Abstract
Objective
To propose a new classification method based on CT three-dimensional reconstruction for inferior articular process injury following percutaneous endoscopic interlaminar lumbar discectomy (PEID), and to analyze the impact of > 50% inferior articular process(IAP) defect on clinical outcomes.
Methods
A retrospective analysis was conducted on 100 PEID patients. IAP injuries were classified into four types based on CT three-dimensional reconstruction, and the inter- and intra-observer reliability was assessed using the Kappa consistency test. Patients were divided into two groups based on IAP defect size: Group A (defect ≤ 50%) and Group B (defect > 50%). VAS, ODI, clinical outcomes, and lumbar instability were compared between the two groups, and IAP changes were observed.
Results
The classification showed good inter- and intra-observer consistency. There were no significant differences in VAS, ODI, clinical outcomes, or lumbar instability between the two groups (P > 0.05). Both groups showed varying degrees of IAP regeneration and remodeling.
Conclusion
The classification of IAP injuries based on CT three-dimensional reconstruction demonstrates good inter- and intra-observer consistency. In the short term, patients with > 50% IAP defects (Types III and IV) show no difference in lumbar stability or clinical outcomes compared to those with ≤ 50% defects (Types I and II).However, for patients with complete IAP loss (Type IV), the potential long-term risk of lumbar instability and related clinical complications remains a concern.
Keywords: Interlaminar approach, Inferior articular process injury, Lumbar instability, CT three-dimensional reconstruction, Classification
Introduction
Lumbar disc herniation (LDH) is a common and frequently encountered condition in spinal surgery, most often affecting the L4/5 and L5/S1 segments, and typically requires surgical intervention when conservative treatment fails [1]. Percutaneous endoscopic interlaminar discectomy (PEID) was first introduced by Ruetten in 2006 [2]. PEID often requires foraminoplasty of the inferior articular process (IAP) during surgery to better expose intraspinal structures and achieve sufficient decompression.However, the extent of IAP resection and its impact on clinical outcomes remain poorly understood.
Previous studies generally suggest that unilateral facet resection of less than 50% has minimal impact on lumbar stability, whereas resection exceeding 50% significantly increases the risk of spinal instability, potentially necessitating additional pedicle screw fixation to enhance stability [3–5], however, some scholars have argued that the actual impact of facet joint resection > 50% on spinal stability may be limited [6], these findings indicate that controversy still exists on this issue.Moreover, most of the aforementioned studies are based on biomechanical experiments or finite element analysis, with a lack of clinical follow-up data. In addition, the evaluation of IAP injury still primarily depends on traditional imaging methods such as MRI and conventional CT, which are limited by poor visualization of bony structures and a rough estimation of injury severity [7, 8]. In contrast, CT three-dimensional reconstruction, with its high resolution and multi-dimensional imaging capabilities, offers a novel and precise method for evaluating IAP injuries.
Therefore, to address the above issues, this study aims to classify the extent of IAP injury after PEID using CT three-dimensional reconstruction, and to investigate the impact of > 50% IAP defect on clinical outcomes, with the goal of providing more evidence-based guidance for clinical practice.
Materials and methods
General information
A total of 100 patients who underwent PEID at our hospital from January 2021 to December 2023 were enrolled.Among them, 54 were male and 46 were female, with ages ranging from 18 to 66 years (mean age 47.5 ± 10.94 years).
Inclusion Criteria: (1) Age ≥ 18years; (2) Definitive diagnosis of single-level lumbar disc herniation (including paracentral, central, and sequestered types) based on clinical symptoms, physical examination, and imaging, with unilateral nerve root symptoms only; (3) The responsible segment is either L4/5 or L5/S1; (4) Ineffective conservative treatment for ≥ 3months; (5)Availability of complete and high-quality imaging data, including lumbar flexion-extension X-rays, plain CT scans, and three-dimensional reconstruction.Exclusion Criteria: (1) Patients with definite pathological changes such as lumbar scoliosis, deformity, fracture, infection, or tumor; (2)Patients with foraminal or far-lateral disc herniation, or disc herniation accompanied by segmental instability; (3) Responsible segments other than L4/5 or L5/S1; (4) History of prior lumbar spine surgery. This study was approved by the hospital ethics committee (Approval No. EC-2023-007), and all patients provided written informed consent.
