Abstract
Declines in skeletal muscle and cognitive function in older adults have been linked to abnormalities in abdominal subcutaneous adipose tissue (ASAT), yet the underlying molecular mediators remain poorly understood. Here, leveraging ASAT transcriptomics and explant-conditioned media proteomics from participants in the Study of Muscle, Mobility and Aging (SOMMA; age ≥70 years, n = 229), we identified ASAT gene clusters and secreted proteins strongly associated with comprehensive assessments of physical and cognitive function in older adults. ASAT inflammation and secreted immunoglobulins were identified as key signatures of aging-associated physical and cognitive performance limitations. Systems genetics analysis confirmed secreted-SERPINF1 as a negative regulator of skeletal muscle contraction and highlighted its potential role in inducing inflammation in the heart in silico. Additionally, novel ASAT-secreted proteins such as NID2 and APOA4 were implicated in mediating ASAT crosstalk with skeletal muscle and brain in silico. Our framework provides insights into ASAT-driven tissue crosstalk underlying physical and cognitive performance in older adults and offers a valuable resource for understanding the role of ASAT in human aging.
Keywords: Aging, Physical function, Cognitive function, Subcutaneous adipose tissue, Secretome, Tissue crosstalk
Highlights
-
•
ASAT inflammation and immunoglobulin secretion are strongly linked to reduced physical and cognitive function in older adults.
-
•
Novel ASAT-secreted proteins were identified to be significantly associated with physical and cognitive performance.
-
•
Systems genetics analysis validated endocrine roles of ASAT-secreted proteins in skeletal muscle, heart, and brain.
1. Introduction
Declining muscle mass, strength, gait speed, cardiorespiratory fitness, and cognitive function are typical with age and can lead to disability and death [[1], [2], [3]]. While significant efforts have focused on uncovering the molecular drivers of aging-related changes within skeletal muscle [4,5], brain [6,7], and the muscle–brain axis [8,9], growing evidence indicates that adipose tissue abnormalities may also contribute to aging-related functional declines [10,11]. Excessive mobilization of fatty acids and the secretion of pro-inflammatory factors from adipose tissue—common in aging adults—can lead to lipotoxicity and inflammation in skeletal muscle [12], contributing to metabolic impairments and functional declines in physical function [13]. Chronic low-grade inflammation, largely attributed to adipose tissue, is associated with cognitive decline [14], and some adipose tissue-derived factors can directly regulate cognitive function [11]. Senescent cells (cells that have reached irreversible cell fate but have not undergone programmed cell death) accumulate in adipose tissue during aging and are associated with physical and cognitive impairments [15,16]. Therefore, understanding the biology of adipose tissue in aging and identifying molecular mediators that link adipose tissue changes to physical and cognitive impairments can help develop novel strategies to address age-related functional declines.
Beyond inflammatory cytokines, adipose tissue secretes a variety of proteins, collectively referred to as ‘adipokines,’ which can exert significant biological effects on neighboring and distant organs [17]. Visceral adipose tissue (VAT) has garnered much attention due to its tendency to increase with age and its higher protein secretory capacity per unit mass compared with subcutaneous adipose tissue (SAT) [18,19]; however, SAT likely serves as a major contributor to adipose tissue-mediated crosstalk because SAT is more susceptible to immune cell infiltrations and is the sentinel site of cellular senescence [20,21]. SAT also comprises the majority of total adipose tissue mass regardless of age [18,22]. Among SAT depots, the relative proportion of abdominal subcutaneous adipose tissue (ASAT) increases during aging [23], suggesting ASAT could be a significant contributor to adipose tissue-derived systemic effects. Therefore, profiling the proteins secreted by ASAT is a first step in understanding its direct contributions to physical and cognitive performance in aging.
The primary aim of this study was to characterize and interrogate the potential endocrine roles of ASAT-secreted proteins in physical and cognitive functions in older adults. To achieve this, we performed multi-omic profiling, including in vivo transcriptomics and ex vivo conditioned media (CM) proteomics in ASAT collected from participants in the Study of Muscle, Mobility and Aging – Adipose Tissue (SOMMA-AT) [24], who underwent comprehensive assessments of physical (i.e., walking and stair climbing speeds, leg power) and cognitive (i.e., executive function, and processing speed, memory, contrast sensitivity) performance. Using systems genetics analysis, we identified the expected and novel endocrine roles of selected secreted proteins in secondary target tissues associated with physical and cognitive function in silico. This workflow integrates ASAT-secreted protein profiling with the investigation of their systemic roles, providing a framework to better understand the link between aging-related adipose tissue abnormalities and physical and cognitive function in older adults thus enhancing the translational potential of clinical observations.
