Abstract
Pneumolysin (PLY) is a major virulence factor of Streptococcus pneumoniae that elicits a variety of proinflammatory responses from cells of the host immune system. Intercellular adhesion molecule 1 (ICAM-1) is a cell adhesion molecule involved in leukocyte trafficking toward inflammatory stimuli in extravascular sites. In this study, we evaluated the effect of PLY on expression of ICAM-1 in THP-1 monocytic cells exposed to S. pneumoniae. Exposure of cells to PLY-expressing S. pneumoniae strain WU2 for 6 h led to significantly higher levels of ICAM-1 message than those in cells exposed to either medium alone or ΔPLY1, a PLY-negative isogenic mutant of WU2. Cells exposed to purified recombinant PLY also showed a dose-dependent increase in ICAM-1 mRNA compared to cells exposed to medium alone. Exposure to recombinant PLY containing a single amino acid substitution (Trp433→Phe) that decreases cytolytic activity did not increase ICAM-1 mRNA to levels seen with wild-type PLY. In addition, THP-1 cells exposed to wild-type strain WU2 or D39 had increased ICAM-1 on their surface compared to cells exposed to medium alone or their PLY-negative isogenic mutants ΔPLY1 and ΔPLY2, respectively. These data indicate that PLY induces transcription and production of a cell adhesion molecule involved in the inflammatory response that may play a role in pneumococcal infection.
Streptococcus pneumoniae (pneumococcus) is responsible for a number of human diseases including pneumonia, bacteremia, and meningitis, as well as being the cause of the less serious but far more common infections of sinusitis and otitis media. Worldwide, the pneumococcus is a significant health threat, especially in children under the age of five and the elderly. Despite this reputation for morbidity and mortality, the pneumococcus is normally a commensal colonizing the nasopharynx of healthy asymptomatic carriers (18). The events which cause the bacterium to become an invasive pathogen have yet to be fully determined. However, it has been proposed that the host immune response, particularly inflammatory factors and mediators, likely plays a role (36).
Colonization of the nasopharynx occurs as pneumococci bind to surface carbohydrates of the respiratory epithelium. This initial adherence is mediated by cell wall-associated proteins including pneumococcal surface protein C (PspC), also known as CbpA or SpsA (5, 18). Aspiration allows the bacterium access to the lower respiratory tract; however, additional events such as the activation of cells by preexisting viral infection and cytokine production are thought to be required for progression to pneumonia (37). The pneumococcus exploits the activated host cells by binding to up-regulated surface receptors, such as platelet-activating factor receptor and polyimmunoglobulin (poly-Ig) receptor, and transiting the mucosal barrier (3, 36). By this route, the bacterium enters the submucosa and vasculature. If unchecked, bacterial replication in the bloodstream can occur, resulting in bacteremia. After successfully entering the blood, the bacteria may transit the blood-brain barrier and enter the cerebrospinal fluid (CSF), leading to meningitis, which is often fatal. As is the case with invasion from the lungs to the bloodstream, a complex interplay between host and pathogen is required for the bacterium to access the CSF and involves activation of the host response by numerous bacterial components including peptidoglycan, lipoteichoic acid, CpG DNA, and proteins. These stimuli trigger intracellular signal transduction pathways resulting in release of cytokines including interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) (33). Chemokines including IL-8, MIP-1α, MIP-1β, Gro-α, and MCP-1 have also been found to be elevated during bacterial meningitis (34). Chemokines attract leukocytes toward inflammation while cytokines and contact with bacterial components lead to up-regulation of integrins and other cell adhesion molecules including macrophage antigen 1 (Mac-1; CD11b/CD18) and intercellular adhesion molecule 1 (ICAM-1) (33).
ICAM-1 is a member of the immunoglobulin superfamily of receptors and is the ligand for the leukocyte integrins Mac-1 and lymphocyte function-associated antigen 1 (LFA-1) (CD11a/CD18) (9). During infection, chemoattractants bind to receptors on leukocytes and the signal produced leads to an increased adhesiveness of integrin proteins, resulting in tighter binding to their ligands (35). The binding that occurs between ICAM-1 and the integrins aids in the rolling of leukocytes along the endothelium near areas of inflammation, a process initiated by members of the carbohydrate-binding selectin family. ICAM-1 is generally expressed at low levels on endothelial cells and on leukocytes; however, upon activation, induction of ICAM-1 can occur and may increase cell-cell interactions, thus promoting leukocyte migration into extravascular locations (9, 35). When properly coordinated, the cellular immune response is very effective against infection; however, immune cell action must be precisely targeted to prevent damage to the host itself (15). Activated leukocytes produce matrix metalloproteases and reactive oxidants which can be potentially destructive to host tissue (33). Damage from leukocyte products could potentially aid bacterial dissemination.