Surgical procedure
After general anesthesia, the patient was placed in the prone position, and the surgical table was adjusted to reduce lumbar lordosis and sufficiently open the interspinous space. After routine sterilization and draping, the responsible segment was localized using C-arm fluoroscopy and marked. A puncture point was made approximately 2 cm lateral to the spinous process, and the needle was advanced to the target intervertebral space. A skin incision was made, and sequential dilators were inserted bluntly, followed by the placement of the working cannula and the endoscopic working channel. Soft tissues on the surface of the ligamentum flavum were thoroughly cleared, and the lower margin of the lamina of the upper vertebra and the IAP were carefully identified. Depending on intraoperative conditions, a high-speed burr or lamina rongeur was used to resect part of the IAP and/or lamina to enlarge the working space. The ligamentum flavum was incised layer by layer with a Basket Forceps to expose the epidural fat, nerve root, and dural sac. A nerve dissector was used to probe for annular rupture or weak areas. Once the intervertebral space was confirmed, the working cannula was rotated to retract and protect the nerve root or dural sac. Protruded nucleus pulposus was removed using nucleus pulposus forceps, and loose disc material within the disc space was also excised as completely as possible. After confirming no residual disc fragments, radiofrequency ablation was applied to shape the annulus fibrosus, followed by meticulous hemostasis and withdrawal of the working cannula.
Radiological classification, grouping, and reliability assessment
Thin-slice lumbar CT scans (in DICOM format) of patients who underwent PEID were extracted and processed using the built-in three-dimensional reconstruction tools of our hospital’s PACS system.Method for classifying IAP injuries: The boundaries of the IAP were first delineated (shaded area outlined in yellow, see Fig. 1), with the superior limit defined as the upper margin of the facet joint, the inferior limit as the tip of the IAP, the medial border as the inner edge, and the lateral border as the outer edge of the IAP. The IAP was then divided into four equal parts (0–100%) at the mid-level of the facet joint (see Fig. 1), and classified into four types based on the degree of bony defect, using the contralateral intact IAP as reference. Type I: Bony defect < 25% of the IAP, with an intact articular surface, with or without lamina involvement. Type II: Bony defect > 25% but ≤ 50%, partial articular surface damage, with or without lamina involvement. Type III: Bony defect > 50% but incomplete (< 100%), with extensive articular surface damage, with or without lamina involvement. Type IV: Complete loss of the IAP (100%).Based on the extent of IAP loss, patients were divided into Group A (defect ≤ 50%, Types I and II) and Group B (defect > 50%, Types III and IV).
Fig. 1.
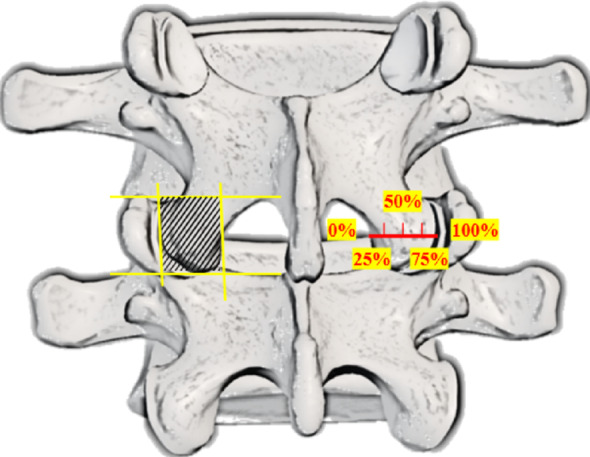
Schematic diagram showing the delineated area of the IAP (shaded region) and the grading of bony defect extent (0–100%)
Three spine surgeons who were not involved in the development of the classification system were selected as observers to evaluate its consistency. No relevant information was provided to the observers during the evaluation process, and communication between them was strictly prohibited. One month later, all patient data were re-randomized using a random number table, and a second round of classification was performed.
Follow-up evaluation indicators
Patients were confined to bed on the day of surgery and allowed to ambulate with a lumbar brace on the following day; heavy physical activity was restricted for three months postoperatively. The follow-up period ranged from 1 to 2 years. All patients underwent immediate postoperative CT and three-dimensional reconstruction to classify IAP injuries.At the final follow-up, repeat CT scans and three-dimensional reconstructions were performed to evaluate changes in the IAP, lumbar flexion-extension radiographs were also performed to assess spinal instability, defined as intervertebral translation > 3 mm or angular change > 10° on standing flexion-extension X-rays [9]. Postoperative recovery was evaluated using the Visual analogue scale (VAS) and Oswestry disability index (ODI), clinical outcomes at the final follow-up were assessed using the modified MacNab criteria.
