Abstract
Background
The prevalence of obesity is steadily increasing in patients with end-stage kidney disease. However, it is still debatable whether obesity affects outcomes after kidney transplantation. This study evaluated the relationship between pretransplant obesity, defined as a body mass index (BMI) ≥ 25 kg/m2, and posttransplant outcomes in Korean kidney transplant recipients (KTRs).
Methods
We investigated prospective nationwide cohort data from the Korean Organ Transplantation Registry (KOTRY) from 2014 to 2021. KTRs were categorized into 4 groups based on pretransplant BMI: underweight (< 18.5), normal weight (18.5–22.9), overweight (23–24.9), and obesity (≥ 25). Posttransplant outcomes, including death-censored allograft loss, cardiovascular events, and all-cause mortality, were compared using Kaplan-Meier curves with the log-rank test. Cox proportional hazard regression analysis was employed to assess associations between BMI and posttransplant outcomes.
Results
A total of 9,130 KTRs were finally analyzed. The mean age was 49.9 ± 11.6 and 60.2% of KTRs were male. The prevalence of obesity in KTRs was 28.6% and continued to increase (24.8% in 2014 to 32.5% in 2021). Obese KTRs were characterized by male predominance, shorter dialysis vintage, and more diabetes as primary kidney disease. Kaplan-Meier curve showed a significant difference in death-censored allograft loss among BMI groups (P = 0.007). Obesity (BMI ≥ 25 kg/m²) was as an independent risk factor for death-censored allograft loss (adjusted hazard ratio 1.511, 95% confidence interval 1.063–2.148, P = 0.021), but not for cardiovascular events or mortality.
Conclusions
Our study evaluated BMI across a spectrum of categories, suggesting that obesity is an independent risk factor for graft survival in KTRs. Risk stratification using BMI and strategies to prevent obesity should be considered in the preparation for kidney transplantation.
Clinical trial number
Not applicable.
Keywords: Body mass index, Graft loss, Kidney transplantation, Obesity
Background
Over the decades, kidney transplantation has demonstrated gradual improvement in graft and patient survival [1]. Nevertheless, some kidney transplant recipients (KTRs) may eventually return to dialysis or face death. Graft loss can result from various causes and risk factors, such as acute or chronic rejection, vascular complications, delayed graft function (DGF), or recipient age [2]. Additionally, the leading cause of recipient mortality was cardiovascular disease, with infection and cancer following behind [1, 3, 4].
Obesity has diverse impacts on kidney transplant outcomes through increased adiposity and adipocytokines-related inflammation [5–7]. Several studies have mentioned associations between obesity and adverse short-term outcomes, especially DGF [8–13]. Furthermore, obesity independently elevates the risk of cardiovascular disease and plays a role in other cardiovascular risk factors such as hypertension and diabetes mellitus [14]. These metabolic diseases can worsen upon post-transplant immunosuppressive therapy and potentially lead to graft damage [8, 15, 16].
The prevalence of obesity has consistently risen in both the general population and those with end-stage kidney disease [17, 18]. Although obesity has its disadvantages, its effect on post-transplant outcomes remains a subject of debate. Some previous meta-analyses have indicated that obesity raises the chance of graft failure and recipient death [7, 9, 19–21]. Other research has suggested a limited significant association between obesity and both graft loss and mortality [10, 22]. These conflicting results suggest the necessity for further investigation into the impact of obesity on kidney transplants.
Ethnic diversity should be considered in evaluating the effect of obesity in KTRs. While the World Health Organization (WHO)’s international classification defines obesity as a body mass index (BMI) ≥ 30 kg/m2, guidelines specific to the Asian-Pacific region propose a lower threshold for general Asians, at a BMI ≥ 25 kg/m2, based on ethnic variations in body fat ratio and associated health risks [23, 24]. Accordingly, some studies have suggested a BMI ≥ 25 kg/m2 as a risk factor for graft loss in Asian KTRs [25–27]. However, research on clinical outcomes in obese Asian KTRs is limited, which may impede appropriate management strategies for obese KTRs.
This study aims to analyze the relationship between the pretransplant obesity (defined as BMI 25 ≥ kg/m2 per Asia-Pacific criteria) and kidney transplant outcomes including death-censored allograft loss, all-cause mortality, and cardiovascular events in Korean KTRs. Secondary analyses were performed across BMI categories to explore broader trends in transplant outcomes by analyzing data from a Korean nationwide study.
Methods
Patient population
This study utilized data from the Korean Organ Transplantation Registry (KOTRY). KOTRY has been collecting prospective transplant data from 59 centers (including 30 centers for kidney) nationwide in Korea since 2014 [28]. All KTRs in the KOTRY database from 2014 to 2021 were included in this study. Recipients under the age of 19 or those having simultaneous multiorgan transplants, other than pancreas-kidney cotransplantation, were not eligible. The detailed design and methodologies of KOTRY have been outlined in a previous article [29].
Data collection
The 9,130 KTRs were accounted for in the analysis. The BMI was assessed using the records available at the time of the kidney transplantation, and if no records were present, the measurement taken closest to the date before the transplantation was utilized. We categorized the subjects into four groups based on their pretransplant BMI: underweight (< 18.5), normal weight (18.5–22.9), overweight (23–24.9), and obesity (≥ 25), following the WHO’s Asia-Pacific guidelines [24]. BMI was calculated using pre-transplant weight divided by height squared (kg/m2). Baseline demographic characteristics of the recipients, dialysis vintage before transplantation, laboratory data, primary renal disease, comorbidities, transplantation type, immunological variables including pretransplant desensitization, types of immunosuppressants, and the occurrence of clinical outcomes were collected. Data on donor demographics, comorbidities, and donor types were also obtained.
Outcomes
The primary clinical outcomes included death-censored allograft loss, all-cause mortality, and cardiovascular events. The secondary outcomes were DGF, acute rejection, and de novo donor-specific antibody (DSA). De novo DSA was detected using a Luminex single-antigen bead assay. The definition of death-censored allograft loss included either the onset of dialysis lasting over three months or re-transplantation, with censoring at the time of patient death with a functioning graft. Cardiovascular events included post-transplant cardiovascular death, ischemic heart disease with pertinent evidence, myocardial infarction, newly diagnosed congestive heart failure requiring inpatient care, and arrhythmia. All-cause mortality was defined as death caused by any issue. For patients who passed away with a functioning graft, graft survival was censored at the moment of death. DGF was characterized as the requirement for at least one dialysis in the first seven days after transplants. Acute rejection included clinical rejection and biopsy-confirmed rejection based on the Banff classification. De novo DSA was defined as the detection of antibodies in the recipient’s panel reactive antibody (PRA) I and PRA II that matched the donor’s human leukocyte antigen (HLA) typing following the transplant.
