Abstract
The facultatively intracellular pathogen Brucella, characterized by its capacity to replicate in professional and non professional phagocytes, also causes abortion in ruminants. This property has been linked to the presence of erythritol in the placenta, as brucellae preferentially utilize erythritol. The ery operon encodes enzymes involved in erythritol metabolism, and a link with virulence has since been discussed. Allelic exchange mutants in eryC of Brucella suis were erythritol sensitive in vitro with a MIC of 1 to 5 mM of erythritol. Their multiplication in macrophage-like cells was 50- to 90-fold reduced, but complementation of the mutant restored wild-type levels of intracellular multiplication and the capacity to use erythritol as a sole carbon source. In vivo, the eryC mutant colonized the spleens of infected BALB/c mice to a significantly lower extent than the wild type and the complemented strain. Interestingly, eryC mutants that were in addition spontaneously erythritol tolerant nevertheless exhibited wild-type-like intramacrophagic and intramurine replication. We concluded from our results that erythritol was not an essential carbon source for the pathogen in the macrophage host cell but that the inactivation of the eryC gene significantly reduced the intramacrophagic and intramurine fitness of B. suis.
Members of the genus Brucella are gram-negative intracellular pathogens that cause abortion and sterility in domestic animals and Malta fever in humans. These bacteria can survive and multiply inside trophoblasts and within a membrane-bound compartment in professional and nonprofessional phagocytic cells (4, 7, 11, 23, 24). In ruminants, Brucella abortus colonizes the placenta and fetus, causing abortion. This tropism for the reproductive organs correlates with the presence of erythritol in the bovine placenta, and it has been shown that, in the presence of erythritol and glucose, the genus Brucella preferentially metabolizes erythritol (31) and that erythritol promotes the growth of some Brucella strains (1, 21). A possible correlation between erythritol oxidation and virulence in animal models has been, however, a matter of controversy (15, 20). The catabolic pathway of erythritol has since been elucidated: the polyalcohol is phosphorylated, and three oxidation steps finally result in the formation of dihydroxyacetone phosphate, which can be metabolized to pyruvic acid (32). In the attenuated B. abortus B19 vaccine strain, the enzyme d-erythrulose-1-phosphate dehydrogenase, which plays an important role in the erythritol metabolism, has been shown to be absent (33). Genetic analysis of the chromosomal region containing the erythritol catabolic genes confirmed a deletion in this strain (29). In the presence of erythritol, ATP is consumed with the accumulation of d-erythrulose-1-phosphate. A general ATP depletion due to high-level erythritol kinase activity and interference with hexose kinase activity involved in glucose metabolism have been suggested as possible explanations for the sensitivity of B. abortus B19 to erythritol (33). However, the defect in erythritol metabolism of this vaccine strain is unrelated to its attenuated virulence in mice, as a strain obtained by gene replacement of the deleted ery region shows residual virulence similar to that of B. abortus B19 (30). A detailed analysis of the sequence and the organization of the ery genes was performed more recently (27). The four genes eryA, -B, -C, and -D form an operon of approximately 5 kb. The EryA protein is the erythritol kinase (19), and EryB and EryC are the two dehydrogenases in the catabolic chain. EryD is a transcriptional repressor, inactivated by erythritol binding (27). These sequences and the ery operon organization are highly conserved in the other two species whose genomes have been entirely sequenced, Brucella melitensis and Brucella suis (6, 22). Tn5 mutagenesis of the B. abortus 2308 genome allowed the isolation of a mutant where the transposon was inserted into eryB (26). As it has been observed for B. abortus B19, this Tn5 mutant is sensitive to erythritol. In addition, it is not significantly less virulent in a murine model of infection than the erythritol-tolerant B. abortus 2308 (30).
In contrast to these results, we have lately reported the isolation of two attenuated Tn5 mutants, obtained from a large-scale screening for genes of B. suis involved in intramacrophagic replication, where the transposon was shown to be integrated in eryB and eryC (16). It therefore appeared that the ery genes were somehow involved in the multiplication of brucellae in macrophages. The aim of this work was to analyze the possible role of erythritol in the virulence of B. suis in the macrophage and in the murine model of infection and to get insights into the possible causes of the attenuation of ery mutants.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. suis 1330 (ATCC 23444) was grown to stationary phase in tryptic soy (TS) broth at 37°C. Kanamycin or chloramphenicol was added at concentrations of 50 or 25 μg/ml, respectively, when appropriate. The Escherichia coli strains CC118λpir (12) and DH5α (Invitrogen, Cergy Pontoise, France) were used as host strains for the cloning experiments with the erythritol gene of B. suis; they were grown in Luria-Bertani broth in the presence of kanamycin or ampicillin at a concentration of 50 μg/ml when appropriate. Constructions were performed using plasmids pUC4K, pUC18 (both Amersham Biosciences, Orsay France), pGEM-T Easy (Promega, Charbonnières, France), pBBR1MCS (18), and pCVD442 (8).
Construction of B. suis ΔeryC deletion mutants.