Statistical analysis
Statistical analyses were performed using SPSS version 26.0. Kappa values were calculated to assess inter- and intra-observer agreement, using the classification system proposed by Landis and Koch [10]: mild confidence (0.01–0.20), mild to moderate confidence (0.21–0.40), moderate confidence (0.41–0.60), basic confidence (0.61–0.80) and complete confidence (> 0.80). Continuous variables were expressed as mean ± standard deviation ( ±s). Between-group comparisons were performed using the independent-samples t test.Repeated measures ANOVA was used for within-group comparisons across time points, with post hoc pairwise comparisons conducted using the LSD method.Categorical data were expressed as frequencies and percentages [n(%)], and intergroup comparisons were made using Fisher’s exact test, with P < 0.05 indicating statistical difference.
±s). Between-group comparisons were performed using the independent-samples t test.Repeated measures ANOVA was used for within-group comparisons across time points, with post hoc pairwise comparisons conducted using the LSD method.Categorical data were expressed as frequencies and percentages [n(%)], and intergroup comparisons were made using Fisher’s exact test, with P < 0.05 indicating statistical difference.
Results
Distribution of the new classification and consistency testing
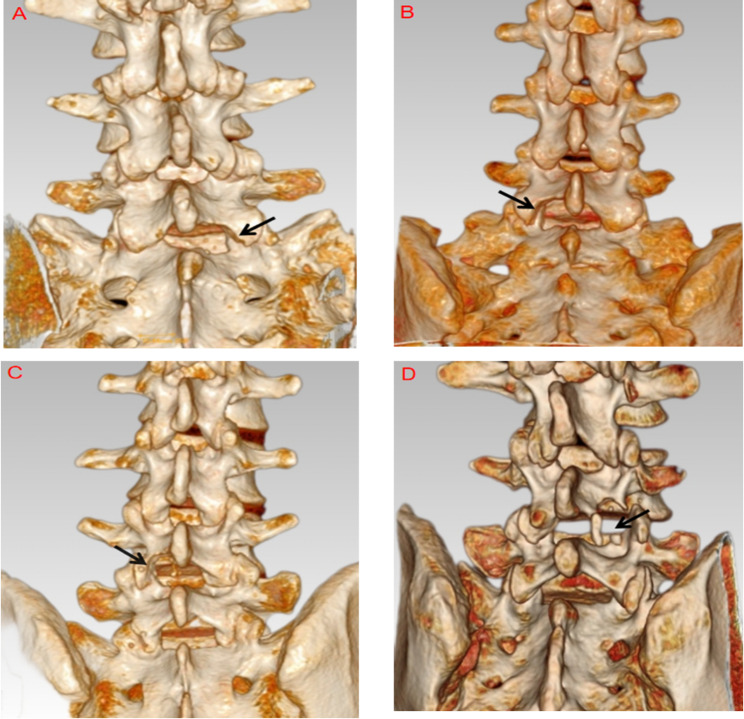
CT three-dimensional reconstruction-based classification of IAP injuries was performed in 100 patients who underwent PEID. The detailed distribution is as follows. Type I: 40 cases, including 7 at L4/5 and 33 at L5/S1; disc herniation types included 38 paracentral, 2 central, and 0 sequestered. Type II: 27 cases, including 18 at L4/5 and 9 at L5/S1; 24 paracentral, 2 central, and 1 sequestered. Type III: 28 cases, including 24 at L4/5 and 4 at L5/S1; 22 paracentral, 4 central, and 2 sequestered. Type IV: 5 cases, all at L4/5 and none at L5/S1;1 paracentral, 3 central, and 1 sequestered.Representative cases of each type are shown in Fig. 2A–D.
Fig. 2.
Type I: Right L5 IAP with < 25% bony defect, intact articular surface, and no lamina injury (A); Type II: Left L5 IAP with > 25% and ≤ 50% defect, partial articular surface damage, and associated lamina injury (B); Type III: Left L4 IAP with > 50% but < 100% defect, major articular surface damage, and lamina injury (C); Type IV: Right L4 IAP with complete loss (100%) (D)
Inter-observer reliability analysis of the new classification showed an average agreement rate of 94.53% (range: 85.71–100%) among the three observers, with a Kappa value of 0.882 (range: 0.795–1). The agreement rates for Types I, II, III, and IV were 95.00%, 93.83%, 89.29%, and 100%, respectively, with corresponding Kappa values of 0.859, 0.856, 0.814, and 1, indicating almost perfect reliability. See Table 1.
Table 1.
