Abstract
Members of the genus Rickettsia possess the ability to invade host cells and promptly escape from phagosomal vacuoles into the host cell cytosol, thereby avoiding destruction within the endosomal pathway. The mechanism underlying rickettsial phagosomal escape remains unknown, although the genomic sequences of several rickettsial species have allowed for the identification of four genes with potential membranolytic activities (tlyA, tlyC, pat1, and pld). This study was undertaken to determine which of the selected genes of Rickettsia prowazekii mediate the escape process. Quantitative ultrastructural analyses indicated that the period of active phagosomal escape was between 30 and 50 min postinfection. Reverse transcriptase PCR analyses determined that tlyC and pld were transcribed during the period of active phagosomal escape but that tlyA and pat1 were not. The functionality of both tlyC and pld was determined by complementation studies of Salmonella, which replicates within endosomes. Complementation of Salmonella organisms with either tlyC or pld resulted in the escape of transformants from endosomal vacuoles into the host cell cytosol demonstrated by quantitative ultrastructural analyses. These data suggest a role for tlyC and pld in the process of phagosomal escape by R. prowazekii.
Rickettsia prowazekii is a small, gram-negative, obligately intracellular bacterium that is the agent of epidemic typhus. It is transmitted by the human-body louse Pediculus humanus corporis and is also associated with flying squirrels and their ectoparasites (9, 11). Infected lice, when feeding, excrete a live, dormant form of the organism onto the cutaneous bite sites in their feces. R. prowazekii enters the host through breaks in the epidermis caused by scratching of the contaminated area. Once within the host, R. prowazekii primarily infects endothelial cells and macrophages, where the organisms proliferate to massive numbers inside the host cells and eventually lyse them. The clinical symptoms of epidemic typhus include fever, severe headache, and myalgia. The nonspecific nature of the symptoms often leads to misdiagnosis, delaying appropriate chemotherapeutic intervention. The disease is fatal in approximately 30% of cases unless it is treated in a timely manner, preferably with a tetracycline drug (5). While the vegetative form of Rickettsia is generally unstable extracellularly, R. prowazekii in louse feces remains stable and infective for months or longer. Furthermore, there have been a multitude of well-documented laboratory-associated infections caused by accidental inhalation of aerosols containing R. prowazekii (18). Owing to its high case fatality rate and the threat of human infection by low-dose, stable aerosol, R. prowazekii is on the select agent list that restricts possession and transfer of the agents, to hinder access to the organisms by would-be bioterrorists (5, 38).
All members of the genus Rickettsia possess the ability to invade host cells and quickly escape phagosomal vacuoles before phagolysosomal fusion occurs and bactericidal mechanisms are activated (12, 17, 34, 40). The mechanism of phagosomal escape remains unknown, although it has been hypothesized to be mediated by a hemolysin or phospholipase enzyme (31, 32). Genomic sequences for R. prowazekii, Rickettsia conorii, and Rickettsia typhi have revealed the presence of four genes with potential membranolytic activities: patatin B1 precursor, pat1 (RP602); hemolysin, tlyA (RP555); hemolysin C, tlyC (RP740); and pld (RP819) (4, 24, 25). The gene product of pld exhibits phospholipase D activity and has significant homology with the phospholipase D family of proteins (32). TlyC has been demonstrated to have hemolytic activity (31). Rickettsiae have proven extremely difficult to manipulate genetically, hindering the production of site-directed knockout clones for the study of gene function (6, 29, 30, 36). We report here the establishment of a novel system for expression of rickettsial virulence factors in another intracellular pathogen, Salmonella enterica serovar Typhimurium. All Salmonella spp. possess the ability to invade host cells but lack the ability to escape phagosomal vacuoles, having adapted to replicating within a vacuole that has the characteristics of a late endosome (10, 33). We hypothesized that expression in Salmonella of the R. prowazekii gene responsible for phagosomal escape would complement the transformants with the ability to escape into the host cell cytosol.
In this study we determined the period of active phagosomal escape. We further demonstrated that both tlyC and pld, but not tlyA or pat1, were transcriptionally expressed in R. prowazekii during the peak time of escape. To further study the membranolytic functions of tlyC and pld, we introduced the genes into Salmonella isolates and demonstrated the expression of each gene in the transformants by reverse transcriptase PCR (RT-PCR). A quantitative ultrastructural study demonstrated that Salmonella organisms expressing either tlyC or pld were able to escape phagosomal vacuoles, resulting in salmonellae exiting into the host cell cytosol.
MATERIALS AND METHODS
Experimental design.