For the cloning of the eryC gene from B. suis (locus BRA0866) (22), chromosomal DNA was prepared from a stationary-phase culture as previously described (2). The entire gene was then amplified by PCR as a 1,181-bp fragment using the primers 5′-CGCCGCACCAAGCATTACC-3′ and 5′-TTGGCGATGAGACGATGCG-3′. For the construction of the ΔeryC::Kanr mutant, the PCR product was cloned into pGEM-T Easy. A 387-bp StuI-HindIII internal fragment was deleted from eryC and replaced by the 1.3-kb HincII Kanr cassette from pUC4K. This construct was amplified in E. coli and then electroporated as a suicide vector into B. suis as described earlier (17). After growth at 37°C for 3 days, colonies were streaked on kanamycin and on ampicillin, and only Kanr/Amps clones were taken into consideration for further analysis. PCR analysis performed with the primers described above on chromosomal DNA isolated from these clones allowed the identification of mutants containing the correct allelic exchange within eryC. The ΔeryC deletion mutant, devoid of any resistance cassette to exclude any possible polar effects on genes located downstream, was constructed as follows. Instead of inserting the Kanr cassette to replace the StuI-HindIII fragment of eryC as described above, the deleted gene was religated and recloned as an SalI-SphI fragment into the vector pCVD442. This vector carries the sacB gene conferring sucrose sensitivity to gram-negative bacteria (8), hence allowing the application of selective pressure for allelic exchange, which would then yield sucrose-resistent clones. To this purpose, following electroporation, brucellae were first plated onto TS agar plates containing ampicillin to select for transformants. Positive clones were restreaked in the presence of 5% sucrose, and sucrose-resistant, isolated colonies were assayed by PCR using the eryC primers. The deleted gene yielded a PCR product of 800 bp. Standard DNA manipulations were performed according to established protocols (25).
Complementation of the unmarked B. suis ΔeryC deletion mutant by eryC.
The gene was amplified by PCR as a 1-kb fragment using the primers 5′-TAACTGCAGTGGAATGGTTCGACAACGCC-3′ and 5′-TAAGGATCCTGAACGCGGTCCGGTTCTGG-3′ and cloned via BamHI/PstI restriction sites into pBBR1MCS in the orientation of the lacZ gene. The recombinant plasmid was isolated from E. coli and introduced into the ΔeryC mutant of B. suis by electroporation, and complemented mutants were selected on TS agar supplemented with chloramphenicol.
Erythritol sensitivity assay with the ΔeryC deletion mutant of B. suis and growth curves in RPMI cell culture medium.
In experiments directly monitoring erythritol sensitivity, ΔeryC mutants grown to stationary phase were diluted in TS broth and incubated in the presence of erythritol at a concentration of 80 mM for 54 h. Growth curves in RPMI 1640 (Cambrex Bio Science, Paris, France) containing 10% fetal calf serum (FCS) were obtained following 100-fold dilution of a stationary-phase culture and incubation at 37°C for 5 days. In both types of experiments, growth was monitored by measuring the optical density at 600 nm (OD600).
Determination of the MIC of erythritol for the ΔeryC deletion mutant of B. suis.
Stationary-phase precultures of wild-type B. suis 1330 containing pBBR1MCS, the ΔeryC deletion mutant with plasmid pBBR1MCS, and ΔeryC complemented with pBBR1-eryC were diluted 1/1,000 in TS broth containing chloramphenicol and erythritol at concentrations of 0.1, 0.5, 1, 5, or 20 mM. At various times of incubation, the OD600 was recorded.
Growth curves of B. suis strains in TS broth or in minimal medium in the presence of erythritol as sole carbon source.
Stationary-phase precultures of wild-type B. suis 1330, the ΔeryC deletion mutant, ΔeryC complemented with pBBR1-eryC, and a spontaneously erythritol-tolerant mutant of B. suis ΔeryC were diluted either 1/1,000 in TS broth with or without erythritol (25 mM) or (following a washing step in saline) 1/100 in Gerhardt's modified minimal medium containing 25 mM erythritol as a sole carbon source (10). Growth was monitored at different time points by OD600 measurements.
Cell culture, infection, and intracellular survival assay of B. suis in macrophage-like cells and in murine bone marrow-derived macrophages.
Experiments with macrophage-like cells were performed as described previously (4). Briefly, THP-1 cells, differentiated by 1,25-dihydroxyvitamin D3 at a concentration of 10−7 M for 72 h, were resuspended at 5 × 105 cells/ml in RPMI 1640 medium supplemented with 10% FCS. Alternatively, murine J774 cells were resuspended at 1.2 × 105 cells/ml in the same medium. Bone marrow-derived macrophages were obtained from BALB/c mice as described previously (5). Adherent cells were incubated for 24 h at 37°C in 5% CO2 prior to infection at a multiplicity of infection of 20 with stationary-phase B. suis grown in the presence of the corresponding antibiotics. After 30 min, cells were washed twice with phosphate-buffered saline (PBS) and reincubated in RPMI 1640-10% FCS with gentamicin (30 μg/ml) for at least 1 h. At 1.5, 7, 24, and 48 h postinfection, cells were washed twice with PBS and lysed in 0.2% Triton X-100. CFU were determined by plating serial dilutions on TS agar and incubation for 3 days at 37°C.
When infection experiments were performed in the absence of FCS, adherent cells were washed once with phosphate-buffered saline and cultivated in RPMI 1640 medium devoid of FCS for the duration of the experiment. All experiments were performed twice in triplicate.
Infection of BALB/c mice by B. suis strains.
