Abstract
The development of an effective subunit vaccine against brucellosis is a research area of intense interest. The outer membrane proteins (Omps) of Brucella spp. have been extensively characterized as potential immunogenic and protective antigens. This study was conducted to evaluate the immunogenicity and protective efficacy of the B. melitensis Omp31 gene cloned in the pCI plasmid (pCIOmp31). Immunization of BALB/c mice with pCIOmp31 conferred protection against B. ovis and B. melitensis infection. Mice vaccinated with pCIOmp31 developed a very weak humoral response, and in vitro stimulation of their splenocytes with recombinant Omp31 did not induced the secretion of gamma interferon. Splenocytes from Omp31-vaccinated animals induced a specific cytotoxic-T-lymphocyte activity, which leads to the in vitro lysis of Brucella-infected macrophages. pCIOmp31 immunization elicited mainly CD8+ T cells, which mediate cytotoxicity via perforins, but also CD4+ T cells, which mediate lysis via the Fas-FasL pathway. In vivo depletion of T-cell subsets showed that the pCIOmp31-induced protection against Brucella infection is mediated predominantly by CD8+ T cells, although CD4+T cells also contribute. Our results demonstrate that the Omp31 DNA vaccine induces cytotoxic responses that have the potential to contribute to protection against Brucella infection. The protective response could be related to the induction of CD8+ T cells that eliminate Brucella-infected cells via the perforin pathway.
Bacteria of the genus Brucella are gram-negative, facultative intracellular pathogens that cause a severe infectious disease in many animal species, including humans. B. melitensis is the most pathogenic species for humans and is the least species specific, infecting goats, sheep, cows, camels, and dogs (63). B. ovis also infects sheep and, together with B. melitensis, causes ovine brucellosis, a disease that induces major economic losses in countries in which sheep husbandry is an important industry (14). Vaccination is the only method of control suitable for countries with a high incidence of this disease (8) and at present, live attenuated B. melitensis Rev 1 is still the strain most widely used for this purpose (10, 45). However, Rev 1 produces serological responses that interfere with the diagnosis of Brucella infections (10, 25); it is resistant to streptomycin, one of the antibiotics of choice used to treat brucellosis (46); it is pathogenic for humans (9); and its use is prohibited in countries free of B. melitensis (35).
Numerous attempts to develop killed vaccines that are as effective as Rev 1 have met with limited success. An inactivated vaccine comprising killed, smooth B. melitensis strain H38 induced protection similar to that given by the Rev 1 strain, but the local lesions that develop at the site of inoculation have prevented further use of this vaccine (4). Inactivated H38 vaccine is, however, a good laboratory reference vaccine for evaluation of the protective properties of vaccinal preparations in mice (11). Subcellular vaccines could avoid the drawbacks of live attenuated vaccines, being safer and not interfering with immunodiagnosis, provided that good protective antigens (Ags), different from those used for testing, are selected.
Protective immunity to Brucella spp. is incompletely understood, but similar to the case for most intracellular bacterial infections, cell-mediated immunity plays a critical role in protection against virulent Brucella infection, although antibodies (Ab) specific for the O polysaccharide of the lipopolysaccharide and certain membrane proteins can confer protection in some host species (12, 33). It is thought that a coordinated response of the cellular immune system is fundamental. Consequently, both CD4+ and CD8+ T lymphocytes are believed to play important roles in immunity to Brucella (6), in part because they secrete gamma interferon (IFN-γ) for the activation of bactericidal functions in macrophages (39). In addition, lysis of infected cells and subsequent killing of Brucella by cytotoxic T lymphocytes (CTLs), including CD4+, CD8+, and γδ T cells, may be important in maintaining continuous immune surveillance. T-helper 1 (Th1)-type antibody isotypes such as immunoglobulin G2a (IgG2a) and IgG3 may also opsonize the pathogen to facilitate phagocytosis (39).
Despite the many mechanisms postulated to be at play in immune protection, the general concept of a subunit vaccine in brucellosis was based on the generation of memory Th1 cells by immunization with T-cell Ags. The majority of the T-cell Ags that have been characterized induce CD4+ T-cell proliferation and IFN-γ secretion. Some of them have been successfully tested as subunit vaccines against B. abortus (2, 51, 57, 59). CD8+ T-cell Ags have been less well studied. C57BL/6 mice infected with B. abortus S19 induced cytotoxic CD8+ T cells and controlled infection (50), suggesting that protective Ags presented to the T-cell receptor through major histocompatibility complex (MHC) class I molecules exist. Yet, information on the development of Brucella-specific CTLs in vaccinated animals and the phenotypic and functional characterization of such CTLs is scant. B. abortus RB51 vaccination induced Brucella-specific CTL activity in both CD4+ and CD8+ lymphocytes (29). On the other hand, instraspleen immunization with a DNA vaccine coding for Brucella superoxide dismutase generated a Th1 response along with CD8+-restricted CTLs and protected mice against B. abortus challenge (47). It is still unknown if there is a correlation between the in vitro CTL activity and the levels of protection in vivo against Brucella challenge.
The outer membrane proteins (Omps) of Brucella spp. have been extensively characterized as potential immunogenic and protective Ags (20). Passive protection experiments in mice have shown that mixtures of monoclonal Abs (MAbs), previously shown to bind individually to several Omps, conferred no or poor protection against smooth Brucella strains in mice (18, 33), whereas they were protective against rough B. ovis (13). Protection was better when anti-Omp31 MAb was present in the mixture of MAbs (12). This could be attributed to the presence of the O-polysaccharide-bearing lipopolysaccharide on smooth strains, which has been shown to hinder deeper Omp epitopes (12, 19). Indeed, vaccination with Omp31-enriched preparations, which induced a strong Ab response but a poor cellular response, provided protection against B. ovis (22) but not against B. melitensis (26) challenge. These findings have added support to the contention that Brucella Omps seem to have little relevance as protective Ags in smooth B. melitensis infections, whereas these proteins, in particular Omp31, appear as important protective Ags against B. ovis infection. Nonetheless, Omp31 could be potentially protective against smooth Brucella strains if an appropriate cell-mediated immunity could be achieved.
DNA vaccination is a relatively novel and powerful method of immunization that induces both humoral and cellular (Th1 and CTL) immune responses and protection against a wide range of pathogens (27). Based on the results obtained with DNA vaccines against other pathogenic intracellular bacteria, many studies of brucellosis have been conducted (3, 16, 41, 43, 47, 52, 59). These vaccines induced strong Th1 responses, and some of them conferred protection against challenge with B. abortus (3, 41, 47, 52, 59).
In this study we evaluated the immunogenicity and protective efficacy of a DNA vaccine coding for the Omp31 protein. This vaccine conferred protection against B. ovis and B. melitensis infection. We also characterized the T-cell responses elicited after DNA vaccination and evaluated in vivo the roles of CD4+ and CD8+ T cells in protection. Our results demonstrate that a subunit vaccine can be efficacious against Brucella infection by eliciting a CTL response mediated by CD8+ T cells via the perforin pathway.
MATERIALS AND METHODS
Mice.
Four- to 6 week-old female BALB/c mice (obtained from Instituto Nacional de Tecnología Agropecuaria, CICV, Castelar, Argentina) were acclimated and randomly distributed into experimental groups. Mice were kept in conventional animal facilities and received water and food ad libitum, in accordance with pertinent U.S. federal regulations and polices.
Bacterial strains.