The pneumococcus possesses multiple virulence factors that aid in its survival and transmission. In addition to PsaA and PspC, there are pneumococcal surface protein A (PspA), neuraminidase A (NanA), autolysin (LytA), and the multifunctional cytotoxin pneumolysin (PLY) (19, 27). Many of these factors have been shown to be up-regulated and differentially expressed in vivo as well as contributing to infection in a tissue-specific fashion (24, 25).
PLY is the primary cytolysin produced by the pneumococcus. It belongs to a family of homologous thiol-activated cytolytic proteins collectively known as cholesterol-binding cytolysins. The cytotoxic mode of action involves the binding of membrane cholesterol, resulting in insertion into the lipid bilayer, oligomerization, and transmembrane pore formation (26). PLY also possesses the ability to activate the classical complement pathway (29) and has been extensively studied with regard to its effects on cells of the immune system. At extremely low concentrations, PLY treatment of polymorphonuclear leukocytes significantly inhibited the respiratory burst, bactericidal activity, and random migration of the leukocytes (28). PLY has also been shown to increase the synthesis of IL-8 (7) and be responsible for calcium-dependent generation of prostaglandin E2 and leukotriene B4 from human neutrophils (8). Exposure of nontoxic PLY concentrations to human monocytes leads to inhibition of the respiratory burst, degranulation, and phospholipid methylation, producing monocytes that have reduced capacity to kill S. pneumoniae (23). Also, monocytes exposed to PLY had increased expression of inflammatory cytokines TNF-α and IL-1β (13). Using cDNA microarray analysis of the THP-1 monocytic cell line exposed to S. pneumoniae, we previously reported PLY-dependent up-regulation of many genes including those for the chemokines IL-8, MCP-3, and MIP-1β (32).
In the present study, we evaluated the ability of the toxin to modulate expression of ICAM-1 in THP-1 monocytes. Using wild-type and PLY-deficient isogenic mutants, we found that ICAM-1 mRNA and protein expression was increased when PLY was present. Additionally, purified recombinant PLY up-regulates ICAM-1 expression in a dose-dependent fashion.
MATERIALS AND METHODS
Bacterial strains.
Strains used included wild-type S. pneumoniae serotype 2 strain D39 and serotype 3 strain WU2. PLY-negative isogenic mutants of D39 and WU2 were created by insertion-deletion mutagenesis and designated ΔPLY2 and ΔPLY1, respectively. Briefly, PCR primers (AF and AR) were designed to amplify approximately 2,586 nucleotides (nt) containing the entire ply gene plus 583 nt upstream of the start codon and 580 nt downstream of the stop codon. Primers AF and AR contain 5′ KpnI and XbaI sites, respectively. This PCR product was directionally cloned into pBluescript KS (Invitrogen, Carlsbad, CA) and transformed into Escherichia coli DH5α (Invitrogen, Carlsbad, CA). This vector served as template for a second “inverse” PCR using primers BF and BR, which were both engineered with 5′ BamHI sites. BF and BR both amplify outward from the ply gene beginning 29 nt inside the initiation and termination codons. The resulting product, containing the entire pBluescript vector and ply flanking regions, was subsequently digested with BamHI and ligated to a trimethoprim (Tmp) cassette BamHI digested from pKOT (1). This vector was designated pKOPLY and contained the Tmp resistance cassette flanked by ply surrounding DNA segments. Upon transformation, a double-crossover event results in replacement of the ply gene with the Tmp cassette, thus greatly decreasing the possibility of reversion and/or polar effects. S. pneumoniae was transformed using competence-stimulating peptide as described previously (30). Transformants were selected by being plated on blood agar plates containing 2% sheep erythrocytes and trimethoprim (50 μg/ml) and incubated overnight at 37°C with 5% CO2. Transformants were screened by PCR (primers AF-AR) and PLY assay (hemolysis). All transformants were negative for the ply gene and hemolysis.
Strains were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) or on BD Trypticase soy agar supplemented with 5% sheep erythrocytes (BD Biosciences, San Diego, CA). Ten-ml cultures were grown at 37°C to an optical density at 600 nm of approximately 1.0. Cultures were then centrifuged, and pellets were suspended in 1 ml RPMI 1640 cell culture medium (BioWhittaker, Walkersville, MD) plus 10% fetal bovine serum (FBS) and 10% glycerol, and 100-μl aliquots were stored at −80°C until use. One aliquot was thawed, and serial dilutions were plated on blood agar plates to confirm bacterial concentration.
Cell culture exposures.
Human acute monocytic leukemia THP-1 cells were maintained in RPMI 1640 cell culture medium containing 10% fetal bovine serum, l-glutamine, and 5 × 10−5 M β-mercaptoethanol. Cells were suspended in 12-well tissue culture plates and exposed to either medium alone, S. pneumoniae strain D39, ΔPLY2, WU2, ΔPLY1, or recombinant PLY (see below). Cells were coincubated at 37°C in 5% CO2 for 1, 3, or 6 h. The bacteria-to-THP-1 ratio for all exposures was approximately 5:1. After exposure, cells were collected by low-speed centrifugation (2,000 rpm) for 3 min. Pelleted cells were then washed once with sterile phosphate-buffered saline (PBS) before RNA isolation.
