Abstract
The contribution of the Staphylococcus aureus surface polysaccharide poly-N-acetylglucosamine (PNAG) to virulence was evaluated in three mouse models of systemic infection: bacteremia, renal abscess formation, and lethality following high-dose intraperitoneal (i.p.) infection. Deletion of the intercellular adhesin (ica) locus that encodes the biosynthetic enzymes for PNAG production in S. aureus strains Mn8, Newman, and NCTC 10833 resulted in mutant strains with significantly reduced abilities to maintain bacterial levels in blood following intravenous or i.p. injection, to spread systemically to the kidneys following i.p. injection, or to induce a moribund/lethal state following i.p. infection. In the bacteremia model, neither growth phase nor growth medium used to prepare the S. aureus inoculum affected the conclusion that PNAG production was needed for full virulence. As the SarA regulatory protein has been shown to affect ica transcription, PNAG synthesis, and biofilm formation, we also evaluated S. aureus strains Mn8 and 10833 deleted for the sarA gene in the renal infection model. A decrease in PNAG production was seen in sarA mutants using immunoblots of cell surface extracts but was insufficient to reduce the virulence of sarA-deleted strains in this model. S. aureus strains deleted for the ica genes were much more susceptible to antibody-independent opsonic killing involving human peripheral blood leukocytes and rabbit complement. Thus, PNAG confers on S. aureus resistance to killing mediated by these innate host immune mediators. Overall, PNAG production by S. aureus appears to be a critical virulence factor as assessed in murine models of systemic infection.
Staphylococcus aureus is well-known for its ability to elaborate a broad range of virulence factors that are thought to be key to this organism's abilities to colonize, infect, and eventually cause disease in a variety of host tissues. Among these factors are bacterial proteins that bind to host extracellular matrix proteins that are referred to as MSCRAMMs (16, 65, 75); capsular polysaccharides (CP) such as CP5 and CP8 (54); extracellular toxins, hemolysins, and superantigens (36, 53, 62); protein A (20); mediators of antibiotic resistance (26, 67); proteases and lipases (11, 22); formation of biofilms (17, 59); and iron acquisition (45, 66, 70). Regulatory proteins also impact the production of virulence factors, with prominent systems including the products of the accessory gene regulator (agr) locus (39), the staphylococcal accessory regulator (sar) locus (6), proteins encoded by two-component regulatory systems (4, 58), and transcriptional factors and their regulators, such as sigB and rsbU (29).
While acknowledging the multifactorial nature of S. aureus pathogenesis, typical virulence studies in animals for new virulence factors usually evaluate changes in tissue levels of mutant strains compared with parental controls or some change in lethality (3, 7, 54, 58). Such studies can be useful for determining the impact of a virulence factor on the organism's overall ability to cause disease, but often the evaluation must be done to take account of the fact that many S. aureus virulence factors are part of redundant systems. In these cases, loss of a single factor does not markedly change the virulence phenotype. Virulence studies are sometimes coupled with investigations into the vaccine potential of certain factors, with the expectation that if the loss of production of the vaccine antigen reduces virulence sufficiently, then escape mutants unable to elaborate the vaccine factor are unlikely to emerge under selective pressure from vaccine-induced immunity (23, 35, 44). Overall, finding antigens that both contribute to virulence and serve as targets for protective immunity in S. aureus could underscore the vaccine potential of such factors.
Prior work has evaluated the vaccine potential of the poly-N-acetylglucosamine (PNAG) surface polysaccharide elaborated by S. aureus (42, 47-49) and Staphylococcus epidermidis (47, 51, 63) and also recently found to be produced by Escherichia coli (74) and members of the genus Actinobacillus (30). Enzymes encoded by the intercellular adhesin (ica) locus are responsible for synthesis of staphylococcal PNAG (8, 24, 49; reviewed in reference 21), and the polysaccharide has also been referred to as the capsular polysaccharide/adhesin of S. epidermidis (50, 51, 69) and polysaccharide intercellular adhesin (PIA) (40, 41) and mistakenly referred to as a poly-N-succinyl glucosamine (PNSG) molecule (47, 49). Clinical isolates of S. epidermidis are more likely to carry the ica genes than are commensal strains (51, 77), and among isolates of S. aureus, the ica locus was one of seven genes found to be more frequently present in invasive strains than in commensal isolates from healthy blood donors (56). Deletion of the ica locus in S. epidermidis decreases virulence in models of foreign-body or device-related infection when bacteria are inoculated onto freshly implanted foreign bodies (60, 61) but not when inoculated onto foreign bodies that had been resident in animal tissues for 2 weeks prior to infection (15). Deletion of the ica locus in S. aureus failed to alter virulence in rats or mice when bacteria were inoculated into tissue cages implanted 2 weeks prior to infection (15, 18, 34). Other types of studies with ica-deleted strains of S. aureus have not been reported.
Therefore, we evaluated the contribution of PNAG to virulence of S. aureus using outcomes wherein systemic spread of the organism would be a key component of the pathological outcomes. These models would require that PNAG contribute to resistance to host innate phagocytic capabilities, as opposed to contributing to biofilm formation on an implanted foreign body. In the series of experiments reported here, we evaluated the effect on virulence of loss of the ica locus in three different S. aureus strains using murine models of bacteremia, renal infection, and lethality. Furthermore, because the sarA locus has been found to regulate transcription of the ica locus and PNAG production in vitro (1, 72) and the SarA protein binds to the promoter of the ica locus (28), we evaluated the virulence of two of the strains deleted for the sarA locus in the murine model of renal infection. Overall, we found that complete loss of the ica locus and inability to produce PNAG severely compromised the virulence of all three S. aureus strains in all three models of infection, whereas partial reduction of PNAG production in strains deleted for the sarA locus had no impact on the virulence of S. aureus in the renal infection model.
MATERIALS AND METHODS
Bacterial strains.
The characteristics of the strains used are shown in Table 1. Staphylococcus aureus strain NCTC 10833 (referred to as 10833 hereafter) (ATCC 25904) is a CP-negative, clumping factor-positive variant of a throat swab isolate. S. aureus strain Mn8 is a CP8 clinical isolate originally obtained from a patient with toxic shock syndrome (33) and kindly provided by Patrick Schlievert, Minneapolis, MN. S. aureus strain Newman is a CP5 strain that has been used for many virulence studies of S. aureus (12, 46, 55) and was kindly provided by Timothy Foster, Dublin, Ireland. Capsule serotypes were confirmed by Robert Solinga in the laboratory of Jean Lee, Boston, MA, using immunodiffusion (54).