Inter-observer consistency analysis of the new classification
| Classification | Physician 1–2 | Physician 1–3 | Physician 2–3 | |||
|---|---|---|---|---|---|---|
| Consistent number of cases(%) | Kappa value | Consistent number of cases(%) | Kappa value | Consistent number of cases(%) | Kappa value | |
| Type I | 38(95.00%) | 0.869 | 37(92.50%) | 0.813 | 39(97.50%) | 0.894 |
| Type II | 25(92.59%) | 0.865 | 26(96.30%) | 0.881 | 25(92.59%) | 0.823 |
| Type III | 24(85.71%) | 0.795 | 25(89.29%) | 0.812 | 26(92.86%) | 0.836 |
| Type IV | 5(100%) | 1 | 5(100%) | 1 | 5(100%) | 1 |
Intra-observer consistency analysis showed an average agreement rate of 97.06% (range: 92.86–100%) across the three observers, with a Kappa value of 0.933 (range: 0.845–1). Agreement rates for Types I, II, III, and IV were 96.67%, 97.53%, 94.05%, and 100%, with corresponding Kappa values of 0.912, 0.947, 0.875, and 1, all indicating almost perfect reliability. See Table 2.
Table 2.
Intra-observer consistency analysis of the new classification
| Classification | Physician 1 | Physician 2 | Physician 3 | |||
|---|---|---|---|---|---|---|
| Consistent number of cases(%) | Kappa value | Consistent number of cases(%) | Kappa value | Consistent number of cases(%) | Kappa value | |
| Type I | 39(97.50%) | 0.934 | 39(97.50%) | 0.934 | 38(95.00%) | 0.869 |
| Type II | 27(100.00%) | 1 | 26(96.30%) | 0.921 | 26(96.30%) | 0.921 |
| Type III | 26(92.86%) | 0.845 | 27(96.43%) | 0.935 | 26(92.86%) | 0.845 |
| Type IV | 5(100.00%) | 1 | 5(100.00%) | 1 | 5(100%) | 1 |
Clinical outcomes analysis of patients with > 50% IAP defect
Patients were divided into two groups based on the extent of IAP defect: Group A (defect ≤ 50%, Types I and II; n = 67) and Group B (defect > 50%, Types III and IV; n = 33). There were no statistically significant differences in baseline characteristics (age, sex, disease duration) between the two groups (P > 0.05), indicating comparability.In both groups, VAS and ODI scores significantly improved at immediate postoperative and final follow-up compared to preoperative values (P < 0.05). A statistically significant difference was also observed between the immediate postoperative period and the final follow-up (P < 0.05). However, intergroup equivalence were found between the two groups at any time point (P > 0.05). At the final follow-up, there were 2 cases and 1 case of lumbar instability in group A and group B, the excellent/good rates of modified MacNab criteria were 98.5% and 96.9%, but no statistical divergence was found between the two groups (P > 0.05). See Table 3.
