Abstract
A number of epitope specificities associated with the cell wall polysaccharide antigen of group A streptococci were identified in a polyclonal rabbit antiserum induced in rabbits by whole group A streptococci and in polyclonal convalescent human antisera from children that had recovered from streptococcal A infections. The identification was achieved by using a series of synthetic oligosaccharides, glycoconjugates, and bacterial polysaccharide inhibitors to inhibit the binding of the group A helical polysaccharide to the polyclonal antisera. The exclusively dominant epitope expressed in the convalescent human antisera was the doubly branched extended helical hexasaccharide with the structure α-l-Rhap(1→2)[β-d-GlcpNAc(1→3)]α-l-Rhap(1→3)α-l-Rhap(1→2)[β-d-GlcpNAc(1→3)]α-l-Rhap. The hexasaccharide epitope also bound with the highest immunoreactivity to the rabbit antiserum. In contrast, the human antisera did not show significant binding to the singly branched pentasaccharide with the structure α-l-Rhap(1→2)α-l-Rhap(1→3)α-l-Rhap(1→2)[β-d-GlcpNAc(1→3)]α-l-Rhap or the branched trisaccharide α-l-Rhap(1→2)[β-d-GlcpNAc(1→3)]α-l-Rhap, although both these haptens bound significantly to the same rabbit antiserum, albeit with less immunoreactivity than the hexasaccharide. Inhibition studies using streptococcal group A and B rabbit antisera and the inhibitors indicated above also suggested that the group A carbohydrate, unlike the group B streptococcal polysaccharide, does not contain the disaccharide α-l-Rhap(1→2)α-l-Rhap motif at its nonreducing chain terminus, stressing the importance of mapping the determinant specificities of these two important streptococcal subcapsular group polysaccharides to fully understand the serological relationships between group A and group B streptococci.
The group A streptococci (GAS) are primary infective agents in humans and cause infectious diseases ranging from mild (pharyngitis [strep throat], impetigo, and cellulites) to very severe (pneumonia, toxic shock syndrome, and necrotizing fasciitis or flesh-eating disease) (23, 26, 30, 37). These organisms have been the subject of investigation in the past because of their sequelae, including rheumatic fever, rheumatic heart disease, and acute glomerulonephritis (1, 5, 17, 35, 39), and more recently because of the identification of pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (32).
The chemical makeup of the group A antigen was first investigated by McCarty, and this antigen was determined to be a group consisting of N-acetylglucosamine and rhamnose (18, 19, 20). The group carbohydrate antigen has remained one of the primary diagnostic tools used to identify group A streptococcal infections and forms the basis of many of the currently used “rapid strep tests” (21, 34). The studies of Riesen et al. with the group A antigen were the first studies to describe the oligoclonal nature of the antibody response in humans to carbohydrate antigens (28). However, interest in this carbohydrate as a vaccine candidate did not arise until recently (6).
The threat of antibiotic-resistant bacteria (38) in the future makes a vaccine protocol an attractive alternative to the present antibiotic protocol (14). In addition, a vaccine strategy might circumvent the current therapy for invasive necrotizing fasciitis and myositis, which involves high doses of antibiotics, aggressive surgery, and administration of intravenous immunoglobulin (30, 37).
We have focused on development of a vaccine based on the GAS cell wall polysaccharide (29). A patent describing the preparation of GAS polysaccharide (GASP) conjugates for use as vaccines has been issued (6). The bactericidal activity of human sera (containing antibodies to the GAS polysaccharide) in vitro against several strains of GAS has been demonstrated, and the phagocytic, serological, and protective properties of streptococcal group A carbohydrate antibodies have also been demonstrated (29, 40). Well-defined oligosaccharide-conjugate vaccines have also been explored with other bacterial systems with a view toward eliciting discriminating immune responses (4, 25). We propose to adopt this strategy for the group A streptococci.
Previous immunochemical work in our laboratory using the cell wall polysaccharide of GAS, synthetic portions of this compound, and glycoconjugates (Fig. 1) has indicated that the branch point and the size of the GAS antigen are crucial elements of the epitope recognized by both rabbit polyclonal and mouse monoclonal antibodies that bind the native polysaccharide antigen (8, 24, 27). Conformational analysis has shown that the branch point represented in the trisaccharide repeating motif of the polysaccharide is a well-defined conformational feature both in the free ligand form and when the compound is bound to an antibody (15, 31, 36). Furthermore, epitope mapping of a branched trisaccharide and a doubly branched hexasaccharide by saturation transfer difference nuclear magnetic resonance methods (12) has confirmed the importance of the branched trisaccharide epitope recognized by a mouse monoclonal antibody (11). The latter studies have served to check and validate previous computed models of the polysaccharide and oligosaccharide-antibody complexes (15, 24, 31). In these models, the polysaccharide exists in a helix in which the rhamnosyl residues form the core of the helix and the immunodominant N-acetylglucosamine residues are disposed on the periphery (16, 24, 31)
FIG. 1.