TABLE 1.
Bacterial strains used in this study
| Strain | Serotype | Reference(s) |
|---|---|---|
| Newman | CP5 | 52 |
| Newman Δica | CP5 | This study |
| Newman Δica complemented | CP5 | This study |
| MN8 | CP8 | 33 |
| MN8 Δica | CP8 | 27 |
| MN8 Δica complemented | CP8 | This study |
| ALC1342 (ΔsarA donor) | Nontypeable | 7 |
| MN8 ΔsarA | CP8 | This study |
| 10833 | Nontypeable | ATCC 25904 |
| 10833 Δica | Nontypeable | 8, 27 |
| 10833 Δica complemented | Nontypeable | This study |
| 10833 ΔsarA | Nontypeable | This study |
The ica locus was replaced with a tetracycline (Tet) resistance cassette by homologous recombination as described previously (8, 27) in S. aureus strains 10833, Mn8, and Newman to produce S. aureus strains 10833ica::tet, MN8ica::tet, and Newman ica::tet, respectively, but referred to as Δica strains. All of the ica::tet strains were complemented with the intact chromosomal ica locus by homologous recombination. Briefly, the ica::tet strains were transformed with plasmid pWT, which was constructed as previously described (27) by inserting the cloned ica locus from S. aureus strain MN8 into the temperature-sensitive shuttle vector pBT9 (5). Transformants were subcultured twice in tryptic soy broth (TSB) at 42°C in the presence of chloramphenicol (Cm) to select for chromosomal integration of the plasmid. Integrants were subcultured twice in TSB at 30°C in the absence of antibiotics to promote excision of the pBT9 plasmid and retention of the ica locus and then grown on tryptic soy agar (TSA) without antibiotics. Single colonies were replica plated onto TSA without antibiotics and TSA containing 5 μg Tet/ml or 5 μg chloramphenicol (Cm)/ml. Tet/Cm-sensitive colonies were analyzed by PCR for the presence of the intact ica locus and assessed for production of PNAG as described below. S. aureus strain ALC1342, which has the sarA locus from S. aureus strain RN6390 replaced by an erythromycin cassette (76), was kindly provided by Ambrose Cheung, Hanover, NH. The mutation was transduced to strains Mn8 and 10833 using phage 80 (28), and loss of the sarA locus in erythromycin-resistant transductants was confirmed by PCR analysis.
Production of PNAG.
Synthesis of the PNAG polysaccharide by the strains in this study was assessed using a semiquantitative immunoblot method as described previously (9) with some modifications. Approximately 5 × 109 CFU from 5 ml of overnight cultures grown in TSB plus 1% glucose was collected by centrifugation, and the cell pellets were resuspended in 250 μl 0.5 M EDTA and then boiled for 5 min. The cells were removed by centrifugation, and the cleared surface extract was treated with 1 mg proteinase K/ml at 65°C for 30 min, after which the protease was inactivated by boiling for 5 min. The material in 200 μl of surface extracts, either undiluted or diluted 1:10 in Tris-buffered saline, was immobilized on nitrocellulose using a slot blot vacuum manifold. The blots were blocked with 5% bovine serum albumin and probed with 200 ng/ml of a goat antibody raised to a conjugate of deacetylated PNAG (dPNAG) and diphtheria toxoid (43) and affinity purified on a column of immobilized dPNAG as described previously (31). After the blots were washed, they were treated with a 1:10,000 dilution of swine anti-goat antibody conjugated to horseradish peroxidase. Immunoreactive bands were detected using the enhanced chemiluminescent reagent (Amersham Biosciences, Piscataway, NJ) and visualized on autoradiographic film.
Preparation of bacteria for animal studies.
For the bacteremia studies, S. aureus strains were grown on TSA plates overnight and inoculated the next morning into TSB to achieve an optical density of 0.1 (optical density at 650 nm [OD650]). The cultures were incubated on a rotor rack at 37°C until an OD650 of 0.4 was reached. Bacteria were washed once with sterile saline, resuspended in phosphate-buffered saline (PBS) to predetermined optical densities, and kept on ice until injected into the animals. The actual inocula were verified by viable counts. The inocula used were between 4.5 × 107 and 5.0 × 108 CFU/mouse.
For some of the bacteremia studies, strain Newman was grown overnight at 37°C on Columbia salt agar plates, then suspended in 2% NaCl, and adjusted to the appropriate challenge dose by OD measurements. The inocula were verified by viable counts.
For the two infection studies initiated by i.p. injection, bacteria were grown overnight on TSA plates and suspended in PBS to an OD of 0.4 (∼2.5 × 109 CFU/ml).
Animal experiments.
Female Swiss-Webster mice (5 to 7 weeks old) were obtained from Charles River Laboratories (Kingston, MA). Female and male inbred FVB mice (4 to 6 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME).
For the bacteremia studies, Swiss-Webster mice were infected by i.v. injection of 0.2 ml of bacteria and sacrificed after 2 and 4 h. A sample of 0.5 ml blood was obtained by using a sterile heart stick, mixed with 20 μl of heparin (Sigma), and plated onto TSA plates. Bacteremia was quantified by colony counts after overnight growth plated from duplicate samples. The lower limit of detection was 2 CFU/ml of blood. Samples with no bacterial colonies were assigned a value of 1 CFU for purposes of statistical analysis.
For the renal abscess model, Swiss-Webster mice were injected i.p. with 0.2 ml of a suspension of bacteria adjusted to contain 5 × 108 CFU of each strain in the inoculum. Plate counts verified the actual infectious dose, and all were within 5% of this target. After 5 days the animals were sacrificed and their kidneys were removed, weighed, and homogenized for determination of bacterial CFU/g kidney following dilution and plating. Dilutions were made in TSB containing 0.05% Tween to prevent bacterial adherence to the pipette tips and walls of the dilution vessels.
For the lethality model, 4- to 6-week-old inbred FVB mice were used, as mice of this strain and age have been found to be more susceptible to S. aureus infection by the i.p. route (T. Maira-Litran and G. B. Pier, unpublished observation). The mice were injected i.p. with ∼5 × 108 to 1 × 109 CFU S. aureus strains in 0.2 ml PBS. Mice were monitored twice daily for signs of illness, and when they were moribund, as determined by an inability to move upon repeated stimulus, inability to right itself after being placed on its side, piloerection, rapid respiration, and overall appearance of illness associated with imminent death, the animals were humanely sacrificed and counted as dead for the outcomes in this experiment. Mice that expired in between observations were also counted as lethal events for data analysis.