Escherichia coli strain JM109 (Promega, Madison, WI) was used as the host during the cloning experiments and for propagation of plasmids. Strain BL21(DE3) (Stratagene, La Jolla, CA) was used for expression of the recombinant protein. Bacterial strains were routinely grown at 37°C in LB broth or agar, supplemented when required with 100 μg/ml of ampicillin. B. melitensis H38S and B. ovis PA76250 (virulent strains) were cultured in tryptose soy agar (Merck, Buenos Aires, Argentina) supplemented with yeast extract (Merck) and incubated as described previously (22, 26).
Cell lines.
J774.A1 and COS-7 cell lines were purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in complete medium (RPMI 1640 supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum) (Gibco-BRL, Life Technologies, Grand Island, NY). A20J cells expressing class I and II MHC molecules were kindly provided by Mauricio Rodriguez (Universidad Federal de Sao Pablo, Brazil) and were cultured in complete medium (Gibco).
Cloning and expression of the B. melitensis Omp31 protein.
Recombinant Omp31 (rOmp31) was cloned, expressed in E. coli, and purified as previously described (15). Purity was assessed by Coomassie blue staining as reported elsewhere (15).
Construction of the Omp31 DNA vaccine.
The omp31 gene from B. melitensis, including restriction sites at the 5′ ends of the primers and the Kozak consensus sequence (40), was cloned in the pCI-neo vector (Promega). The sequence information previously reported (61) was used to design the oligonucleotides Omp31 for (5′ACCGGATCCACCACCATGAAATCCGTAATTTTG3′) and Omp31 back (5′AGGCGTCTAGACATTCAGGACAATTCCCGCC3′). The Kozak sequence is underlined. To ensure that the plasmid construct was intact and functional, it was sequenced across the gene insert. The plasmid (pCIOmp31) was amplified in E. coli JM109 cells and isolated using Megaprep plasmid isolation columns (QIAGEN, Dorking, United Kingdom). The purity and concentration of DNA were determined by spectrophotometry at 260/280 nm. To corroborate Omp31 expression, COS-7 cells were transfected with 2 μg of pCIOmp31 and 20 μl Lipofectamine reagent (Gibco) according to the manufacturer's specifications. Expression was confirmed by Western blotting, developed with the anti-Omp31 MAb A59/F09/G10 (21). Omp31 was detected in cell lysates but not in supernatants after 24 and 48 h of culture (data not shown).
Immunization.
Mice were anesthetized with methoxyfuorane (Mallinckrodt, Phillipsburg, NJ) and immunized by the intramuscular route with 100 μg of pCIOmp31 or pCI as a control. Each mouse was injected at days 0, 15, 30, and 45. As a positive control, another group was immunized once subcutaneously at day 0 with 8 × 108 formalin-killed B. melitensis H38S (H38) organisms in incomplete Freund's adjuvant. As a negative control, an additional group was inoculated with phosphate-buffered saline (PBS). Sera were obtained at 15, 30, 45, 60, 75, and 105 days after the first immunization (eight mice per group). Thirty days after the last DNA injection, mice were challenged intravenously with virulent Brucella organisms (eight mice per group) or were sacrificed to conduct the analysis of immune responses, including proliferation, cytokine production, and CTL induction (five mice per group).
Protection experiments.
pCIOmp31-, pCI-, PBS-, or H38-immunized mice were challenged, by intravenous injection, with 1.1 × 104 B. melitensis H38S or 1 × 104 B. ovis organisms. Mice were killed by cervical dislocation 30 days after being challenged, and their spleens and livers were removed aseptically. Each spleen or liver was homogenized in a stomacher bag, serially diluted, plated on tryptose soy agar supplemented with yeast extract, and incubated as described previously (22, 26). The number of CFU per spleen or liver was counted, and the results were represented as the mean log CFU ± standard deviation (SD) per group. Log units of protection were obtained by subtracting the mean log CFU for the vaccinated group from the mean log CFU for the control immunized group.
Omp31 ELISA.
Serum reactivities against rOmp31 were determined by indirect enzyme-linked immunosorbent assay (ELISA) as described previously (15). The cutoff value for the assay was calculated as the mean specific optical density plus 3 SDs from 20 sera from nonimmunized mice assayed at a 1:100 dilution. Serum titers were established as the reciprocal of the last serum dilution yielding an optical density higher than the cutoff.
Proliferative and cytokine responses.
Spleen cell suspensions from immunized or control mice were prepared in complete medium and plated at 4 × 106/well in 24-well flat-bottom plates (Nunclon; Nunc, Roskilde, Denmark) for cytokine assays and at 2 × 105/well in 96-well flat-bottom plates (Nunc) for proliferation assays. Cells were stimulated in vitro at 37°C in 5% CO2 with rOmp31 (0.1, 1, or 10 μg/ml), concanavalin A (ConA) (2.5 μg/ml), or medium alone. Supernatants were taken after 24, 48, or 72 h of culture and stored at −70°C until assayed for cytokine production. Interleukin-2 (IL-2), IL-4, IL-10, and IFN-γ in culture supernatants were measured by sandwich ELISA using paired cytokine-specific monoclonal antibodies according to the manufacturer's instructions (PharMingen, San Diego, CA). Alternatively, after 5 days, the cultures were pulsed with 1 μCi of [3H]thymidine (ICN Pharmaceuticals Inc., Costa Mesa, CA) for 18 h and harvested, and radioactivity was counted in a liquid scintillation counter. Results were expressed as a stimulation index (S.I.), which is counts per minute of stimulated cultures divided by counts per minute of unstimulated cultures.
Generation of CTL targets.
A20J cells were transfected with 2 μg of pCIOmp31 or pCI and 20 μl Lipofectamine reagent (Gibco) according to the manufacturer's specifications. Stable transfectants were selected in RPMI 1640 complete medium containing 800 μg/ml of Geneticin (Gibco). These cells were cloned by limiting dilution in 96-well tissue culture dishes at a cell density of 0.3 to 0.5 cells/well. Individual clones were expanded in medium containing Geneticin and tested for the expression of Omp31 by reverse transcription-PCR. For generation of CTL targets, pCIOmp31-transfected cells were incubated for 16 h with 10 μg/ml of rOmp31 (A20JOmp31) or pCI-transfected cells were cultured in medium alone (A20pCI). Alternatively, J774.A1 macrophages at confluent growth were infected with opsonized live B. ovis at a ratio of 1:100 (cells to bacteria) for 6 h. Extracellular bacteria were rinsed away with RPMI containing 100 μg/ml of gentamicin and 50 μg/ml of streptomycin. Macrophages (J774 B. ovis) were scraped off with a sterile rubber policeman and centrifuged at 200 × g for 5 min. Target cell were harvested and labeled with 0.1 mCi 51Cr/2 × 106 cells (Amersham, Arlington Heights, IL) in 80 μl of complete RPMI for 1 h at 37°C. Cells were washed three times with RPMI and their viability determined by trypan blue exclusion.
Stimulator cells.
Stimulator cells were A20JOmp31 cells treated with 25 μg/ml of mitomycin C (Sigma) in a 37°C water bath for 45 min. Cells were washed three times by centrifugation and resuspended in complete RPMI.
Generation of effector cells.
For the generation of CTLs or IFN-γ-producing cells, 2.5 × 107 splenocytes were cocultured with 0.5 × 106 stimulator cells for 5 days in 15 ml of complete RPMI plus 2.5% (vol/vol) RAT-T-STIM without ConA (Becton Dickinson Labware, Bedford, MA).
51Cr release CTL assay.
