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peeling, R. W., and D. C. Mabey. 1999. Heat shock protein expression and immunity in chlamydial infections. Infect. Dis. Obstet. Gynecol. 7:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesonen, E., I. Paakkari, and J. Rapola. 1999. Infection-associated intimal thickening in the coronary arteries of children. Atherosclerosis 142:425-429. [DOI] [PubMed] [Google Scholar]
- 40.Rottenberg, M. E., A. C. Gigliotti Rothfuchs, D. Gigliotti, C. Svanholm, L. Bandholtz, and H. Wigzell. 1999. Role of innate and adaptive immunity in the outcome of primary infection with Chlamydia pneumoniae, as analyzed in genetically modified mice. J. Immunol. 162:2829-2836. [PubMed] [Google Scholar]
- 41.Simms, I., and J. M. Stephenson. 2000. Pelvic inflammatory disease epidemiology: what do we know and what do we need to know? Sex. Transm. Infect. 76:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson, D. A., S. Feeney, C. Boyle, and A. W. Stitt. 2000. Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol. Vis. 6:178-183. [PubMed] [Google Scholar]
- 43.Törmäkangas, L., H. Alakärppä, D. Bem David, M. Leinonen, and P. Saikku. 2004. Telithromycin treatment of chronic Chlamydia pneumoniae infection in C57BL/6J mice. Antimicrob. Agents Chemother. 48:3655-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wick, G., H. Perschinka, and G. Millonig. 2001. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 22:665-669. [DOI] [PubMed] [Google Scholar]
- 45.Wiedeman, J. A., R. Kaul, L. S. Heuer, N. N. Thao, K. E. Pinkerton, and W. M. Wenman. 2004. Tobacco smoke induces persistent infection of Chlamydophila pneumoniae in HEp-2 cells. Microb. Pathog. 37:141-148. [DOI] [PubMed] [Google Scholar]
- 46.Zhu, J., A. A. Quyyumi, J. E. Norman, G. Csako, M. A. Waclawiw, G. M. Shearer, and S. E. Epstein. 2000. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am. J. Cardiol. 85:140-146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.