Opsonophagocytic assay.
Analyses of the susceptibility of ica-deleted, sarA-deleted wild-type (WT) and ica-deleted then ica-complemented strains to the opsonic killing activities of human polymorphonuclear neutrophils (PMN) and different concentrations of complement (infant rabbit serum; Accurate Chemical and Scientific, Westbury, NY) were carried out as described previously (42). All components were prepared in RPMI medium containing 15% fetal calf serum (HyClone, Logan, UT). Briefly, a 100 μl suspension of bacteria at 2 × 107 to 4 × 107 CFU/ml was mixed with 100 μl of 2 × 106 human leukocytes purified by dextran sedimentation and 100 μl of infant rabbit serum diluted from 1:5 to 1:25, and this mixture was rotated for 90 min at 37°C. Surviving CFU were determined by dilution and plating for bacterial enumeration. Survival was calculated in comparison to the mean number of CFU surviving in tubes without PMN or without complement.
Statistical analysis.
Statistical significance for two-way comparisons was determined by an unpaired t test. Analysis of variance (ANOVA) for multigroup comparisons was used on log-transformed data, and the Tukey's multiple-comparison test was used for posthoc analysis for pairwise comparisons. Results from Fisher's exact test were calculated in a Microsoft Excel spreadsheet containing the appropriate formula. Remaining statistical results were calculated using the Prism 3 software package on a Macintosh computer.
RESULTS
PNAG production by S. aureus strains.
We evaluated the production of PNAG by the three S. aureus strains and their Δica and ica-complemented isogenic variants, as well as by the two strains deleted for the sarA gene that we used. Using a semiquantitative immunoblot method (Fig. 1), we showed that the Δica strains made no detectable PNAG, the ica-complemented strains made WT levels of PNAG, and the ΔsarA strains had reduced but detectable synthesis of PNAG.
FIG. 1.
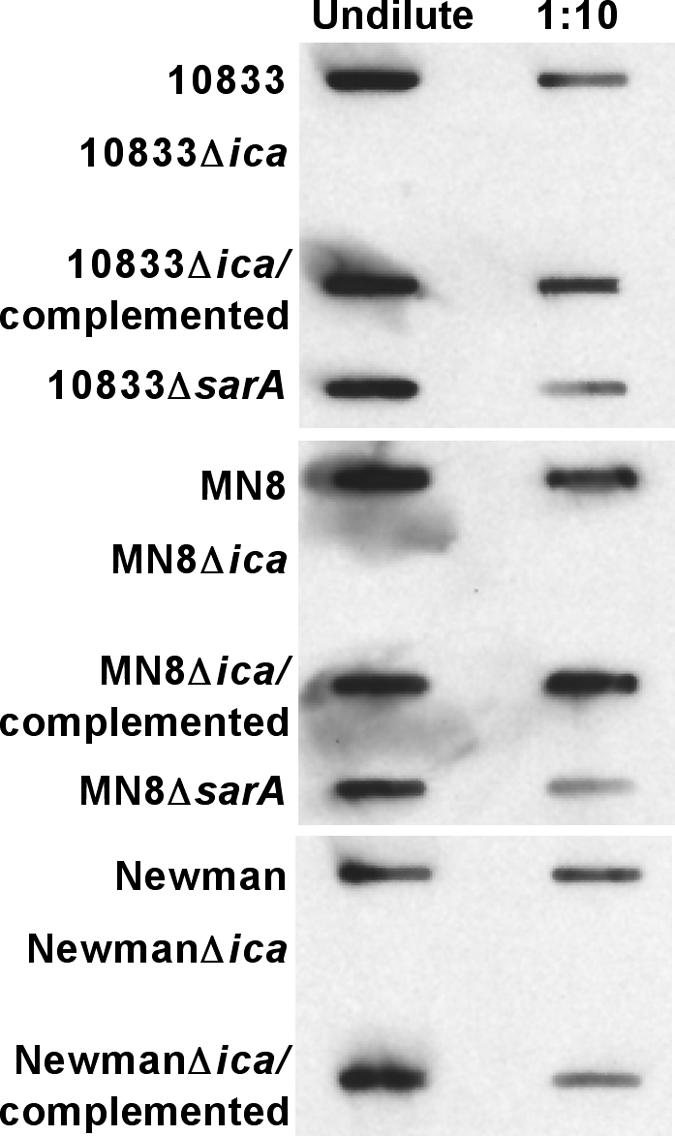
Immunoblot analysis of PNAG expression by strains of S. aureus (listed along the left-hand side of the blot) evaluated in animal models of infection. Undiluted and 1:10 diluted boiled EDTA extracts from 5 × 109 cells were applied by a vacuum manifold to the membrane which was probed with 200 ng/ml of affinity-purified goat antibody raised to a conjugate of dPNAG and diphtheria toxoid (43) followed by swine anti-goat immunoglobulin G conjugated to horseradish peroxidase.
Reduced levels of Δica strains in the blood of i.v. infected mice.