Structures of the inhibitors and glycoconjugates and symbolic representations.
Before further evaluation of oligosaccharide-conjugate vaccines for GAS is warranted, it is critical that the GAS carbohydrate epitopes recognized by human sera in particular are delineated. Here we describe the determinant specificities of the GAS polysaccharide binding to rabbit polyclonal antisera raised against whole GAS bacteria and to human convalescent-phase sera. The possible topographies presented to the human immune system are also discussed below.
MATERIALS AND METHODS
Growth of group A streptococcus wild type and GAS variant.
Wild-type GAS strain D58X and GAS variant strain M8 expressing a linear group A polysaccharide lacking terminal N-acetylglucosaminyl residues were provided by John Zabriskie of The Rockefeller University, New York, N.Y. Both GAS strains were grown in Columbia broth containing glucose.
Preparation of group A polysaccharide and group A variant polysaccharide.
The cell wall group A polysaccharide (Fig. 1, compound 6) and its variant form (compound 7) were extracted from wet GAS cell paste obtained after fermentation of the bacteria and separation of the cells from the culture supernatant by centrifugation. The polysaccharides were released from the cell wall by treatment at room temperature with sodium nitrite and were purified by a series of size exclusion chromatography procedures. The final products were characterized by 500-MHz nuclear magnetic resonance analysis using a Bruker AMX-500 spectrometer. The sizes and polydispersity of the polysaccharides were determined using size exclusion chromatography on line with a multiangle laser light scattering detector (miniDawn; Wyatt Technology) (7).
Preparation of the pentasaccharide-tetanus toxoid conjugate (compound 5).
A solution of the allyl glycoside of the pentasaccharide (compound 2) (23 mg) (9) and cysteamine hydrochloride (44 mg) in methanol (MeOH) (1.0 ml) was irradiated with a Hanovia UV lamp for 50 min. The solution was passed through a Dowex 1 (OH−) column (1.5 cm by 8 cm) with MeOH, and the combined fractions that contained the product, as assessed by thin-laver chromatography (Rf, 0.8) with MeOH-concentrated NH3-H2O (7:2:1), were concentrated to obtain the cysteamine adduct (18 mg) as a colorless solid. A portion (15 mg) was dissolved in MeOH (2 ml) and treated with a stock solution of diethyl squarate in MeOH (0.14 M, 200 μl) for 1 h at room temperature. The mixture was concentrated and purified by column chromatography on silica gel with ethyl acetate-MeOH-H2O (6:3:1) to obtain the monoethyl squarate derivative (14 mg) as a colorless glass. A portion (2.8 mg; 40 equivalents/tetanus toxoid [TT] molecule) was dissolved in H2O (100 μl) and added to tetanus toxoid (9.6 mg) in 0.1 M carbonate buffer, pH 10 (400 μl). After incubation for 7 days at room temperature, analysis by matrix-assisted laser desorption ionization mass spectrometry (sinapinic acid matrix) indicated that no further increase in mass had occurred, and the observed mass (177,000 Da) was used to calculate that an average of 23 pentasaccharide units was present on each tetanus toxoid molecule. The reaction mixture was dialyzed against distilled water (three times, 5 ml) using an Amicon ultrafiltration cell equipped with a Diaflo membrane. The residue was taken up in water and lyophilized to obtain the tetanus toxoid-pentasaccharide conjugate (compound 5) as a white powder (9.1 mg).
Group A polysaccharide-HSA conjugate.
The purified group A polysaccharide was conjugated to human serum albumin (HSA) by direct reductive amination using sodium cyanoborohydride (NaBH3CN), as described previously (10), to obtain a conjugate (compound 8) with an average of three polysaccharide chains per HSA molecule.
Antisera.
Reference typing rabbit antisera specific for GAS and group B streptococci (GBS) were obtained from Difco. Convalescent-phase sera containing high levels of anti-group A polysaccharide antibody, obtained from two children (ages, 4 to 8 years) in a group of 20 children (University of Virginia at Charlottesville) who had confirmed group A streptococcal infections, were chosen for further study; their profiles were representative of the collection of samples.
ELISA for binding of antibody.
Microtiter plates (Nunc Polysorp) were passively coated with either hexasaccharide-bovine serum albumin (BSA) (compound 4), pentasaccharide-TT (compound 5), or GASP-HSA (compound 8) conjugates (approximately 100 ng in 100 μl/well) diluted in phosphate-buffered saline (PBS) (950 mM sodium phosphate, 150 mM NaCl, pH 7.4) for 1 h at 37°C. After the plates were washed with PBS containing 0.05% Tween 20 (PBS-Tween) (pH 7.4), they were postcoated with 150 μl/well PBS containing 0.1% bovine serum albumin (pH 7.4) for 1 h at room temperature. After the postcoating, the plates were washed and stored at 2 to 8°C until they were used.