Brucella suspensions were prepared as described elsewhere (14). Eight- to 9-week-old female BALB/c mice (University of Granada, Granada, Spain) were distributed in three groups and then injected intraperitoneally with 5 × 104 CFU (in a volume of 100 μl) of one of the following strains: wild-type B. suis 1330 containing pBBR1MCS, B. suis ΔeryC deletion mutant with pBBR1MCS, or ΔeryC complemented with pBBR1-eryC. The actual infectious dose was confirmed later by diluting and plating the inocula on TS agar (14). Five mice from each group were killed by CO2 asphyxiation at 3 days, 7 days, 4 weeks, or 8 weeks postinoculation. Spleens were aseptically removed, weighed, and homogenized with 10 ml of PBS (Gibco BRL Life Technologies) with a homogenizer (Stomacher 80 lab blender; Seward, London, United Kingdom) for the determination of Brucella counts. The homogenates were serially 10-fold diluted in PBS and plated on TS agar containing chloramphenicol (13). To assess intramurine behavior of the spontaneously erythritol-tolerant ΔeryC mutant, an additional infection experiment was performed as described with the B. suis 1330 wild type, the ΔeryC deletion mutant, and the erythritol-tolerant ΔeryC strain. A total of 105 CFU were effectively injected intraperitoneally. Per strain, five mice were killed at 7 days postinoculation, and survival of brucellae in the spleens was determined as described above.
Brucella colonies were counted after incubation for 3 to 5 days. Data are presented as log10 values of CFU (log CFU)/spleen ± standard deviation of the mean (SD). The limit of detection in these experiments was ≤10 CFU per spleen.
Statistical analysis.
Analysis of variance using the Fisher protected least-significant-difference test and the Tukey-Kramer test was performed to determine the level of significance of differences in CFU and in spleen weights observed with the mouse infection experiments. In macrophage infection experiments, Student's t test was used for statistical analysis. In both cases, P values of ≤0.05 were considered significant.
RESULTS
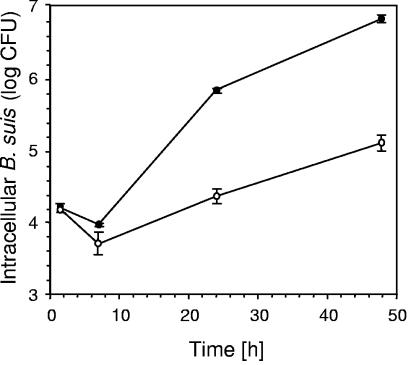
The eryC knockout mutant of B. suis was attenuated in human and murine macrophage-like cells. Our previously published results described the isolation of independent Tn5 mutants of B. suis where the transposon was shown to be localized in the eryB and eryC genes, and both mutants were attenuated in the human macrophage-like THP-1 cells (16). To know whether this observation was limited to the use of human cells or if was a general phenomenon, we performed infection experiments using the murine macrophage-like J774 cells. The results obtained with J774 cells confirmed those previously obtained with human THP-1 macrophages: the number of intracellular B. suis eryC::Tn5 mutants was significantly lower at 24 and 48 h postinfection than with the wild-type B. suis 1330 (P ≤ 0.001). At 48 h, the difference between both strains was 50 fold (Fig. 1). Thus, the lower replication rate observed for the mutant in macrophage-like cells was independent of the type of macrophagic cell line used. Similar results of reduced intracellular multiplication were obtained with both macrophage-like cell lines when eryB was inactivated (data not shown).
FIG. 1.
Multiplication of B. suis 1330 wild-type (•) and B. suis eryC::Tn5 (○) in murine J774A.1 macrophage-like cells over a time course of 48 h. Values represent means of one out of two experiments performed in triplicate (each), and error bars indicate SD.
The construction of allelic exchange mutants was performed to verify that the transposon-based inactivation of genes of the ery operon was at the origin of the reduced intramacrophagic multiplication of B. suis. In the first allelic exchange mutant, a 387-bp internal fragment was deleted from eryC and replaced by a cassette carrying a kanamycin resistance gene. The gene eryC encodes the enzyme d-erythrulose-1-phosphate dehydrogenase; it is the last of the three genes (eryA, -B, and -C) located within the ery operon and involved in erythritol degradation (27). It was conceivable, however, that the introduction of the cassette with the resistance marker would exert a polar effect on the gene eryD located immediately downstream, which has been described as encoding a putative repressor of the ery operon (27), and therefore might affect the correct functioning of the enzymes encoded by the gene cluster. To verify this hypothesis, we constructed an additional, nonpolar deletion mutant of eryC in B. suis, which contained an internal deletion devoid of any selection marker. Infection experiments with THP-1 macrophage-like cells revealed that both mutants, ΔeryC::Kanr and ΔeryC, showed a very similar intracellular behavior, resulting in an approximately 90-fold-lower intracellular replication than the wild type at 48 h postinfection (Fig. 2A and data not shown). Differences between the wild type and the mutants were significant at 7, 24, and 48 h (P ≤ 0.001). Thus, the lower replication rate observed for the mutants in macrophage-like cells was clearly linked to eryC inactivation, and the attenuated phenotype of the ΔeryC::Kanr mutant was not due to a polar effect on eryD.
FIG. 2.
(A) Comparison of the intracellular survival of B. suis 1330 wild-type containing plasmid pBBR1MCS (•), the ΔeryC deletion mutant with plasmid pBBR1MCS (▵), and ΔeryC complemented with pBBR1-eryC (▴) in human THP-1 macrophage-like cells. Results are displayed as means ± SD, and one out of two experiments performed in triplicate (each) is shown. (B) Growth curves of wild-type B. suis 1330 (•) and B. suis ΔeryC (▵) in RPMI 1640 medium with 10% FCS, where bacterial growth was measured by monitoring OD600 increase.