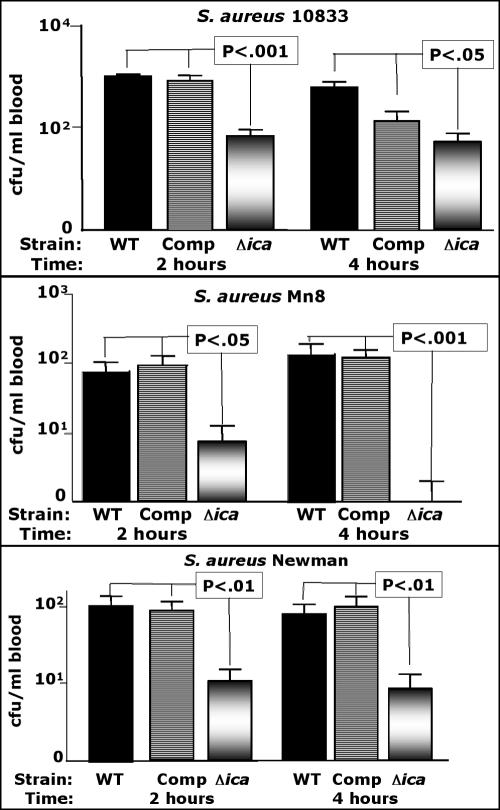
We first evaluated the ability of three strains of S. aureus with serotype CP5 (Newman), CP8 (Mn8), or CP-nontypeable (10833) isolates with intact or deleted ica loci for their ability to be cleared from mouse blood 2 and 4 h after intravenous (i.v.) injection. As shown in Fig. 2, at both 2 and 4 h postinjection, the strains deleted for the ica genes had significantly (P < 0.05) reduced blood levels compared to both the WT and ica-complemented strains. There was no significant difference (P > 0.05) between the blood levels of the WT or complemented strains. Table 2 shows the results of a comparison of the proportion of animals with any detectable S. aureus in their blood (i.e., documented bacteremia) with those that had no detectable bacteria in their blood samples (<2 CFU/ml). Although there were reduced proportions of animals with documented bacteremia in all groups challenged with Δica strains, the proportion was significantly lower compared to the WT parental strain only in strain 10833 at 4 h postinfection and strain Mn8 at both 2 and 4 h postinfection. However, for strain Newman at both 2 h and 4 h postinfection, two of the mice in each of the Δica groups had only 1 CFU on the blood culture plate (2 CFU/ml blood), meaning that four of eight mice in the Δica groups had ≤2 CFU/ml blood, compared with all eight WT or ica-complemented mice with ≥3 CFU/ml blood. Using this as a basis for comparing rates of bacteremia, a P value of 0.038 (Fisher's exact test) is achieved. Thus, when analyzing all six of the comparisons of S. aureus Δica strains with their isogenic WT counterparts (Fig. 1), five of the six comparisons showed significantly reduced rates of bacteremia (≤2 CFU/ml compared to ≥3 CFU/ml) in an evaluation of WT and Δica S. aureus strains.
FIG. 2.
Comparison of CFU/ml of blood 2 and 4 h postinfection using three strains of S. aureus with either a WT ica locus, the ica locus deleted and replaced with a tetracycline resistance cassette (Δica), or with the WT ica locus placed back into the chromosome (complemented [Comp] strain) of the Δica strain. Bars represent mean CFU/ml blood, error bars the standard deviations. Eight mice per group were used. The lower limit of detection is 2 CFU/ml. P values for strain 10833 represent pairwise comparisons between the Δica strain and both the WT and Comp strains determined by Tukey's multiple-comparison test. Overall ANOVA for all three strains yielded a P value of <0.001. There was no significant difference (P > 0.05) in CFU/ml blood comparing any of the WT and isologous S. aureus Comp strains.
TABLE 2.
Proportion of mice with positive blood cultures (≥ 2 CFU/ml) after infection under the indicated conditions with different strains of S. aureus
| S. aureus strain and injection route | Time postinfection (h) | No. of mice with positive blood cultures/total no.
|
||
|---|---|---|---|---|
| WT | Complemented | Δica | ||
| Newman, i.v. | 2 | 8/8 | 8/8 | 6/8 |
| 4 | 8/8 | 8/8 | 6/8 | |
| 10833, i.v. | 2 | 8/8 | 8/8 | 5/8 |
| 4 | 8/8 | 8/8 | 4/8a | |
| MN8, i.v. | 2 | 8/8 | 8/8 | 3/8b |
| 4 | 8/8 | 8/8 | 1/8c | |
| Newman, i.v./CSAd | 2 | NDe | 8/8 | 4/8a |
| 4 | ND | 8/8 | 3/8b | |
| Newman, i.p. | 2 | ND | 8/8 | 4/8a |
| 4 | ND | 8/8 | 4/8a | |
Significantly different from the value obtained for the WT strain (P = 0.038 by Fisher's exact test).
Significantly different from the value obtained for the WT strain (P = 0.013 by Fisher's exact test).
Significantly different from the value obtained for the WT strain (P = 0.0007 by Fisher's exact test).
CSA, Columbia salt agar used to prepare the inoculum.
ND, not done.
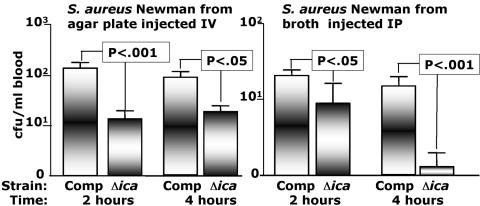
Previous reports indicated that demonstrating differences in the virulence of WT and CP mutant strains of S. aureus in a mouse bloodstream infection system, as well as the binding of complement, are affected by the method of growth of the organisms (10, 68). To determine whether similar in vitro growth conditions affected the demonstration of a role for PNAG in S. aureus virulence, we compared the blood levels of Δica and ica-complemented strains of S. aureus Newman taken directly from a Columbia agar plate after overnight growth, which enhances CP expression (68). As shown in Fig. 3, at 2 h and 4 h postinjection the Δica strain still had significantly reduced blood levels compared with the complemented strain. The proportion of infected animals with documented bacteremia (≥2 CFU/ml) following injection of bacteria grown on Columbia salt agar medium is shown in Table 2. Along the same lines, because the route of injection could affect virulence, we compared the blood levels of the complemented and Δica S. aureus Newman strains 2 h and 4 h following i.p. injection of bacteria taken from log-phase growth in broth. The levels of the complemented strains in blood were lower when the strains were injected by the i.p. route compared to the i.v. route (Fig. 2), but again the Δica strain had significantly reduced levels in blood even following i.p. injection (Fig. 3) and significantly reduced rates of bacteremia (Table 2). Overall, the Δica strains had reduced levels in the blood of mice 2 h and 4 h postinjection regardless of whether the bacteria were injected i.p. or i.v., and growth on Columbia salt agar medium to promote CP expression did not affect the virulence of the Δica mutant of S. aureus Newman.
FIG. 3.
Comparison of the method of growth of the challenge inoculum or the route of injection on the CFU/ml in blood 2 and 4 h postinfection achieved by S. aureus strain Newman either deleted for the ica locus (Δica) or with the WT ica locus placed back into the chromosome (complemented [Comp] strain). Bars represent mean CFU/ml blood, error bars the standard deviations. Eight mice per group were used. The lower limit of detection is 2 CFU/ml. P values were determined by t test.
Evaluation of the virulence of Δica and ΔsarA mutants of S. aureus strains in a renal infection model.