Rabbit antisera or human convalescent-phase sera were diluted serially in PBS-Tween in wells of coated microtiter plates to obtain a final volume of 100 μl/well and incubated for 1 h at room temperature. The plates were washed with PBS-Tween, and 100 μl of an appropriate primary conjugate was added to each well. For the rabbit antisera, the conjugate was a goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP) conjugate (KPL Laboratories), and for the human convalescent-phase sera, the conjugate was either goat anti-human IgG-HRP or goat anti-human IgM-HRP (KPL Laboratories) diluted 1:2,500 in PBS-Tween. Following 1 h of incubation at room temperature, the plates were washed again, and 100 μl of TMB substrate solution (KPL) was added to each well. The plates were incubated for 5 to 10 min at room temperature, and color development was stopped by addition of 100 μl of one-component stop solution (KPL) to each well. The optical density at 450 nm of each well was determined, and titration curves were generated for each condition.
ELISA for inhibition of antibody binding.
Microtiter plates were coated as described above. Rabbit antisera or representative GASP-specific human convalescent-phase sera exhibiting high immunoreactivity were titrated on plates coated with the coating conjugate of interest (either hexasaccharide-BSA [compound 4], pentasaccharide-TT [compound 5], or GASP-HSA [compound 8]). The dilution of serum corresponding to approximately one-half the maximal signal was chosen as the appropriate dilution for the inhibition studies. The sera were diluted in PBS-Tween. Inhibitors were serially diluted in buffer containing the dilute sera in Titertubes (Bio-Rad), and 100 μl of each sample was taken from the Titertubes and added directly to wells of coated microtiter plates. Samples of sera and inhibitors were incubated in the microtiter plates for 1 h at room temperature. The microtiter plates were washed with PBS-Tween, and then 100 μl of the appropriate HRP conjugate (as described above) diluted 1:2,500 in PBS-Tween was added to each well. The plates were incubated for 1 h at room temperature and washed with PBS-Tween. One hundred microliters of TMB substrate solution (KPL) was added to each well. The plates were incubated for 5 to 10 min at room temperature, color development was stopped by addition of 100 μl/well of one-component stop solution (KPL), and the absorbance at 450 nm was determined. Inhibition was expressed as a percentage of the maximum signal achieved with dilute serum in the absence of any inhibitor.
RESULTS AND DISCUSSION
Haptens and glycoconjugates.
The trisaccharide B(C)A′ (compound 1) (3), pentasaccharide B(C)A′B′A (compound 2) (9), and hexasaccharide B(C)A′B′(C′)A (compound 3) (3) haptens, present as their allyl glycosides, and the hexasaccharide-BSA conjugate (compound 4) (2) were available from our previous work. The pentasaccharide-TT conjugate (compound 5) was prepared by conversion of the allyl glycoside (compound 2) (9) to the corresponding cysteamine adduct, as described previously (2). This amine was linked to 3,4-diethoxy-3-cyclobutene-1,2-dione (diethyl squarate) in methanol to obtain the monoethyl squarate adduct, which was then coupled to tetanus toxoid in carbonate buffer, as described previously for the coupling of oligosaccharides to various protein carriers (2, 13, 33). For convenience, the structures of the oligosaccharide haptens (compounds 1 to 3), the polysaccharides (compounds 6 and 7), and the glycoconjugates (compounds 4, 5, and 8) used in this study are summarized in Fig. 1.
Specificity of the GAS rabbit antiserum for the group A cell wall polysaccharides.
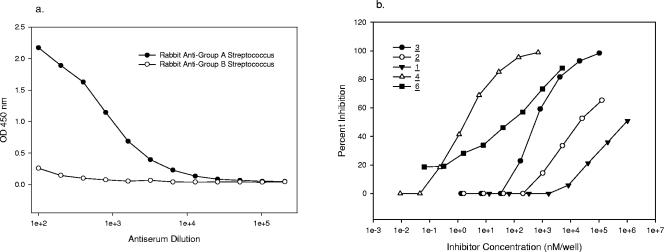
The immune responses of rabbit antisera raised to both group A and group B streptococcal organisms (whole-cell preparations) to group A cell wall polysaccharide were examined by analysis on a microtiter plate coated with GASP-HSA conjugate (compound 8). ELISA titration of the polyclonal streptococcal group A rabbit antiserum revealed that there was high-titer binding of this antibody to the solid-phase coated antigen (Fig. 2a). In contrast, a group B streptococcus-specific antiserum did not show any reactivity with the group A streptococcal antigen (Fig. 2a).
FIG. 2.
(a) ELISA titration of GAS-specific rabbit antiserum on a GASP-HSA (compound 8)-coated plate. OD 450 nm, optical density at 450 nm. (b) Inhibition of GAS-specific rabbit antiserum with GAS inhibitors on a GASP-HSA (compound 8)-coated plate. The numbers for the symbols indicate compounds shown in Fig. 1.