Statistical analysis
Continuous variables were summarized with mean and standard deviation, while categorical variables were outlined using frequency and percentage. To assess statistical differences, one-way analysis of variance was used for continuous variables, and Pearson’s chi-squared test or Fisher’s exact test was applied for categorical variables. To evaluate the trend of obesity prevalence among KTRs, linear regression analysis was employed. Differences among sub-BMI groups in death-censored allograft loss, cardiovascular events, and all-cause mortality were assessed using Kaplan-Meier curves and the log-rank test. The associations between BMI and these primary outcomes were assessed through Cox proportional hazard regression analysis, with adjustments for clinically relevant covariates and baseline variables that significantly differed across BMI groups. In the multivariable Cox proportional hazard regression models, we controlled for confounding variables associated with KTRs and donor baseline characteristics in the following manner: Model 1 was without adjustments; Model 2 was adjusted for age, sex, and BMI in KTRs; Model 3 added donor type into the variables from Model 2; and Model 4 included additional adjustments for the donor’s gender, BMI, and cardiovascular disease, recipient’s diabetes, hypertension, smoking, dialysis vintage, and acute rejection along with the variables established in Model 3. Subgroup analyses for death-censored allograft loss were carried out based on age, sex, comorbidities, donor type, and desensitization status. To identify factors associated with pretransplant obesity, we performed logistic regression analyses. Variables were selected based on clinical relevance and statistically significant differences between BMI groups in the baseline characteristics. Factors that showed significant associations in univariate analyses (P < 0.05) were included in the multivariate model. Statistical analyses were conducted using R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) and SPSS version 22.0 (IBM Corp., Armonk, NY). A P-values < 0.05 was deemed statistically significant.
Results
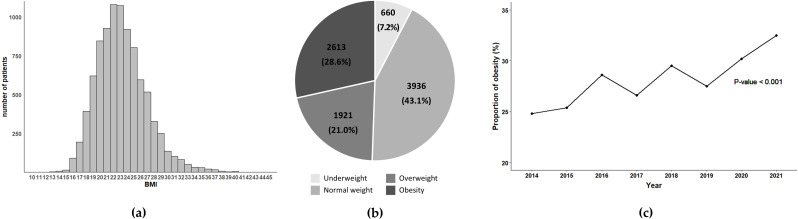
Figure 1a shows the overall distribution of BMI in 9,130 patients. The prevalence of overweight and obesity was 21.0% and 28.6%, respectively (Fig. 1b). The proportion of obesity gradually increased from 24.8% in 2014 to 32.5% in 2021, showing significant changes over the years (Fig. 1c).
Fig. 1.
Distribution and trends of BMI among KTRs. (a) Distribution of BMI; (b) Proportion of BMI categories; (c) annual changes in the proportion of KTRs with obesity. Abbreviations: BMI, body mass index; KTRs, kidney transplant recipients
The demographics, categorized by BMI groups, are provided in Table 1. The mean age of all KTRs was 49.9 ± 11.6 years, with a male ratio of 60.2% (n = 5,499). Statistically significant differences were identified across BMI groups in recipient characteristics, including age, systolic and diastolic blood pressure, and the number of HLA mismatches. Notably, the obesity group was characterized by a higher prevalence of male sex, more frequent smoking, and shorter duration of dialysis, as well as higher fasting glucose levels, lower total and high-density lipoprotein cholesterol, and higher triglyceride levels. In this group, diabetes mellitus was the leading cause of primary kidney disease, while hypertension was the most common comorbidity. Most transplants were from living donors, and donor gender and BMI varied significantly across recipient BMI groups.
Table 1.
Baseline characteristics
| Underweight (n = 660) |
Normal weight (n = 3936) |
Overweight (n = 1921) |
Obesity (n = 2613) |
P-value | |
|---|---|---|---|---|---|
| Recipient Demographics | |||||
| Age (y) | 43.0 ± 12.9 | 49.5 ± 11.6 | 51.9 ± 10.8 | 50.6 ± 11.0 | < 0.001 |
| Gender (male n, %) | 192 (29.1) | 2133 (54.2) | 1335 (69.5) | 1839 (70.4) | < 0.001 |
| Body mass index (kg/m2) | 17.4 ± 1.0 | 21.0 ± 1.2 | 23.9 ± 0.6 | 27.8 ± 2.6 | < 0.001 |
| Dialysis vintage before KT (months) | 54.3 ± 70.7 | 57.4 ± 67.9 | 48.7 ± 57.2 | 42.9 ± 52.7 | < 0.001 |
| Smoking, n (%) | 64 (9.8) | 743 (19.1) | 528 (27.9) | 812 (31.8) | < 0.001 |
| Systolic BP (mmHg) | 135.2 ± 21.8 | 139.4 ± 21.0 | 140.8 ± 20.4 | 140.0 ± 21.0 | < 0.001 |
| Diastolic BP (mmHg) | 84.3 ± 13.7 | 83.6 ± 12.6 | 83.2 ± 12.4 | 82.8 ± 12.6 | 0.026 |
| Fasting glucose (mg/dL) | 109.7 ± 39.0 | 117.9 ± 53.7 | 125.6 ± 56.2 | 129.5 ± 60.1 | < 0.001 |
| Total cholesterol (mg/dL) | 162.3 ± 41.0 | 153.2 ± 40.9 | 148.8 ± 41.5 | 147.8 ± 43.6 | < 0.001 |
| LDL (mg/dL) | 85.3 ± 29.0 | 84.3 ± 33.5 | 83.2 ± 34.2 | 81.8 ± 35.7 | 0.056 |
| HDL (mg/dL) | 60.1 ± 19.9 | 52.0 ± 16.5 | 46.0 ± 15.3 | 40.5 ± 13.6 | < 0.001 |
| Triglyceride (mg/dL) | 100.9 ± 54.0 | 107.7 ± 67.5 | 126.3 ± 76.7 | 158.3 ± 103.7 | < 0.001 |
| Primary renal disease, n (%) | |||||
| Diabetes mellitus | 60 (11.8) | 749 (23.8) | 579 (37.2) | 959 (43.8) | < 0.001 |
| Hypertensive nephrosclerosis | 80 (15.8) | 581 (18.5) | 293 (18.8) | 424 (19.4) | < 0.001 |
| Glomerulonephritis | 288 (56.8) | 1407 (44.7) | 538 (34.6) | 618 (28.2) | < 0.001 |
| Others | 79 (15.6) | 409 (13.0) | 145 (9.3) | 190 (8.7) | < 0.001 |
| Comorbid conditions, n (%) | |||||
| Hypertension | 530 (80.3) | 3446 (87.6) | 1766 (92.0) | 2423 (92.7) | < 0.001 |
| Diabetes mellitus | 86 (13.1) | 959 (24.4) | 718 (37.4) | 1187 (45.4) | < 0.001 |
| Cardiovascular disease | 43 (6.5) | 410 (10.4) | 243 (12.7) | 402 (15.4) | < 0.001 |