Effector and target cells were incubated at different effector/target cell ratios in 96-well round-bottom plates (Costar Corporation, Cambridge, MA) for 6 h at 37°C in a final volume of 200 μl. After culture, the supernatants were harvested, and radioactivity was counted in a gamma counter (Clinigamma; LKB, Turku, Finland). Spontaneous release was determined by culturing target cells in medium alone, and maximum release was determined by lysis of target cells in 5% (vol/vol) Triton X-100-containing medium. The percentage of specific lysis was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Data given are the means of triplicate determinations. Spontaneous release values were always <10% maximum release values. Where indicated, in vitro-generated effector cells were preincubated with 10 μl/ml of culture supernatants from anti-mouse CD4 (GK1.5; American Type Culture Collection [ATCC]) or anti-mouse CD8 (53-6.72; ATCC) hybridomas for 2 h. Nonspecific rat IgG hybridomas were used as isotype controls. In some experiments effector cells were previously depleted of CD4+ or CD8+ T cells by using mouse CD4 (L3T4) or mouse CD8 (Lyt2) Dynabeads according to the manufacturer's instructions (Dynal Biotech, Oslo, Norway). Efficacy of cell depletion was greater than 99% as determined by flow cytometric analysis of effector cells (not shown). After being depleted, effector cells were resuspended in the original volume and used in the cytotoxic assay. Where indicated, depleted or nondepleted effector cells were pretreated with brefeldin A (BFA) (Sigma) (5 μg/ml) or 200 nM concanamycin A (CCA) (Sigma) for 2 h as described previously (37, 44, 58). Treated cells were washed thoroughly before being used. Concentration of 0.5 μg/ml of BFA and 100 nM CCA was kept throughout the assay.
Flow cytometry for intracellular IFN-γ and cell surface marker staining.
Intracellular cytokine staining was used to determine the IFN-γ production at the single-cell level. Briefly, after 5 days of primary stimulation, effector cells from pCIOmp31- or pCI-immunized mice were extensively washed and restimulated with mitomycin C-treated A20JOmp31 cells and rOmp31 (10 μg/ml) or phorbol myristate acetate (20 ng/ml) (Sigma) plus ionomycin (750 ng/ml) (Sigma) and then incubated 4 h with 10 μg/ml of BFA (Sigma). Cells were then harvested, stained for surface expression with fluorescein isothiocyanate-conjugated anti-CD4+ (clone GK1.5) and Cy-Chrome-conjugated anti-CD8+ (clone 53-6.7) (PharMingen), and washed with PBS-2% fetal bovine serum. Intracellular IFN-γ staining was performed with phycoerythrin-conjugated anti-IFN-γ (clone XMG1.2) (PharMingen), using the Fix & Perm kit (PharMingen) according to the manufacturer's instructions. Negative control samples were incubated with irrelevant, isotype-matched Abs in parallel with all experimental samples. Samples were analyzed for a total of 300,000 events on a FACScan flow cytometer (BD Biosciences, Mountain View, CA).
In vivo T-cell depletion.
Vaccinated mice were depleted of CD4+ or CD8+ T cells by intraperitoneal injection of 200 μg of purified GK1.5 or 2.43 (ATCC) MAbs, respectively, on days −2, 1, 4, 7, and 10 after bacterial challenge. Efficacy of cell depletion was greater than 99% as determined by flow cytometric analysis of splenocytes (not shown). Nonspecific rat IgG purified MAb was used as an isotype control.
Statistical analysis.
The CFU data were normalized by log transformation and evaluated by analysis of variance followed by Dunnett's post hoc test. The cellular and antibody responses were compared using the nonparametric Mann-Whitney U test (InStat; GraphPadv4).
RESULTS
The Omp31 DNA vaccine protects BALB/c mice against B. melitensis and B. ovis infection.
Protection experiments were carried out by challenging pCIOmp31-vaccinated and control mice with B. melitensis or B. ovis, and the level of infection was evaluated by determining CFU in spleens and livers. In the spleen, mice given pCIOmp31 exhibited a significant degree of protection against B. melitensis and B. ovis (P < 0.001) compared with controls receiving PBS (1.30 and 2.24 log protection, respectively) (Table 1). Inactivated H38, the control vaccine, induced 2 and 2.61 log protection against B. melitensis and B. ovis, respectively. No reduction in the number of CFU was seen in animals injected with pCI compared to the number in control animals. pCIOmp31 and H38 were also protective against B. melitensis and B. ovis infection in the liver (Table 1). These results indicate that immunization with pCIOmp31 afforded a significant degree of protection against smooth and rough Brucella infection.
TABLE 1.
Protection against B. melitensis or B. ovis in BALB/c mice immunized with the Omp31 DNA vaccine
| Treatment group (n = 8) |
B. melitensis
|
B. ovis
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean log10 CFU ± SD in:
|
Log U of protection in:
|
Mean log10 CFU ± SD in:
|
Log U of protection in:
|
|||||
| Spleen | Liver | Spleen | Liver | Spleen | Liver | Spleen | Liver | |
| PBS | 5.75 ± 0.16 | 2.53 ± 0.16 | 0 | 0 | 4.83 ± 0.17 | 3.12 ± 0.08 | 0 | 0 |
| pCI | 5.74 ± 0.30 | 2.20 ± 0.68 | 0.01 | 0.33 | 4.82 ± 0.30 | 3.02 ± 0.08 | 0.01 | 0.10 |
| pCIOmp31 | 4.45 ± 0.60a | 1.53 ± 0.23a | 1.30 | 1.00 | 2.59 ± 0.25a | 2.49 ± 0.18a | 2.24 | 0.63 |
| H38 | 3.75 ± 0.57a | 1.41 ± 0.08a | 2.00 | 1.12 | 2.22 ± 0.57a | 2.29 ± 0.02a | 2.61 | 0.83 |
Significantly different (P < 0.001) from PBS-immunized mice as estimated by Dunnett's test.
The Omp31 DNA vaccine induces weak humoral and T-helper responses.
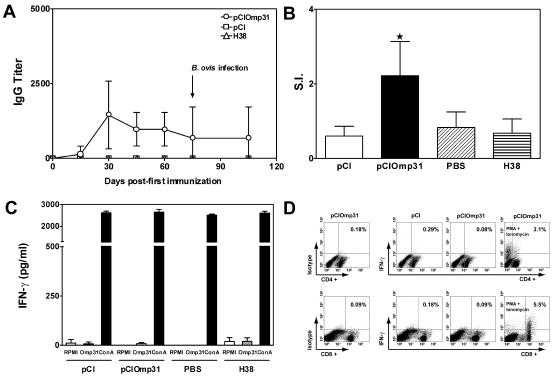
Omp31 is an excellent model to evaluate the roles of different immune mechanisms elicited in protection, since it is well exposed in B. ovis but hindered in B. melitensis (12). To evaluate the humoral response, IgG anti-Omp31 titers in sera from immunized mice were measured by ELISA. Immunization with pCIOmp31 elicited a weak IgG response that was detectable only 30 days after the first immunization (IgG titer range, 400 to 3,200) and declined thereafter. B. ovis challenge was unable to boost this response (Fig. 1A). IgG1 Abs were not detected throughout the experiment. Low titers of IgG2a were detected in only three out of eight mice (data not shown). Similar reactivities were detected in an ELISA using hot-saline extract from B. ovis (not shown). Neither the animals inoculated with pCI nor the H38-inoculated animals showed any specific anti-Omp31 antibodies (Fig. 1A).
FIG. 1.