2. Results
2.1. Subject characteristics
Out of the 351 participants who were assessed for eligibility in SOMMA-AT [24], 241 ASAT biopsy samples were acquired (Figure 1A) and 229 samples were subsequently analyzed with at least one biochemical assay (i.e., bulk RNAseq, n = 192 or CM measurements (ELISA, n = 168; proteomics, n = 61)) (Figure 1B). Participants were 76 ± 4 years old and 52.4% (n = 120) were females (Table 1). Average BMI was 28.1 ± 4.3 kg/m2, and participants with higher ASAT volume had higher ectopic fat mass (i.e., visceral adipose tissue (VAT), liver fat, thigh fat) and lower muscle mass and aerobic fitness (i.e., VO2peak) (Table 1). Significant intercorrelations among anthropometric and functional traits were identified (Figure 1C). For example, adiposity measurements such as BMI, waist circumference (WC), ASAT volume, VAT volume, liver fat %, and thigh fat % exhibited high positive intercorrelations (adjusted p < 0.001) (Figure 1C). Similarly, muscle mass, walking speed, stair climbing, leg power, and aerobic fitness were significantly positively intercorrelated (all adjusted p < 0.001). While strong associations between body composition and physical function were observed in both females and males, the relationship between body composition and cognitive function was far less evident (Fig. S1). In males, significant intercorrelations were detected among several cognitive measures—including processing speed, memory, and contrast sensitivity (a visual processing metric known to be linked to cognitive ability [25]) — but these relationships were not apparent in females (Fig. S1). Notably, aerobic fitness (ml/kg/min) was significantly associated with cognitive performance in males (all adjusted p < 0.05), whereas this link was absent in females (Fig. S1). Age was negatively correlated with physical and cognitive function measurements, consistent with the age-associated decline in physical and brain function [26,27].
Figure 1.
Study design and inter-clinical trait associations.
A) Flow chart of subject recruitment, sample collection, and sample analysis. B) Venn diagram showing the distribution of sample analyses. C). Inter-clinical trait correlation of enrolled participants (n = 229). MSO, Management service organization; MD, Medical doctor; ASAT, Abdominal subcutaneous adipose tissue; ELISA, Enzyme-Linked Immunosorbent Assay; LC/MS, Liquid Chromatography Mass Spectrometry. ∗adjusted p < 0.05, ∗∗adjusted p < 0.01, ∗∗∗adjusted p < 0.001.
Table 1.
Baseline anthropometric characteristics of study participants.
| Overall |
Tertile 1 |
Tertile 2 |
Tertile 3 |
||
|---|---|---|---|---|---|
| (N = 229) | (N = 74) | (N = 74) | (N = 74) | P-value (ANOVA) | |
| Age, y | 76.1 (4.4) | 76.7 (4.9) | 76.1 (4.1) | 75.2 (3.8) | 0.109 |
| Sex | 1.88E-08 | ||||
| F | 120 (52.4%) | 22 (29.7%) | 38 (51.4%) | 57 (77.0%) | |
| M | 109 (47.6%) | 52 (70.3%) | 36 (48.6%) | 17 (23.0%) | |
| Weight, kg | 78.2 (14.7) | 69.7 (11.9) | 78.0 (12.4) | 86.0 (14.7) | 7.57E-12 |
| BMI, kg/m2 | 28.1 (4.3) | 24.5 (2.8) | 27.9 (2.8) | 31.8 (3.5) | <2e-16 |
| Waist circumference, cm | 95.9 (13.3) | 88.0 (11.5) | 96.4 (12.4) | 102.4 (11.5) | 6.96E-11 |
| ASAT, L | 7.84 (3.06) | 4.62 (0.99) | 7.56 (0.78) | 11.35 (1.91) | <2e-16 |
| VAT, L | 4.57 (2.21) | 3.71 (1.96) | 4.74 (2.19) | 5.24 (2.21) | 7.20E-05 |
| Liver fat, % | 4.60 (4.23) | 3.38 (2.83) | 4.60 (3.78) | 6.78 (5.78) | 0.00387 |
| Thigh muscle fat infiltration, % | 7.32 (2.12) | 6.65 (2.24) | 7.12 (1.78) | 8.20 (2.02) | 1.87E-05 |
| D3CR muscle Mass, kg/weight | 29.2 (6.9) | 33.9 (6.9) | 28.5 (4.7) | 25.6 (6.1) | 4.10E-14 |
| VO2peak, L/min | 1.58 (0.44) | 1.68 (0.49) | 1.51 (0.39) | 1.55 (0.42) | 5.50E-02 |
| VO2peak, ml/kg/min | 20.3 (4.8) | 23.9 (5.3) | 19.4 (3.7) | 17.8 (2.9) | 2.34E-16 |
Data given as mean (SD) except sex; n (%). P-values reflect significance for linear trend following one-way ANOVA for differences among ASAT tertiles. Tertile 1, 2.18–6.09 L ASAT; Tertile 2, 6.16–8.97 L ASAT; Tertile 3, 9.01–18.2 L ASAT. SD, Standard Deviation; BMI, Body Mass Index; ASAT, Abdominal Subcutaneous Adipose Tissue; VAT; Visceral Adipose Tissue; MFI, Muscle Fat Infiltration. Subjects without SAT measurements (n = 7) are included in the total, but not in tertiles.