| Transplant-related Variables | |||||
| Living donor transplantation, n (%) | 484 (73.3) | 2566 (65.2) | 1240 (64.5) | 1830 (70.0) | < 0.001 |
| Deceased donor transplantation, n (%) | 176 (26.7) | 1370 (34.8) | 681 (35.5) | 783 (30.0) | < 0.001 |
| Desensitization, n (%) | 177 (26.8) | 1004 (25.5) | 472 (24.6) | 628 (24.0) | 0.362 |
| ABOi KT, n (%) | 121 (18.3) | 723 (18.4) | 361 (18.8) | 478 (18.3) | 0.975 |
| HLA mismatch number | 3.0 ± 1.7 | 3.3 ± 1.8 | 3.3 ± 1.8 | 3.4 ± 1.8 | < 0.001 |
| Basiliximab induction, n (%) | 527 (79.8) | 3054 (77.6) | 1513 (78.8) | 2070 (79.3) | 0.305 |
| ATG induction, n (%) | 143 (21.7) | 955 (24.3) | 441 (23.0) | 564 (21.6) | 0.069 |
| Tacrolimus, n (%) | 636 (96.4) | 3832 (97.4) | 1858 (96.8) | 2528 (96.9) | 0.317 |
| Mycophenolate, n (%) | 599 (90.8) | 3691 (93.8) | 1800 (93.8) | 2459 (94.2) | 0.012 |
| Corticosteroid, n (%) | 649 (98.3) | 3878 (98.6) | 1886 (98.3) | 2542 (97.4) | 0.006 |
| Donor Characteristics | |||||
| Age (y) | 47.4 ± 12.3 | 48.1 ± 12.6 | 48.0 ± 13.1 | 47.9 ± 12.7 | 0.572 |
| Gender (male n, %) | 272 (41.2) | 1810 (46.0) | 949 (49.4) | 1392 (53.3) | < 0.001 |
| Body mass index (kg/m2) | 23.4 ± 3.4 | 23.8 ± 3.3 | 24.2 ± 3.5 | 24.5 ± 3.5 | < 0.001 |
| Hypertension, n (%) | 105 (16.2) | 617 (16.0) | 288 (15.3) | 447 (17.4) | 0.264 |
| Diabetes mellitus, n (%) | 18 (2.8) | 182 (4.7) | 86 (4.6) | 129 (5.0) | 0.109 |
| Cardiovascular disease, n (%) | 9 (1.5) | 62 (1.7) | 50 (2.8) | 37 (1.5) | 0.010 |
Data are presented as mean ± standard deviation or number (%)
Abbreviations: KT, kidney transplantation; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ABOi, ABO incompatible; HLA, human leukocyte antigen; ATG, anti–thymocyte globulin
Patients were followed for a mean duration of 2.5 years with a maximum of 7.4 years. Table 2 summarizes the occurrence of clinical outcomes in KTRs. Acute rejection rates revealed a significant difference among the BMI groups, with a higher incidence observed in the obesity group (20.3%, P = 0.031). The obesity group also exhibited the highest rates of DGF and death-censored allograft loss, though the differences between groups were not statistically meaningful. Cardiovascular events and all-cause mortality rates were similar in the different BMI classifications.
Table 2.
Clinical outcomes
| Underweight (n = 660) |
Normal weight (n = 3936) |
Overweight (n = 1921) |
Obesity (n = 2613) |
P-value | |
|---|---|---|---|---|---|
| DGF | 21 (3.2%) | 170 (4.3%) | 63 (3.3%) | 120 (4.6%) | 0.079 |
| Acute rejection | 132 (20.0%) | 706 (17.9%) | 333 (17.3%) | 530 (20.3%) | 0.031 |
| de novo DSA | 61 (9.2%) | 375 (9.5%) | 217 (11.3%) | 239 (9.1%) | 0.082 |
| Death-censored allograft loss | 18 (2.7%) | 101 (2.6%) | 55 (2.9%) | 96 (3.7%) | 0.073 |
| Cardiovascular events | 48 (7.3%) | 228 (5.8%) | 128 (6.7%) | 163 (6.2%) | 0.371 |
| All-cause mortality | 15 (2.3%) | 83 (2.1%) | 41 (2.1%) | 61 (2.3%) | 0.935 |
Data are presented as number (%)
Abbreviations: DGF, delayed graft function; DSA, donor specific antigen
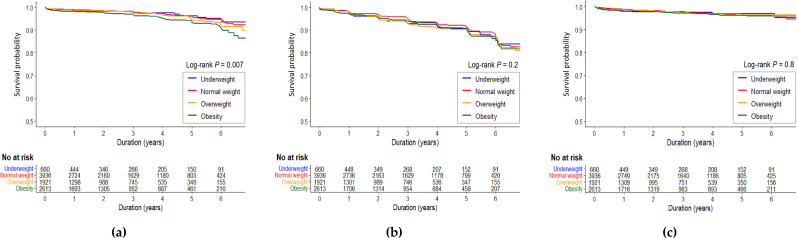
Figure 2 depicts the survival curves of the three clinical outcomes: death-censored allograft loss, cardiovascular events, and all-cause mortality. A significant difference in death-censored allograft loss was found between the groups according to the Kaplan-Meier curve (P = 0.007), showing the lowest allograft survival in the obesity KTRs (Fig. 2a, Log-rank P = 0.007). No differences were observed in the cumulative rates of cardiovascular events or all-cause mortality across BMI groups (Fig. 2b and c).
Fig. 2.
Kaplan-Meier curves for posttransplant outcomes according to BMI categories. (a) Death-censored allograft loss; (b) Cardiovascular events; (c) All-cause mortality. Abbreviations: BMI, body mass index
In univariate Cox regression analysis, the obesity group demonstrated a higher risk of death-censored allograft loss relative to the normal weight group (hazard ratio [HR] 1.529, confidence interval [CI] 1.097–2.130, P = 0.012). Multivariate analysis confirmed obesity as an independent risk factor of death-censored allograft loss (adjusted hazard ratio [aHR] 1.511, 95%, CI 1.063–2.148, P = 0.021) (Table 3). The underweight and overweight groups did not show significant changes in the incidence of death-censored allograft loss. However, the obesity group was not linked to the risk of cardiovascular events or all-cause mortality (Tables 4 and 5).
Table 3.
Cox regression analysis for death-censored allograft loss
| Model 1 1) | Model 2 2) | Model 3 3) | Model 4 4) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | aHR (95% CI) |
P-value | aHR (95% CI) |
P-value | aHR (95% CI) |
P-value | |
| Underweight |
0.802 (0.401–1.606) |
0.534 |
0.900 (0.446–1.818) |
0.769 |
0.956 (0.473–1.934) |
0.901 |
0.996 (0.490–2.023) |
0.990 |
| Normal weight | Reference | Reference | Reference | Reference | ||||
| Overweight |
1.118 (0.748–1.669) |
0.587 |
1.053 (0.702–1.580) |
0.803 |
1.043 (0.691–1.574) |
0.841 |
1.086 (0.712–1.656) |
0.701 |
| Obesity |
1.529 (1.097–2.130) |
0.012 |
1.457 (1.041–2.039) |
0.028 |
1.407 (0.996–1.988) |
0.053 |
1.484 (1.039–2.119) |
0.030 |
(1) unadjusted. (2) adjusted for age and sex. (3) adjusted for age, sex, primary renal disease, number of HLA mismatch (ref 0 ~ 3), and transplantation type. (4) adjusted for age, sex, primary renal disease, diabetes, hypertension, smoking, acute rejection, number of HLA mismatch (ref 0 ~ 3), transplantation type, donor gender, donor body mass index and donor cardiovascular disease
Table 4.