A. Kinetics of the humoral response elicited after immunization with the Omp31 DNA vaccine. Mice were immunized with pCIOmp31, pCI, or H38 and bled retro-orbitally at the indicated days. IgG-specific antibodies against rOmp31 were evaluated by ELISA. Each symbol represents the mean ± SD for eight animals. The arrow indicates the time of B. ovis infection. B. Proliferative responses of spleen cells from mice immunized with pCIOmp31. Cells from immunized mice (2 × 105 cells/well) were stimulated with rOmp31 (1 μg/ml) for 5 days, and incorporation of [3H] thymidine was measured. The S.I. corresponds to the counts per minute of stimulated cells divided by counts per minute of unstimulated cells. Results are expressed as the mean ± SD for five mice. ★, significantly different from PBS-immunized group (P < 0.05). Data are representative of three separate experiments. C. Determination of IFN-γ production in cells from pCI-, pCIOmp31-, PBS-, or H38-immunized mice. Spleen cells (4 × 106/ml) from mice were stimulated with RPMI, rOmp31 (1 μg/ml), or ConA (2.5 μg/ml) for 48 h. IFN-γ in cell supernatants was quantified by antibody capture ELISA. Each value represents the mean ± SD of the responses of spleen cells from five individual mice. Data are representative of four separate experiments. D. Expression of IFN-γ versus cell surface markers in spleen cells from pCI- or pCIOmp31-immunized mice. Spleen cells from pCI- or pCIOmp31-immunized mice were cultured for 5 days with mitomycin C-treated A20JOmp31. On day 5 they were restimulated with mitomycin C-treated A20JOmp31 cells and rOmp31 (10 μg/ml) or, where indicated, with phorbol myristate acetate (20 ng/ml) plus ionomycin (750 ng/ml) and then incubated for 4 h with 10 μg/ml of BFA before analysis by flow cytometry. Numbers within quadrants represent the percentage of positive gated cells.
To evaluate the cellular immune response induced by pCIOmp31, the ability of spleen cells from PBS-, pCI-, pCIOmp31-, or H38-immunized mice to proliferate in vitro against rOmp31 was tested at the time of bacterial challenge. Cells from mice injected with pCIOmp31 showed a weak but significant (P < 0.05) Ag-specific proliferative response (S.I. = 2.3) compared with splenocytes from PBS- or pCI-immunized mice (S.I. = 0.83 and 0.6, respectively). In contrast, no significant response was detected in spleen cells from mice injected with H38 (S.I. = 0.67) (Fig. 1B). Spleen cells from all mice proliferated in response to ConA, with no significant differences observed among the groups (data not shown). To get further information on the type of immune response induced by pCIOmp31 immunization, cytokine secretion in culture supernatants of spleen cells from PBS-, pCI-, pCIOmp31-, or H38-immunized mice was evaluated by ELISA. Different concentrations of rOmp31 (0.1, 1, and 10 μg/ml) were unable to induce antigen-specific production of IFN-γ in spleen cells from immunized mice at 24, 48, or 72 h (Fig. 1C and data not shown). Under the same experimental conditions, cells from mice immunized with rOmp31 secreted IFN-γ (unpublished observations). Spleen cells from all mice produced IFN-γ in response to ConA, with no significant differences observed among the groups (Fig. 1C). The inability of T cells from pCIOmp31-immunized mice to produce IFN-γ upon restimulation with Omp31 was further confirmed by intracellular analysis of IFN-γ production. Neither CD4+ nor CD8+ T cells produced IFN-γ when cultured with mitomycin C-treated A20JOmp31 cells and 10 μg/ml of rOmp31 (Fig. 1D). Spleen cells from immunized mice were also unable to produce IL-4 or IL-10. A weak but not significant IL-2 secretion was observed in the supernatants of spleen cells from pCIOmp31-immunized mice (not shown).
Taken together, these results indicate that pCIOmp31 immunization afforded significant protection in mice against smooth and rough Brucella strains without inducing a detectable Th1 cytokine response and in the presence of a weak humoral response, suggesting that other mechanisms are involved in the pCIOmp31-elicited immune protection.
The Omp31 DNA vaccine induces a specific cytotoxic response.
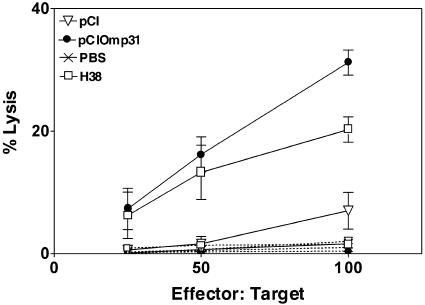
DNA immunization has been shown to be effective in inducing CTLs (27). Therefore, the induction of Omp31-specific CTLs in pCIOmp31-immunized mice was examined and compared with the responses in mice immunized with the H38 vaccine. Splenocytes from pCI-, PBS-, pCIOmp31-, or H38-immunized mice were harvested at the time of bacterial challenge. CTL responses were assessed by the 51Cr release assay after in vitro stimulations with mitomycin C-treated A20JOmp31 stimulator cells. Target cells were A20JOmp31 or A20JpCI (control) cells. Specific lysis of A20JOmp31 cells was observed at effector/target cell ratios of 25:1 or higher using CTLs from mice that were immunized with pCIOmp31 or H38 and that had been protected against bacterial challenge. Splenocytes from pCI-immunized mice minimally lysed A20JOmp31 cells and failed to lyse A20JpCI cells (Fig. 2). These results demonstrate that Omp31-specific CTL activity could be detected in spleen cell cultures derived from pCIOmp31- and H38-vaccinated mice and suggest that Omp31 cytotoxic responses are a key mechanism in the protection against smooth and rough Brucella infection.
FIG. 2.
Induction of Omp31-specific CTLs in spleen cells from pCIOmp31-immunized mice. Cytotoxicity was detected in a standard 6-h 51Cr release assay. Target cells were A20JpCI (dashed lines) or A20JOmp31 (solid lines) cells labeled with 51Cr. Effector cells were the splenocytes from immunized mice previously cultured for 5 days with mitomycin C-treated A20JOmp31.The cytotoxicity was measured at the indicated effector/target cell ratios. Each value represents the mean ± SD of the responses of spleen cells from five individual mice. Data are representative of three separate experiments.
pCIOmp31 immunization elicits CD8+ T cells that mediate cytotoxicity via perforins and CD4+ T cells that mediate lysis via Fas-FasL pathways.
The above results prompted us to further characterize the Omp31-specific cytotoxic responses. To investigate whether CD4+ and/or CD8+ T cells were involved in the cytotoxic response elicited by pCIOmp31, effector cells were preincubated with anti-CD4 or anti-CD8 MAbs and cytotoxic activity against A20JOmp31 cells was assessed by the 51Cr release assay. Either treatment significantly abrogated (P < 0.05) the cytotoxic activity of spleen cells from pCIOmp31- or H38-vaccinated animals, indicating that both cell types were involved in cytotoxicity (Fig. 3).
FIG. 3.
CD4+ T and CD8+ T cells are involved in the Omp31-specific cytotoxic activity elicited by pCIOmp31 or H38 immunization. 51Cr release CTL assay was undertaken as described for Fig. 2. Effector cells from pCIOmp31- or H38-immunized mice were previously incubated for 2 h with culture supernatants from anti-mouse CD4+ or anti-mouse CD8+ hybridomas or an IgG isotype control (-). The effector/target cell ratio was 100:1. Each value represents the mean ± SD of the responses of spleen cells from five individual mice. Data are representative of two separate experiments. ★, significantly different from isotype control-treated cells (P < 0.05).
To further determine the contributions of CD8+ and CD4+ T cells in the Omp31-specific cytotoxic responses, effector cells were depleted of CD4+ T cells or CD8+ T cells by using immunobeads. When CTLs from pCIOmp31-immunized animals were depleted of CD4+ T cells, lysis of A20JOmp31 cells was reduced 30%. In contrast, depletion of CD8+ T cells reduced the Omp31-specific lysis 70% (Fig. 4). Thus, pCIOmp31 immunization induces mainly CD8+ T cells but also elicits CD4+-specific CTLs.