2.2. Association of ASAT transcripts with clinical traits
To investigate how ASAT biology relates to anthropometric and functional traits at the transcriptional level, we conducted bulk RNAseq on whole frozen ASAT samples (n = 192) and correlated gene expressions with clinical traits. Among all traits, walking speed – a key functional outcome trait for mobility disability in SOMMA [28] showed the greatest number of significantly correlated ASAT genes, followed by stair climbing (p < 0.05, Figure 2A). Overrepresentation analysis (ORA) from significantly associated transcripts with walking speed revealed upregulation of pathways related to protein quality control (e.g., ‘multivesicular body assembly’, ‘proteasome complex’, ‘response to unfolded protein’) and downregulation of ion transportation and detection of stimulus (Fig. S2A). Notably, few overlapping transcripts were observed across traits, indicating distinct gene–trait associations (Figure 2A). To further characterize gene networks associated with clinical traits, we conducted Weighted Gene Co-expression Network Analysis (WGCNA) and identified nine distinct modules (modules 1–9, see details in the Methods), each composed of co-expressed genes (Figure 2B). Correlation between module eigengene (ME, the first principal component of the module representing overall gene expression) and clinical traits revealed unique associations between gene clusters and clinical traits (Figure 2B, S2C). Module 9 was strongly linked with greater adiposity and poorer physical performance across sexes, and was inversely associated with executive function and processing speed in females (Fig. S2C). Hub genes in module 9 were enriched for immune-related genes (e.g., JAML, LAT2, RIPOR2, FGR, SPN, LILRB2) (Figure 2C), with enrichment analysis highlighting immune activation pathways (Figure 2B). Cell-type analysis, using adipose tissue cell markers from Emont et al., 2022 [29], further supported these findings, showing that module 9 was predominantly composed of immune cell marker genes compared with non-immune cells (Figure 2D). Given the established link between inflammation and cellular senescence [30], we also examined whether module 9 membership genes may be associated with cellular senescence. An integrated list of senescence genes (n = 1369 genes) was curated by combining four different databases: CellAge [31], SenMayo [32], AgingAtlas [33], and GO:0090398 (cellular senescence). Module 9 showed the greatest overlap with the senescence genes, suggesting that cellular senescence may also have a role in poorer physical and cognitive functions (Figure 2E). In males, module 3 was associated with liver fat and walking speed—associations absent in females (Fig. S2C). Module 3 hub genes included transcripts involved in cell growth (e.g., ACTN4, PDAP1, APCDD1) and metabolism (e.g., LIPE, FURIN, FTH1), with pathway enrichment in structural (i.e., actin) assembly, protein metabolism, apical junction, and p53 signaling (Figure 2C, S2B). Principal component analysis of gene–trait correlations revealed that ASAT gene–liver fat associations in post-menopausal females clustered with other fat depots (ASAT, VAT, thigh), a pattern not observed in males, suggesting potentially altered ASAT–liver crosstalk in men (Fig. S2D).
Figure 2.
ASAT transcript clusters are associated with skeletal muscle and cognitive functions.
A) UpSet plot showing intersecting ASAT genes significantly correlated with clinical traits. Node represents the number of genes that are associated with the given trait (p < 0.05). Edge represents an intersection. B) The left heatmap represents the top two overrepresented Gene Ontology terms for each module. ∗FDR<0.05. The middle heatmap represents the correlation between clinical traits and module eigengenes. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. The right bar plots represent the module size (i.e., number of genes per module). C) Top 20 hub genes for modules 3 and 9. Hub genes indicate module member genes with the highest intermodular connectivity, significantly contributing to the module eigengene. Edges indicate a significant correlation between member genes. D) Distribution of Module 9 member genes across different adipose tissue cell types. A total of 90 genes were mapped using cell-type marker genes from Emont et al., 2022. Genes were allowed to overlap across multiple cell types. E) An integrated senescence gene list (1369 genes) was curated by combining four different datasets. Then, the percentage of senescence genes per module was calculated. ME, Module eigengene; FDR, False Discovery Rate. Sample N = 192.