In parallel, bone marrow-derived macrophages from BALB/c mice were infected by the wild type and the ΔeryC deletion mutant under experimental conditions identical to those described above. At 48 h postinfection, the number of intramacrophagic bacteria was 90-fold higher with the wild type than with the ΔeryC mutant (data not shown), confirming that this phenomenon was independent of the macrophage infection model used.
Complementation in trans of ΔeryC with the intact eryC gene restored wild-type levels of intramacrophagic replication.
The definite proof that eryC was responsible for the attenuated phenotype described above was obtained by complementation with an intact copy of the gene on plasmid pBBR1MCS. Following insertion of the gene in the proper orientation, the lacZ promoter of the plasmid was used to express eryC, as the gene is devoid of a promoter, because it is the third gene of the ery operon. In macrophage infection experiments with THP-1 cells, the complemented mutant of B. suis reached wild-type levels of intracellular multiplication, confirming that the erythritol metabolism pathway was in some way involved in optimal adaptation of Brucella to the intramacrophagic environment (Fig. 2A). In bone marrow-derived macrophages, similar intracellular replication results were obtained with the complemented mutant (data not shown).
Extracellular factors were not involved in reduced intramacrophagic survival of B. suis ΔeryC.
It has been known for several decades that the absence of d-erythrulose-1-phosphate dehydrogenase in the live vaccine strain B. abortus B19 inhibits the growth of the strain in the presence of erythritol (33). The generation of a transposition mutant in eryB of the virulent B. abortus strain mimics the erythritol sensitive response of the B19 strain (26). We therefore had to consider the possibility that extramacrophagic factors present in the infection model, namely, in the FCS used in the cell culture medium, could be responsible for the attenuated phenotype observed with our mutant. To address this question, two complementary experiments were performed: (i) in vitro growth curves of the wild-type and the ΔeryC deletion mutant in the cell culture medium used during infection, i.e., RPMI 1640 containing 10% FCS; and (ii) infection experiments with THP-1 cells in the absence of fetal calf serum.
In the cell culture medium, despite the fact that growth rates were considerably lower than in TS broth, where an optical density of 1.5 was reached in stationary phase after 20 h of culture, both the wild-type strain and the ΔeryC mutant showed very similar growth rates over the entire duration of the experiment, which lasted for 5 days (Fig. 2B). The possibility that factors inhibiting growth of the mutant could be present in the cell culture medium used was therefore excluded. In the THP-1 infection model, the absence of fetal calf serum during the infection by B. suis strains did not alter the intracellular growth curves of both the wild type and the ΔeryC mutant strain (data not shown). At 48 h, a 50-fold difference between both strains was measured, which was comparable to the results obtained in the presence of FCS (Fig. 1 and 2A). As the RPMI 1640 medium and the FCS used cannot be implicated, it appeared evident that the intramacrophagic environment itself was responsible for the reduced replication observed with the eryC mutants.
In vivo replication of the ΔeryC mutant of B. suis in a murine model was affected in the early phase of infection.
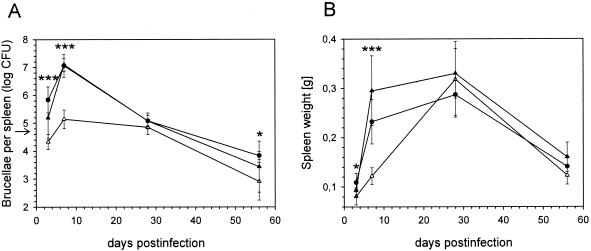
The results presented above, describing the intramacrophagic attenuation of the eryC mutants, were obtained with cell culture infection models. It was therefore of interest to study the fate of an eryC mutant in vivo, and we chose the well-established BALB/c murine model of infection. At 3 and 7 days postinfection, the number of intrasplenic, viable ΔeryC brucellae was significantly lower (P ≤ 0.001) than the number of wild-type bacteria, which increased rapidly during that period (Fig. 3A). The observed difference at 3 days (a factor of 33) was similar to the difference obtained in J774 macrophages at 48 h postinfection (Fig. 1). The strong and rapid replication of the wild-type strain during the first 7 days of infection was followed by a decline in the number of intrasplenic brucellae, confirming our previously published results with B. suis 1330 (9), whereas the level of the mutant strain was maintained during the following 3 weeks. Numbers of residual bacteria in the mice infected with B. suis 1330 or ΔeryC were again significantly different (P ≤ 0.05) at 8 weeks postinfection (Fig. 3A). The ΔeryC mutant was therefore characterized by its reduced capacity to set up an infection in the mouse, as opposed to the wild-type strain and the complemented mutant, which were not significantly different from each other during the course of the experiment. Evolution of the spleen weights of the infected animals confirmed these observations. The increase in weight, indicator of an inflammatory response of the organism, was significantly delayed in the mice infected by the mutant and hence corroborated the reduced capacity of infection of this strain (Fig. 3B).
FIG. 3.
Infection of BALB/c mice with the following B. suis strains over a period of 56 days. Wild-type B. suis 1330 containing plasmid pBBR1MCS (•), the ΔeryC deletion mutant with plasmid pBBR1MCS (▵), and ΔeryC complemented with pBBR1-eryC (▴). (A) Survival of B. suis strains in the spleens. Viable bacteria were counted at different time points, and results are represented as means ± SD. The infection dose of 5 × 104 viable bacteria for each strain is indicated by an arrow. (B) Weights of the infected spleens removed for the determination of viable Brucella counts. In both panels, statistically significant differences between the wild-type and the ΔeryC mutant were marked by asterisks (*, P ≤ 0.05; ***, P ≤ 0.001).