RNA isolation and cDNA synthesis.
Total RNA was purified using the RNeasy minikit (QIAGEN, Valencia, CA) according to the manufacturer's instructions and frozen at −80°C. A DNase treatment step was included to eliminate contaminating genomic DNA. RNA was quantitated spectrophotometrically, and quality was determined by A260/A280 and by agarose gel electrophoresis. One μg of RNA from each sample was used for cDNA synthesis. cDNA was synthesized using the Advantage RT for PCR kit (Clontech Laboratories Inc., Palo Alto, CA) according to manufacturer's specifications. Oligo(dT) primers were used to ensure amplification of eukaryotic message.
Cloning and expression.
Chromosomal DNA from S. pneumoniae D39 was purified using the DNeasy tissue kit (QIAGEN, Valencia, CA) and used as template for PCR. Primers LSM 520 (5′-CACCATGGCAAATAAAGCATGAAATGAC-3′) and LSM 523 (5′-GTCATTTTCTACCTTATCCTCTACC-3′) were used to amplify the full-length ply gene, and the product was subsequently cloned into pET101/D-TOPO expression vector (Invitrogen, Carlsbad, CA). This construct was transformed into E. coli TOP-10 competent cells, screened by PCR, and subsequently cloned into E. coli BL21star competent cells (Invitrogen, Carlsbad, CA) for expression. Clones were screened by PCR, and a positive clone was designated PlyEx101. The pET101/D-TOPO vector produces a fusion protein possessing a six-histidine tag at the C terminus of the expressed protein. Protein expression was induced by the addition of 60 mM isopropyl-β-d thiogalactopyranoside (IPTG) to exponentially growing E. coli PlyEx101.
Site-directed mutagenesis of the PlyEx101 vector was achieved using the GeneTailor site-directed mutagenesis system (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. The amino acid tryptophan 433 was changed to a phenylalanine, a mutation previously demonstrated to decrease the cytotoxic activity to approximately 1% of wild-type PLY (12). The new expression vector was named ΨPlyEx, and the mutation was confirmed by sequencing of the vector and hemolytic assay of the recombinant protein.
Recombinant proteins containing the His tag were purified from E. coli by affinity chromatography using the B-PER six-His fusion protein purification kit (Pierce, Rockford, IL) per manufacturer's instructions. The purity of the proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 4 to 20% Tris-glycine precast gels (Cambrex BioScience, Rockland, ME). To eliminate any potentially contaminating lipopolysaccharide, recombinant protein was incubated twice for 1 h with polymyxin B immobilized on agarose (AffinityPak Detoxi-Gel endotoxin-removing gel; Pierce, Rockford, IL). Endotoxin removal was confirmed by Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
Real-time PCR.
Gene-specific oligonucleotide primers were designed using Beacon Designer 2.1 software (Premier Biosoft International, Palo Alto, CA) and synthesized commercially (Integrated DNA Technologies, Coralville, IA). Primers were designed to amplify a gene segment of human 18S rRNA, which served as a constitutively expressed internal control, and human ICAM-1, our gene of interest. Primer sequences were as follows: 18S forward (5′-TCCATTATTCCTAGCTGCGGTATC-3′), 18S reverse (5′-CACCCATGCGACAGAGAACGGACT-3′), ICAM-1 forward (5′-GCACATTGGTTGGCTATCTTCT-3′), ICAM-1 reverse (5′-GCCCGAAGCGTTTACTTTGA-3′).
Quantification of ICAM-1 mRNA levels was achieved by real-time PCR analysis using an iCycler thermocycler (Bio-Rad Laboratories, Hercules, CA). Optimization trials resulted in a single product for each primer set and indicated what dilution of cDNA yielded optimal results. Reaction reagents (iQ SYBR Green Supermix; Bio-Rad Laboratories, Hercules, CA) contained iTaq DNA polymerase (2× concentration), 0.4 mM of each deoxynucleoside triphosphate, 6 mM MgCl2, and reaction buffers. The optimized reaction mix included 0.5 μM forward primer, 0.5 μM reverse primer, SYBR Green Supermix, and PCR-grade H2O to adjust the final reaction volume. For each sample 3.5 μl of diluted cDNA (1:10) was added to 76 μl of master mix containing the appropriate primers and mixed well by pipette. PCR was carried out in triplicate using 96-well plates with a 25-μl reaction volume. PCR cycling conditions were as follows: denaturation at 96°C for 1 min and 55 amplification cycles of 95°C for 10 s, 60°C for 1 min, and 73°C for 3 min. Real-time results were analyzed using iQ optical system software, version 3.0a (Bio-Rad Laboratories, Hercules, CA). Relative gene expression was determined by using the PFaFEl method and normalized to rRNA expression.