FIG. 4.
pCIOmp31 elicits effector CD8+ T cells that mediate cytotoxicity via perforin and CD4+-specific T cells that use the Fas-FasL pathway. 51Cr release CTL assay was performed as described for Fig. 2. Target cells were A20JOmp31 cells. The effector/target cell ratio was 100:1. pCIOmp31-specific effector cells were depleted of CD4+ T cells (−CD4+) or CD8+ T cells (−CD8+) by using mouse CD4 (L3T4) Dynabeads or mouse CD8 (Lyt2) Dynabeads or were not depleted (total cells). Depleted and nondepleted effector cells were preincubated for 2 h with RPMI (-), brefeldin A (BFA), or concanamycin A (CCA). Each value represents the mean of triplicates ± SD of the response of a pool of spleen cells from five mice. Data are representative of two separate experiments.
T cells may induce cytotoxicity by two distinct molecular pathways: the granule exocytosis pathway, which is dependent on the pore-forming molecule perforin, or the up-regulation of FasL (CD95L), which may initiate programmed cell death through interaction with Fas (CD95) on target cells (36). To investigate the roles of both pathways in the Omp31-specific cytotoxicity, CD4+- or CD8+-depleted effector cells were previously incubated for 2 h with CCA, a specific inhibitor of the secretory pathway (perforin, granzymes, etc.) (37), or with BFA, a drug that preferentially affects the Fas/FasL pathway (37, 44), and then used as CTLs against A20JOmp31 cells in the 51Cr release assay. CCA strongly inhibited the cytotoxic activity elicited by CD4+-depleted effector cells, whereas BFA had no effect on these cells. Conversely, BFA completely abrogated the cytotoxicity elicited by CD8+-depleted effector cells, while CCA had no effect (Fig. 4). These results indicate that Omp31-specific CD8+ T cells mediate specific lysis of target cells via the perforin pathway, while CD4+ T cells use the Fas-FasL pathway.
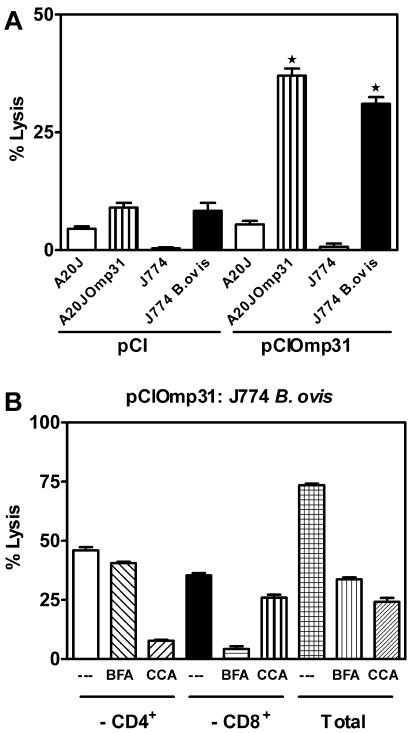
CTLs from pCIOmp31-immunized mice lyse Brucella-infected macrophages.
We next investigated whether pCIOmp31 vaccination could induce a cytotoxic response against Brucella-infected macrophages. Splenocytes from pCIOmp31-immunized mice were cultured for 5 days with mitomycin C-treated A20JOmp31 stimulator cells, and then their ability to recognize and kill a macrophage cell line (J774) infected with B. ovis was analyzed. Uninfected J774 cells served as a control. CTLs from pCIOmp31-immunized mice were able to lyse J774-infected cells but did not lyse uninfected J774 cells (Fig. 5A). As with the Omp31-specific response, CD8+ T cells elicited lysis of Brucella-infected cells via the perforin pathway, while CD4+ T cells did so via the Fas-FasL pathway (Fig. 5B). The same results were obtained when Brucella-infected A20J cells were used (data not shown). These results indicate that Omp31-specific CTLs have the capacity to recognize and destroy Brucella-infected cells. This cytotoxicity is mediated mainly by CD8+ T cells via the perforin pathway.
FIG. 5.
Vaccination with pCIOmp31 elicit CD8+- and CD4+-specific T cells that lyse Brucella-infected macrophages in vitro. Cytotoxicity was detected in a standard 6-h 51Cr release assay. The effector/target cell ratio was 100:1. A. Target cells were A20J, A20JOmp31, J774, or J774 B. ovis. Effector cells were the splenocytes from pCIOmp31- or pCI-immunized mice previously cultured for 5 days with mitomycin C-treated A20JOmp31. Each value represents the mean ± SD of the responses of spleen cells from five individual mice. Data are representative of two separate experiments. ★, significantly different from pCI-immunized mice (P < 0.05). B. Target cells were J774 B. ovis. Effector cells from pCIOmp31-immunized mice were depleted of CD4+ T cells (−CD4+) or CD8+ T cells (−CD8+) or were not depleted (total cells) as indicated in Fig. 4. Depleted and nondepleted effector cells were preincubated for 2 h with RPMI (-), BFA, or CCA. Each value represents the mean of triplicates ± SD of the response of a pool of spleen cells from five mice. Data are representative of three separate experiments.
Protection against Brucella infection in vivo is mediated predominantly by anti-Omp31 CD8+ T cells but also by CD4+T cells.
We next focused on the role of CD4+ or CD8+ T cells in the protective immunity induced by the Omp31 DNA vaccine. pCIOmp31-vaccinated mice were injected intraperitoneally with MAb 2.43 to deplete CD8+ T cells or with MAb GK1.5 to deplete CD4+ T cells. In the absence of immune depletion, mice given pCIOmp31 exhibited a significant (P < 0.001) degree of protection against B. melitensis compared with controls receiving pCI (1.45 log protection). pCIOmp31 vaccination induced 0.63 log protection in mice depleted of CD8+ T cells (P > 0.05) and 1.10 log protection in CD4+-depleted mice (P < 0.05). Isotype control IgG injections had no effect on the degree of protection induced by pCIOmp31 (Table 2). These results suggest a major role of CD8+ T cells and a minor contributive role of CD4+ T cells in the pCIOmp31-mediated immunity against Brucella.
TABLE 2.
Protection against B. melitensis infection induced by pCIOmp31 immunization is mediated mainly by CD8+ T cells
| Vaccine group (n = 8) | Treatment | Mean log10B. melitensis CFU ± SD in spleen | Log U of protection |
|---|---|---|---|
| pCI | None | 5.43 ± 0.51 | 0 |
| pCIOmp31 | None | 3.98 ± 0.84b | 1.45 |
| Anti-CD4+ | 4.33 ± 0.63a | 1.10 | |
| Anti-CD8+ | 4.80 ± 0.60c | 0.63 | |
| IgG | 4.01 ± 0.52b | 1.42 | |
| H38 | None | 3.11 ± 0.40b | 2.32 |
Significantly different from pCI-immunized mice (P < 0.05) as estimated by Dunnett's test.
Significantly different from pCI-immunized mice (P < 0.001) as estimated by Dunnett's test.
Significantly different from pCIOmp31-immunized mice (P < 0.05) as estimated by Dunnett's test.
DISCUSSION
Control of brucellosis in domestic animals has markedly reduced the incidence of human infection, but this disease still represents an important cause of morbidity worldwide. A human vaccine would be valuable for individuals who may be occupationally exposed to Brucella spp. and for persons who consume unpasteurized dairy products from areas in which this bacterium is endemic. Several attempts have been made to control human disease by vaccination with attenuated Brucella strains (54). However, practical acceptance of these preparations has been very limited, mainly because of the unacceptable local inflammatory reactions elicited by them, together with their limited protection. Clearly, there is a need for a better vaccine for brucellosis eradication (5). Due to its epidemic potential, the absence of a human vaccine, the drawbacks of current vaccine strains in terms of safety, and the efficiency of aerosol infection, this airborne pathogen is classified as a biosafety level 3 pathogen and considered to be a potential bioterrorism agent (38), further supporting the need to develop effective medical protective measures against it.