Cox regression analysis for cardiovascular events
| Model 1 1) | Model 2 2) | Model 3 3) | Model 4 4) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | aHR (95% CI) |
P-value | aHR (95% CI) |
P-value | aHR (95% CI) |
P-value | |
| Underweight |
1.062 (0.716–1.575) |
0.765 |
1.175 (0.787–1.755) |
0.431 |
1.205 (0.806–1.803) |
0.364 |
1.261 (0.541–1.890) |
0.261 |
| Normal weight | Reference | Reference | Reference | Reference | ||||
| Overweight |
1.212 (0.940–1.561) |
0.138 |
1.148 (0.888–1.485) |
0.291 |
1.141 (0.880–1.480) |
0.319 |
1.166 (0.896–1.516) |
0.254 |
| Obesity |
1.094 (0.865–1.383) |
0.455 |
1.049 (0.827–1.331) |
0.693 |
1.012 (0.793–1.291) |
0.925 |
0.989 (0.769–1.271) |
0.931 |
(1) unadjusted. (2) adjusted for age and sex. (3) adjusted for age, sex, primary renal disease, number of HLA mismatch (ref 0 ~ 3), and transplantation type. (4) adjusted for age, sex, primary renal disease, diabetes, hypertension, smoking, acute rejection, number of HLA mismatch (ref 0 ~ 3), transplantation type, donor gender, donor body mass index and donor cardiovascular disease
Table 5.
Cox regression analysis for all-cause mortality
| Model 1 1) | Model 2 2) | Model 3 3) | Model 4 4) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | aHR (95% CI) |
P-value | aHR (95% CI) |
P-value | aHR (95% CI) |
P-value | |
| Underweight |
0.934 (0.464–1.881) |
0.849 |
1.450 (0.716–2.937) |
0.302 |
1.540 (0.761–3.119) |
0.230 |
1.355 (0.643–2.853) |
0.424 |
| Normal weight | Reference | Reference | Reference | Reference | ||||
| Overweight |
0.968 (0.619–1.515) |
0.888 |
0.788 (0.502–1.238) |
0.302 |
0.798 (0.508–1.253) |
0.327 |
0.834 (0.530–1.314) |
0.434 |
| Obesity |
1.246 (0.857–1.811) |
0.249 |
1.130 (0.775–1.647) |
0.525 |
1.115 (0.760–1.636) |
0.577 |
1.142 (0.776–1.683) |
0.500 |
(1) unadjusted. (2) adjusted for age and sex. (3) adjusted for age, sex, primary renal disease, number of HLA mismatch (ref 0 ~ 3), and transplantation type. (4) adjusted for age, sex, primary renal disease, diabetes, hypertension, smoking, acute rejection, number of HLA mismatch (ref 0 ~ 3), transplantation type, donor gender, donor body mass index and donor cardiovascular disease
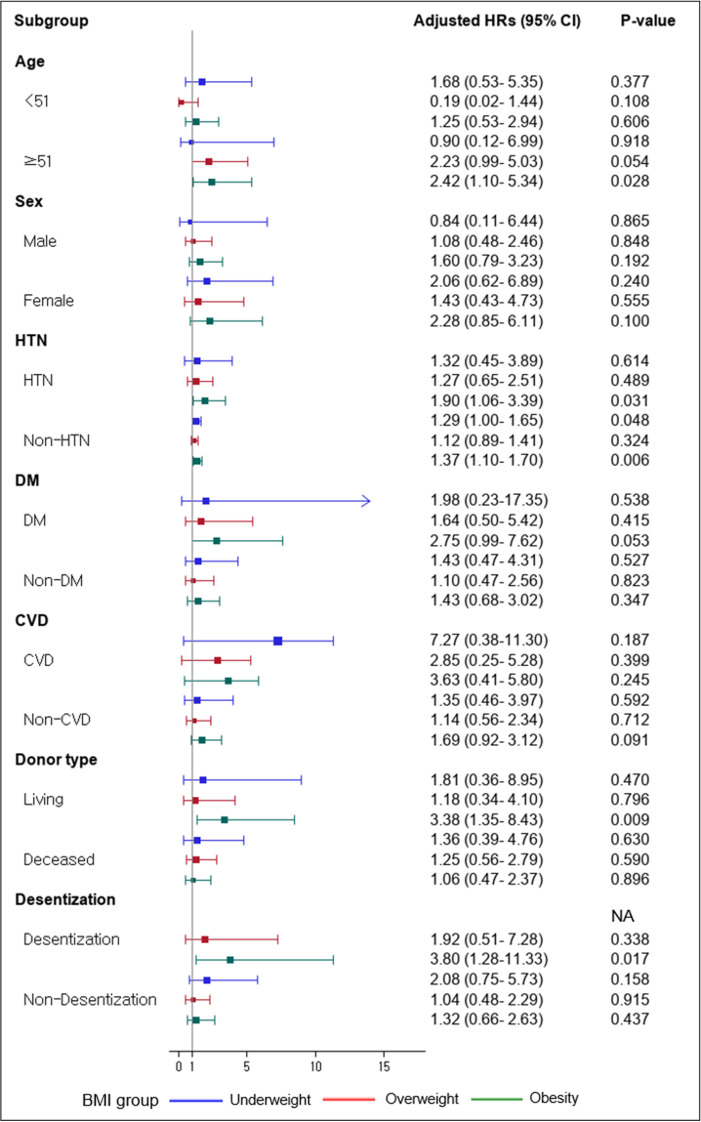
Subgroup analysis verified the influence of demographic factors on death-censored allograft loss in each BMI group (Fig. 3). In the obesity group, death-censored allograft loss was increased in KTRs with age older than 51 (aHR 2.42, CI 1.10–5.34, P = 0.028), living donor (aHR 3.38, CI 1.35–8.43, P = 0.009), and desensitization (aHR 3.80, CI 1.28–11.33, P = 0.017). Obesity in KTRs was linked to a higher hazard of death-censored allograft loss regardless of the presence of hypertension.
Fig. 3.
Subgroup analysis for death-censored allograft loss. Abbreviations: HTN, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease
Multivariate logistic regression identified male gender, smoking, lower high-density lipoprotein (HDL) levels, higher triglycerides, diabetes and cardiovascular disease (all P < 0.05) as significant factors associated with pretransplant obesity (Table 6).