In order to determine the period of active escape from the phagosome, Vero cells were infected with R. prowazekii and placed in Ito's fixative (a mixture of 1.25% formaldehyde, 2.5% glutaraldehyde, 0.03% trinitrophenol, 0.03% CaCl2, and 0.05 M cacodylate buffer, pH 7.3 [21]) for electron microscopy or in RNAlater (Ambion, Austin, TX) for RT-PCR analysis at 30 and 50 min postinfection. The fixed cells were further studied by quantitative ultrastructural analysis. The proportion of rickettsiae within the host cell cytosol to the total number (at least 50 for each time point) of rickettsiae evaluated was determined. Rickettsiae in the process of escape were considered intracytosolic. The experiment was repeated in order to ensure reproducibility, and the results were calculated as the mean percentages ± standard deviations for rickettsiae within the cytosol.
To determine which of the four membranolytic genes (pat1, tlyA, tlyC, or pld) were expressed during the period of active rickettsial phagosomal escape, RT-PCR was performed using mRNA isolated from the cells fixed in RNAlater. The genes found to be transcribed during rickettsial escape were amplified from the genome by PCR, along with the putative promoter regions upstream of the open reading frame, and cloned into the pcr2.1-TOPO vector (Invitrogen, Carlsbad, CA). The plasmids were propagated in Top 10F′ E. coli (Invitrogen, Carlsbad, CA). E. coli clones that transcribed the rickettsial genes (tlyC or pld) as determined by RT-PCR were chosen for plasmid purification using the High Pure plasmid isolation kit (Roche, Mannheim, Germany). The plasmids containing the genes were each electroporated into Salmonella enterica serovar Typhimurium (SB109) to determine whether or not the cloned genes could be involved in phagosomal escape. Salmonella was chosen for this experiment as it enters Vero cells but does not escape from phagosomal vacuoles, thereby allowing for the possibility of complementation of the ability to escape phagosomal vacuoles. RT-PCR was used again to confirm the transcriptional expression of the selected genes in Salmonella isolates. A quantitative ultrastructural analysis was performed exactly as described for the rickettsial study, except that observations were made at 4 hours after inoculation of cells. In both experiments, care was taken to avoid the counting of the same bacteria in serial sections.
Cells.
Vero cells (African green monkey fibroblast cell line from the kidney) were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultivated in Dulbecco's minimum essential medium (Gibco, Grand Island, NY), which contained 5% bovine calf serum (HyClone Inc., Logan, UT) and 10 mM HEPES, in 150-cm2-surface-area flasks or 24-well plates (approximately 2 × 105 cells per well) and were incubated at 37°C in 5% CO2. Vero cells were chosen for this study as they are nonphagocytic and therefore do not ingest dead organisms.
Rickettsia.
Rickettsia prowazekii (Breinl strain), provided by G. A. Dasch, Naval Medical Research Institute, Bethesda, Maryland, was passaged twice in yolk sacs of embryonated chicken eggs in our laboratory; 100 μl of infected yolk sac (105 PFU per flask) was used to infect three 150-cm2-surface-area flasks of confluent Vero cells. The flasks were then incubated at 37°C until they were 100% infected as determined by Protocol (Fisher, Pittsburgh, PA) staining of infected cells scraped from the monolayer. Stocks consisting of 1-ml aliquots of infected Vero cells were then prepared by scraping the infected cells from the monolayer and storing them in Dulbecco's minimum essential medium at −80°C. Five 1-ml aliquots were used to infect 10 150-cm2-surface-area flasks of confluent Vero cells, and the infection was monitored by Protocol staining of slides containing smears of infected cells from each flask. Once the infected cells were observed to contain more than 100 rickettsiae per cell, the monolayer was scraped from the flasks, and the rickettsiae were purified using Renografin density gradient centrifugation as previously reported (19). The light and heavy bands were combined into a 10-ml suspension of sucrose-phosphate-glutamic acid medium (0.128 M sucrose, 0.0038 M KH2PO4, 0.0072 M K2HPO4, 0.0049 M monosodium l-glutamic acid). The purified rickettsiae (4 × 109 PFU/ml) were immediately used to infect 24-well plates containing confluent Vero cells for both determination of expression of selected genes by RT-PCR and quantitative electron microscopic studies. Vero cells were incubated at 37°C, harvested, and placed in either RNAlater for RT-PCR analyses or Ito's fixative for electron microscopy.
Salmonella.
Salmonella enterica serovar Typhimurium strain SB109 was kindly provided to us by Jorge Galan, Department of Cell Biology, Yale University (New Haven, CT). This Salmonella strain contains a mutation in the invE gene and has been demonstrated to be attenuated in the invasion of epithelial cells (15). A qualitative ultrastructural study performed by our laboratory indicated that this Salmonella strain does invade Vero cells, with only a small portion of the organisms escaping from phagosomal vacuoles. Furthermore, it has been documented that several Salmonella species do efficiently invade Vero cells (7). Salmonella was propagated in either imMedia Kan liquid (nontransformed Salmonella), imMedia Amp liquid (transformed Salmonella), or imMedia Amp plates (transformed Salmonella).