In this study we investigated the immune response and protection elicited by a DNA vaccine coding for Omp31, an outer membrane protein from Brucella. Mice immunized with the Omp31 DNA vaccine were significantly protected against B. ovis and B. melitensis infection. Protection was observed in the spleen and also in the liver, an important site for the control of Brucella infection (17, 32). Levels of protection afforded in the spleen and the liver after B. ovis challenge were comparable to the ones achieved by the H38 control vaccine. After B. melitensis challenge, pCIOmp31 elicited a lower degree of protection in the spleen than H38. However, in the liver it induced levels of protection similar to those induced by the control vaccine, suggesting that using this vaccine preparation the liver seems to be better for protection against B. melitensis. Until now there have been no reports on a subunit vaccine that is able to confer protection against smooth B. melitensis, the most virulent strain of Brucella spp. Thus, the Omp31 DNA vaccine could be included in the development of a multisubunit vaccine in the immunoprophylaxis of brucellosis.
Although there were detectable levels of IgG anti-Omp31, the antibody response to pCIOmp31 vaccination was low compared to those with other immunization systems using the same antigen (22). Moreover, titers of IgG1 or IgG2a isotypes were heterogeneous, with some animals producing detectable levels of antigen-specific antibodies, whereas the majority appeared not to respond; this is a phenomenon already reported for other DNA vaccines (16, 53). Surprisingly, B. ovis infection was unable to boost this response. This could be because (i) the response was too low and could not generate memory B cells or (ii) the levels of Omp31 expressed on the surface of B. ovis were insufficient to boost the response.
Splenocytes from pCIOmp31-protected mice were not able to mount an Omp31-specific secondary Th1 recall response. In a previous report we have shown that a DNA vaccine coding for a ribosome recycling factor induced a specific Th1 response but did not confer protection against B. melitensis infection (16). Our results and numerous examples in the literature (2, 43, 60) indicate that the levels of IFN-γ produced in response to a candidate vaccine do not always correlate with the efficacy of this vaccine during Brucella challenge. It appears that many Brucella proteins to which infected or vaccinated animals develop appropriate immune responses may not play a determinant role in host-acquired protective immunity to brucellosis. It is also possible that these antigens are not able to develop another arm of the cell-mediated immune response that is important for protection against Brucella infection. Therefore, the Omp31 DNA vaccine could activate another cell-mediated protective mechanism without inducing antigen specific IFN-γ induction, as was demonstrated in different protocols for DNA vaccination against intracellular pathogens (7, 28, 62).
We are interested in determining other immune mechanisms that could contribute to the control of Brucella intracellular growth, particularly because understanding these mechanisms may provide improved correlates of protection for vaccine research. Previous reports indicated that mice immunized with B. abortus conjugated to a peptide derived from the third variable loop of the human immunodeficiency virus type1 envelope containing a CTL epitope developed MHC class I-restricted CD8+ T cells capable of killing target cells expressing human immunodeficiency virus type 1 envelope (23). Importantly, these responses were also evident in mice deficient in CD4+ T cells, suggesting that the conjugates were being taken up by dendritic cells and stimulating CD8+ T cells to differentiate into memory cells in the absence of help from CD4+ T cells (24). As the Ag-specific CTL control of Brucella infection has not been extensively studied, we evaluated CTL responses in vaccinated mice. Both CD4+- and CD8+-specific cytotoxic T cells were induced after Omp31 DNA vaccination. Significant Omp31-specific CTL responses were also detected in H38-immunized mice. Since effector CTL responses against complex infectious antigens are restricted to a few epitopes compared to the total number of epitopes potentially available (1), this result suggests that Omp31 harbors, at least, an immunodominant T cytotoxic epitope. Our results are in agreement with previous reports indicating that a certain level of cytotoxic activity by antigen-specific CD4+ T cells is induced after strain RB51 immunization, although their cytolytic ability was much less than that of CD8+ T cells (29).
The potential of cytotoxic T-lymphocyte-mediated mechanisms in the regulation and control of Brucella infection is largely unexplored. Omp31-specific CTLs were able to lyse Brucella-infected macrophages. Mainly CD8+, but also CD4+, T cells were involved in the CTL activity induced by the Omp31 DNA vaccine, yet by a different cytotoxic mechanism. CD8+ T cells elicit lysis of Brucella-infected macrophages via the perforin pathway, while CD4+ T cells use the Fas-FasL pathway. This suggests that, similarly to the situation observed in the vaccine-mediated defense against other intracellular bacteria (55), the Omp31-specific CTL responses might serve different purposes in the control of infection. Cytotoxic CD8+ T cells may have a predominantly antimicrobial function against this intracellular pathogen, whereas the cytotoxic CD4+ T cells, through the Fas-mediated pathway, may have a predominantly immunomodulatory role in removing the cells that sustain immune help by presenting antigen on MHC class II (56). In that respect, a recent study suggests that Fas is involved in termination of the antigen-specific response of T lymphocytes to Listeria monocytogenes after the resolution of infection (34).
The in vivo T-cell subset depletion experiments strongly correlate with the in vitro CTL induction in that they clearly show that protection against B. melitensis elicited by pCIOmp31 was mainly attributed to CD8+ T cells but also that CD4+ T cells are involved. Although protection in Omp31-vaccinated mice depleted of CD8+ T cell was significantly decreased, it was not completely abrogated. In the same way, CD4+ T-cell depletion affects, albeit minimally, the level of protection elicited by the Omp31 DNA vaccine. This is consistent with the contention that induction of both CD8+ and CD4+ T cells is needed to obtain the maximum level of protection by the DNA vaccine. Our results are compatible with previous results that shows that B. abortus infection was greatly exacerbated in mice that were deficient in MHC class I molecules. In contrast, mice that lacked functional MHC II were able to control B. abortus infection (50). These experiments demonstrate that CTLs, but not Th cells, are critical for protection against B. abortus and are in agreement with findings showing that B. abortus can induce CTLs even in the absence of functional CD4+ T cells. Altogether, these data suggest that vaccines that elicit both CD8+ and CD4+ T cytotoxic responses are sufficient to confer protection against Brucella in mice. Others, however, using a different strain of B. abortus, indicated that CTLs were not important after the first week of infection in C57BL/6 mice (49). The same authors in another paper reported that at 3 weeks postinfection, CD8+ T cells are important in controlling bacterial numbers in BALB/c mice (48).
Thus, if cytotoxicity can make a contribution to protection, brucellosis vaccines should be designed to promote this aspect of cellular immunity, not just Th1 responses and macrophage activation. In particular, antigen-specific delivery of cytotoxic bactericidal proteins may be highly desirable. Unfortunately, little is currently known about the mechanisms of regulation of the development of cytotoxicity (42), and still less is known about the differential development of cells expressing the Fas-FasL versus the perforin pathway. However, DNA vaccination seems predisposed to generate responses with a strong bias towards cytotoxicity (31). Moreover, it has been reported that DNA vaccination could induce CTL responses in cattle (30), further reinforcing the probable use of this vaccine in larger animals.
In conclusion, our results indicate that Omp31 could be a useful candidate for the development of subunit vaccines against brucellosis, since it elicits high levels of protection against smooth and rough species of Brucella. Protection takes place by virtue of the induction of cytotoxic CD8+ (mainly) and CD4+ T cells. A weak humoral response was also observed (Table 3). The results also demonstrate that lysis of infected cells via perforins/granzymes is relevant for the induction of protective anti-Brucella immunity.