With respect to the above-mentioned results, two major hypotheses had to be taken into consideration as possible explanations for the observed phenomenon of reduced intramacrophagic replication of the eryC mutants of B. suis. (i) Erythritol contributed as a carbon or energy source to the capacity of multiplication within the macrophage host cell. (ii) The possible intracellular presence of erythritol at low concentrations partially inhibited the growth of ΔeryC. To address these points, the properties of the ΔeryC mutant of B. suis were further characterized.
The ΔeryC mutant of B. suis was sensitive to erythritol, and spontaneously resistant mutants occurred readily.
It has been previously described that ery mutants of B. abortus are sensitive to erythritol (26). However, erythritol-tolerant mutants unable to oxidize erythritol appear regularly in cultures of strain B. abortus B19 at a frequency of 10−4 to 10−6, and the most likely explanation for this observation is a mutation in the erythritol uptake system (28). We confirmed in our assays that a ΔeryC mutant of B. suis was also sensitive to erythritol, by incubation of the strain in broth in the presence of 1% erythritol (80 mM) for 54 h (Fig. 4A). No growth was measurable until 30 h of culture, when growth of resistant mutants started, as determined by an increase in optical density. The erythritol-tolerant character of the mutants was confirmed by the demonstration of rapid growth of a subculture in erythritol-containing broth (Fig. 4B). The experiments described below involving such an erythritol-tolerant ΔeryC mutant were performed with a single, isolated mutant. In several cases, different individual erythritol-tolerant mutants were used in parallel experiments, and the results were always identical for all mutants used (not shown).
FIG. 4.
Growth curves of B. suis ΔeryC (A) and spontaneously erythritol-tolerant mutants of B. suis ΔeryC (B) in TS broth containing 80 mM erythritol. The growth curve in panel B was obtained from a subculture of bacteria harvested at the end of the experiment shown in panel A. The OD600 value was measured in both sets of experiments, and one representative curve is shown for each.
The MIC of erythritol for the B. suis ΔeryC mutant was in the millimolar range.
The erythritol-sensitive character of the ΔeryC mutant of B. suis justified a thorough analysis of the conditions under which this sensitivity was observed. To this end, the approximate MIC for erythritol was determined during Brucella growth in TS broth in the presence of various concentrations of the compound. The wild-type strain and the complemented mutant grew well at concentrations of at least up to 25 mM, with a slightly higher growth rate of the complemented strain during the log phase (Fig. 5 and 6B). In contrast, the growth rate of the ΔeryC mutant was clearly affected at erythritol concentrations of 0.5 to 1 mM, and it was totally inhibited at concentrations of 5 mM and above. At the concentration of 0.1 mM, growth in the early log phase was slower than for the wild type (Fig. 5).
FIG. 5.
Determination of the MIC of erythritol for the ΔeryC mutant of B. suis. A growth curve measuring the OD600 value of the culture was performed at concentrations of 0.1 (○), 0.5 (⋄), 1 (□), 5 (▵), and 20 mM (▿) erythritol in TS broth. In addition, growth of the wild-type strain (•), and of the complemented ery mutant (▴) was followed in the presence of 20 mM erythritol.
FIG. 6.
Growth curves of B. suis strains in TS broth (A), in TS broth supplemented with 25 mM erythritol (B), and in minimal medium containing only erythritol (25 mM) as a carbon source (C). The strains used were the wild type (•), the ΔeryC deletion mutant (▵), the complemented ΔeryC mutant containing pBBR1-eryC (▴), and an isolated, spontaneously erythritol-tolerant mutant of ΔeryC (○). One typical set of curves out of three independent experiments performed is shown for each.
Growth of a spontaneously erythritol-tolerant mutant of B. suis ΔeryC was not inhibited by erythritol, but this polyalcohol could not be used as a carbon source.
To compare the properties of the ΔeryC mutant and of a selected, spontaneously erythritol-tolerant ΔeryC mutant with respect to tolerance and use of erythritol, growth experiments were performed with rich TS broth and in minimal medium in the absence or presence of erythritol, respectively. In rich medium and in the absence of erythritol, all strains grew comparably well, excluding any general growth defect of the mutants (Fig. 6A). In the same culture medium containing erythritol, the wild type, the complemented ΔeryC mutant, and the spontaneously erythritol-tolerant ΔeryC mutant grew well; only the ΔeryC mutant was inhibited (Fig. 6B). Finally, although all strains grew in standard Gerhardt's minimal medium (not shown), the use of erythritol as a sole carbon source in this minimal medium prevented growth not only of the ΔeryC mutant but also of the erythritol-tolerant ΔeryC mutant (Fig. 6C). This finding was consistent with the assumption that the internal deletion of a 400-bp fragment of the eryC gene in the latter mutant, verified by PCR (not shown), could not spontaneously revert into a functional gene allowing complete erythritol metabolism. Altogether, these results made clear that the erythritol-tolerant ΔeryC deletion mutant, as expected, did not recover the capacity to metabolize erythritol, suggesting therefore a defect in the uptake of the polyalcohol.
A spontaneously erythritol-tolerant mutant of B. suis ΔeryC exhibited wild-type-like replication in THP-1 cells and in BALB/c mice.