Flow cytometry.
THP-1 expression of surface ICAM-1 was determined by flow cytometry. After bacterial exposure, THP-1 cells were washed twice with sterile 1× PBS and incubated at room temperature (25°C) for 1 h in 100 μl of mouse anti-human CD54 (ICAM-1) monoclonal antibody (IgG1κ; BD Biosciences, San Diego, CA) followed by three 1-ml washes with 1× PBS. Cells were then incubated at room temperature for 30 min in 100 μl of biotinylated goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL), washed three times with 1 ml 1× PBS, and suspended in 1.0 μg/ml streptavidin conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR) on ice for 30 min. Cells were again washed three times with PBS, suspended in 750 μl 1× PBS, and analyzed with a FACScan cytometer (Becton Dickinson, Franklin Lakes, NJ).
Statistical analysis.
Results from real-time analysis of three different conditions were analyzed using analysis of variance with Tukey's correction for multiple comparisons (P < 0.05).
RESULTS
Expression of ICAM-1 mRNA after exposure to S. pneumoniae.
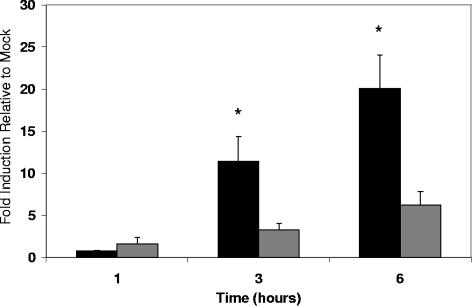
Real-time PCR analysis was used to determine the effect of S. pneumoniae exposure on THP-1 monocytes with regard to ICAM-1 mRNA levels. Exposure to S. pneumoniae led to a time-dependent increase in ICAM-1 mRNA message as seen in Fig. 1. Relative to medium alone, both WU2 and ΔPLY1 led to an increase in ICAM-1 mRNA by 3 h, and this increased further by 6 h. Exposure to WU2, however, led to significantly higher levels of ICAM-1 message compared to cells exposed to ΔPLY1 at 3 h, and this continued to increase by the 6-h time point. At 6 h, exposure to WU2 led to a 20-fold increase compared to a sixfold increase for exposure to ΔPLY1. This indicates that the presence of pneumolysin enhances the transcription of ICAM-1 in THP-1 monocytes under the conditions tested. Later time points were not evaluated due to loss of viability of cells exposed to WU2. THP-1 viability remained above 85% for all exposures as qualitatively determined by microscopic examination and trypan blue exclusion.
FIG. 1.
Real-time analysis of ICAM-1 message levels in THP-1 cells exposed to S. pneumoniae strain WU2 (black bars) and its PLY-negative isogenic mutant, ΔPLY1 (gray bars). THP-1 cells were exposed to either medium alone, WU2, or ΔPLY1 for 1, 3, and 6 h of incubation. Data represent fold induction relative to cells receiving medium alone. The experiment was repeated three times with each quantitative PCR being done in triplicate. Bars represent the means of all experiments with error bars representing standard errors of the means. Statistical analysis was performed by using analysis of variance with Tukey's correction for multiple comparisons (P < 0.05). *, significantly different from exposure to ΔPLY1 and medium alone.
Surface expression of ICAM-1 after exposure to S. pneumoniae.
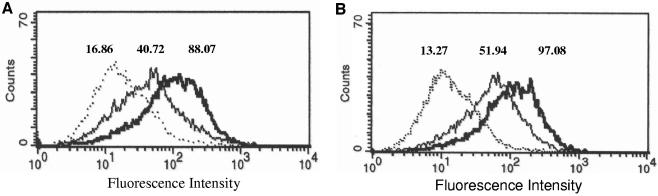
To determine if increased mRNA levels correlated with increased protein expression, we used fluorescence-activated cell sorting analysis to examine surface ICAM-1 on THP-1 cells exposed to S. pneumoniae for 10 h. We used strains D39 and WU2 and their isogenic PLY deletion mutants. Chloramphenicol was added at 3 h (D39 and ΔPLY2) and at 4.5 h (WU2 and ΔPLY1) to slow bacterial growth, allowing for longer incubations. This was required due to the closed system and the autolytic properties of S. pneumoniae in late stationary phase. Differences in time of chloramphenicol addition are due to differences in growth rate between strains in RPMI 1640 medium. Representative data are shown in Fig. 2. THP-1 cells exposed to each pneumococcal strain tested had increased ICAM-1 expression; however, cells exposed to PLY-expressing strains WU2 and D39 expressed ICAM-1 to greater levels than cells exposed to the PLY-deficient strain ΔPLY1 or ΔPLY2. Cells exposed to D39 had a geometric mean fluorescence of 88.07 compared to 40.72 for cells exposed to ΔPLY2 and 16.86 for cells exposed to medium alone (Fig. 2A). Cells exposed to WU2 had a geometric mean fluorescence of 97.08 compared to 51.94 for cells exposed to ΔPLY1 and 13.27 for cells exposed to medium alone (Fig. 2B).