TABLE 3.
Omp31-specific immune response and protection in BALB/c mice against Brucella challenge
| Group | Response
|
||||||
|---|---|---|---|---|---|---|---|
| Antibody | Proliferative | Th1 | CTL | CD4 CTL | CD8 CTL | Protection | |
| PBS | − | − | − | − | − | − | − |
| pCI | − | − | − | − | − | − | − |
| pCIOmp31 | ± | + | − | +++ | + | ++ | ++ |
| H38 | − | − | − | ++ | ++ | + | +++ |
Finally, our results together with previous work (39) indicate that several distinct bactericidal mechanisms may operate in cellular immunity against brucellosis. Different mechanisms may be featured at distinct stages of infection, to kill both actively multiplying and dormant bacteria and cumulatively decrease the duration of persistence of the infection.
Acknowledgments
We thank Mauricio Rodriguez from Universidad Federal de Sao Pablo, Brazil, for providing A20J cells.
This work was supported by grant 05-06324 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-Argentina) and grants 14116-160 and 14156-33 from Fundación Antorchas. J.C. is a recipient of a postdoctoral fellowship from CONICET (Argentina). K.A.P. is a recipient of a doctoral fellowship from CONICET (Argentina). S.D.L.B, C.A.F., and G.H.G. are members of the Research Career of CONICET. S.M.E is a member of CIC (Argentina). C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Editor: J. D. Clements
REFERENCES
- 1.Adorini, L., E. Appella, G. Doria, and Z. A. Nagy. 1988. Mechanisms influencing the immunodominance of T cell determinants. J. Exp. Med. 168:2091-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godefroid, K. Walravens, and J. J. Letesson. 2001. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun. 69:4816-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godfroid, K. Walravens, and J. J. Letesson. 2001. Induction of immune response in BALB/c mice with a DNA vaccine encoding bacterioferritin or P39 of Brucella spp. Infect. Immun. 69:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alton, G. 1985. Rev. 1 and H38 Brucella melitensis vaccines, p. 215-227. In P. M. Verger (ed.), Brucella melitensis. Martinus Nijho, Dordrecht, The Netherlands.
- 5.Anonymous. 1986. Joint FAO/W.H.O. expert committee on brucellosis. World Health Org. Tech. Rep. Ser. 740:1-132. [PubMed] [Google Scholar]
- 6.Araya, L. N., P. H. Elzer, G. E. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 7.Barry, R. A., H. G. Archie Bouwer, T. R. Clark, K. A. Cornell, and D. J. Hinrichs. 2003. Protection of interferon-gamma knockout mice against Listeria monocytogenes challenge following intramuscular immunization with DNA vaccines encoding listeriolysin O. Vaccine 21:2122-2132. [DOI] [PubMed] [Google Scholar]
- 8.Blasco, J. 1990. Brucella ovis, p. 352-378. In K. Nielson and R. J. Duncan (ed.), Animal brucellosis. CRC Press, Boca Raton, Fla.
- 9.Blasco, J. M., and R. Diaz. 1993. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. Lancet 342:805. [DOI] [PubMed] [Google Scholar]
- 10.Blasco, J. M., C. M. Marin, M. Barberan, I. Moriyon, and R. Diaz. 1987. Immunization with Brucella melitensis Rev. 1 against Brucella ovis infection of rams. Vet. Microbiol. 14:381-392. [DOI] [PubMed] [Google Scholar]
- 11.Bosseray, N., and M. Plommet. 1983. A laboratory reference vaccine to titrate immunogenic activity of antibrucella vaccines in mice. Ann. Rech. Vet. 14:163-168. [PubMed] [Google Scholar]
- 12.Bowden, R. A., A. Cloeckaert, M. S. Zygmunt, and G. Dubray. 1995. Outer-membrane protein- and rough lipopolysaccharide-specific monoclonal antibodies protect mice against Brucella ovis. J. Med. Microbiol. 43:344-347. [DOI] [PubMed] [Google Scholar]
- 13.Bowden, R. A., S. M. Estein, M. S. Zygmunt, G. Dubray, and A. Cloeckaert. 2000. Identification of protective outer membrane antigens of Brucella ovis by passive immunization of mice with monoclonal antibodies. Microbes Infect. 2:481-488. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter, T. E., S. L. Berry, and J. S. Glenn. 1987. Economics of Brucella ovis control in sheep: epidemiologic simulation model. J. Am. Vet. Med. Assoc. 190:977-982. [PubMed] [Google Scholar]
- 15.Cassataro, J., K. Pasquevich, L. Bruno, J. C. Wallach, C. A. Fossati, and P. C. Baldi. 2004. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough brucellae. Clin. Diagn. Lab Immunol. 11:111-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassataro, J., C. A. Velikovsky, G. H. Giambartolomei, S. Estein, L. Bruno, A. Cloeckaert, R. A. Bowden, M. Spitz, and C. A. Fossati. 2002. Immunogenicity of the Brucella melitensis recombinant ribosome recycling factor-homologous protein and its cDNA. Vaccine 20:1660-1669. [DOI] [PubMed] [Google Scholar]
- 17.Cheville, N. F., R. A. Kunkle, A. E. Jensen, and M. V. Palmer. 1995. Persistence of Brucella abortus in the livers of T cell-deficient nude mice. Lab Investig. 73:96-102. [PubMed] [Google Scholar]
- 18.Cloeckaert, A., I. Jacques, N. Bosseray, J. N. Limet, R. Bowden, G. Dubray, and M. Plommet. 1991. Protection conferred on mice by monoclonal antibodies directed against outer-membrane-protein antigens of Brucella. J. Med. Microbiol. 34:175-180. [DOI] [PubMed] [Google Scholar]
- 19.Cloeckaert, A., P. Kerkhofs, and J. N. Limet. 1992. Antibody response to Brucella outer membrane proteins in bovine brucellosis: immunoblot analysis and competitive enzyme-linked immunosorbent assay using monoclonal antibodies. J. Clin. Microbiol. 30:3168-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloeckaert, A., N. Vizcaino, J. Y. Paquet, R. A. Bowden, and P. H. Elzer. 2002. Major outer membrane proteins of Brucella spp.: past, present and future. Vet. Microbiol. 90:229-247. [DOI] [PubMed] [Google Scholar]
- 21.Cloeckaert, A., M. S. Zygmunt, P. de Wergifosse, G. Dubray, and J. N. Limet. 1992. Demonstration of peptidoglycan-associated Brucella outer-membrane proteins by use of monoclonal antibodies. J. Gen. Microbiol. 138:1543-1550. [DOI] [PubMed] [Google Scholar]
- 22.Estein, S. M., J. Cassataro, N. Vizcaino, M. S. Zygmunt, A. Cloeckaert, and R. A. Bowden. 2003. The recombinant Omp31 from Brucella melitensis alone or associated with rough lipopolysaccharide induces protection against Brucella ovis infection in BALB/c mice. Microbes Infect. 5:85-93. [DOI] [PubMed] [Google Scholar]
- 23.Golding, B., J. Inman, P. Highet, R. Blackburn, J. Manischewitz, N. Blyveis, R. D. Angus, and H. Golding. 1995. Brucella abortus conjugated with a gp120 or V3 loop peptide derived from human immunodeficiency virus (HIV) type 1 induces neutralizing anti-HIV antibodies, and the V3-B. abortus conjugate is effective even after CD4+ T-cell depletion. J. Virol. 69:3299-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golding, B., D. E. Scott, O. Scharf, L. Y. Huang, M. Zaitseva, C. Lapham, N. Eller, and H. Golding. 2001. Immunity and protection against Brucella abortus. Microbes Infect. 3:43-48. [DOI] [PubMed] [Google Scholar]
- 25.Gradwell, D. V., and F. E. Van Zyl. 1975. Effectivity of Rev. 1 vaccine in rams against Brucella ovis infection. J. S. Afr. Vet. Assoc. 46:349-351. [PubMed] [Google Scholar]