Primers for RT-PCR and PCR.
Primers used in PCRs were designed to amplify regions within the potential membranolytic genes from DNA and RNA isolated from R. prowazekii: tlyA forward primer 5′-TGGGATTAAGGTGAAATAGACATGATCC-3′ (nucleotides 125 to 151); tlyA reverse primer 5′-GCCAATTCTTAATTTTATCACATACCT-3′ (nucleotides 616 to 643); pat1 forward primer 5′-CTTATTCTTATTTTACTTAATTTGCCG-3′ (nucleotides 169 to 195); pat1 reverse primer 5′-TATTCCTCCACCACAACAAACAGT-3′ (nucleotides 724 to 747); tlyC forward primer 5′-ATTGAAGCTGGAAGATAAAATTGTTGAAGATA-3′ (nucleotides 207 to 223); tlyC reverse primer 5′-TAGCTTTTCACCTATTATTTCTTCAAGC-3′ (nucleotides 693 to 720); pld forward primer 5′-ATGAAGAGCAAAAATAATAAATTTA-3′ (nucleotides 1 to 25); and pld reverse primer 5′-CTAAAAATGTACTGCATTACTCGTTGTT-3′ (nucleotides 591 to 618).
PCR amplification of entire open reading frames with the upstream endogenous promoters.
A previous study by Policastro and Hackstadt indicated that the promoters for both of the rickettsial genes ompA and ompB function in E. coli, justifying the approach of using the endogenous rickettsial promoters (28). Primers used to amplify both tlyC and pld with the putative endogenous rickettsial promoters were designed based on the genomic nucleotide sequence of R. prowazekii (GenBank accession no. AJ235273): tlyC forward primer (nucleotides −163 to −135) 5′-ACTTTTGAGAATCATTTTATTCATATGT-3′ and reverse primer (nucleotides 888 to 912) 5′-CTATGTTAAATTATCACTATTCAA-3′ and pld forward primer (nucleotides −300 to −274) 5′-AGTAATGAGTGGTTTATGCAACGA-3′ and reverse primer (nucleotides 590 to 618) 5′-CTAAAAATGTACTGCATTACTCGTTGTT-3′. For PCR, 0.60 μg of purified R. prowazekii (Breinl strain) DNA was used as the template. Thermal cycling conditions for all PCRs were as follows: 1 denaturation cycle at 95°C for 5 min; 35 cycles of denaturation at 95°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 2 min; followed by 1 extension cycle at 72°C for 5 min. Following PCR, 12 μl of the reaction mixture was electrophoretically separated on a 1% agarose gel and visualized by ethidium bromide staining. Accuracy of the DNA sequences was confirmed by sequencing using an ABI automated sequencer.
Cloning of tlyC and pld into E. coli and Salmonella.
The PCR products, along with the promoters of both tlyC and pld, were cloned into the pcr2.1-TOPO vector (Invitrogen, Carlsbad, CA) and propagated in Top 10 F′ chemically competent E. coli according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Transformed E. coli organisms were plated onto imMedia Amp agar (Invitrogen, Carlsbad, CA). Positive clones were selected by blue/white screening.
Plasmids expressing pld or tlyC were isolated using the High Pure plasmid isolation kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Salmonella cells for transformation were grown overnight in imMedia Kan liquid (Invitrogen, Carlsbad, CA). Briefly, 1 ml of Salmonella organisms was mixed with at least 0.05 μg of pld plasmids, tlyC plasmids, or plasmids with no insert. Salmonellae were incubated with plasmids for 1 h and were then centrifuged for 1 min at 16,100 × g in an Eppendorf 5415D centrifuge. The pellets were resuspended in 100 μl of a 10% glycerol solution and electroporated at 1.6 kV, 200 Ω, and 25 μF for 4.0 milliseconds in 0.1-cm-gapped electroporation cuvettes (Eppendorf, Hamburg, Germany). The transformants were then grown on imMedia Amp agar plates overnight at 37°C. Salmonella clones were selected and grown overnight in imMedia Amp liquid (Invitrogen, Carlsbad, CA). RNA was isolated from 1-ml broth samples containing transformed Salmonella cells and tested for transcription of the rickettsial genes by RT-PCR.
RNA extraction and RT-PCR.
Transformed E. coli colonies or transformed Salmonella colonies were selected and grown in imMedia Amp liquid overnight at 37°C. RNA isolation was performed by using an RNeasy kit according to the manufacturer's instructions (QIAGEN, Valencia, CA). RNA from R. prowazekii-infected Vero cells fixed in RNAlater was also extracted by the RNeasy kit. RNA extractions from all samples were separated electrophoretically on a 1% agarose gel, stained with ethidium bromide, and analyzed to ensure that the RNA was not degraded. All RT-PCRs were performed with the Titanium one-step RT-PCR kit (BD Biosciences, San Jose, CA). The PCR mixtures were incubated at 50°C for 1 h and 94°C for 5 min, followed by 30 cycles of 95°C (1 min), 50°C (30 s), and 72°C (1 min), and then 1 cycle at 72°C (5 min). RT-PCRs were carried out with an RNA concentration of 10 ng/μl.