To examine the fine specificity of the rabbit anti-GAS antiserum to group A polysaccharide epitopes, inhibition of ELISA binding of this antiserum to the GASP conjugate-coated plate was carried out with a series of synthetic GAS oligosaccharide inhibitors (Fig. 1). The inhibitory activities of the oligosaccharide haptens (compounds 1 to 3) are shown in Fig. 2b. The doubly branched hexasaccharide (compound 3) was a significantly (approximately 20-fold) better inhibitor than the branched pentasaccharide (compound 2), which in turn was a better (approximately 15-fold) inhibitor than the branched trisaccharide (compound 1). The native GAS polysaccharide (compound 6) containing an average of 20 contiguous branched trisaccharide repeating units was a significantly better inhibitor (2 orders of magnitude) than the hexasaccharide (compound 3), presumably due to its multivalency. Presumably because of its pentavalency, the doubly branched hexasaccharide-BSA glycoconjugate (compound 4) had inhibitory properties similar to those of the polysaccharide.
To further examine the fine specificity of the rabbit anti-GAS antiserum, a similar binding and inhibition study was conducted on microtiter plates coated with the doubly branched hexasaccharide-BSA conjugate (compound 4) as the coating antigen in place of the GASP-HSA conjugate (compound 8) used in the experiment described above. The titration profile of the two anti-group A antisera with the hexasaccharide-BSA conjugate (compound 4) (Fig. 3a) was similar to the pattern seen with the GASP-HSA conjugate (compound 8), in that the rabbit anti-group A streptococcus antiserum bound with a high titer and very little reactivity was displayed by the anti-group B antiserum. A similar pattern of inhibition of the rabbit anti-GAS antiserum was observed when the doubly branched hexasaccharide-BSA conjugate (compound 4) was used as the coating antigen in place of the GASP-HSA conjugate (compound 8) (Fig. 3b). That is, the doubly branched hexasaccharide (compound 3) was a 40-fold-better inhibitor than the branched pentasaccharide (compound 2), which in turn was a 40-fold-better inhibitor than the branched trisaccharide (compound 1). However, it is worth noting that in this case the conjugate (compound 4) seemed to be better than the GASP as an inhibitor of the binding between the rabbit antiserum and the coating antigen, as might be expected since the coating antigen and inhibitor were identical.
FIG. 3.
(a) ELISA titration of rabbit antisera specific for GAS and GBS on a hexasaccharide-BSA (compound 4)-coated plate. OD 450 nm, optical density at 450 nm. (b) Inhibition of GAS-specific rabbit antiserum with GAS inhibitors on a hexasaccharide-BSA (compound 4)-coated plate. The numbers for the symbols indicate compounds shown in Fig. 1.
Binding relationships between GAS and GBS rabbit antisera toward shared epitopes on the branched pentasaccharide.
ELISA titration of the polyclonal streptococcal group B rabbit antiserum (raised against whole GBS organisms) on a microtiter plate coated with the pentasaccharide-TT glycoconjugate (compound 5) (containing ca. 23 pentasaccharide units per molecule of TT) was performed. The titration curve indicated that there was high-titer binding of the antibody to the antigen-coated plate (Fig. 4a). The same group B antiserum did not bind to the GASP-HSA-coated plate and the hexasaccharide-BSA (compound 4)-coated plate, as shown in Fig. 2a and 3a, respectively. The polyclonal rabbit group A antiserum bound to the pentasaccharide-TT-coated plate and produced a titration curve nearly identical to that of the group B antiserum (Fig. 4a). These findings can be rationalized as follows. Structural and immunochemical studies have shown that a terminal α-l-(1→2)-trirhamnopyranoside is the dominant epitope on the streptococcal group B-specific polysaccharide (22). The pentasaccharide-TT glycoconjugate (compound 5) contains an α-l-(1→2)-dirhamnopyranoside chain terminus that binds to the group B antibody but also contains the GAS branched trisaccharide that binds to the group A antibody.
FIG. 4.
(a) ELISA titration of rabbit antisera specific for GAS and GBS on a pentasaccharide-TT (compound 5)-coated plate. OD 450 nm, optical density at 450 nm. (b) Inhibition of GBS-specific rabbit antiserum with GAS inhibitors on a pentasaccharide-TT (compound 5)-coated plate. The numbers for the symbols indicate compounds shown in Fig. 1.
To further examine the fine specificity of the rabbit anti-group B streptococcus antiserum, inhibition of binding of this antiserum to the pentasaccharide-TT-coated plate was carried out with the following inhibitors: pentasaccharide (compound 2), hexasaccharide (compound 3), GASP (compound 6), and the corresponding pentasaccharide-TT (compound 5) and hexasaccharide-BSA (compound 4) conjugates (Fig. 4b). The pentasaccharide is a 50-fold-better inhibitor of this antiserum than the hexasaccharide in its monovalent form. The conjugated form of the pentasaccharide is also a much more potent inhibitor (by 3 orders of magnitude) of the rabbit group B antiserum than the hexasaccharide glycoconjugate. The GASP (compound 6) is also clearly not as effective at competing with the pentasaccharide solid-phase antigen with the group B antiserum as the pentasaccharide conjugate (compound 5) (by over 3 orders of magnitude).