Table 6.
Factors related to pretransplant obesity in logistic regression model
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| B (SE) | β | P-value | B (SE) | β | P-value | |
| Age | –0.91 (0.02) | 0.40 | < 0.001 | –0.003 (0.00) | 1.00 | 0.316 |
| Male gender | 0.01 (0.00) | 1.01 | < 0.001 | 0.20 (0.08) | 1.22 | 0.017 |
| Dialysis vintage before KT | –0.00 (0.00) | 0.99 | < 0.001 | –0.001 (0.00) | 1.00 | 0.090 |
| Smoking | 0.58 (0.05) | 1.78 | < 0.001 | 0.31 (0.08) | 1.36 | < 0.001 |
| Systolic BP | 0.001 (0.00) | 1.00 | 0.181 | |||
| Diastolic BP | –0.01 (0.00) | 1.00 | 0.015 | –0.001 (0.00) | 1.00 | 0.803 |
| Fasting glucose | 0.003 (0.00) | 1.00 | < 0.001 | –0.001 (0.00) | 1.00 | 0.052 |
| Total cholesterol | –0.003 (0.00) | 1.00 | < 0.001 | 0.001 (0.00) | 1.00 | 0.268 |
| HDL | –0.05 (0.00) | 0.95 | < 0.001 | –0.04 (0.00) | 0.97 | < 0.001 |
| Triglyceride | 0.01 (0.00) | 1.01 | < 0.001 | 0.004 (0.00) | 1.00 | < 0.001 |
| Hypertension | 0.54 (0.09) | 1.72 | < 0.001 | 0.21 (0.13) | 1.23 | 0.103 |
| Diabetes mellitus | 0.81 (0.05) | 2.24 | < 0.001 | 0.54 (0.08) | 1.72 | < 0.001 |
| Cardiovascular disease | 0.42 (0.07) | 1.52 | < 0.001 | 0.31 (0.10) | 1.36 | 0.002 |
Abbreviations: B, unstandardized regression coefficient; SE, standard error; β, standardized coefficient; KT, kidney transplantation; BP, blood pressure; HDL, high-density lipoprotein
Discussion
This study investigated the relationship between the pretransplant BMI and post-transplant outcomes in Korean KTRs. Clinical outcomes across different BMI groups were compared based on the rate of death-censored allograft loss, cardiovascular events, and all-cause mortality. Obesity, defined as BMI over 25 kg/m2, was related to a higher hazard of allograft failure. The pretransplant BMI did not affect cardiovascular events and mortality.
Obesity is a widely acknowledged independent risk factor with a positive linear relationship to overall mortality and cardiovascular issues in the general population [14, 30]. However, in our study, obesity was not significantly related to death or cardiovascular events in KTRs. The meta-analysis examining BMI in KTRs revealed varying results for these outcomes. Regarding mortality, while some studies have indicated a higher risk of mortality associated with obesity [7, 9, 20], others have found no significant link between BMI and patient survival [10, 11]. Additionally, some studies have reported inconsistencies in mortality results depending on the statistical methods used [9, 19]. Research on the relationship between BMI and cardiovascular events in KTRs is relatively limited and shows similarly inconsistent results [31, 32]. These inconsistencies in patient mortality and cardiovascular events may be attributed to patient variables such as age, underlying comorbidities, and immune status, which can have a more substantial and complex impact on survival rates and cardiovascular issues, despite the established risk of obesity for cardiovascular disease and general mortality [15].
Regarding allograft outcomes, most meta-analyses have identified obesity as a risk factor for allograft loss [7, 9, 11, 19, 20], which aligns with our results. However, one meta-analysis reported no link between obesity and allograft loss [10]. This study focused on KTRs who underwent kidney transplantation after the 2000s and attributed the lack of association to advancements in transplant medical practices. Our study, based on a nationwide multicenter cohort of over 9,000 KTRs, offers robust statistical power and improved generalizability within an ethnically homogeneous population. In addition, our cohort reflects contemporary transplant practices, including updated immunosuppressive regimens and metabolic management strategies. These contextual improvements may clarify prior conflicting results by controlling for evolving clinical factors.
When considering BMI, it is essential to account for ethnic differences. Compared to Western populations, Asians are inclined to have a greater body fat percentage at the same BMI and are more susceptible to obesity-related health issues even at lower BMI thresholds [23]. Most previous studies on obese KTRs have been conducted in Western countries. Our study reflects ethnic-specific risk stratification by using the Asia-Pacific BMI criterion (≥ 25 kg/m²), which better accounts for differences in body composition and metabolic risk among Asian populations. Research in China [25], Hong Kong [26], and Japan [27] has consistently shown that obese KTRs with an Asian-specific BMI cutoff of ≥ 25 kg/m2 revealed reduced graft survival rates. Some of these studies have reported no statistically significant association with patient survival [25, 27]. The present study also confirmed obesity as an independent factor associated with death-censored allograft loss, consistent with several prior studies, including a meta-analysis [11] and an analysis of Asian populations [25].
The pathogenic impact of obesity on transplanted organs can be explained through multiple mechanisms. The most extensively researched mechanism is the metabolic disturbances associated with obesity. Obesity-related metabolic issues, such as insulin resistance, dyslipidemia, hypertension, and chronic inflammation, are recognized risk factors for allograft damage due to their hemodynamic and proinflammatory changes [33–35]. Our data indicate that the obese group, which showed a higher risk of death-censored allograft loss, has elevated fasting glucose levels and abnormal lipid profiles, in addition to a greater prevalence of hypertension and diabetes, in contrast to the other BMI groups. These findings support the association between abnormal metabolic conditions and allograft failure in the KTRs.
Several studies have linked obesity to acute rejection [1, 9, 27] and DGF [1, 7, 9–12, 21], both of which heighten the risk of allograft failure [1, 7]. One possibility is that obesity-related inflammation may exacerbate graft rejection pathways. Obesity is reported to be associated with alloimmune dysregulation through elevated leptin and lowered adiponectin levels, activating both innate and adaptive immunity [5, 6]. This association can be supported by experimental studies in obese mice with heart transplants [36, 37]. Additionally, recent clinical research has reported a link between hyperleptinemia and the production of de novo DSA [38], which can contribute to allograft rejection. However, the risk of death-censored allograft loss remained higher in obese recipients, even after adjusting for acute rejection, highlighting a potential independent effect of obesity on graft failure that warrants further investigation. This suggests that obesity may affect graft survival not only through immunologic pathways but also via non-immunologic mechanisms. These findings underscore the multifactorial impact of obesity on graft outcomes and support the need for comprehensive perioperative management in obese KTRs. Given this association between pretransplant obesity and adverse graft outcomes, efforts to optimize body weight prior to kidney transplantation may be beneficial.