Table 3.
Clinical outcomes of the two groups of patients
| Group A (n = 67) | Group B (n = 33) | Statistical value | |
|---|---|---|---|
| VAS(score) | |||
| Preoperative | 5.64 ± 1.069 | 5.73 ± 1.098 |
t= − 0.373 p = 0.71 |
| Immediate postoperative | 1.61 ± 0.758 | 1.39 ± 0.659 |
t = 1.410 p = 0.162 |
| Final follow-up | 0.85 ± 0.609 | 0.82 ± 0.584 |
t= − 0.255 p = 0.799 |
| Statistical Value |
F = 638.064 p = 0.000 |
F = 360.222 p = 0.000 |
|
| ODI(%) | |||
| Preoperative | 61.2 ± 10.0 | 61.3 ± 7.3 |
t= − 0.033 p = 0.974 |
| Immediate postoperative | 27.0 ± 5.9 | 26.8 ± 4.1 |
t= − 0.195 p = 0.846 |
| Final follow-up | 9.6 ± 2.5 | 9.9 ± 2.4 |
t= − 0.558 p = 0.578 |
| Statistical value |
F = 989.445 p = 0.000 |
F = 900.016 p = 0.000 |
|
| Modified MacNab criteria (n) | p = 1.000 | ||
| Excellent | 60 | 29 | |
| Good | 6 | 3 | |
| Fair | 1 | 1 | |
| Poor | 0 | 0 | |
| Lumbar instability (n) | p = 1.000 | ||
| Yes | 2 | 1 | |
| No | 65 | 32 |
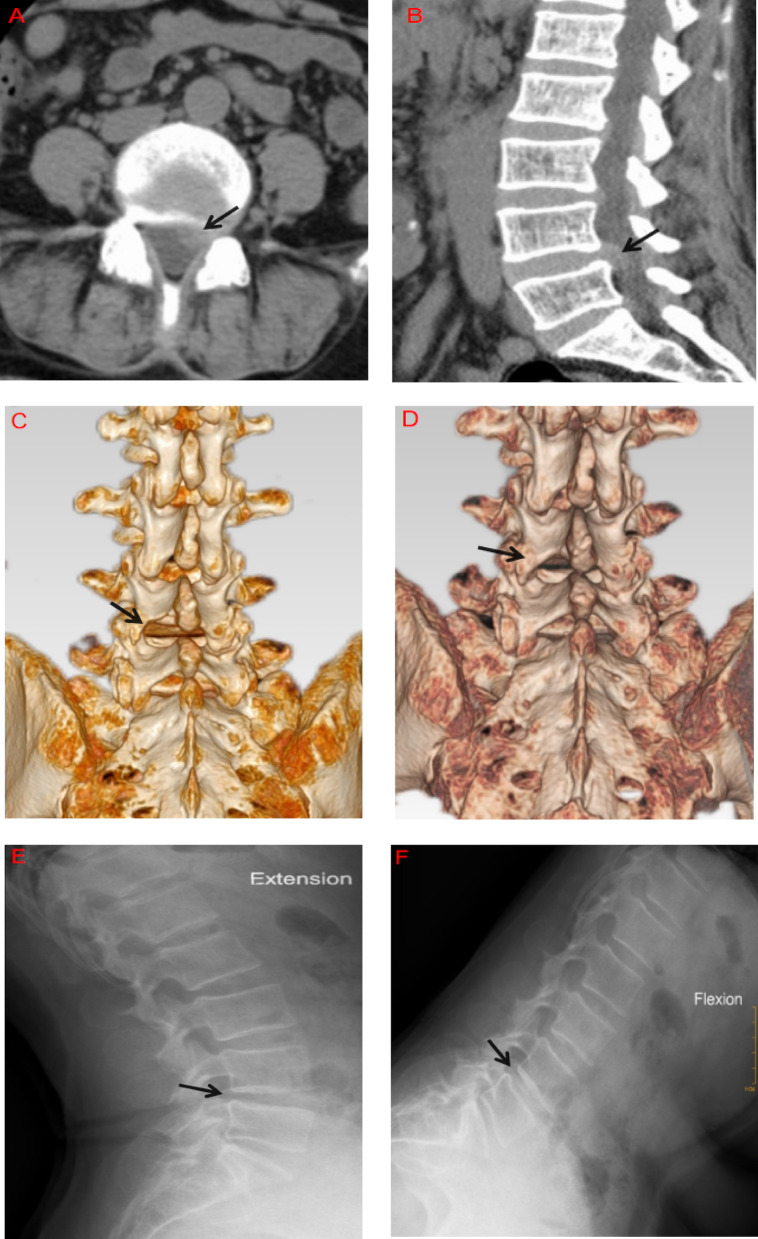
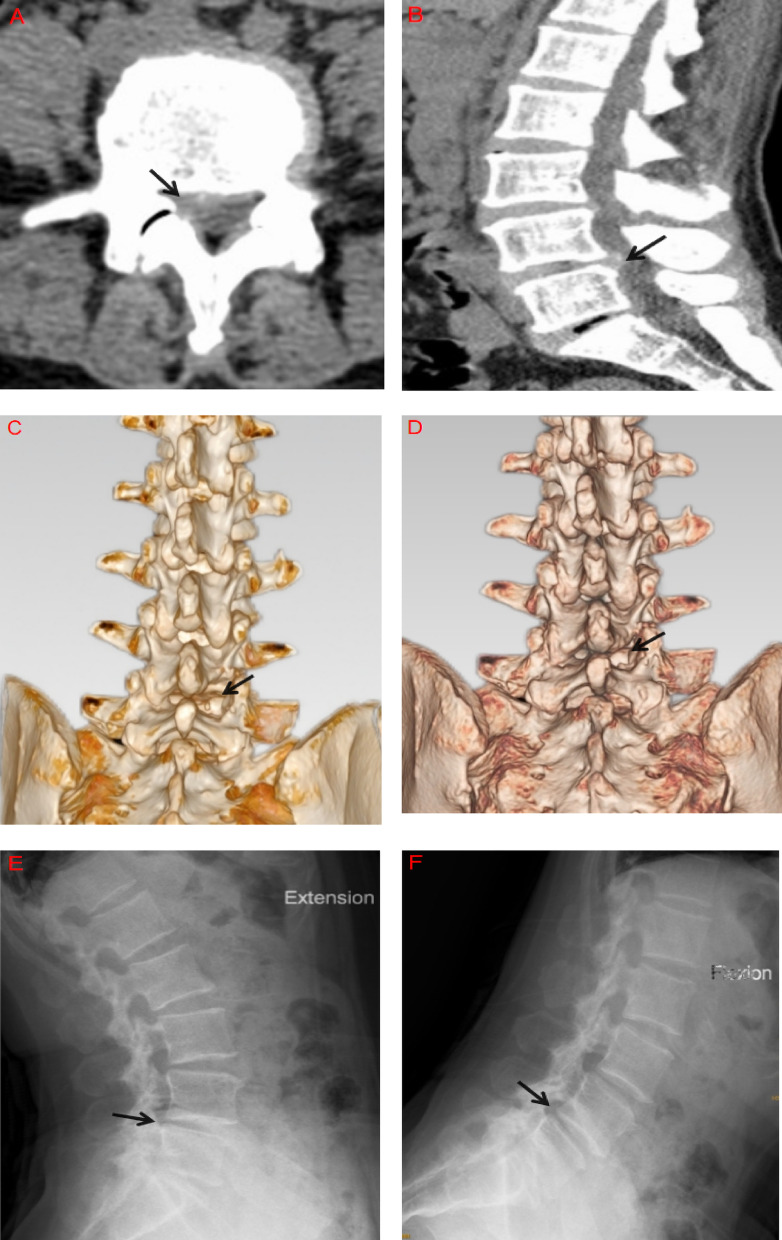
At the final follow-up, 78 patients showed varying degrees of IAP regeneration and remodeling (56 in Group A and 22 in Group B): Among 40 cases of Type I injury, 35 had restored IAP morphology comparable to the contralateral intact side.Among 27 Type II cases, 21 had remodeled to Type I. Of the 28 Type III cases, 21 remodeled to Type II, and 1 case to Type I.In contrast, all 5 patients with Type IV injury still showed complete IAP loss at the final follow-up. Representative follow-up cases of IAP defects > 50% (Types III and IV) are shown in Figs. 3A–F and 4A–F.
Fig. 3.
A 51-year-old female. Axial and sagittal CT images show L4/5 paracentral disc herniation with compression of the left L5 nerve root (A, B). Postoperative three-dimensional CT reconstruction reveals a Type III injury of the left IAP (C). At 1-year follow-up, evidence of regeneration and remodeling is observed at the defect site, classified as Type II (D). Flexion-extension lumbar X-rays show no instability at L4/5 (E, F)