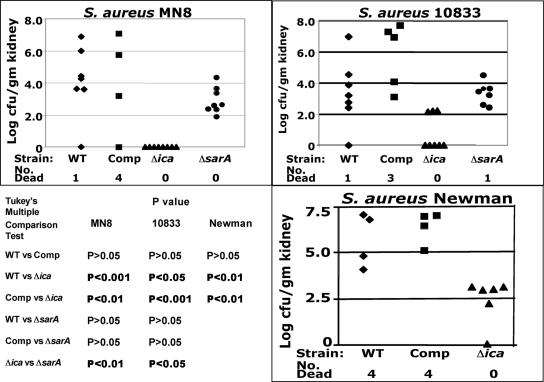
We next analyzed the virulence of the WT, Δica, and ica-complemented variants of S. aureus Mn8, Newman, and 10833 in a renal infection model by measuring the levels of bacteria in the kidneys 5 days after i.p. infection with ∼5 × 108 CFU/mouse. As shown in Fig. 4, for all three of the Δica variants, the levels of bacteria in the kidneys were significantly reduced or undetectable compared to the WT and ica-complemented strains. For strain Mn8, none of eight mice had detectable Δica mutant bacteria in their kidneys, and for strain 10833, five of eight mice infected with the Δica mutant had apparently sterile kidneys. For strain Newman, while five of six mice infected with the Δica mutant had detectable bacteria in their kidneys, the numbers were quite low, with less than 1,000 CFU/gram measured. In contrast, surviving mice infected with the WT or ica-complemented strains had bacterial levels ranging from 10,000 to 10,000,000 CFU/g kidney. The statistical analyses of these results are also shown in Fig. 4.
FIG. 4.
Comparison of CFU/g kidney 5 days after i.v. infection with ∼5 × 108 CFU of three strains of S. aureus with either a wild-type (WT) ica locus (⧫), the Δica strain with the WT ica locus placed back into the chromosome (complemented [Comp] strain) (▪), or the Δica strain (▴). For S. aureus strains MN8 and 10833, we also performed infections with the sarA::erm strain (ΔsarA) (•). Each point is the result from one mouse. The lower limit of detection is 10 CFU/g kidney. Kidneys in mice found dead were not analyzed for CFU/g due to postmortem effects on bacterial levels. Overall ANOVA for results with all three strains gave a P value of <0.001. The results of pairwise comparisons for each strain using Tukey's multiple-comparison test are shown. P values of <0.05 are highlighted in boldface type.
As the dose of the WT and ica-complemented S. aureus strains injected into the mice to cause renal infections was lethal for a proportion of the animals, we also analyzed lethal outcomes, comparing the combined results of mice infected with all three WT and ica-complemented strains with the results of mice infected with all three of the Δica mutants. Overall, 17 of 48 (35%) mice infected with the WT or ica-complemented strains died, compared with 0 of 22 mice infected with the Δica mutants (P = 0.0005, Fisher's exact test).
Because SarA has been reported both to bind to the ica promoter (28) and to promote ica transcription and biofilm formation in S. aureus (1, 72), we investigated the virulence of ΔsarA mutants of S. aureus strains Mn8 and 10833 in the renal infection model. The levels of these mutants in the kidneys 5 days after i.p. injection were not significantly reduced compared to the levels of either WT or ica-complemented S. aureus strains (Fig. 4). The ΔsarA strains were recovered from the kidneys at significantly higher levels than the Δica strains were (Fig. 4). Thus, although the ΔsarA mutants of S. aureus Mn8 and 10833 showed somewhat reduced production of PNAG (Fig. 1), this reduction was insufficient to affect virulence, consistent with other reports that strains in which only sarA was deleted maintain virulence in a kidney infection model (6, 7).
Effect of deleting the ica locus on virulence of S. aureus in a high-dose i.p. injection model.
As a third determination of the roles of the ica locus and PNAG production in virulence, we examined lethality following i.p. injection of inbred 4- to 6-week-old FVB mice with Δica mutant and ica-complemented S. aureus strains. Preliminary analysis indicated that in these mice, as well as strain A/J mice, a 1-log-unit-lower dose of S. aureus was needed for a lethal infection in most of the mice compared to the lethal doses determined for outbred Swiss-Webster mice and inbred BALB/c, C3H/HeN, C57BL/6, and SJ/L mice (T. Maira-Litran and G. B. Pier, unpublished observations). As shown in Table 3, for all three S. aureus strains injected i.p. at a dose of ∼5 × 108 −109 CFU, survival rates were only 0 to 12.5% in animals injected with the ica-complemented strains versus 83.3% to 100% survival in mice injected with the Δica strains.
TABLE 3.
Comparison of the lethality of ica-complemented and Δica strains of S. aureus following i.p. injection into FVB mice
| Strain | No. of survivors/total no. of challenged mice (%) (challenge dose per mouse)a
|
P valueb | |
|---|---|---|---|
| ica-complemented strain | Δica mutant | ||
| Mn8 | 0/6 (0) (5.0 × 108) | 5/6 (83.3) (5.0 × 108) | 0.008 |
| Newman | 0/6 (0) (4.3 × 108) | 6/6 (100) (6.7 × 108) | 0.001 |
| 10833 | 1/8 (12.5) (1.0 × 109) | 8/8 (100) (1.0 × 109) | 0.0007 |
Challenge dose in CFU per mouse.
P value by Fisher's exact test.
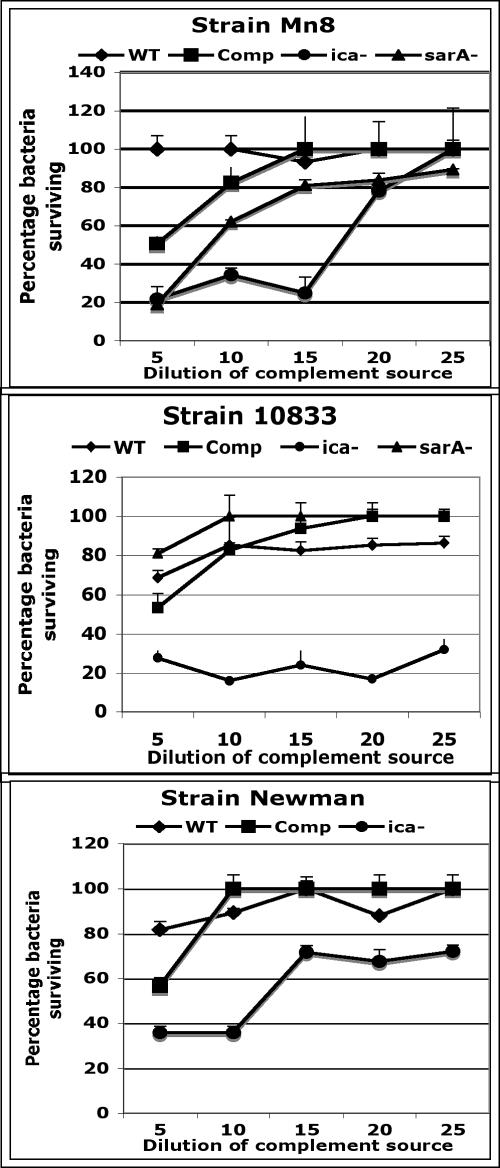
Role of PNAG in S. aureus resistance to complement-mediated phagocytosis.