- 26.Guilloteau, L. A., K. Laroucau, N. Vizcaino, I. Jacques, and G. Dubray. 1999. Immunogenicity of recombinant Escherichia coli expressing the omp31 gene of Brucella melitensis in BALB/c mice. Vaccine 17:353-361. [DOI] [PubMed] [Google Scholar]
- 27.Gurunathan, S., C. Y. Wu, B. L. Freidag, and R. A. Seder. 2000. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 12:442-447. [DOI] [PubMed] [Google Scholar]
- 28.Hassett, D. E., J. Zhang, and J. L. Whitton. 1999. Plasmid DNA vaccines are effective in the absence of IFNgamma. Virology 263:175-183. [DOI] [PubMed] [Google Scholar]
- 29.He, Y., R. Vemulapalli, A. Zeytun, and G. G. Schurig. 2001. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect. Immun. 69:5502-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, Y., L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2005. Immunization with a bovine herpesvirus 1 glycoprotein B DNA vaccine induces cytotoxic T-lymphocyte responses in mice and cattle. J. Gen. Virol. 86:887-898. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki, A., C. A. Torres, P. S. Ohashi, H. L. Robinson, and B. H. Barber. 1997. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 159:11-14. [PubMed] [Google Scholar]
- 32.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 68:5314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacques, I., A. Cloeckaert, J. N. Limet, and G. Dubray. 1992. Protection conferred on mice by combinations of monoclonal antibodies directed against outer-membrane proteins or smooth lipopolysaccharide of Brucella. J. Med. Microbiol. 37:100-103. [DOI] [PubMed] [Google Scholar]
- 34.Jensen, E. R., A. A. Glass, W. R. Clark, E. J. Wing, J. F. Miller, and S. H. Gregory. 1998. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect. Immun. 66:4143-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez de Bagues, M. P., P. H. Elzer, J. M. Blasco, C. M. Marin, C. Gamazo, and A. J. Winter. 1994. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect. Immun. 62:632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14:207-232. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka, T., N. Shinohara, H. Takayama, K. Takaku, S. Kondo, S. Yonehara, and K. Nagai. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678-3686. [PubMed] [Google Scholar]
- 38.Kaufmann, A. F., M. I. Meltzer, and G. P. Schmid. 1997. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg. Infect. Dis. 3:83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko, J., and G. A. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16:65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozak, M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurar, E., and G. A. Splitter. 1997. Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine 15:1851-1857. [DOI] [PubMed] [Google Scholar]
- 42.Lanzavecchia, A. 1998. Immunology. Licence to kill. Nature 393:413-414. [DOI] [PubMed] [Google Scholar]
- 43.Leclerq, S., J. S. Harms, G. M. Rosinha, V. Azevedo, and S. C. Oliveira. 2002. Induction of a Th1-type of immune response but not protective immunity by intramuscular DNA immunisation with Brucella abortus GroEL heat-shock gene. J. Med. Microbiol. 51:20-26. [DOI] [PubMed] [Google Scholar]
- 44.Li, J. H., D. Rosen, D. Ronen, C. K. Behrens, P. H. Krammer, W. R. Clark, and G. Berke. 1998. The regulation of CD95 ligand expression and function in CTL. J. Immunol. 161:3943-3949. [PubMed] [Google Scholar]
- 45.Marin, C. M., M. Barberan, M. P. Jimenez de Bagues, and J. M. Blasco. 1990. Comparison of subcutaneous and conjunctival routes of Rev. 1 vaccination for the prophylaxis of Brucella ovis infection in rams. Res. Vet. Sci. 48:209-215. [PubMed] [Google Scholar]
- 46.Moriyon, I., M. J. Grillo, D. Monreal, D. Gonzalez, C. Marin, I. Lopez-Goni, R. C. Mainar-Jaime, E. Moreno, and J. M. Blasco. 2004. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet. Res. 35:1-38. [DOI] [PubMed] [Google Scholar]
- 47.Munoz-Montesino, C., E. Andrews, R. Rivers, A. Gonzalez-Smith, G. Moraga-Cid, H. Folch, S. Cespedes, and A. A. Onate. 2004. Intraspleen delivery of a DNA vaccine coding for superoxide dismutase (SOD) of Brucella abortus induces SOD-specific CD4+ and CD8+ T cells. Infect. Immun. 72:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy, E. A., M. Parent, J. Sathiyaseelan, X. Jiang, and C. L. Baldwin. 2001. Immune control of Brucella abortus 2308 infections in BALB/c mice. FEMS Immunol. Med. Microbiol. 32:85-88. [DOI] [PubMed] [Google Scholar]
- 49.Murphy, E. A., J. Sathiyaseelan, M. A. Parent, B. Zou, and C. L. Baldwin. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira, S. C., and G. A. Splitter. 1995. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25:2551-2557. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 52.Onate, A. A., S. Cespedes, A. Cabrera, R. Rivers, A. Gonzalez, C. Munoz, H. Folch, and E. Andrews. 2003. A DNA vaccine encoding Cu, Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peet, N. M., J. A. McKeating, B. Ramos, T. Klonisch, J. B. De Souza, P. J. Delves, and T. Lund. 1997. Comparison of nucleic acid and protein immunization for induction of antibodies specific for HIV-1 gp120. Clin. Exp. Immunol. 109:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schurig, G. G., N. Sriranganathan, and M. J. Corbel. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479-496. [DOI] [PubMed] [Google Scholar]
- 55.Silva, C. L., V. L. Bonato, K. M. Lima, A. A. Coelho-Castelo, L. H. Faccioli, A. Sartori, A. O. De Souza, and S. C. Leao. 2001. Cytotoxic T cells and mycobacteria. FEMS Microbiol. Lett. 197:11-18. [DOI] [PubMed] [Google Scholar]
- 56.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 57.Tabatabai, L. B., and G. W. Pugh, Jr. 1994. Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 58.Traidl, C., S. Sebastiani, C. Albanesi, H. F. Merk, P. Puddu, G. Girolomoni, and A. Cavani. 2000. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J. Immunol. 165:3058-3064. [DOI] [PubMed] [Google Scholar]
- 59.Velikovsky, C. A., J. Cassataro, G. H. Giambartolomei, F. A. Goldbaum, S. Estein, R. A. Bowden, L. Bruno, C. A. Fossati, and M. Spitz. 2002. A DNA vaccine encoding lumazine synthase from Brucella abortus induces protective immunity in BALB/c mice. Infect Immun. 70:2507-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vemulapalli, R., S. Cravero, C. L. Calvert, T. E. Toth, N. Sriranganathan, S. M. Boyle, O. L. Rossetti, and G. G. Schurig. 2000. Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin. Diagn. Lab Immunol. 7:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vizcaino, N., A. Cloeckaert, M. S. Zygmunt, and G. Dubray. 1996. Cloning, nucleotide sequence, and expression of the Brucella melitensis omp31 gene coding for an immunogenic major outer membrane protein. Infect Immun. 64:3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida, A., T. Nagata, M. Uchijima, and Y. Koide. 2001. Protective CTL response is induced in the absence of CD4+ T cells and IFN-gamma by gene gun DNA vaccination with a minigene encoding a CTL epitope of Listeria monocytogenes. Vaccine 19:4297-4306. [DOI] [PubMed] [Google Scholar]
- 63.Young, E. J. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21:283-289. [DOI] [PubMed] [Google Scholar]