Fig. 4.
A 52-year-old female. Axial and sagittal CT images show L4/5 paracentral disc herniation with compression of the right L5 nerve root (A, B). Postoperative three-dimensional CT reconstruction reveals a Type IV injury of the right IAP (C). At 1.5-year follow-up, the right IAP remained absent with no morphological change (D). Flexion-extension lumbar X-rays show no instability at the L4/5 level (E, F)
Discussion
During PEID procedures, partial resection of the IAP is often necessary to facilitate exposure and decompression, particularly in cases of sequestered disc herniation, elongated or hypertrophic facet joints, where IAP injury is more likely to occur. The severity of IAP injury is commonly evaluated by measuring the volume of the defect and calculating the facet joint preservation ratio—defined as the residual length of the IAP as a percentage of the SAP length on coronal CT images, or the postoperative to preoperative facet joint surface area ratio on axial CT images [8, 11, 12], however, variations in imaging slice selection or interference from surrounding tissues can result in considerable measurement bias.In this study, the severity of IAP injury was quantitatively assessed using CT three-dimensional reconstruction, providing more intuitive and precise results. The classification demonstrated high inter- and intra-observer consistency (Kappa values > 0.80), confirming its reliability. Its clinical utility is reflected in the following aspects: (1) In preoperative planning, especially for complex cases requiring extended exposure, pre-estimation of the IAP resection range can help optimize the surgical approach and reduce unnecessary bone removal due to blind intraoperative expansion. (2) For patients classified as Type III or IV, where > 50% of the facet is resected, follow-up should include focused monitoring to assess potential long-term risk of lumbar instability. (3) Previous studies have often used vague or inconsistent descriptions of IAP resection extent [13, 14], making it difficult to standardize patient inclusion. The proposed classification helps address discrepancies caused by heterogeneity in assessment methods across studies.