FIG. 2.
Increased expression of ICAM-1 protein on the surface of THP-1 cells exposed to S. pneumoniae. THP-1 cells were exposed for 10 h to either medium alone (Mock), D39, or PLY-deficient ΔPLY2 (A) or medium alone (Mock), WU2, or ΔPLY1 (B). Dotted line, mock; thick line, wild type; thin line, PLY mutant. At 3 h for D39 and ΔPLY2 and at 4.5 h for WU2 and ΔPLY1, chloramphenicol (2 μg/ml) was added to slow bacterial growth to allow time for protein expression. Mock controls were also treated with chloramphenicol. Representative data are shown as fluorescence intensity of the total cell population, and the geometric mean of each peak is indicated.
Expression of ICAM-1 mRNA after exposure to recombinant PLY.
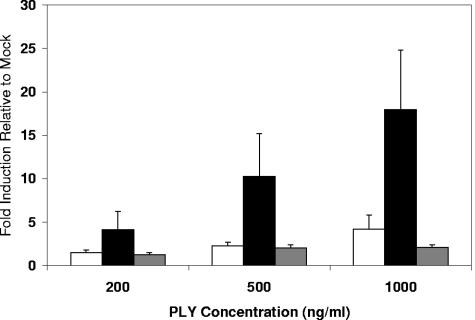
To determine the effect that PLY alone has on ICAM-1 expression, THP-1 cells were exposed to various concentrations of purified recombinant PLY for 6 h. Real-time PCR results are shown in Fig. 3. A dose-dependent response was seen. Six hours of exposure to 500 ng/ml PLY resulted in approximately a 2.2-fold increase in ICAM-1 mRNA compared to mock-exposed cells, and exposure to 1 μg/ml led to approximately a 4.2-fold increase. Cells exposed to 200 ng/ml increased expression by only 1.5-fold. Exposure of THP-1 cells to recombinant PLY resulted in relatively low increases in ICAM-1 message compared to increases for cells incubated with the PLY-expressing WU2 strain. Since the presence of free serum cholesterol (from FBS in medium) could be inhibiting the action of the recombinant PLY, exposures were repeated in RPMI 1640 medium without FBS, and higher levels of ICAM-1 up-regulation were observed. Results are shown in Fig. 3. Although the differences in ICAM-1 message levels among THP-1 cells exposed to increasing concentrations of PLY were not statistically significant when averaged, a dose-dependent response was seen in each of three independent experiments. In each experiment ICAM-1 mRNA expression was proportionate to the concentration of PLY.
FIG. 3.
Real-time analysis of ICAM-1 message levels in THP-1 cells exposed to purified recombinant PLY and ΨPLY. THP-1 cells were exposed for 6 h to recombinant PLY in RPMI containing (white bars) or lacking (black bars) 10% FBS or to recombinant ΨPLY protein in RPMI lacking 10% FBS (gray bars) at concentrations of 200 ng/ml, 500 ng/ml, and 1,000 ng/ml. Data represent fold induction relative to that of cells receiving medium alone. The experiment was repeated three times with each quantitative PCR being done in triplicate. Bars represent the means of all experiments with error bars representing standard errors of the means.
Effect of cytolytic activity on ICAM-1 up-regulation.
In an effort to determine if the cytolytic property of PLY was responsible for the increased ICAM-1 up-regulation, we exposed THP-1 cells to an altered recombinant PLY (ΨPLY), in which a single amino acid had been changed (Trp433→Phe). This mutation decreases the cytolytic activity by 99% (12). THP-1 cells were exposed to ΨPLY for 6 h in RPMI without FBS, and mRNA levels were determined by real-time PCR. Results are shown in Fig. 3. In contrast to wild-type PLY, exposure to the cytolysis-deficient protein did not greatly increase ICAM-1 mRNA levels. Even at 1000 ng/ml, exposure to ΨPLY resulted in only a 2.1-fold increase in ICAM-1 mRNA compared to cells exposed to medium alone.
DISCUSSION
Throughout the last couple of decades, virulence factors of Streptococcus pneumoniae have been extensively studied and new ones have been discovered. Much has been learned about how the pneumococcus interacts with the host and how these virulence factors aid the bacterium in transmission, carriage, and disease. However, many questions remain regarding the intimate interactions between the bacterium and host cells and how coordinate gene regulation by both the pathogen and the host contributes to disease. In this study, we demonstrate that exposure of THP-1 monocytes to Streptococcus pneumoniae leads to increased expression of ICAM-1. ICAM-1 is an important component of the leukocyte trafficking system involved in tight binding of leukocytes to the endothelium (9). Along with P-selectin, ICAM-1 has been shown to be important for neutrophil emigration during S. pneumoniae-induced peritonitis in mice (6, 21). Additionally, mice lacking ICAM-1 ligands (LFA-1 and Mac-1) were shown to have increased mortality after S. pneumoniae infection, with mice lacking LFA-1 having decreased emigration of neutrophils into the peritoneal cavity and complications associated with late pneumococcal disease (31).