Infection assay.
Salmonella (SB109 nontransformed) or the transformants were used to infect Vero cells in a 24-well plate at a multiplicity of infection of approximately 70:1. The infected cells were incubated for 4 hours at 37°C in a 5% CO2 atmosphere.
Electron microscopy.
Vero cells infected with R. prowazekii for 30 or 50 min or with Salmonella for 4 h were harvested and immersed in Ito's fixative overnight (21). Pellets were dehydrated in ethanol, embedded in epoxy resin (Poly/Bed 812; Polysciences, Inc., Warrington, PA), and polymerized at 60°C overnight. Ultrathin sections (70 nm) were cut using a Reichert Ultracut S ultramicrotome, placed on copper grids, stained with uranyl acetate and lead citrate, and examined in a Phillips CM 100 electron microscope at 60 kV.
Quantitative electron microscopy.
Rickettsia or Salmonella organisms were identified as being either completely within vacuoles or completely within the cytosol of the host cell. Bacteria within ruptured phagosomal vacuoles (identified as being in the process of escape) were counted as intracytosolic. Electron photomicrographs of infected Vero cells were taken at low magnifications between ×2,950 and ×5,200, and after prints were made, the ultrastructural locations of intracellular bacteria were determined. The experiment was performed three times, and the material from each experiment was examined to ensure reproducibility. At least 50 bacteria were examined at each time point in each experiment. The data were calculated as the mean percentages of intravacuolar and cytosolic bacteria, and standard deviations were calculated for each time point using Microsoft Excel. The proportion of intracytosolic rickettsiae at 30 min postinfection was statistically compared to that at 50 min postinfection (χ2 test) with the program epiInfo 2002. The proportions of Salmonella organisms expressing either tlyC or pld that were within the host cell cytosol were statistically compared to numbers of Salmonella strain SB109 organisms by the χ2 test with the program epiInfo 2002. Values from both experiments were considered significantly different when P was <0.05.
RESULTS
Period of phagosomal escape.
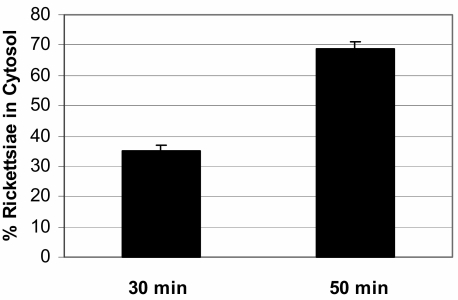
The quantitative ultrastructural examination of Vero cells infected with R. prowazekii demonstrated that at 30 min postinfection, 35% ± 2.8% of rickettsiae had escaped from vacuoles, whereas at 50 min postinfection, 69% ± 2.1% of rickettsiae had escaped into the cytosol (Fig. 1). The differences between the percentages were statistically significant (P < 0.001). These data demonstrated that between 30 and 50 min postinfection was a period of active phagosomal escape by R. prowazekii.
FIG. 1.
Proportions of R. prowazekii organisms within Vero cell cytosol at 30 and 50 min postinfection. The difference between proportions at 30 min and 50 min postinfection was statistically significant (P < 0.001).
Evaluation of the transcription of potentially membranolytic genes and cloning of transcriptionally active genes into Salmonella.
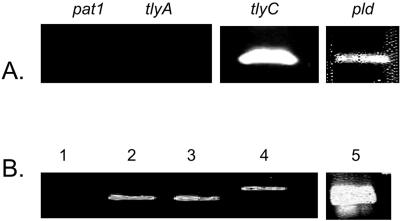
RT-PCR of RNA extracted from R. prowazekii-infected Vero cells with primers specific for pat1, tlyA, tlyC, and pld at 30 min postinfection revealed that only tlyC and pld were transcribed at 30 min postinfection and that tlyA and pat1 were not (Fig. 2). These results demonstrated that tlyC and pld, but not tlyA or pat1, were transcriptionally expressed during the period that most rickettsiae escape from phagosomal vacuoles into the host cell cytosol.
FIG. 2.
mRNA expression of four potentially membranolytic genes of Rickettsia prowazekii in Vero cell culture at 30 min postinfection. (A) RT-PCR using primers specific for tlyA, pat1, tlyC, and pld. (B) PCRs using R. prowazekii DNA demonstrated the effectiveness of the primers. Lane 1, negative control (tlyA primers with water as the template); lane 2, primers specific for tlyA; lane 3, primers specific for pat1; lane 4, primers specific for tlyC; lane 5, primers specific for pld.