During PEID, determining the appropriate extent of IAP resection is often challenging. Inadequate resection may result in poor exposure of the nerve root and dural sac, increasing the risk of inadvertent neural injury or incomplete removal of compressive disc material. Conversely, excessive resection may compromise facet joint stability and predispose to long-term lumbar instability [15, 16]. Erbulut DU [17] using finite element analysis, demonstrated that resection of more than 50% of a unilateral facet joint significantly alters lumbar biomechanics during extension and axial rotation, thereby increasing the risk of spinal instability. Zhou et al. [18] also reported that unilateral facet joint resection exceeding 50% significantly compromises lumbar spine stability. The results of this study showed that although patients in Group B (Types III and IV) underwent > 50% unilateral facet joint resection, there were no meaningful differences in lumbar instability or clinical outcomes compared to Group A ( Types I and II) (P > 0.05),this finding contrasts with traditional assumptions. Several factors may account for this discrepancy: (1) Only a single lumbar segment was treated, leaving the overall biomechanical chain intact. Moreover, the IAP is just one of several structures contributing to lumbar stability [19, 20]; compensatory mechanisms from surrounding ligaments, paraspinal muscles, and intervertebral discs may mitigate the effects of partial bony loss. (2) According to the theory of dynamic stabilization [21], postoperative use of a lumbar brace and functional exercises may enhance core musculature, contributing to segmental stability and reducing the incidence of lumbar instability. (3) In this study, 78% of patients with IAP injury exhibited evidence of bone regeneration and morphological remodeling at the defect site by final follow-up, partially or completely restoring the integrity of the facet joint, thereby providing an anatomical basis for maintained spinal stability.Similar bone regeneration phenomena have been reported in previous studies [22–24], although the underlying mechanisms remain poorly understood.Based on the above findings, this study provides valuable reference for determining the extent of IAP resection during PEID. It allows clinicians to appropriately expand the resection range in selected cases to ensure adequate exposure and decompression, without excessive concern over postoperative lumbar instability. Furthermore, we believe that routine supplementary fixation and fusion may not be necessary when facet joint resection exceeds 50%, however, this does not imply that unrestricted IAP removal is acceptable during surgery. Our follow-up also revealed that in patients with complete IAP resection (Type IV), the defect site underwent fibrous healing without evidence of bone regeneration. These cases were often associated with axial vertebral rotation and osteophyte formation, increasing the risk of long-term lumbar instability or functional impairment.
Conclusion
The CT-based three-dimensional classification system proposed in this study allows for more accurate evaluation of the extent and severity of IAP injury following PEID.Short-term follow-up revealed that IAP defects greater than 50% did not negatively impact lumbar stability or clinical outcomes. However, for patients with complete IAP loss (Type IV), the potential long-term risk of lumbar instability and related clinical complications remains a concern. This study has several limitations: the proposed classification does not account for associated damage to the facet capsule, ligaments, or other soft tissues, which may compromise its comprehensiveness; in addition, this was a single-center retrospective study with a relatively small sample size and short follow-up duration, which may affect the generalizability of the findings. Therefore, future studies with larger sample sizes, multicenter designs, prospective approaches, and longer follow-up are needed to validate these findings.
Acknowledgements
We thank the staff of Zigong Fourth People’s Hospital for their assistance in data collection and imaging analysis.
Abbreviations
- CT
Computed tomography
- PEID
Percutaneous endoscopic interlaminar discectomy
- IAP
Inferior articular process
- LDH
Lumbar disc herniation
- VAS
Visual analogue scale
- ODI
Oswestry disability index
Author contributions
Haigang Hu: study design, data collection, statistical analysis, manuscript drafting, Chao Wu: radiographic evaluation, clinical data acquisitionTao Li: radiographic evaluation, patient follow-up, clinical data acquisitionHong Li: radiographic evaluation, patient follow-up, clinical data acquisition.
Funding
This study was supported by the Zigong Key Science and Technology Program-Zigong Academy for Medical Big Data and Artificial Intelligence Joint Project, Sichuan Province, China (Project No.: 2023-YGY-2-05).