The involvement of cell adhesion molecules in the early phases of pneumococcal meningitis has also been well established. Tuomanen et al. showed that monoclonal antibody against the beta subunit (CD18) of LFA-1 and Mac-1 inhibited influx of leukocytes and protein into the CSF, thus greatly reducing inflammation and mortality in rabbits challenged with S. pneumoniae (38). Also, anti-ICAM-1 monoclonal antibody has been shown to attenuate regional cerebral blood flow and leukocyte invasion into the CSF in a rat model of pneumococcal cell wall-induced meningitis (40). More recently, pneumococcal cell wall has been shown to induce TNF-α, nitric oxide, and ICAM-1 expression by rat primary brain microvascular endothelial cells (10). The increased ICAM-1 expression was found to be completely abrogated by the addition of anti-TNF-α antibody, indicating TNF-α-dependent autocrine stimulation of ICAM-1 expression (10). The possibility of inflammatory cytokine-induced ICAM-1 expression in our study cannot be ruled out. The quantity and effect of inflammatory cytokines were not determined in this study and may contribute to ICAM-1 up-regulation since pneumolysin has previously been shown to stimulate the expression of TNF-α and IL-1β from monocytes (13). If this is the reason for the increase in ICAM-1 expression, THP-1 cells exposed to PLY-expressing strains must release more ICAM-1-inducing cytokines than cells exposed to either medium alone or mutant strains. This study indicates that PLY increases the up-regulation of ICAM-1 on THP-1 monocytes but that it is clearly not the only factor leading to increased expression. Although not as significantly as PLY-expressing strains, the mutant strains also led to increased ICAM-1 expression compared to cells receiving medium alone. It has recently been shown that PspC from S. pneumoniae elicits cytokine and ICAM-1 expression from human alveolar epithelial cells (22). This could be the reason for the PLY-independent increase in ICAM-1 seen in our study. While we did not evaluate the contribution of PspC to ICAM-1 expression, it is likely that there are multiple factors contributing synergistically to the increased expression of cell adhesion molecules during the infection process.
The presence of FBS in our cell culture medium inhibited the up-regulation of ICAM-1 after exposure to recombinant PLY. This was likely due to the presence of free serum cholesterol, which is a potent inhibitor of PLY. The PLY hemolysis assay indicated that the presence of 10% FBS was sufficient to partially inhibit cytolytic activity (data not shown). When recombinant PLY exposures were done in medium lacking FBS, the values corresponded better to those seen with cells exposed to PLY-expressing pneumococci. Since FBS was present during the bacterial exposures where higher levels of ICAM-1 mRNA expression were seen, this may indicate that intimate contact between the bacterium and THP-1 cells was required and allowed PLY to exert its effect with less cholesterol inhibition. Although the presence of serum and free cholesterol more accurately resembles in vivo conditions, PLY likely exerts its effect over short distances requiring direct or nearly direct contact between bacterium and host cell in vivo, in which case PLY binding free host cholesterol would be diminished. The concentrations of recombinant PLY used in this study correlate well with the amount produced by pneumococci and are within the physiological range.
PLY is known to have both cytolytic and complement-activating properties. It has also been shown to interact with toll-like receptor 4 (TLR4), contributing to proinflammatory responses of macrophages (17). Any of these properties could potentially be involved in the up-regulation of ICAM-1. It is doubtful that the activation of complement resulted in the increased ICAM-1 expression in our studies, since heat-inactivated FBS was used in our culture medium and since we saw higher levels of ICAM-1 during exposures in medium lacking serum. We therefore examined whether the cytolytic property of PLY contributed to the up-regulation. Interestingly, THP-1 cells exposed to the cytolysis-deficient ΨPLY protein did not display increased ICAM-1 mRNA levels. This indicates that the cytolytic activity of PLY may play a role in the up-regulation of ICAM-1. Although the concentrations of PLY used did not significantly decrease the viability of the THP-1 cells, PLY could have led to leakage of ions such as extracellular calcium. The cytolytic activity of PLY has previously been shown to be important for neutrophil accumulation in lung tissue during pneumococcal pneumonia (14).