The spontaneously erythritol-tolerant mutant was then used to study its intramacrophagic behavior in THP-1 cells, compared to the wild-type strain and the original ΔeryC mutant. In contrast to the original, erythritol-sensitive ΔeryC mutant of B. suis, the tolerant mutant showed an intracellular replication rate over a period of 48 h that was identical to the growth rate observed with the wild-type strain (Fig. 7). Identical results were obtained with spontaneously erythritol-tolerant mutants of the ΔeryC::Kanr deletion mutant and of the original eryC::Tn5 mutant (not shown). These results suggested (i) that although an intact erythritol degradation pathway was essential for normal intramacrophagic multiplication of the B. suis wild-type strain, erythritol was not an essential carbon source under these conditions; and (ii) that the reduced intracellular replication of the erythritol-sensitive ΔeryC mutants might be due to the presence of erythritol in the host cell. In this context, we verified that the reduced intracellular replication of the original ery mutants was not due to the occurrence of spontaneously erythritol-tolerant mutants that might allow a certain degree of multiplication of a mixed population in the macrophages: ery mutants reisolated from infected macrophages at 48 h postinfection were all sensitive to erythritol (data not shown).
FIG. 7.
Intracellular multiplication of B. suis 1330 wild-type (•), of an isolated, spontaneously erythritol-tolerant mutant of B. suis ΔeryC (○), and of the ΔeryC deletion mutant (▵) in human THP-1 cells. The experiment was performed twice in triplicate (each), and results of one of the experiments are shown, presented as the means ± SD.
An additional infection experiment using BALB/c mice was performed with the Brucella wild-type strain, the erythritol-sensitive ΔeryC mutant, and the erythritol-tolerant ΔeryC mutant to address the question of the behavior of the latter in the murine model of infection at 7 days postinfection, where differences between the wild type and the erythritol-sensitive ΔeryC mutant were most significant (Fig. 3A). With effective infection doses of 105 bacteria, we obtained the following results for intramurine survival (log10 total counts of Brucella CFU in the spleens): 7.49 ± 0.15 for the wild type, 6.0 ± 0.39 for the ΔeryC mutant, and 7.30 ± 0.1 for the erythritol-tolerant strain. Statistical analysis confirmed that the difference between wild-type and erythritol-tolerant bacteria was not significant (P = 0.096), whereas it remained significant between the wild type or the erythritol-tolerant strain and the ery mutant (P < 0.0001). The erythritol-tolerant strain therefore survived as well as the wild type in the mouse model of infection, confirming our results obtained with macrophages (Fig. 7).
DISCUSSION
We have recently described for the first time the observation that eryB and eryC mutants of B. suis obtained in a Tn5 mutagenesis screen are characterized by reduced levels of intramacrophagic replication in human macrophage-like cells (16). This rather unexpected finding led to a more profound analysis of the interaction between an eryC mutant and the macrophage host cell. Allelic exchange mutants of eryC confirmed the results obtained with the original Tn5 mutants: the lack of an enzyme of the catabolic pathway of erythritol affects intracellular B. suis multiplication, and the effect of the mutation was entirely reversible by complementation of the mutant. The observed attenuation could therefore be attributed to the inactivation of eryC. We extended the choice of in vitro infection models to a murine macrophage-like cell line and to bone marrow-derived macrophages, as attenuation could have been specific to human THP-1 cells. Nevertheless, a very similar degree of attenuation was recorded in all cell culture models used. However, if one considers that eryB and eryC are part of the same metabolic pathway (27, 32), there was contradiction with previously published results by Sangari et al., who reported the lack of attenuation of an eryB mutant of B. abortus in a murine model of infection (30); it was therefore possible that our results obtained in cell culture models of infection were not reproduced in vivo. Infection of BALB/c mice eliminated this concern, as we showed that an eryC mutant of B. suis was impaired in replication in this model. One possible explanation for this discrepancy may be differences in the strategy of infection for both species, as intramurine survival curves of the respective wild-type strains differed, notably the lack of an infection peak and the absence of decrease in the case of B. abortus (30). Results obtained with the complemented ΔeryC mutant showed a good complementation in all infection models used, confirming that the observed attenuation was indeed linked exclusively to the inactivation of eryC by deletion of an internal fragment.
Previously published observations reported that the live vaccine strain B. abortus B19 and ery mutants of B. abortus are sensitive to erythritol (3, 26, 33) and that erythritol-tolerant mutants unable to oxidize erythritol appear regularly in cultures of strain B. abortus B19 (28). We confirmed erythritol sensitivity for the eryC mutant of B. suis and spontaneous occurrence of erythritol-tolerant phenotypes among these mutants. This tolerance was most likely due to a defect in the erythritol uptake system, as described and discussed previously (28). The possibility that traces of erythritol were present in fetal calf serum during infection experiments, leading to partial inhibition of mutant replication, could be excluded experimentally, and it was therefore straightforward to conclude that the intracellular environment of the host cell itself was the cause of the reduced intramacrophagic replication of the ΔeryC mutant.