In S. epidermidis it has been shown that loss of PNAG production leads to increased sensitivity to antibody-independent opsonophagocytosis by white blood cells (64, 73). To determine whether the same mechanism is operative in S. aureus, where production of other capsular polysaccharides could compensate for loss of PNAG synthesis, we analyzed the susceptibility to killing by complement and human phagocytes of WT, ica-complemented, Δica, and ΔsarA S. aureus strains. As shown in Fig. 5, all three Δica mutant strains were much more readily killed, particularly in the presence of the highest complement concentrations tested, whereas the WT, ica-complemented, and ΔsarA strains were clearly less susceptible to complement-mediated phagocytic killing. Notably, the two ica-deleted strains with classic S. aureus CP, Mn8 (CP8), and Newman (CP5), were somewhat more resistant to complement-mediated opsonic killing than was the ica-deleted unencapsulated strain 10833, as this strain was killed by all concentrations of complement tested.
FIG. 5.
Susceptibility of WT, ica-complemented (Comp), Δica (ica−) and ΔsarA (sarA−) strains of S. aureus to opsonic killing mediated by various concentrations of infant rabbit serum as a source of complement.
DISCUSSION
The virulence properties of S. aureus are clearly multifactorial, involving a large array of surface and secreted factors encompassing a range of biochemical entities, including proteins, polysaccharides, peptidoglycans, and teichoic acids. Production of virulence factors is regulated by complex genetic interactions whose properties are only minimally understood at present (4, 6, 14, 19). Thus, there is usually not a global or complete loss of virulence when a single factor is interrupted, and the effects on virulence of loss of single genes or single genetic loci are quite contextual, depending on the S. aureus strain, the animal system, the tissue infected, and the outcome measured (57). With these caveats, we consistently found, in three mouse models of infection, using three divergent S. aureus strains, that the loss of PNAG via deletion of the ica locus had a profound effect on virulence. The Δica bacterial mutants were poorly able to sustain bacteremia in blood, poorly able to disseminate from the peritoneum to the kidney, and unable to mediate lethal events following high-dose peritoneal infection. Overall, our results indicate that PNAG production by S. aureus is important for high-level virulence in murine models of systemic infection, likely due to PNAG protecting the bacterial cells from innate host defenses, encompassing phagocytes and complement.
It should be noted that chromosomally complemented strains had to be used, since many S. aureus plasmids containing antibiotic resistance genes that are used for transcomplementation cannot be maintained in vivo, even with antibiotic selection (2). Also, most plasmids used for S. aureus complementation are multicopy plasmids (12) which could result in increased transcription of the ica genes which leads to increased production of PNAG (27, 72). This would likely affect experimental outcomes, as happened in a study on the role of clumping factor B (ClfB) in a rat model of endocarditis (12), wherein there was no difference in virulence between a WT and ClfB mutant but overexpression of clfB from a multicopy plasmid resulted in increased virulence. Although achieving a WT phenotype by chromosomal complementation of the initial Δica strains ensured that no secondary mutations were introduced during strain manipulations, we cannot totally exclude the possibility that the tet gene inserted into the Δica strains did not have an effect on transcription of other genes that might have impacted the phenotypes seen here with the Δica strains.
Prior studies in S. epidermidis on strains unable to produce PNAG indicated a loss of virulence in models of both systemic infection (63, 64) and local, foreign-body-related infection (60, 61, 71). However, in S. aureus, interruption of the ica locus did not materially affect pathogenesis in rat or mouse models of foreign-body infection (15, 18, 34). Thus, there appears to be some differences in the role PNAG plays in S. epidermidis versus S. aureus foreign-body infection. In addition, we also found that even though SarA is a transcriptional activator of ica, production of PNAG is not dependent enough on SarA to render a sarA mutant deficient in virulence to the same degree as is loss of the ica locus. The reduced amount of PNAG made by ΔsarA strains appears to be sufficient for S. aureus to maintain resistance to host defenses and retain essentially WT levels of virulence in the mouse renal infection model used to compare the pathogenesis of Δica and ΔsarA strains to WT S. aureus Mn8 and Newman strains.
Among S. aureus strains, the most studied surface polysaccharides are the CP5 and CP8 antigens (54), chemically related but immunologically distinct polymers that have been shown to play important roles in S. aureus virulence (13, 54). As with many bacterial capsular polysaccharides, S. aureus CP antigens appear to protect the bacterial cells from innate immunity mediated by complement and phagocytes (10, 54, 68), similar to what we report in this work for PNAG expression. These results suggest both CP expression and PNAG expression may be needed for full resistance of S. aureus strains to innate immunity mediated by phagocytes and complement. Supporting this conclusion are the data generated here with the ica-deleted strains Mn8 (CP8) and Newman (CP5), which both retained more resistance to complement-mediated phagocytic killing than did the ica-deleted and unencapsulated strain 10833. Thus, both PNAG and CP expression appear to protect S. aureus cells from innate host phagocytic effectors, consistent with findings that these two surface polysaccharides have somewhat similar functions in regard to S. aureus resistance to host immune effectors.
Virtually 100% of analyzed S. aureus human clinical strains carry an ica locus (8, 32). We have shown that with sensitive enough methods, reliant on good immunologic reagents, all examined human clinical bacteremic strains of S. aureus produce detectable PNAG (42). However, some commensal strains of S. aureus do not carry an ica locus as shown in the study that compared invasive and noninvasive isolates of S. aureus (56). Peacock et al. (56) found that the invasive strains were much more likely to carry an intact ica locus than the commensal S. aureus strains. When these findings are placed in the context of the animal studies reported here describing a significant loss of virulence for three S. aureus strains lacking the ica genes in three models of systemic infection, it appears that PNAG expression may be critical for disease manifestations to emerge in individuals with systemic S. aureus infection.