Taken together, this evidence argues against the presence of a chain terminus consisting of a nonreducing α-l-(1→2)-dirhamnopyranoside on the GASP, as was previously suggested (11).
Specificity of human convalescent sera for the GASP.
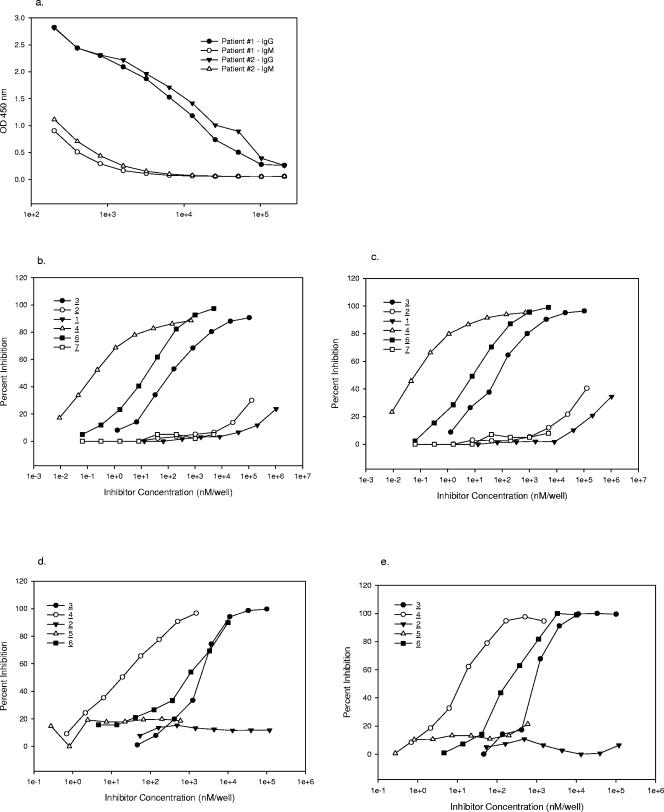
To examine the fine specificity of the immune response in humans to group A streptococcal infection, convalescent-phase sera from two children that had confirmed GAS infections were tested for their specificity for the group A polysaccharide. The results of ELISA titration of these two sera on a GASP-HSA-coated plate are shown in Fig. 5a. The two sera used in this study exhibited high-titer GASP-specific IgG binding but significantly lower-titer (approximately 2 orders of magnitude) IgM binding. The high-titer IgG anti-GASP response could be indicative of a thymus-dependent-like presentation of this carbohydrate in the context of the whole organism, or it could be due to the fact that the GASP is a rather low-molecular-weight cell wall carbohydrate that could also interact with T cells through proteins bound in close proximity on the cell wall. In contrast, typical thymus-independent high-molecular-weight capsular polysaccharides that are shed by the organisms are usually unable to elicit a significant IgG response but rather lead to an IgM response of short duration.
FIG. 5.
(a) ELISA titration (IgG, IgM) of human convalescent-phase sera on a GASP-HSA (compound 8)-coated plate. OD 450 nm, optical density at 450 nm. (b) Inhibition of human convalescent-phase serum 1 with GAS inhibitors on a GASP-HSA (compound 8)-coated plate. (c) Inhibition of human convalescent-phase serum 2 with GAS inhibitors on a GASP-HSA (compound 8)-coated plate. (d) Inhibition of human convalescent-phase serum 1 with GAS inhibitors on a hexasaccharide-BSA (compound 4)-coated plate. (e) Inhibition of human convalescent-phase serum 2 with GAS inhibitors on a hexasaccharide-BSA (compound 4)-coated plate. The numbers for the symbols indicate compounds shown in Fig. 1.
Further delineation of the fine specificity of the human response was examined by competitive inhibition of the binding of the two human sera to GASP-HSA (compound 8) with GAS synthetic inhibitors (compounds 1 to 4) and polysaccharides (compounds 6 and 7) (Fig. 1). The inhibition curves for the two sera are shown in Fig. 5b and 5c. It is evident from these curves that the singly branched trisaccharide (compound 1) and pentasaccharide (compound 2) are not very effective in inhibiting the binding of these antisera compared to the doubly branched hexasaccharide (compound 3) (more than 6 and 5 orders of magnitude, respectively). The GASP (by approximately 10-fold in both cases) and the pentavalent hexasaccharide-BSA glycoconjugate (compound 4) (by at least 2 orders of magnitude) are significantly better inhibitors than the unconjugated hexasaccharide (compound 3). The variant GASP (compound 7), which lacks terminal N-acetylglucosamine residues, does not inhibit either of these antisera at the concentrations used in the experiment.