PLY is primarily thought of as a cytoplasmic protein released by the action of autolysin. This is because, unlike the other thiol-activated cytolysins, PLY lacks a secretory N-terminal signal sequence (39). However, Balachandran et al. demonstrated that some pneumococcal strains including WU2 actively release PLY during log-phase growth in an autolysin-independent manner (4). This is one of the reasons that WU2 was selected for use in our study. Also, WU2 was found to grow slowly in complete RPMI 1640 medium, allowing for longer incubations of high bacterial concentrations with THP-1 cells. PLY released from strain WU2 did not reach concentrations high enough to significantly decrease THP-1 cell viability in 6 h. Growth rates of D39 and its isogenic mutant did not allow for 6-h exposures due to pneumococcal autolysis and subsequent loss of THP-1 viability.
Previously, we used THP-1 cells to look at monocyte gene expression in response to S. pneumoniae D39 and its PLY-negative mutant PLN (32). Although ICAM-1 was not found to be significantly up-regulated in that study, recent microarray analysis indicated a significant increase in ICAM-1 expression in THP-1 cells exposed to D39 (data not shown). We therefore chose to continue use of the THP-1 cell line model. The importance of ICAM-1 expression on endothelial and epithelial cells is well established with regard to the role in leukocyte trafficking, extravasation, and respiratory burst. However, it is less clear how increased ICAM-1 expression on monocytes may relate to the inflammatory process. Leukocyte-leukocyte interactions would likely be the major benefit of increased ICAM-1 expression on a cell of the monocyte/macrophage lineage. Streptococcus suis, a major pathogen of swine and a zoonotic agent for humans, was shown to up-regulate ICAM-1 along with CD11a/CD18 and CD11c/CD18 in the THP-1 cell line (2). It has been reported that Mycobacterium tuberculosis also enhances expression of ICAM-1 on monocytic cells and that this increased expression may enhance monocyte recruitment and enhance antigen presentation of M. tuberculosis (16) since ICAM-1 is an integral component of the immunological synapse between a T lymphocyte and antigen-presenting cell, resulting in T-cell receptor/major histocompatibility complex-peptide interaction (11). Increased ICAM-1 expression on antigen-presenting cells could therefore enhance the adaptive immune response. Additionally, splenic monocytes were shown to have increased expression of ICAM-1 and major histocompatibility complex class II compared to blood monocytes, indicating potential involvement with retention in the tissue via cell-cell or cell-extracellular matrix interactions (20). During pneumococcal infection, such congregation of leukocytes in tissues where bacteria replicate to high numbers would likely prove beneficial in clearance of the infection.
We have demonstrated that exposure of S. pneumoniae to the human monocytic cell line THP-1 leads to increased expression of ICAM-1 and that this up-regulation is enhanced by the presence of PLY. These results add yet another immunological effect attributable to PLY and may have important implications in the pathogenesis and progression of pneumococcal disease.
Acknowledgments
This work was supported by National Institutes of Health grant AI43653.
We thank Ed Swiatlo, Dave Rogers, and Stephanie Carmicle for critical reading of the manuscript and Scott Long, Lashundra Johnson, and Lisa Smith for technical assistance and helpful insight. We also thank Nan Harvey and Cecile Snell for fluorescence-activated cell sorting assistance.
Editor: J. N. Weiser
REFERENCES
- 1.Adrian, P. V., C. J. Thomson, K. P. Klugman, and S. G. Amyes. 2000. New gene cassettes for trimethoprim resistance, dfr13, and streptomycin-spectinomycin resistance, aadA4, inserted on a class 1 integron. Antimicrob. Agents Chemother. 44:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Numani, D., M. Segura, M. Dore, and M. Gottschalk. 2003. Up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 on human THP-1 monocytes stimulated by Streptococcus suis serotype 2. Clin. Exp. Immunol. 133:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollingshead, and D. E. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert, D., R. de Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 6.Bullard, D. C., L. Qin, I. Lorenzo, W. M. Quinlin, N. A. Doyle, R. Bosse, D. Vestweber, C. M. Doerschuk, and A. L. Beaudet. 1995. P-selectin/ICAM-1 double mutant mice: acute emigration of neutrophils into the peritoneum is completely absent but is normal into pulmonary alveoli. J. Clin. Investig. 95:1782-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockeran, R., C. Durandt, C. Feldman, T. J. Mitchell, and R. Anderson. 2002. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J. Infect. Dis. 186:562-565. [DOI] [PubMed] [Google Scholar]
- 8.Cockeran, R., H. C. Steel, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Pneumolysin potentiates production of prostaglandin E2 and leukotriene B4 by human neutrophils. Infect. Immun. 69:3494-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etzioni, A. 1996. Adhesion molecules in leukocyte endothelial interaction. Adv. Exp. Med. Biol. 408:151-157. [DOI] [PubMed] [Google Scholar]