Data availability
The data supporting the findings of this study are not publicly available due to privacy concerns and their intended use in future research. However, de-identified and anonymized data may be obtained from the corresponding author upon reasonable request, subject to Institutional Review Board (IRB) approval and compliance with ongoing research requirements.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Zigong Fourth People’s Hospital (Approval No.: EC-2023-007). All procedures were conducted in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments. The requirement for informed consent was waived by the Ethics Committee of Zigong Fourth People’s Hospital due to the retrospective nature of the study and the use of anonymized patient data.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Ercole M, Innocenzi G, Ricciardi F, et al. Prognostic value of Michigan state university (MSU) classification for lumbar disc herniation: is it suitable for surgical selection?? Int J Spine Surg. 2021;15(3):466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruetten S, Komp M, Godolias G. A new full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: prospective 2-year results of 331 patients. Minim Invasive Neurosurg. 2006;49(2):80–7. [DOI] [PubMed] [Google Scholar]
- 3.Lee KK, Teo EC, Qiu TX, et al. Effect of facetectomy on lumbar spinal stability under sagittal plane loadings. Spine (Phila Pa 1976). 2004;29(15):1624–31. [DOI] [PubMed] [Google Scholar]
- 4.Zhi-Li Z, Rui Z, Yang-Chun W, et al. Effect of graded facetectomy on lumbar biomechanics. J Healthc Eng. 2017;2017:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enyo Y, Yamada H, Kim JH, et al. Microendoscopic lateral decompression for lumbar foraminal stenosis: a biomechanical study. J Spinal Disord Tech. 2014;27(5):257–62. [DOI] [PubMed] [Google Scholar]
- 6.Haufe SM, Mork AR. Effects of unilateral endoscopic facetectomy on spinal stability. J Spinal Disord Tech. 2007;20(2):146–8. [DOI] [PubMed] [Google Scholar]
- 7.Heo DH, Lee DC, Park CK. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis:biportal endoscopy,uniportal endoscopy, and microsurgery. Neurosurg Focus. 2019;46(5):E9. [DOI] [PubMed]
- 8.Ito Z, Shibayama M, Nakamura S et al. Clinical comparison of unilateral biportal endoscopic laminectomy versus microendoscopic laminectomy for Single-Level laminectomy: a single-center, retrospective analysis. World Neurosurg. 2021;148:e581–8. [DOI] [PubMed]
- 9.Elmose SF,Andersen GO, Carreon LY et al. Radiological definitions of sagittal plane segmental instability in the degenerative lumbar spine a systematic review. Global Spine J. 2023;13(2):523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 11.Doi T, Hirai S, Horii C, et al. Effect of facet joint resection on postoperative radiographic and clinical outcomes after microendoscopic laminectomy for single-Level lumbar spinal stenosis. World Neurosurg. 2024;192:e565–71. [DOI] [PubMed] [Google Scholar]
- 12.Li DY, Su QJ, Zhang XN, et al. Clinical study of lumbar stability after unilateral biportal endoscopy in the treatment of degenerative lumbar diseases. Chin J Surg. 2024;62(3):187–93. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Xie YZ, Zhou Q, et al. The Biomechanical effect of the relevant segments after facet-disectomy in different diameters under posterior lumbar percutaneous endoscopes: a three-dimensional finite element analysis. J Orthop Surg Res. 2021;16(1):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976). 1990;15(11):1142–7. [DOI] [PubMed] [Google Scholar]
- 15.Fukuhara D, Ono K, Kenji T, et al. A narrative review of full-endoscopic lumbar discectomy using interlaminar approach. World Neurosurg. 2022;168:324–32. [DOI] [PubMed] [Google Scholar]
- 16.Ju CI, Lee SM. Complications and management of endoscopic spinal surgery. Neurospine. 2023;20(1):56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erbulut DU. Biomechanical effect of graded facetectomy on asymmetrical finite element model of the lumbar spin. Turk Neurosurg. 2014;24(6):923–8. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Luo G, Chu TW, et al. The Biomechanical change of lumbar unilateral graded facetectomy and strategies of its microsurgical reconstruction: report of 23 cases. Zhonghua Yi Xue Za Zhi. 2007;87(19):1334–8. [PubMed] [Google Scholar]
- 19.Inoue N, Orías AAE, Segami K. Biomechanics of the lumbar facet joint. Spine Surg Relat Res. 2019;4(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapetanakis S, Gkantsinikoudis N. Anatomy of lumbar facet joint: a comprehensive review. Folia Morphol (Warsz). 2021;80(4):799–805. [DOI] [PubMed] [Google Scholar]
- 21.Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5(4):390–6. discussion 397. [DOI] [PubMed] [Google Scholar]
- 22.Dohzono S, Matsumura A, Terai H, et al. Radiographic evaluation of postoperative bone regrowth after microscopic bilateral decompression via a unilateral approach for degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2013;18(5):472–8. [DOI] [PubMed] [Google Scholar]
- 23.Guigui P, Barre E, Benoist M, et al. Radiologic and computed tomography image evaluation of bone regrowth after wide surgical decompression for lumbar stenosis. Spine (Phila Pa 1976). 1999;24(3):281–8. discussion 288-9. [DOI] [PubMed] [Google Scholar]
- 24.Dohzono S, Toyoda H, Matsumura A, et al. Clinical and radiological outcomes after microscopic bilateral decompression via a unilateral approach for degenerative lumbar disease: minimum 5-year follow-up. Asian Spine J. 2017;11(2):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to privacy concerns and their intended use in future research. However, de-identified and anonymized data may be obtained from the corresponding author upon reasonable request, subject to Institutional Review Board (IRB) approval and compliance with ongoing research requirements.