Two explanations were conceivable for the observed reduced intramacrophagic replication of the eryC mutants of B. suis. (i) The possible intracellular presence of erythritol at low concentrations partially inhibited the growth of ΔeryC. (ii) Erythritol contributed as a carbon or energy source to the capacity of multiplication within the macrophage host cell. To further elucidate the possible reason(s) for intramacrophagic attenuation of the ΔeryC deletion mutant, we made use of the availability of the spontaneously erythritol-tolerant mutants of ΔeryC. In vitro growth experiments allowed us to determine the minimal erythritol concentrations that affected or inhibited growth of the erythritol-sensitive ΔeryC deletion mutant and confirmed that the complemented mutant grew at least as well as the wild type and the erythritol-tolerant mutant at high concentrations of erythritol. Whereas a concentration of 5 mM was needed to completely inhibit growth of the ΔeryC deletion mutant, concentrations as low as 100 μM reduced the rate of bacterial multiplication in the logarithmic growth phase in rich medium. As suggested earlier, a general ATP depletion due to high-level erythritol kinase activity and interference with hexose kinase activity may be explanations for the sensitivity of this mutant to erythritol (33). Additional experiments with minimal medium containing erythritol as a sole carbon source demonstrated the capacity of the B. suis wild-type strain and the complemented mutant to grow under these conditions and the absence of any growth for the erythritol-sensitive and -tolerant ΔeryC deletion mutants. These results made clear that the capacity of B. suis to use erythritol as a C source depended on the presence of an intact eryC gene and that the disruption of this gene resulted in sensitivity of the mutant to erythritol, as described for eryB inactivation in B. abortus (26).
Phenotypically, the eryC deletion mutants had in common their incapacity to grow with erythritol as a C source, but in addition, the erythritol-tolerant mutants grew in rich medium in the presence of this polyalcohol. These properties were the starting point for macrophage infection and survival experiments with the ΔeryC erythritol-tolerant mutant, experiments aimed at eventually explaining the attenuated intracellular phenotype linked to the eryC deletion. We reasoned that if erythritol was a molecule essential for intramacrophagic replication of brucellae, the reduced survival would persist. If, in contrast, the phenotype was only due to the erythritol sensitivity of the mutant, then wild-type-like multiplication would be observed with this spontaneously erythritol-tolerant mutant. The results presented in this work confirmed the latter hypothesis. We therefore speculated that brucellae were in contact with erythritol inside the macrophage, as it has never been reported that substrates other than erythritol were catabolized by these enzymes. The measurement of erythritol in tissue samples is, however, technically difficult and requires the application of very sensitive methods such as high-performance liquid chromatography coupled to gas chromatography-mass spectrometry (34). Although the spontaneously erythritol-tolerant mutants were not further characterized genetically, several independent isolates obtained from the three types of eryC mutants (eryC::Tn5, ΔeryC::Kanr, and ΔeryC) showed identical phenotypes in the described growth experiments in different media and in macrophage infection experiments, and it therefore appeared plausible that mutations in the as-yet-unknown erythritol uptake system were at the origin of the erythritol tolerance observed with the ΔeryC mutants.
It has been previously described that brucellae utilize the carbon source erythritol in preference to glucose (1). In pregnant ungulates, B. abortus, B. melitensis, and B. suis have a marked tropism for the placenta where they preferentially replicate, causing acute placentitis, resulting in fetal death and abortion. The presence of erythritol in these tissues has therefore been linked to this tropism, and erythritol catabolism has been suggested to increase virulence in this host environment (15, 31). On the other hand, the erythritol-sensitive vaccine strain B. abortus B19 is attenuated and induces only few or no abortions. It carries a deletion in the ery operon (26, 27). These evidences suggested a role of erythritol catabolism in virulence of B. abortus, but studies of a murine model of infection using complementation experiments showed that the defect in erythritol metabolism of B. abortus B19 is not linked to its attenuated virulence in mice (30). Nevertheless, a stimulatory action of erythritol in vivo was described several decades ago for the infection of guinea pigs with B. melitensis or B. suis (15); injected erythritol increased both the proportion of animals with detectable splenic infection and the level of the infection. Larger amounts of erythritol than those needed to produce enhancement of B. suis growth, however, tend to result in a smaller increase in the infection rate with this species. In addition, the authors mention that the erythritol content in tissue extracts of the placenta is three times higher in the cow than in the sow, and it is therefore conceivable that mutants of B. suis affected in the ery operon are generally more sensitive to the polyol than corresponding mutants of B. abortus or B. melitensis. As-yet-unknown differences in basic metabolic processes yielding, for example, various concentrations of ATP could be the cause of the observed discrepancies between Brucella strains and might explain why our results differed from those described earlier by Sangari et al. for B. abortus (30). Unfortunately, no data are yet available concerning the replication of a defined ery mutant of Brucella spp. in placenta trophoblasts and the rate of abortion it induces in ungulates or on the in vivo behavior of B. abortus B19 complemented with the ery operon. Future work in this direction may help to elucidate the questions remaining open today with respect to the role of erythritol in Brucella infection.
The general conclusion of our work was that the inactivation of the erythritol degradation pathway by mutation of eryC reduced the fitness of B. suis in the intracellular environment of the macrophage, although we could not show any direct benefit of its presence for the pathogen in this specific niche. The brucellae must be able to completely metabolize any erythritol they encounter in their environment to avoid toxicity and to maintain wild-type levels of survival in the host phagocytes. This constraint may be considered a safeguard, since maintaining constant pressure on the presence of a functional erythritol degradation pathway in Brucella appears to represent a selective advantage for rapid and intense colonization of erythritol-rich tissues such as the placenta of host animals, an origin for the further spread of disease and preservation of the pathogen.
Acknowledgments
This work was supported in part by grant QLK2-CT-1999-00014 from the European Union. M. P. Jiménez de Bagües was the recipient of a grant from the Region Languedoc-Roussillon (CTPFDG/C568).