Competitive inhibition of the binding of the two human sera to the hexasaccharide-BSA conjugate (compound 4) coated on a plate was carried out with the GAS synthetic inhibitors (compounds 2 to 5) and the polysaccharides (compounds 6 and 7) (Fig. 1). The inhibition curves for the two sera are shown in Fig. 5d and 5e. Again, in an even more pronounced fashion, neither the pentasaccharide (compound 2) nor the pentasaccharide-TT conjugate (compound 5) was able to compete in the binding of either antiserum to the glycoconjugate-coated plate, whereas the unconjugated and conjugated hexasaccharides (compounds 3 and 4, respectively) were excellent inhibitors. The native GASP (compound 6) was approximately equivalent or slightly more efficient, depending on the individual antiserum examined, than the hexasaccharide (compound 3) in inhibiting the binding of both antisera to the hexasaccharide-BSA conjugate (compound 4).
Summary.
The results presented above seem to indicate that the human antibody generated upon exposure to a streptococcal organism has a much narrower epitope specificity for GASP than the rabbit antibody. The rabbit antibody does not exhibit such a pronounced difference in recognition between the singly branched (tri- and pentasaccharide) and the doubly branched (hexasaccharide) epitopes (only 1 and 2 orders of magnitude difference, respectively). The human subjects were children who were 4 to 8 years of age and were likely exposed several times to infectious group A streptococcus. It is plausible that antibody maturation upon repeated exposure in the human subjects was responsible for the narrow epitope specificities. As previously observed (8, 24, 27), the results also indicate that the branch point and the size of the GAS antigen are crucial elements of the epitope recognized by both polyclonal human and rabbit antibodies.
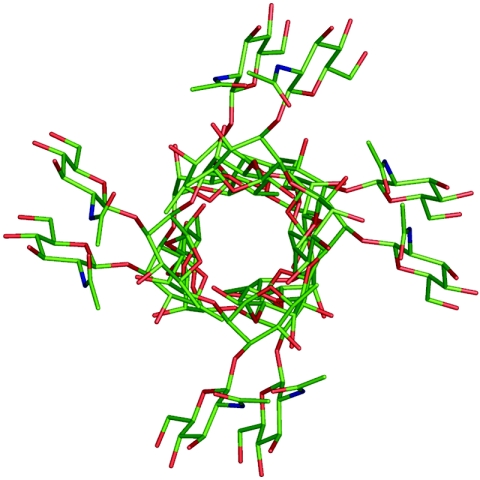
Conformational analysis of the oligosaccharides (compounds 1 to 3) showed that the branch point represented in the trisaccharide (compound 1) and pentasaccharide (compound 2) is a well-defined conformational feature both in the free ligand and when the compound is bound to an antibody (11, 15, 24, 31). The polysaccharide exists in a helix (Fig. 6) in which the rhamnosyl residues form the core of the helix and the N-acetylglucosamine residues are disposed on the periphery. The helix of GASP presents the branched trisaccharide motif to the polyclonal rabbit antisera, as well as an accessible extended surface (24) in both the pentasaccharide (compound 2) and doubly branched hexasaccharide (compound 3), but the extended helices of the hexasaccharide (compound 3) and the polysaccharide (compound 6) are recognized significantly better by the human antisera.
FIG. 6.
Model of the cell wall polysaccharide of GAS, looking down the barrel of the helix. The core of the helix is composed of α-l-rhamnose residues, and the β-d-N-acetylglucosamine moieties are displayed on the periphery. Reproduced with permission of Elsevier from reference 12.
These results are significant for the design of a human glycoconjugate vaccine based on fragments of the GAS cell wall polysaccharide. We concluded that an effective oligosaccharide conjugate vaccine would require presentation to the immune system of a contiguous series of GASP helical motifs and would likely be comprised of structures at least as large as a doubly branched hexasaccharide.
Acknowledgments
We are grateful to the Natural Sciences and Engineering Research Council of Canada and Baxter Healthcare for financial support.
We thank Theresa Schlager and J. Owen Hendley, Department of Emergency Medicine, University of Virginia at Charlottesville, for providing the human sera.
Editor: J. D. Clements
REFERENCES
- 1.Anonymous. 1980. World Health Organization: community control of rheumatic heart disease in developing countries. 1. A major public health problem. W. H. O. Chron. 34:336-345. [PubMed] [Google Scholar]
- 2.Auzanneau, F.-I., and B. M. Pinto. 1996. Preparation of antigens and immunoadsorbents corresponding to the Streptococcus group A cell-wall polysaccharide. Bioorg. Med. Chem. 4:2003-2010. [DOI] [PubMed] [Google Scholar]
- 3.Auzanneau, F.-I., F. Forooghian, and B. M. Pinto. 1996. Efficient, convergent syntheses of oligosaccharide allyl glycosides corresponding to the Streptococcus group A cell-wall polysaccharide. Carbohydr. Res. 291:21-41. [PubMed] [Google Scholar]
- 4.Benaissa-Trouw, B., D. J. Lefeber, J. P. Kamerling, J. F. G. Vliegenthart, K. Kraaijeveld, and H. Snippe. 2001. Synthetic polysaccharide type 3-related di-, tri-, and tetrasaccharide-CRM197 conjugates induce protection against Streptococcus pneumoniae type 3 in mice. Infect. Immun. 69:4698-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisno, A. L. 1985. Nonsuppurative poststreptococcal sequelae: rheumatic fever and glomerulonephritis, p. 1133-1142. In G. L. Mandrell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious diseases, 2nd ed. Wiley, New York, N.Y.