Primers specific for both tlyC and pld, including the entire gene and the upstream putative promoter regions, were used to amplify both genes by PCR from the genome of R. prowazekii. The PCR products were then ligated into the pcr2.1 vector. The vectors containing each gene were then separately electroporated into S. enterica serovar Typhimurium, and transcription was confirmed by RT-PCR (Fig. 3). The presence of mRNA of each gene demonstrated that both genes were actively transcribed in the transformed Salmonella.
FIG. 3.
mRNA clonal expression of rickettsial genes in Salmonella after overnight incubation. (A) RT-PCR using primers specific for tlyC. (B) RT-PCR using primers specific for pld. Lane 1, negative control (RT-PCR using RNA extracted from SB109); lane 2, recombinant Salmonella RNA expressing tlyC (A) or pld (B); lane 3, RT-PCR of RNA with no reverse transcriptase enzyme.
Infection of Vero cells and ultrastructural analyses.
Vero cells were chosen for this study, as they are nonphagocytic and are therefore less likely to ingest dead organisms, an event that could skew the quantitative data because these bacteria would remain within host cell vacuoles. Vero cells infected with Salmonella transformants expressing either tlyC or pld were studied by electron microscopy at 4 hours postinfection (Fig. 4). Qualitative analyses revealed that while a portion of the tlyC-Salmonella transformants were observed free in the host cell cytosol, all pld-Salmonella transformants had relocated from phagosomal vacuoles to the cytosolic compartment. Nontransformed salmonellae and salmonellae with the nonrecombinant plasmid only were still predominantly located within vacuoles at 4 h postinfection. The quantitative ultrastructural analyses demonstrated that while approximately 20% ± 2.0% of the Salmonella organisms expressing tlyC escaped phagosomal vacuoles, 100% of Salmonella organisms expressing pld were present within the cytosol (Fig. 5). These findings were significantly different from each other (P < 0.001). When percentages of tlyC-expressing or pld-expressing Salmonella transformants in the cytosol were statistically compared to the percentage of nontransformed Salmonella SB109 organisms in the cytosol, they were determined to be significantly different (P < 0.001 and P < 0.0000001, respectively); 5% ± 2.1% of nontransformed salmonellae and 6% ± 2.8% of salmonellae with vector alone were present in the cytosol. The difference between the controls was not statistically significant (P = 0.08). These results demonstrated that Salmonella clones transcriptionally expressing either pld or tlyC escaped from phagosomal vacuoles and resided within the host cell cytosol.
FIG. 4.
Electron photomicrographs of Salmonella organisms and transformants. (A) Two wild-type salmonellae 4 hours postinfection. One is entering the cell (arrowhead), and the other is completely surrounded by a host cell vacuolar membrane (arrow). Bar, 0.5 μm. (B) Salmonella transformed with plasmid only located completely within a host cell vacuole. Bar, 0.5 μm. (C) Salmonella tlyC transformants at the site of the remnants of a host cell vacuole. Vacuolar membrane breaks are indicated by arrowheads. Bar, 0.5 μm. (D) Salmonella pld transformants are all located free within the cytosol of the host cell. Two organisms are identified with arrows. Bar, 1.0 μm. (E) Higher magnification of two Salmonella tlyC transformants (arrows) present within the host cell cytosol. Bar, 0.5 μm. (F) Higher magnification of Salmonella pld transformants within the host cell cytosol. Bar, 0.5 μm.
FIG. 5.
Quantification of vacuolar escape by the Salmonella transformants. SB109, nontransformed Salmonella; tlyC, Salmonella tlyC transformants; pld, Salmonella pld transformants; NI (not infected), Salmonella containing plasmid only. The percentage of intracytosolic salmonellae transcriptionally expressing tlyC or pld was significantly different from that of the nontransformed salmonellae.