Editor: D. L. Burns
REFERENCES
- 1.Anderson, J. D., and H. Smith. 1965. The metabolism of erythritol by Brucella abortus. J. Gen. Microbiol. 38:109-124. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Brown, G. M., E. L. Love, D. E. Pietz, and C. R. Ranger. 1972. Characterization of Brucella abortus strain 19. Am. J. Vet. Res. 33:759-764. [PubMed] [Google Scholar]
- 4.Caron, E., J. P. Liautard, and S. Köhler. 1994. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J. Leukoc. Biol. 56:174-181. [DOI] [PubMed] [Google Scholar]
- 5.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekaza, E., L. Guilloteau, J. Teyssier, J. P. Liautard, and S. Köhler. 2000. Functional analysis of the ClpATPase ClpA of Brucella suis, and persistence of a knockout mutant in BALB/c mice. Microbiology 146:1605-1616. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardt, P., and J. B. Wilson. 1948. The nutrition of brucellae: growth in simple chemically defined media. J. Bacteriol. 56:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmon, B. G., L. G. Adams, and M. Frey. 1988. Survival of rough and smooth strains of Brucella abortus in bovine mammary gland macrophages. Am. J. Vet. Res. 49:1092-1097. [PubMed] [Google Scholar]
- 12.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez de Bagüés, M. P., P. H. Elzer, J. M. Blasco, C. M. Marín, C. Gamazo, and A. J. Winter. 1994. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect. Immun. 62:632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez de Bagues, M. P., C. M. Marin, M. Barberan, and J. M. Blasco. 1993. Evaluation of vaccines and of antigen therapy in a mouse model for Brucella ovis. Vaccine 11:61-66. [DOI] [PubMed] [Google Scholar]
- 15.Keppie, J., A. E. Williams, K. Witt, and H. Smith. 1965. The role of erythritol in the tissue localization of the brucellae. Br. J. Exp. Pathol. 46:104-108. [PMC free article] [PubMed] [Google Scholar]
- 16.Köhler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhler, S., J. Teyssier, A. Cloeckaert, B. Rouot, and J. P. Liautard. 1996. Participation of the molecular chaperone DnaK in intracellular growth of Brucella suis within U937-derived phagocytes. Mol. Microbiol. 20:701-712. [DOI] [PubMed] [Google Scholar]
- 18.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 19.Lillo, A. M., C. N. Tetzlaff, F. J. Sangari, and D. E. Cane. 2003. Functional expression and characterization of eryA, the erythritol kinase of Brucella abortus, and enzymatic synthesis of l-erythritol-4-phosphate. Bioorg. Med. Chem. Lett. 13:737-739. [DOI] [PubMed] [Google Scholar]
- 20.Meyer, M. E. 1966. Metabolic characterization of the genus Brucella. V. Relationship of strain oxidation rate of i-erythritol to strain virulence for guinea pigs. J. Bacteriol. 92:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer, M. E. 1967. Metabolic characterization of the genus Brucella. VI. Growth stimulation by i-erythritol compared with strain virulence for guinea pigs. J. Bacteriol. 93:996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price, R. E., J. W. Templeton, R. Smith III, and L. G. Adams. 1990. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect. Immun. 58:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sangari, F. J., and J. Aguero. 1994. Identification of Brucella abortus B19 vaccine strain by the detection of DNA polymorphism at the ery locus. Vaccine 12:435-438. [DOI] [PubMed] [Google Scholar]
- 27.Sangari, F. J., J. Aguero, and J. M. Garcia-Lobo. 2000. The genes for erythritol catabolism are organized as an inducible operon in Brucella abortus. Microbiology 146:487-495. [DOI] [PubMed] [Google Scholar]
- 28.Sangari, F. J., J. Aguero, and J. M. Garcia-Lobo. 1996. Improvement of the Brucella abortus B19 vaccine by its preparation in a glycerol based medium. Vaccine 14:274-276. [DOI] [PubMed] [Google Scholar]
- 29.Sangari, F. J., J. M. Garcia-Lobo, and J. Aguero. 1994. The Brucella abortus vaccine strain B19 carries a deletion in the erythritol catabolic genes. FEMS Microbiol. Lett. 121:337-342. [DOI] [PubMed] [Google Scholar]
- 30.Sangari, F. J., M. J. Grillo, M. P. Jimenez De Bagues, M. I. Gonzalez-Carrero, J. M. Garcia-Lobo, J. M. Blasco, and J. Aguero. 1998. The defect in the metabolism of erythritol of the Brucella abortus B19 vaccine strain is unrelated with its attenuated virulence in mice. Vaccine 16:1640-1645. [DOI] [PubMed] [Google Scholar]
- 31.Smith, H., A. E. Williams, J. H. Pearce, J. Keppie, P. W. Harris-Smith, R. B. Fitz-George, and K. Witt. 1962. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature 193:47-49. [DOI] [PubMed] [Google Scholar]
- 32.Sperry, J. F., and D. C. Robertson. 1975. Erythritol catabolism by Brucella abortus. J. Bacteriol. 121:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperry, J. F., and D. C. Robertson. 1975. Inhibition of growth by erythritol catabolism in Brucella abortus. J. Bacteriol. 124:391-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng, C. C., S. Tjoa, P. V. Fennessey, R. B. Wilkening, and F. C. Battaglia. 2002. Transplacental carbohydrate and sugar alcohol concentrations and their uptakes in ovine pregnancy. Exp. Biol Med. (Maywood) 227:189-195. [DOI] [PubMed] [Google Scholar]