- 10.Freyer, D., R. Manz, A. Ziegenhorn, M. Weih, K. Angstwurm, W. D. Docke, A. Meisel, R. R. Schumann, G. Schonfelder, U. Dirnagl, and J. R. Weber. 1999. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J. Immunol. 163:4308-4314. [PubMed] [Google Scholar]
- 11.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 12.Hill, J., P. W. Andrew, and T. J. Mitchell. 1994. Amino acids in pneumolysin important for hemolytic activity identified by random mutagenesis. Infect. Immun. 62:757-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jounblat, R., A. Kadioglu, T. J. Mitchell, and P. W. Andrew. 2003. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect. Immun. 71:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr, J. R. 1999. Cell adhesion molecules in the pathogenesis of and host defence against microbial infection. Mol. Pathol. 52:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez Ramirez, G. M., W. N. Rom, C. Ciotoli, A. Talbot, F. Martiniuk, B. Cronstein, and J. Reibman. 1994. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect. Immun. 62:2515-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel, L. S., and E. Swiatlo. 2004. Pneumococcal disease: pathogenesis, treatment, and disease. Infect. Dis. Clin. Pract. 12:93-98. [Google Scholar]
- 19.McDaniel, L. S., J. Yother, M. Vijayakumar, L. McGarry, W. R. Guild, and D. E. Briles. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milicevic, N. M., K. Nohroudi, Z. Milicevic, and J. Westermann. 2004. Blood lymphocytes, monocytes and NK cells modulate their expression of CD44, ICAM-1, LFA-1 and MHC class II after arrival into lymphoid organs. Immunol. Investig. 33:439-452. [DOI] [PubMed] [Google Scholar]
- 21.Mizgerd, J. P., W. M. Quinlan, B. W. LeBlanc, G. J. Kutkoski, D. C. Bullard, A. L. Beaudet, and C. M. Doerschuk. 1998. Combinatorial requirements for adhesion molecules in mediating neutrophil emigration during bacterial peritonitis in mice. J. Leukoc. Biol. 64:291-297. [DOI] [PubMed] [Google Scholar]
- 22.Murdoch, C., R. C. Read, Q. Zhang, and A. Finn. 2002. Choline-binding protein A of Streptococcus pneumoniae elicits chemokine production and expression of intercellular adhesion molecule 1 (CD54) by human alveolar epithelial cells. J. Infect. Dis. 186:1253-1260. [DOI] [PubMed] [Google Scholar]
- 23.Nandoskar, M., A. Ferrante, E. J. Bates, N. Hurst, and J. C. Paton. 1986. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology 59:515-520. [PMC free article] [PubMed] [Google Scholar]
- 24.Ogunniyi, A. D., P. Giammarinaro, and J. C. Paton. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045-2053. [DOI] [PubMed] [Google Scholar]
- 25.Orihuela, C. J., G. Gao, K. P. Francis, J. Yu, and E. I. Tuomanen. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661-1669. [DOI] [PubMed] [Google Scholar]
- 26.Palmer, M. 2001. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon 39:1681-1689. [DOI] [PubMed] [Google Scholar]
- 27.Paton, J. C., P. W. Andrew, G. J. Boulnois, and T. J. Mitchell. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 47:89-115. [DOI] [PubMed] [Google Scholar]
- 28.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43:1085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Havarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince, J. E., C. F. Brayton, M. C. Fossett, J. A. Durand, S. L. Kaplan, C. W. Smith, and C. M. Ballantyne. 2001. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J. Immunol. 166:7362-7369. [DOI] [PubMed] [Google Scholar]
- 32.Rogers, P. D., J. Thornton, K. S. Barker, D. O. McDaniel, G. S. Sacks, E. Swiatlo, and L. S. McDaniel. 2003. Pneumolysin-dependent and -independent gene expression identified by cDNA microarray analysis of THP-1 human mononuclear cells stimulated by Streptococcus pneumoniae. Infect. Immun. 71:2087-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheld, W. M., U. Koedel, B. Nathan, and H. W. Pfister. 2002. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J. Infect. Dis. 186(Suppl. 2):S225-S233. [DOI] [PubMed] [Google Scholar]
- 34.Spanaus, K. S., D. Nadal, H. W. Pfister, J. Seebach, U. Widmer, K. Frei, S. Gloor, and A. Fontana. 1997. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J. Immunol. 158:1956-1964. [PubMed] [Google Scholar]
- 35.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 36.Tuomanen, E. I. 1997. The biology of pneumococcal infection. Pediatr. Res. 42:253-258. [DOI] [PubMed] [Google Scholar]
- 37.Tuomanen, E. I., R. Austrian, and H. R. Masure. 1995. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 332:1280-1284. [DOI] [PubMed] [Google Scholar]
- 38.Tuomanen, E. I., K. Saukkonen, S. Sande, C. Cioffe, and S. D. Wright. 1989. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 170:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, J. A., R. L. Allen, P. Falmagne, M. K. Johnson, and G. J. Boulnois. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 55:1184-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber, J. R., K. Angstwurm, W. Burger, K. M. Einhaupl, and U. Dirnagl. 1995. Anti-ICAM-1 (CD 54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J. Neuroimmunol. 63:63-68. [DOI] [PubMed] [Google Scholar]