In this context, it is important to recognize that obesity is often accompanied by a cluster of modifiable cardiometabolic risk factors that may collectively influence transplant outcomes. Our multivariate logistic regression analysis suggests that pretransplant obesity is not an isolated phenomenon, but is closely linked to components of metabolic syndrome. These include modifiable risk factors such as smoking, dyslipidemia, diabetes, and cardiovascular disease, all of which warrant comprehensive clinical assessment. Clinicians should consider metabolic risk screening and targeted interventions to optimize pretransplant health and improve posttransplant outcomes in obese transplant candidates.
Another finding from our study was that the impact of obesity on death-censored allograft loss was modified by old age over 51 years, desensitization, and living donors. The elevated risk persisted regardless of hypertension, which may suggest obesity’s independent role, possibly through immunometabolic mechanisms. Aging can contribute to allograft loss through altered metabolic and immunologic factors [39, 40], which may be compounded by obesity. The altered inflammatory state in obesity may also explain the poor allograft outcome observed in the high BMI, HLA-presensitized recipients [41]. Additionally, lower graft survival rates have been reported in obese KTRs who received living donor transplants in previous studies [42, 43]. These findings suggest that obese recipients of living donor kidneys may benefit from intensified immunologic monitoring or tailored immunosuppressive strategies, even in settings traditionally considered lower-risk. However, further research may be warranted to explore the significant impact of obesity on allograft loss in living donor groups, given the similar immunologic barriers between living and deceased donor transplants [44]. In summary, our subgroup analysis highlights the need for tailored management strategies for obese KTRs, especially those with specific risk factors.
There are several limitations to this study. First, the number of patients with extreme obesity is relatively small, and the average follow-up period was short. Post-hoc power analyses indicated that these comparisons were underpowered (power < 40%), likely due to low event rates and small subgroup sizes. Thus, the absence of significant associations should be interpreted with caution. The limited follow-up duration may have restricted our ability to assess long-term outcomes, and the impacts based on the obesity grade could not be compared to the studies of Western patients. Second, discrepancies in our findings may arise from ethnic differences, as Asians have different body fat profiles and obese-related disease rates than Western populations. Accordingly, we used the Asia-Pacific BMI criterion (≥ 25 kg/m²), which more accurately reflects obesity-related risk in Asian populations [23]. However, this ethnic-specific definition may limit the generalizability of our findings to non-Asian populations, where obesity is typically defined using the WHO cutoff of ≥ 30 kg/m². Third, the explanation supporting our results remains ambiguous, as the mechanisms through which obesity impacts kidney transplant outcomes are not yet clearly defined. The lack of data on immunosuppressive drug dosages and trough levels prevented direct assessment of whether obesity-related graft loss was mediated by suboptimal immunosuppression. Sustained data collection and further clinical and experimental research are required to gain a clearer understanding of how obesity affects kidney transplants. Next, BMI is a highly confounded variable that may reflect various underlying clinical and behavioral factors, including nutritional status, inflammation, and comorbidities. Although we adjusted for multiple covariates in our Cox regression models, the possibility of residual or unmeasured confounding cannot be excluded. In addition, posttransplant BMI data were not available in the KOTRY dataset, which precluded us from assessing the duration or persistence of obesity after transplantation. This limits our ability to distinguish between transient and sustained obesity, which may have differing impacts on long-term transplant outcomes.
Conclusion
Our research with the nationwide data from Korea identifies obesity as an independent risk factor for death-censored allograft loss using Asian BMI cut-offs. Pretransplant BMI showed no association with cardiovascular events or mortality rates. The risk of death-censored allograft loss is influenced by specific patient characteristics including age, desensitization, and donor type. These findings highlight the importance of assessing BMI-related risks and implement personalized management strategies for patients considering kidney transplantation.
Acknowledgements
The Korean Organ Transplantation Registry Study Group: Jae Berm Park1, Jung Hwan Park2, Su Hyung Lee3, Ji Yoon Choi4, Cheol Woong Jung5, Jun Young Lee6, Yeong Hoon Kim7, Joong Kyung Kim8, Eun Jeoung Ko9, Sik Lee10, Yeon Ho Park11, Seok Hui Kang12, Tae Hyun Ban13, Sang Heon Song14, Seung Hwan Song15, Ho Sik Shin16, Byung Ha Chung17, Hye Eun Yoon18, Ki-Ryang Na19, Dong Ryeol Lee20, Dong Won Lee21, Jieun Oh22, Su Woong Jung23, Yu Ho Lee24, Hyejin Mo25, Jeong-Hoon Lee26, Jin Seok Jeon27, Sang Youb Han28, Jin Sug Kim29, Jong Soo Lee30, Man Ki Ju31, Jong Cheol Jeong32, Soo Jin Na Choi33, Sung Shin34, Seungyeup Han35, Young Soo Chung36, Young Joo Kwon37. 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; 2Department of Nephrology, Konkuk University School of Medicine, Seoul, Republic of Korea; 3Department of Surgery, Ajou University School of Medicine, Suwon, Republic of Korea; 4Department of Surgery, College of Medicine, Han Yang University, Seoul, Republic of Korea; 5Department of Surgery, Korea University Anam Hospital, Seoul, Republic of Korea; 6Department of Nephrology, Yonsei University Wonju College of Medicine, Wonju Severance Christian Hospital, Wonju, Republic of Korea; 7Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Republic of Korea; 8Department of Internal Medicine, Bongseng Memorial Hospital, Busan, Republic of Korea; 9Division of Nephrology, Department of Internal Medicine, Bucheon St. Mary's Hospital, Seoul, Republic of Korea; 10Department of Internal Medicine, Jeonbuk National University Hospital, Jeonju, Republic of Korea; 11Department of Surgery, Gil Medical Center, Gachon University College of Medicine, Incheon, Republic of Korea; 12Department of Nephrology, Yeungnam University Hospital, Daegu, Republic of Korea; 13Division of Nephrology, Department of Internal Medicine, Eunpyeong St. Mary's hospital, Seoul, Republic of Korea; 14Department of Internal Medicine, Pusan National University Hospital, Busan, Republic of Korea; 15Department of Surgery, Ewha Womans University Seoul Hospital, Seoul, Republic of Korea; 16Department of Internal Medicine, Devision of Nephrology, Kosin University College of Medicine, Busan, Republic of Korea; 17Division of Nephrology, Department of Internal Medicine, Seoul St. Mary's hospital, Seoul, Republic of Korea; 18Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea College of Medicine, Incheon, Republic of Korea; 19Department of Nephrology, Chungnam National University Hospital, Daejeon, Republic of Korea; 20Division of Nephrology, Department of Internal Medicine, Maryknoll Medical Center, Busan, Republic of Korea; 21Division of Nephrology, Department of Internal Medicine, Pusan National University School of Medicine, Busan, Republic of Korea; 22Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Republic of Korea; 23Division of Nephrology, Department of Internal Medicine, College of Medicine, Kyung Hee University, Seoul, Republic of Korea; 24Division of Nephrology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea; 25Department of Surgery, SMG-SNU Boramae Medical Center, Seoul, Republic of Korea; 26Department of Surgery, Myongji Hospital, Goyang, Republic of Korea; 27Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Seoul, Republic of Korea; 28Division of Nephrology, Inje University Ilsan-Paik Hospital, Goyang, Republic of Korea; 29Division of Nephrology, Department of Internal Medicine, College of Medicine, Kyung Hee University Hospital, Kyung Hee University, Seoul, Republic of Korea; 30Department of Internal Medicine, Ulsan University Hospital, Ulsan, Republic of Korea; 31Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; 32Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul, Republic of Korea; 33Department of Surgery, Chonnam National University Medical School, Gwangju, Republic of Korea; 34Department of Surgery, Asan Medical Center, Seoul, Republic of Korea; 35Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Republic of Korea; 36Department of Surgery, Dong-A University College of Medicine, Busan, Republic of Korea; 37Division of Nephrology, Korea University, College of Medicine, Guro Hospital, Seoul, Republic of Korea.