- 6.Blake, M. S., J. B. Zabriskie, J. Y. Tai, and F. Michon. 2February1999. U.S. patent 5.866,135.
- 7.D'Ambra, A., J. E. Baugher, P. E. Concannon, R. A. Pon, and F. Michon. 1997. Direct and indirect methods for molar-mass analysis of fragments of the capsular polysaccharide of Haemophilus influenzae type b. Anal. Biochem. 250:228-236. [DOI] [PubMed] [Google Scholar]
- 8.Harris, S. L., L. Craig, J. S. Mehroke, M. Rashed, M. B. Zwick, K. Kenar, E. J. Toone, N. Greenspan, F.-I. Auzanneau, J. R. Marino Albernas, B. M. Pinto, and J. K. Scott. 1997. Exploring the basis of peptide-carbohydrate crossreactivity: evidence for discrimination by peptides between closely related anti-carbohydrate antibodies. Proc. Natl. Acad. Sci. USA 94:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoog, C., A. Rotondo, B. D. Johnston, and B. M. Pinto. 2002. Synthesis and conformational analysis of a pentasaccharide corresponding to the cell-wall polysaccharide of the group A Streptococcus. Carbohydr. Res. 337:2023-2036. [DOI] [PubMed] [Google Scholar]
- 10.Jennings, H. J., and C. Lugowski. 1981. Immunochemistry of group-A, group-B, and group-C meningococcal polysaccharide tetanus toxoid conjugates. J. Immunol. 127:1011-1018. [PubMed] [Google Scholar]
- 11.Johnson, M. A., and B. M. Pinto. 2002. Saturation transfer difference 1D-TOCSY experiments to map the topography of oligosaccharides recognized by a monoclonal antibody directed against the cell-wall polysaccharide of group A Streptococcus. J. Am. Chem. Soc. 124:15368-15374. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, M. A., and B. M. Pinto. 2004. NMR spectroscopic and molecular modeling studies of protein-carbohydrate and protein-peptide interactions. Carbohydr. Res. 339:907-928. [DOI] [PubMed] [Google Scholar]
- 13.Kamath, V. P., P. Diedrich, and O. Hindsgaul. 1996. Use of diethyl squarate for the coupling of oligosaccharide amines to carrier proteins and characterization of the resulting neoglycoproteins by MALDI-TOF mass spectrometry. Glycoconj. J. 13:315-319. [DOI] [PubMed] [Google Scholar]
- 14.Kehoe, M. A. 1991. Group A streptococcal antigens and vaccine potential. Vaccine 9:797-806. [DOI] [PubMed] [Google Scholar]
- 15.Kreis, U. C., V. Varma, and B. M. Pinto. 1995. Application of 2-dimensional NMR-spectroscopy and molecular-dynamics simulations to the conformational-analysis of oligosaccharides corresponding to the cell-wall polysaccharide of Streptococcus group-A. Int. J. Biol. Macromol. 17:117-130. [DOI] [PubMed] [Google Scholar]
- 16.Lancefield, R. C. 1932. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 57:571-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markowitz, M. 1981. Observations on the epidemiology and preventability of rheumatic-fever in developing countries. Clin. Ther. 4:240-251. [PubMed] [Google Scholar]
- 18.McCarty, M. 1952. The lysis of group A hemolytic streptococci by extracellular enzymes of Streptomyces albus. II. Nature of the cellular substrate attacked by the lytic enzymes. JEM 96:569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty, M. 1956. Variation in the group-specific carbohydrate of group A streptococci. II. Studies on the chemical basis for serological specificity of the carbohydrates. JEM 104:629-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarty, M., and R. C. Lancefield. 1955. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. JEM 102:11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miceika, B. G., A. S. Vitous, and K. D. Thompson. 1985. Detection of group A streptococcal antigen directly from throat swabs with a ten-minute latex agglutination test. J. Clin. Microbiol. 21:467-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michon, F., R. Chalifour, R. Feldman, M. Wessels, D. L. Kasper, A. Gamian, V. Pozsgay, and H. J. Jennings. 1991. The alpha-l-(1-2)-trirhamnopyranoside epitope on the group-specific polysaccharide of group B streptococci. Infect. Immun. 59:1690-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak, R. 1994. Bacteriology—flesh-eating bacteria—not new, but still worrisome. Science 264:1665. [DOI] [PubMed] [Google Scholar]
- 24.Pitner, J. B., W. F. Beyer, T. M. Venetta, C. Nycz, M. J. Mitchell, S. L. Harris, J. R. Marino-Albernas, F.-I. Auzanneau, F. Forooghian, and B. M. Pinto. 2000. Bivalency and epitope specificity of a high-affinity IgG3 monoclonal antibody to the Streptococcus group A carbohydrate antigen. Molecular modeling of a Fv fragment. Carbohydr. Res. 324:17-29. [DOI] [PubMed] [Google Scholar]
- 25.Pozsgay, V. 2001. Oligosaccharide-protein conjugates as vaccine candidates against bacteria. Adv. Carbohydr. Chem. Biochem. 56:153-199. [DOI] [PubMed] [Google Scholar]
- 26.Read, S. E., and J. B. Zabriskie (ed.) 1980. Streptococcal diseases and the immune response. Proceedings of a Symposium Held in Trinidad, November 7-11, 1977. Academic Press, New York, N.Y.