DISCUSSION
The life cycle of Rickettsia spp. has been extensively studied by electron microscopy since the mid-1960s, resulting in the identification of several steps crucial to their intracellular survival and replication (2, 3, 34, 40). There are two main groups of rickettsiae, the typhus group (R. prowazekii and R. typhi), and the spotted fever group (e.g., R. conorii and R. rickettsii). Rickettsiae from both groups adhere to host cells and induce phagocytosis by unknown mechanisms (41). Rickettsial entry into host cells occurs by induced phagocytosis in nonphagocytic cells and by phagocytosis by phagocytic cells. Two outer membrane proteins have been hypothesized to function in adhesion, outer membrane protein A (OmpA; present only in the spotted fever group rickettsiae) and outer membrane protein B (OmpB; present in both the typhus and spotted fever groups) (23, 37). Rickettsia may also adhere and enter by antibody-mediated opsonization and phagocytosis dependent on the presence of the Fc receptor on macrophages and endothelium and the Fc region of the antibody (13, 14). Once within the host cell phagosomal vacuole, the microcapsular layer adjacent to the cell walls of nonopsonized rickettsiae appears to interact with the host cell phagosomal membrane. During this interaction, the vacuolar membrane becomes thicker and more osmiophilic. Eventually, large gaps appear in the host phagosomal membrane around the rickettsiae. Subsequent to this step, rickettsiae can be identified exiting the phagosomal vacuole into the host cell cytosol (13, 14, 34, 40). Once free in the cytosol, rickettsiae begin to multiply by binary fission. Spotted fever group rickettsiae polymerize actin by Arp2/3 nucleation that is dependent on the rickettsial protein RickA and use this ability to move intracellularly and to exit the cell via membrane-bound protrusions, both into adjacent cells and extracellularly (20). It has been observed that spotted fever rickettsiae lyse the tips of the cell protrusions (39). Among typhus group rickettsiae, only R. typhi polymerizes short actin tails that do not create filopodia (35). Once typhus group rickettsiae multiply to high concentrations, they lyse the host cell membrane, also by an unknown mechanism.
Recent work in our laboratory has demonstrated that monoclonal antibodies, directed against OmpA or OmpB, or polyclonal antibodies are protective in the R. conorii mouse model, and cell culture experiments have demonstrated that the likely mechanism of protection is by inhibiting phagosomal escape (13, 14). Ultrastructural and functional studies documented that rickettsiae unable to escape from the phagosomal vacuole undergo destruction by reactive oxygen species and reactive nitrogen species and by limitation of available tryptophan (12).
Phagosomal escape is a crucial mechanism of intracellular survival for many bacterial pathogens, including Shigella spp. and Listeria spp. (16). Shigella spp. utilize the proteins IpaB, IpaC, and IpaD to mediate phagosomal escape and to disseminate to other cells (26, 27). Phagosomal escape by Listeria spp. has been the most widely studied of that of any set of bacteria. Listeriolysin O, encoded by the hlyA gene, is one mechanism of phagosomal escape, as demonstrated by complementation of this activity in Bacillus subtilis (8). HlyA is hypothesized to function by causing oligomers to form pores within phagosomal vacuolar membranes, resulting in their rupture (1, 22). Other studies have suggested that two different phospholipase C enzymes may also be involved (16).
The mechanism of rickettsial phagosomal escape was originally hypothesized to be phospholipase A dependent (40, 43). But to date, no phospholipase A gene has been identified in any of the sequenced rickettsial genomes (4, 24, 25).
The most direct technique to determine rickettsial gene function would be the creation of clones of rickettsiae with the genes of interest inactivated. However, members of the genus Rickettsia have been very resistant to site-directed genetic manipulation (6, 29, 30, 36). This obstacle has required researchers to employ novel approaches to study rickettsial gene function, namely, the expression of rickettsial genes in other bacteria (31). Bacteria that lack specific rickettsial functions are chosen with the aim of complementing the function by introducing the appropriate rickettsial gene. In keeping with this strategy, we report here the transformation and expression of two R. prowazekii genes in Salmonella enterica serovar Typhimurium (SB109 strain). Only two of the four potentially membranolytic genes, tlyC and pld, were expressed in R. prowazekii during the period of rickettsial escape from the phagosome. TlyC was first characterized by Radulovic et al., who demonstrated that the transformation of a nonhemolytic bacterium (Proteus mirabilis) with tlyC resulted in a hemolytic phenotype (31). Renesto et al. expressed Pld in vitro and demonstrated it to possess phospholipase D activity by the catalysis of phosphatidyl choline, leading to the byproduct choline (32). Both TlyC and Pld are secreted and have been hypothesized to play a role in phagosomal escape by rickettsiae (31, 32). We have extended the knowledge of these genes by transcriptionally expressing them in Salmonella, resulting in transformants that escape phagosomal vacuoles by 4 h postinfection. TlyC probably plays a less important role in phagosomal escape, as suggested by our studies, and Pld is likely to be the major effector of rickettsial phagosomal escape (Fig. 5). The possibility that two rickettsial genes can carry out the crucial function of phagosomal escape suggests that redundancy and possibly synergy may exist in the performance of this specific function.
The presence of orthologs of tlyC in R. typhi (RT0725), R. conorii (RC1141), and Rickettsia rickettsii (Rick02001360) and of pld orthologs in R. typhi (RT0807), R. conorii (RC1270), and R. rickettsii (Rick02001502) indicates that antibodies or drugs targeting these proteins could be protective against other rickettsial species.