Abbreviations
- ABOi
ABO incompatible
- aHR
Adjusted hazard ratio
- BMI
Body mass index
- BP
Blood pressure
- CI
Confidence interval
- CVD
Cardiovascular disease
- DGF
Delayed graft function
- DM
Diabetes mellitus
- DSA
Donor specific antigen
- HDL
High-density lipoprotein
- HLA
Human leukocyte antigen
- HR
Hazard ratio
- HTN
Hypertension
- KOTRY
Korean Organ Transplantation Registry
- KT
Kidney transplantation
- KTRs
Kidney transplant recipients
- LDL
Low-density lipoprotein
- PRA
Panel reactive antibody
- WHO
World Health Organization
Author contributions
Conceptualization, J.-H.C. and C.-D.K.; methodology, Y.H.J. and J.-H.L.; formal analysis, Y.J.S.; investigation, Y.H.J.; data curation, Y.J.S.; writing—original draft preparation, E.-Y.C.; writing—review and editing, Y.H.J., J.-H.C. and C.-D.K.; supervision, K.H.H., S.D.H., S.M., H.-Y.J., J.-Y.C., S.-H.P., and Y.-L.K.; funding acquisition, J.Y. and M.S.K. All authors have read and approved the final manuscript.
Funding
This research was supported by the "National Institute of Health"(NIH) research project (2014-ER6301–00, 2014-ER6301–01, 2014-ER6301–02, 2017-ER6301–00, 2017-ER6301–01, 2017-ER6301–02, 2020-ER7201–00, 2020-ER7201–01, 2020-ER7201–02) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute(KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2022-KH130593 and RS-2025-02303987).
Data availability
The data of this study are available from the corresponding author on reasonable request. The data are not publicly available as they contain information that could compromise the privacy of research participants, and data sharing is subject to ethical and registry constraints in accordance with KOTRY policies.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Kyungpook National University Hospital (2020-11-056). Informed consent was obtained from all subjects involved in the study.
Ethics Statement
All procedures involving human participants were conducted in accordance with the 2008 Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The authors of The Korean Organ Transplantation Registry Study Group and their affiliations are provided in the Acknowledgments section.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eun-Young Cho and You Hyun Jeon contributed equally to this work.
Contributor Information
Jang-Hee Cho, Email: jh-cho@knu.ac.kr.
Chan-Duck Kim, Email: drcdkim@knu.ac.kr.
References
- 1.Hariharan S, Israni AK, Danovitch G. Long-Term survival after kidney transplantation. N Engl J Med. 2021;385(8):729–43. [DOI] [PubMed] [Google Scholar]
- 2.Foroutan F, Friesen EL, Clark KE, Motaghi S, Zyla R, Lee Y, Kamran R, Ali E, De Snoo M, Orchanian-Cheff A, et al. Risk factors for 1-Year graft loss after kidney transplantation: systematic review and Meta-Analysis. Clin J Am Soc Nephrol. 2019;14(11):1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C-D. Kidney transplantation. Korean J Med. 2014;86(2):142–51. [Google Scholar]
- 4.Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, Navaneethan SD, Ramanathan V. Trends in the causes of death among kidney transplant recipients in the united States (1996–2014). Am J Nephrol. 2018;48(6):472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinbokel T, Floerchinger B, Schmiderer A, Edtinger K, Liu G, Elkhal A, Tullius SG. Obesity and its impact on transplantation and alloimmunity. Transplantation. 2013;96(1):10–6. [DOI] [PubMed] [Google Scholar]
- 6.Wu D, Dawson NAJ, Levings MK. Obesity-Associated adipose tissue inflammation and transplantation. Am J Transpl. 2016;16(3):743–50. [DOI] [PubMed] [Google Scholar]
- 7.Chan WM, Bosch JAP, Jones DP, McTernan PGP, Phillips ACP, Borrows RMA. Obesity in kidney transplantation. J Ren Nutr. 2014;24(1):1–12. [DOI] [PubMed] [Google Scholar]
- 8.Jindal RM, Zawada ET. Obesity and kidney transplantation. Am J Kidney Dis. 2004;43(6):943–52. [DOI] [PubMed] [Google Scholar]
- 9.Lafranca JA, Ijermans JNM, Betjes MGH, Dor FJMF. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 2015;13(1):111–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicoletto BB, Fonseca NKO, Manfro RC, Gonçalves LFS, Leitão CB, Souza GC. Effects of obesity on kidney transplantation outcomes: A systematic review and Meta-Analysis. Transplantation. 2014;98(2):167–76. [DOI] [PubMed] [Google Scholar]
- 11.Hill CJ, Courtney AE, Cardwell CR, Maxwell AP, Lucarelli G, Veroux M, Furriel F, Cannon RM, Hoogeveen EK, Doshi M, et al. Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transpl. 2015;30(8):1403–11. [DOI] [PubMed] [Google Scholar]
- 12.Fellmann M, Balssa L, Clément E, Frey P, Frontczak A, Bernardini S, Chabannes É, Guichard G, Bittard H, Kleinclauss F. Effects of obesity on postoperative complications and graft survival after kidney transplantation. Transpl Proc. 2020;52(10):3153–9. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-M, Kwon HE, Ko Y, Jung JH, Kwon H, Kim YH, Kim EK, Shin S. Robot-assisted kidney transplantation in a morbidly obese patient with incisional hernia reconstruction and abdominoplasty: a case report. In. 2023;37:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill JS, Abichandani R, Kausz AT, Pereira BJG. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62(5):1875–83. [DOI] [PubMed] [Google Scholar]
- 16.Pischon T, Sharma AM. Obesity as a risk factor in renal transplant patients. Nephrol Dial Transpl. 2001;16(1):14–7. [DOI] [PubMed] [Google Scholar]
- 17.Shelton BA, Reed RD, MacLennan PA, McWilliams D, Mustian MN, Sawinski D, Kumar V, Ong S, Locke JE. Increasing obesity prevalence in the united States End-Stage renal disease population. J Health Sci Educ 2018, 2(5). [PMC free article] [PubMed]
- 18.Yang YS, Han B-D, Han K, Jung J-H, Son JW. Taskforce team of the obesity fact sheet of the Korean society for the study of O: obesity fact sheet in korea, 2021: trends in obesity prevalence and obesity-Related comorbidity incidence stratified by age from 2009 to 2019. J Obes Metabolic Syndrome. 2022;31(2):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin SF, Wu LY, Huang ZL, Fan Y, Lin T, Song TR. Nonlinear relationship between body mass index and clinical outcomes after kidney transplantation: A dose-response meta-analysis of 50 observational studies. Surgery. 2022;171(5):1396–405. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi SF, Zahmatkesh G, Streja E, Molnar MZ, Rhee CM, Kovesdy CP, Gillen DL, Steiner S, Kalantar-Zadeh K. Body mass index and mortality in kidney transplant recipients: A systematic review and Meta-Analysis. Am J Nephrol. 2014;40(4):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prudhomme T, Bento L, Frontczak A, Timsit M-O, Boissier R. Effect of recipient body mass index on kidney transplantation outcomes: A systematic review and Meta-analysis by the transplant committee from the French association of urology. European urology focus; 2023. [DOI] [PubMed]
- 22.Järv L, Pechter Ü, Kuudeberg A, Lember M, Ots-Rosenberg M. Effect of pretransplant body mass index on kidney transplant recipient and graft Long-term survival. Transpl Proc. 2021;53(10):2879–87. [DOI] [PubMed] [Google Scholar]
- 23.Barba C, Cavalli-Sforza T, Cutter J, Darnton-Hill I, consultation WHOe, Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (British edition). 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 24.Organization WH. The Asia-Pacific perspective: redefining obesity and its treatment. 2000.