In addition to S. aureus and strains of coagulase-negative staphylococci, it has been recently demonstrated that E. coli has an ica-homologous locus, designated ycdSRQP in the E. coli K-12 sequenced genome but renamed pga, that encodes proteins that synthesize PNAG (74). Also, Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae (30) possess an ica-homologous genetic locus encoding production of PNAG, and it further appears that the Yersinia pestis hms locus is another ica homologue (38). More ica homologues are found in other gram-negative bacteria (74). Whether PNAG plays a similar role in virulence for these other organisms is not currently known, although deletion of the hms locus in Y. pestis did not affect virulence in a mouse model of bubonic plague (37). However, the hms locus is essential for transmission of Y. pestis from flea vectors to mammalian hosts (25), indicating a role in the natural transmission cycle of Y. pestis.
Overall, for S. aureus, while we were able to demonstrate an essential role for PNAG production in the virulence of this organism in settings of systemic spread using mice as hosts, it is anticipated that the role of PNAG in the virulence of disease in humans will be dependent on the site of infection, the organism's ability to elaborate other virulence factors, and the state of host immunity to multiple S. aureus antigens. With more and more information emerging about the virulence of S. aureus infections, we can expect that these data will be useful for devising new and appropriate intervention strategies, such as multicomponent vaccines, that could help blunt the impact of infection and disease by one of the most common nosocomial pathogens isolated in the early 21st century.
Acknowledgments
This work was supported by NIH grants AI46706 (G.B.P.) and AI061590 (K.J.).
Editor: J. B. Bliska
REFERENCES
- 1.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhasin, N., A. Albus, F. Michon, P. J. Livolsi, J. S. Park, and J. C. Lee. 1998. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol. Microbiol. 27:9-21. [DOI] [PubMed] [Google Scholar]
- 3.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65:1550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronner, S., H. Monteil, and G. Prevost. 2004. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol. Rev. 28:183-200. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramton, S. E., M. Ulrich, F. Gotz, and G. Doring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunnion, K. M., J. C. Lee, and M. M. Frank. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect. Immun. 69:6796-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubin, G. 2002. Extracellular proteases of Staphylococcus spp. Biol. Chem. 383:1075-1086. [DOI] [PubMed] [Google Scholar]
- 12.Entenza, J. M., T. J. Foster, D. Ni Eidhin, P. Vaudaux, P. Francioli, and P. Moreillon. 2000. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 68:5443-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fattom, A. I., and R. Naso. 1996. Staphylococcal vaccines: a realistic dream. Ann. Med. 28:43-46. [DOI] [PubMed] [Google Scholar]
- 14.Fedtke, I., F. Gotz, and A. Peschel. 2004. Bacterial evasion of innate host defenses—the Staphylococcus aureus lesson. Int. J. Med. Microbiol. 294:189-194. [DOI] [PubMed] [Google Scholar]
- 15.Fluckiger, U., M. Ulrich, A. Steinhuber, G. Döring, D. Mack, R. Landmann, C. Goerke, and C. Wolz. 2005. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 73:1811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 17.Fowler, V. G., Jr., P. D. Fey, L. B. Reller, A. L. Chamis, G. R. Corey, and M. E. Rupp. 2001. The intercellular adhesin locus ica is present in clinical isolates of Staphylococcus aureus from bacteremic patients with infected and uninfected prosthetic joints. Med. Microbiol. Immunol. (Berlin) 189:127-131. [DOI] [PubMed] [Google Scholar]
- 18.Francois, P., P. H. Tu Quoc, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, S. E. Cramton, F. Gotz, and P. Vaudaux. 2003. Lack of biofilm contribution to bacterial colonisation in an experimental model of foreign body infection by Staphylococcus aureus and Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 35:135-140. [DOI] [PubMed] [Google Scholar]
- 19.Goerke, C., and C. Wolz. 2004. Regulatory and genomic plasticity of Staphylococcus aureus during persistent colonization and infection. Int. J. Med. Microbiol. 294:195-202. [DOI] [PubMed] [Google Scholar]
- 20.Gomez, M. I., A. Lee, B. Reddy, A. Muir, G. Soong, A. Pitt, A. Cheung, and A. Prince. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10:842-848. [DOI] [PubMed] [Google Scholar]
- 21.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 22.Gotz, F., H. M. Verheij, and R. Rosenstein. 1998. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem. Phys. Lipids 93:15-25. [DOI] [PubMed] [Google Scholar]
- 23.Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J. Berger, F. Randazzo, and G. Grandi. 2002. Gene expression profile in Neisseria meningitidis and Neisseria lactamica upon host-cell contact: from basic research to vaccine development. Ann. N. Y. Acad. Sci. 975:202-216. [DOI] [PubMed] [Google Scholar]
- 24.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 25.Hinnebusch, B. J., R. D. Perry, and T. G. Schwan. 1996. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273:367-370. [DOI] [PubMed] [Google Scholar]
- 26.Hiramatsu, K. 2001. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect. Dis. 1:147-155. [DOI] [PubMed] [Google Scholar]
- 27.Jefferson, K. K., S. E. Cramton, F. Gotz, and G. B. Pier. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 48:889-899. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson, K. K., D. B. Pier, D. A. Goldmann, and G. B. Pier. 2004. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J. Bacteriol. 186:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson, I. M., S. Arvidson, S. Foster, and A. Tarkowski. 2004. Sigma factor B and RsbU are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect. Immun. 72:6106-6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan, J. B., K. Velliyagounder, C. Ragunath, H. Rohde, D. Mack, J. K. Knobloch, and N. Ramasubbu. 2004. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J. Bacteriol. 186:8213-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly-Quintos, K., A. Kropec, S. Briggs, C. Ordonez, D. A. Goldmann, and G. B. Pier. The role of epitope specificity in the human opsonic antibody response to the staphylococcal surface polysaccharide PNAG. J. Infect. Dis, in press. [DOI] [PubMed]
- 32.Knobloch, J. K., M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. (Berlin) 191:101-106. [DOI] [PubMed] [Google Scholar]