- 27.Reimer, K. B., M. A. J. Gidney, D. R. Bundle, and B. M. Pinto. 1992. Immunochemical characterization of polyclonal and monoclonal Streptococcus group-A antibodies by chemically defined glycoconjugates and synthetic oligosaccharides. Carbohydr. Res. 232:131-142. [DOI] [PubMed] [Google Scholar]
- 28.Riesen, W.F., F Skvaril, and D. G. Braun. 1976. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand. J. Immunol. 5:383-390. [DOI] [PubMed] [Google Scholar]
- 29.Salvadori, L. G., M. S. Blake, M. McCarty, J. Y. Tai, and J. B. Zabriskie. 1995. Group-A Streptococcus-liposome ELISA antibody-titers to group-A polysaccharide and opsonophagocytic capabilities of the antibodies. J. Infect. Dis. 171:593-600. [DOI] [PubMed] [Google Scholar]
- 30.Stevens, D. L. 1997. The toxins of group A Streptococcus, the flesh eating bacteria. Immunol. Investig. 26:129-150. [DOI] [PubMed] [Google Scholar]
- 31.Stuike-Prill, R., and B. M. Pinto. 1995. Conformational analysis of oligosaccharides corresponding to the cell-wall polysaccharide of the Streptococcus group A by Metropolis Monte Carlo simulations. Carbohydr. Res. 279:59-73. [DOI] [PubMed] [Google Scholar]
- 32.Swedo, S. E., H. Leonard, M. Garvey, B. Mittleman, A. Allen, S. Perlmutter, L. Lougee, S. Dow, J. Zamkoff, and B. Dubbert. 1998. Pediatric autoimmune neuropsychiatric disorders associated streptococcal infections: clinical description of the first 50 cases. Am. J. Psychiatry 155:264-271. [DOI] [PubMed] [Google Scholar]
- 33.Tietze, L. F., C. Schroter, S. Gabius, U. Brinck, A. Goerlachgraw, and H. J. Gabius. 1991. Conjugation of para-aminophenyl glycosides with squaric acid diester to a carrier protein and the use of neoglycoprotein in the histochemical detection of lectins. Bioconjug. Chem. 2:148-153. [DOI] [PubMed] [Google Scholar]
- 34.Venezia, R. A., A. Ryan, S. Alward, and W. A. Kostun. 1985. Evaluation of a rapid method for the detection of streptococcal group A antigen directly from throat swabs. J. Clin. Microbiol., 21:395-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wannamaker, L. W. 1979. Changes and changing concepts in the biology of group A-streptococci and in the epidemiology of streptococcal infections. Rev. Infect. Dis. 1:967-975. [DOI] [PubMed] [Google Scholar]
- 36.Weimar, T., S. L. Harris, J. B. Pitner, K. Bock, and B. M. Pinto. 1995. Transferred nuclear Overhauser enhancement experiments show that the monoclonal-antibody Strep-9 selects a local minimum conformation of a Streptococcus group-A trisaccharide-hapten. Biochemistry 34:13672-13681. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, K. A., and M. Laverdiere. 1997. Group A Streptococcus invasive infections: a review. Can. J. Surg. 40:18-25. [PMC free article] [PubMed] [Google Scholar]
- 38.Wright, K. 1990. Bad news bacteria. Science 249:22-24. [DOI] [PubMed] [Google Scholar]
- 39.Zabriskie, J. B. 1985. Rheumatic-fever—the interplay between host, genetics, and microbe. Circulation 71:1077-1086. [DOI] [PubMed] [Google Scholar]
- 40.Zabriskie, J. B., T. PoonKing, M. S. Blake, F. Michon, and M. Yoshinaga. 1997. Phagocytic, serological, and protective properties of streptococcal group A carbohydrate antibodies. Adv. Exp. Med. Biol. 418:917-919. [DOI] [PubMed] [Google Scholar]