- 25.Chow KM, Szeto CC, Leung CB, Lui SF, Tong YF, Li PK-T. Body mass index as a predictive factor for long-term renal transplant outcomes in Asians. Clin Transplant. 2006;20(5):582–9. [DOI] [PubMed] [Google Scholar]
- 26.Cheung CY, Chan YH, Chan HW, Chau KF, Li CS. Optimal body mass index that can predict long-term graft outcome in Asian renal transplant recipients. Nephrology. 2010;15(2):259–65. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa S, Tasaki M, Ikeda M, Nakagawa Y, Saito K, Tomita Y. Pretransplant BMI should be < 25 in Japanese kidney transplant recipients: A Single-Center experience. Transpl Proc. 2023;55(1):72–9. [DOI] [PubMed] [Google Scholar]
- 28.Ahn C, Koo TY, Jeong JC, Kim M, Yang J, Lee J, Min SI, Lee JE, Kim MS, Kwon OJ et al. Initial Report of the Korean Organ Transplant Registry: The First Report of National Kidney Transplantation Data. Transplantation proceedings 2014, 46(2):425–430. [DOI] [PubMed]
- 29.Yang J, Jeong JC, Lee J, Kim YH, Paik HC, Kim J-J, Park H-Y, Kim MS, Ahn C. Design and methods of the Korean organ transplantation registry. Transplantation Direct. 2017;3(8):e191–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet (British edition). 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lentine KL, Rocca-Rey LA, Bacchi G, Wasi N, Schmitz L, Salvalaggio PR, Abbott KC, Schnitzler MA, Neri L, Brennan DC. Obesity and cardiac risk after kidney transplantation: experience at one center and comprehensive literature review. Transplantation. 2008;86(2):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladhani M, Craig JC, Irving M, Clayton PA, Wong G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32(3):439–49. [DOI] [PubMed] [Google Scholar]
- 33.Czaja-Stolc S, Potrykus M, Stankiewicz M, Kaska Ł, Małgorzewicz S. Pro-Inflammatory profile of adipokines in obesity contributes to pathogenesis, nutritional disorders, and cardiovascular risk in chronic kidney disease. Nutrients. 2022;14(7):1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf G, Chen S, Han DC, Ziyadeh FN. Leptin and renal disease. Am J Kidney Dis. 2002;39(1):1–11. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Montoro JI, Morales E, Cornejo‐Pareja I, Tinahones FJ, Fernández‐García JC. Obesity‐related glomerulopathy: current approaches and future perspectives. Obes Rev. 2022;23(7):e13450. -n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molinero LL, Yin D, Lei YM, Chen L, Wang Y, Chong AS, Alegre M-L. High-Fat Diet–Induced obesity enhances allograft rejection. Transplantation. 2016;100(5):1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto Y, Christen T, Shimizu K, Asano K, Kihara S, Mitchell RN, Libby P. Adiponectin inhibits allograft rejection in murine cardiac transplantation. Transplantation. 2009;88(7):879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dedinská I, Kleinová P, Macháleková K, Graňák K, Vnučák M, Beliančinová M. The role of hyperleptinaemia and low values of Interleukin 10 in de Novo DSA production after kidney transplantation. Transpl Immunol. 2024;83:101982–101982. [DOI] [PubMed] [Google Scholar]
- 39.Meier-Kriesche HU, Ojo AO, Cibrik DM, Hanson JA, Leichtman AB, Magee JC, Port FK, Kaplan B. Relationship of recipient age and development of chronic allograft failure. Transplantation. 2000;70(2):306–10. [DOI] [PubMed] [Google Scholar]
- 40.Peeters LEJ, Andrews LM, Hesselink DA, de Winter BCM, van Gelder T. Personalized immunosuppression in elderly renal transplant recipients. Pharmacol Res. 2018;130:303–7. [DOI] [PubMed] [Google Scholar]
- 41.Park Y, Lee H, Ko EJ, Lee S, Ban TH, Min J-w, Yoon H-e, Oh E-j, Yang CW, Chung BH. Impact of high body mass index on allograft outcomes in kidney transplant recipients with presensitization to human leukocyte antigen. Kidney Res Clin Pract. 2021;40(2):304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarznau A, Matevossian E, Novotny A, Stangl M. Outcome of living donor renal transplantation in obese recipients. Transpl Proc. 2008;40(4):921–2. [DOI] [PubMed] [Google Scholar]
- 43.Yemini R, Rahamimov R, Nesher E, Anteby R, Ghinea R, Hod T, Mor E. The impact of obesity and associated comorbidities on the outcomes after renal transplantation with a living donor vs. Deceased donor grafts. J Clin Med. 2022;11(11):3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najarian JS, Gillingham KJ, Sutherland DER, Reinsmoen NL, Payne WD, Matas AJ. The impact of the quality of initial graft function on cadaver kidney transplants. In: 1994; Hagerstown, MD: Lippincott: 812–6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author on reasonable request. The data are not publicly available as they contain information that could compromise the privacy of research participants, and data sharing is subject to ethical and registry constraints in accordance with KOTRY policies.