- 33.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 34.Kristian, S. A., T. Golda, F. Ferracin, S. E. Cramton, B. Neumeister, A. Peschel, F. Gotz, and R. Landmann. 2004. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb. Pathog. 36:237-245. [DOI] [PubMed] [Google Scholar]
- 35.Kroll, J. S., and R. Booy. 1996. Haemophilus influenzae: capsule vaccine and capsulation genetics. Mol. Med. Today 2:160-165. [DOI] [PubMed] [Google Scholar]
- 36.Ladhani, S. 2003. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 39:181-189. [DOI] [PubMed] [Google Scholar]
- 37.Lillard, J. W., Jr., S. W. Bearden, J. D. Fetherston, and R. D. Perry. 1999. The haemin storage (Hms+) phenotype of Yersinia pestis is not essential for the pathogenesis of bubonic plague in mammals. Microbiology 145:197-209. [DOI] [PubMed] [Google Scholar]
- 38.Lillard, J. W., Jr., J. D. Fetherston, L. Pedersen, M. L. Pendrak, and R. D. Perry. 1997. Sequence and genetic analysis of the hemin storage (hms) system of Yersinia pestis. Gene 193:13-21. [DOI] [PubMed] [Google Scholar]
- 39.Lyon, G. J., J. S. Wright, T. W. Muir, and R. P. Novick. 2002. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 41:10095-10104. [DOI] [PubMed] [Google Scholar]
- 40.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mack, D., M. Haeder, N. Siemssen, and R. Laufs. 1996. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J. Infect. Dis. 174:881-884. [DOI] [PubMed] [Google Scholar]
- 42.Maira-Litran, T., A. Kropec, D. Goldmann, and G. B. Pier. 2004. Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide. Vaccine 22:872-879. [DOI] [PubMed] [Google Scholar]
- 43.Maira-Litran, T., A. Kropec, D. A. Goldmann, and G. B. Pier. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 73:6752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makela, P. H. 2003. Conjugate vaccines—a breakthrough in vaccine development. Southeast Asian J. Trop. Med. Public Health 34:249-253. [PubMed] [Google Scholar]
- 45.Mazmanian, S. K., H. Ton-That, K. Su, and O. Schneewind. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 99:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAleese, F. M., and T. J. Foster. 2003. Analysis of mutations in the Staphylococcus aureus clfB promoter leading to increased expression. Microbiology 149:99-109. [DOI] [PubMed] [Google Scholar]
- 47.McKenney, D., J. Hubner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 66:4711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenney, D., K. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 2000. Vaccine potential of poly-1-6-beta-D-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis. J. Biotechnol. 83:37-44. [DOI] [PubMed] [Google Scholar]
- 49.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 50.Muller, E., J. Huebner, N. Gutierrez, S. Takeda, D. A. Goldmann, and G. B. Pier. 1993. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect. Immun. 61:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller, E., S. Takeda, H. Shiro, D. Goldmann, and G. B. Pier. 1993. Occurrence of capsular polysaccharide adhesin among clinical isolates of coagulase-negative staphylococci. J. Infect. Dis. 168:1211-1218. [DOI] [PubMed] [Google Scholar]
- 52.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 53.Novick, R. P. 2003. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid 49:93-105. [DOI] [PubMed] [Google Scholar]
- 54.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmqvist, N., T. Foster, A. Tarkowski, and E. Josefsson. 2002. Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death. Microb. Pathog. 33:239-249. [DOI] [PubMed] [Google Scholar]
- 56.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pragman, A. A., and P. M. Schlievert. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42:147-154. [DOI] [PubMed] [Google Scholar]
- 58.Pragman, A. A., J. M. Yarwood, T. J. Tripp, and P. M. Schlievert. 2004. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J. Bacteriol. 186:2430-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohde, H., J. K. Knobloch, M. A. Horstkotte, and D. Mack. 2001. Correlation of Staphylococcus aureus icaADBC genotype and biofilm expression phenotype. J. Clin. Microbiol. 39:4595-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schafer, R., and J. M. Sheil. 1995. Superantigens and their role in infectious disease. Adv. Pediatr. Infect. Dis. 10:369-390. [PubMed] [Google Scholar]
- 63.Shiro, H., G. Meluleni, A. Groll, E. Muller, T. D. Tosteson, D. A. Goldmann, and G. B. Pier. 1995. The pathogenic role of Staphylococcus epidermidis capsular polysaccharide/adhesin in a low-inoculum rabbit model of prosthetic valve endocarditis. Circulation 92:2715-2722. [DOI] [PubMed] [Google Scholar]
- 64.Shiro, H., E. Muller, N. Gutierrez, S. Boisot, M. Grout, T. D. Tosteson, D. Goldmann, and G. B. Pier. 1994. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J. Infect. Dis. 169:1042-1049. [DOI] [PubMed] [Google Scholar]
- 65.Simpson, K. H., G. Bowden, M. Hook, and B. Anvari. 2003. Measurement of adhesive forces between individual Staphylococcus aureus MSCRAMMs and protein-coated surfaces by use of optical tweezers. J. Bacteriol. 185:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skaar, E. P., M. Humayun, T. Bae, K. L. DeBord, and O. Schneewind. 2004. Iron-source preference of Staphylococcus aureus infections. Science 305:1626-1628. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasan, A., J. D. Dick, and T. M. Perl. 2002. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 15:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 70.Trivier, D., and R. J. Courcol. 1996. Iron depletion and virulence in Staphylococcus aureus. FEMS Microbiol. Lett. 141:117-127. [DOI] [PubMed] [Google Scholar]
- 71.Ulphani, J. S., and M. E. Rupp. 1999. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab. Anim. Sci. 49:283-287. [PubMed] [Google Scholar]
- 72.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 73.Vuong, C., J. M. Voyich, E. R. Fischer, K. R. Braughton, A. R. Whitney, F. R. DeLeo, and M. Otto. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 6:269-275. [DOI] [PubMed] [Google Scholar]
- 74.Wang, X., J. F. Preston III, and T. Romeo. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186:2724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wann, E. R., S. Gurusiddappa, and M. Hook. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275:13863-13871. [DOI] [PubMed] [Google Scholar]
- 76.Wolz, C., P. Pohlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36:230-243. [DOI] [PubMed] [Google Scholar]
- 77.Ziebuhr, W., C. Heilmann, F. Gotz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]