The functions of tlyA and pat1 remain to be determined. Although these genes are not transcriptionally expressed early in the rickettsial life cycle, the possibility that they play a role in phagosomal escape cannot be excluded as it is possible that they could have been produced prior to rickettsial entry and stored until needed. Future experiments will be designed to test this hypothesis. Furthermore, the potential later expression of tlyA and pat1 genes should be evaluated, as they may perform other membranolytic functions crucial to rickettsial survival, e.g., filipodial escape or host cell lysis. While tlyC and pld clearly confer the ability to escape vacuoles onto the transformed Salmonella, further studies must be performed in order to confirm that the proteins expressed by these genes possess the same function in the rickettsiae themselves.
These findings offer the opportunity to identify compounds that interrupt the functions of TlyC or Pld. We believe that the development of drugs or antibodies that inhibit the function of TlyC or Pld could provide a novel therapeutic approach to treat infection with R. prowazekii which may have been engineered or selected to be resistant to tetracyline and chloramphenicol, as would potentially occur in a bioterrorist attack (42).
Acknowledgments
We thank Julie Wen and Violet Han for their expert assistance in electron microscopy. We also thank Alfredo Torres for his helpful insight.
This work was supported by the National Institutes of Health grants U54 A1057156 (to D.H.B.), RO1 AI21242 (to D.H.W.), and the T32 training grant 418151 (to T.W.) in emerging infectious diseases.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alouf, J. E. 2000. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 290:351-356. [DOI] [PubMed] [Google Scholar]
- 2.Anacker, R. L., E. G. Pickens, and D. B. Lackman. 1967. Details of the ultrastructure of Rickettsia prowazekii grown in the chick yolk sac. J. Bacteriol. 94:260-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, D. R., H. E. Hopps, M. F. Barile, and B. C. Bernheim. 1965. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J. Bacteriol. 90:1387-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 5.Azad, A. F., and S. Radulovic. 2003. Pathogenic rickettsiae as bioterrorism agents. Ann. N. Y. Acad. Sci. 990:734-738. [DOI] [PubMed] [Google Scholar]
- 6.Baldridge, G. D., N. Burkhardt, M. J. Herron, T. J. Kurtti, and U. G. Munderloh. 2005. Analysis of fluorescent protein expression in transformants of Rickettsia monacensis, an obligate intracellular tick symbiont. Appl. Environ. Microbiol. 71:2095-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrow, P. A., and M. A. Lovell. 1989. Invasion of Vero cells by Salmonella species. J. Med. Microbiol. 28:59-67. [DOI] [PubMed] [Google Scholar]
- 8.Bielecki, J., P. Youngman, P. Connelly, and D. A. Portnoy. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175-176. [DOI] [PubMed] [Google Scholar]
- 9.Bozeman, F. M., D. E. Sonenshine, M. S. Williams, D. P. Chadwick, D. M. Lauer, and B. L. Elisberg. 1981. Experimental infection of ectoparasitic arthropods with Rickettsia prowazekii (GvF-16 strain) and transmission to flying squirrels. Am. J. Trop. Med. Hyg. 30:253-263. [DOI] [PubMed] [Google Scholar]
- 10.Catron, D. M., M. D. Sylvester, Y. Lange, M. Kadekoppala, B. D. Jones, D. M. Monack, S. Falkow, and K. Haldar. 2002. The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cell. Microbiol. 4:315-328. [DOI] [PubMed] [Google Scholar]
- 11.Duma, R. J., D. E. Sonenshine, F. M. Bozeman, J. M. Veazey, B. L. Elisberg, D. P. Chadwick, N. I. Stocks, T. M. McGill, G. B. Miller, and J. N. MacCormack. 1981. Epidemic typhus in the United States associated with flying squirrels. JAMA 245:2318-2323. [PubMed] [Google Scholar]
- 12.Feng, H.-M., and D. H. Walker. 2000. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 68:6729-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, H.-M., T. Whitworth, J. P. Olano, V. L. Popov, and D. H. Walker. 2004. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 72:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, H.-M., T. Whitworth, V. Popov, and D. H. Walker. 2004. Effect of antibody on the rickettsia-host cell interaction. Infect. Immun. 72:3524-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginocchio, C., J. Pace, and J. E. Galán. 1992. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc. Natl. Acad. Sci. USA 89:5976-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goebel, W., and M. Kuhn. 2000. Bacterial replication in the host cell cytosol. Curr. Opin. Microbiol. 3:49-53. [DOI] [PubMed] [Google Scholar]
- 17.Hackstadt, T. 1996. The biology of rickettsiae. Infect. Agents Dis. 5:127-143. [PubMed] [Google Scholar]
- 18.Halle, S., and G. A. Dasch. 1980. Use of a sensitive microplate enzyme-linked immunosorbent assay in a retrospective serological analysis of a laboratory population at risk to infection with typhus group rickettsiae. J. Clin. Microbiol. 12:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson, B. A., C. L. Wisseman, Jr., A. Waddell, and D. J. Silverman. 1981. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect. Immun. 34:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, S., and Y. Rikihisa. 1981. Techniques for electron microscopy of rickettsiae, p. 213-227. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 22.Lety, M. A., C. Frehel, P. Berche, and A. Charbit. 2002. Critical role of the N-terminal residues of listeriolysin O in phagosomal escape and virulence of Listeria monocytogenes. Mol. Microbiol. 46:367-379. [DOI] [PubMed] [Google Scholar]
- 23.Li, H., and D. H. Walker. 1998. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24:289-298. [DOI] [PubMed] [Google Scholar]
- 24.McLeod, M. P., X. Qin, S. E. Karpathy, J. Gioia, S. K. Highlander, G. E. Fox, T. Z. McNeill, H. Jiang, D. Muzny, L. S. Jacob, A. C. Hawes, E. Sodergren, R. Gill, J. Hume, M. Morgan, G. Fan, A. G. Amin, R. A. Gibbs, C. Hong, X.-J. Yu, D. H. Walker, and G. M. Weinstock. 2004. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J. Bacteriol. 186:5842-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogata, H., S. Audic, P. Renesto-Audiffren, P. E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J. M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 26.Page, A. L., H. Ohayon, P. J. Sansonetti, and C. Parsot. 1999. The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell. Microbiol. 1:183-193. [DOI] [PubMed] [Google Scholar]
- 27.Picking, W. L., H. Nishioka, P. D. Hearn, M. A. Baxter, A. T. Harrington, A. Blocker, and W. D. Picking. 2005. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect. Immun. 73:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Policastro, P. F., and T. Hackstadt. 1994. Differential activity of Rickettsia rickettsii ompA and ompB promoter regions in a heterologous reporter gene system. Microbiology 140:2941-2949. [DOI] [PubMed] [Google Scholar]
- 29.Qin, A., A. M. Tucker, A. Hines, and D. O. Wood. 2004. Transposon mutagenesis of the obligate intracellular pathogen Rickettsia prowazekii. Appl. Environ. Microbiol. 70:2816-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rachek, L. I., A. M. Tucker, H. H. Winkler, and D. O. Wood. 1998. Transformation of Rickettsia prowazekii to rifampin resistance. J. Bacteriol. 180:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radulovic, S., J. M. Troyer, M. S. Beier, A. O. T. Lau, and A. F. Azad. 1999. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect. Immun. 67:6104-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renesto, P., P. Dehoux, E. Gouin, L. Touqui, P. Cossart, and D. Raoult. 2003. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J. Infect. Dis. 188:1276-1283. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi, A. 1967. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109-136. [PMC free article] [PubMed] [Google Scholar]
- 34.Teysseire, N., J.-A. Boudier, and D. Raoult. 1995. Rickettsia conorii entry into Vero cells. Infect. Immun. 63:366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teysseire, N., C. Chiche-Portiche, and D. Raoult. 1992. Intracellular movements of Rickettsia conorii and R. typhi based on actin polymerization. Res. Microbiol. 143:821-829. [DOI] [PubMed] [Google Scholar]
- 36.Troyer, J. M., S. Radulovic, and A. F. Azad. 1999. Green fluorescent protein as a marker in Rickettsia typhi transformation. Infect. Immun. 67:3308-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchiyama, T. 2003. Adherence to and invasion of Vero cells by recombinant Escherichia coli expressing the outer membrane protein rOmpB of Rickettsia japonica. Ann. N. Y. Acad. Sci. 990:585-590. [DOI] [PubMed] [Google Scholar]
- 38.Walker, D. H. 2003. Principles of the malicious use of infectious agents to cause terror: reasons for concern for organisms of the genus Rickettsia. Ann. N. Y. Acad. Sci. 990:739-742. [DOI] [PubMed] [Google Scholar]
- 39.Walker, D. H., and B. G. Cain. 1980. The rickettsial plaque. Evidence for direct cytopathic effect of Rickettsia rickettsii. Lab. Investig. 43:388-396. [PubMed] [Google Scholar]
- 40.Walker, D. H., H.-M. Feng, and V. L. Popov. 2001. Rickettsial phospholipase A2 as a pathogenic mechanism in a model of cell injury by typhus and spotted fever group rickettsiae. Am. J. Trop. Med. Hyg. 65:936-942. [DOI] [PubMed] [Google Scholar]
- 41.Walker, T. S., and H. H. Winkler. 1978. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki [sic]. Infect. Immun. 22:200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss, E., and H. R. Dressler. 1962. Increased resistance to chloramphenicol in Rickettsia prowazekii with a note on failure to demonstrate genetic interaction among strains. J. Bacteriol. 83:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler, H. H., and E. T. Miller. 1982. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells). Infect. Immun. 38:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]