2.3. Association of ASAT secreted proteins with clinical traits
To characterize ASAT-secreted protein profiles from older adults, we measured the abundance of well-known adipokines – adiponectin and leptin – via targeted immunoassay (ELISA) from ASAT explant conditioned media (CM) in a subset of participants (n = 168) (Figure 3A). Adiponectin is typically inversely associated with adiposity and has insulin-sensitizing and anti-inflammatory effects on tissues such as the liver and muscle [34,35]. In contrast, leptin regulates satiety, is strongly correlated with adiposity, and its excess is often associated with adverse cardiometabolic health outcomes [36]. The abundance of secreted adiponectin was negatively correlated with adiposity measures (i.e., WC and BMI) and ectopic fat (i.e., VAT and liver fat) and positively correlated with walking speed and executive function (all adjusted p < 0.05) (Figure 3B,C). In contrast, the abundance of secreted leptin was greater in females and was associated with higher ASAT volume and lower muscle mass (Figure 3B). Leptin was also strongly correlated with worse walking speed, stair climbing, leg power, and aerobic fitness (all adjusted p < 0.05) (Figure 3C).
Figure 3.
ASAT-secreted proteins are associated with skeletal muscle and cognitive functions.
A) Schematics of ASAT CM collection and secretome measurements. Correlation matrix between ELISA-measured adipokines (adiponectin and leptin) and B) anthropometric and C) functional traits. Sample N = 168. D) Upset plots depicting intersections of anthropometric/functional traits on significantly associated secreted proteins measured by proteomics (p < 0.05). Bars corresponding to black dots represent the count of significantly associated proteins. Connecting lines represent the intersection of significantly associated proteins among traits. Sample N = 61. Correlation matrix between proteomics-measured secreted proteins and E) anthropometric or F) functional traits. ‘Non-marker’ represents transcripts that were not identified as cell-type specific markers (i.e., |log2FC|<1). Top 5 secreted proteins are shown for each trait if there were more than 5 significantly associated secreted proteins. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. Black outline indicates Benjamini-Hochberg adjusted p < 0.05. Sample N = 61. CM, Conditioned media; Bicor, Biweight midcorrelation coefficient.
To more globally understand the impact of ASAT-secreted proteins on clinical traits, we conducted untargeted global proteomics on ASAT-CM in 61 participants (Figure 3A). A total of 2366 proteins were identified, with 1276 proteins expressed in at least 25% of the samples (Figure 3A). Secreted proteins were annotated using the secreted protein database from The Human Protein Atlas (Supporting information 1), resulting in the retention of 275 ‘secreted proteins’ (Figure 3A). Final secreted proteins were also mapped for cell types and senescence using the marker gene lists used in Figure 2.
BMI and ASAT volume were the top 2 anthropometric traits that were mostly associated with the abundance of secreted proteins, intersecting with other traits (Figure 3D), suggesting a tight link between adiposity and ASAT-secreted proteins. For example, the secreted abundance of SERPINF1 (also known as pigment epithelium-derived factor (PEDF)), a marker for adipose stem and progenitor cells (ASPC) and known for its inhibitory effect on angiogenesis [37], was associated with higher BMI and ASAT volume (adjusted p < 0.05) (Figure 3E). Surprisingly, some secreted proteins more well-known to be produced from the liver, such as PON1, a high-density lipoprotein (HDL)-associated enzyme that contributes to anti-inflammatory effects in endothelial cells [38], and GC, a vitamin D-binding protein, were detected from ASAT-CM, and their abundance was also significantly correlated with higher ASAT volume (Figure 3E). The abundance of secreted DPT, also a marker for ASPC and an adipokine that can induce fibrosis and inflammation in the liver and VAT [39,40], was strongly associated with higher liver fat (bicor = 0.79, adjusted p < 0.05) and also with VAT (p = 0.019) (Figure 3E). Many of the secreted proteins associated with anthropometric traits were markers for ASPCs and macrophages (Figure 3E).
We then examined how ASAT-secreted proteins may relate to functional traits in older adults. Poorer stair climbing was associated with numerous secreted proteins, including complement proteins (e.g., C5, C9, and C4BP4), SERPINF1, A2M, A1BG, CLU, VTN, and PROS1 (all adjusted p < 0.05) (Figure 3F). Many of these secreted proteins were also negatively associated with walking speed and leg power (p < 0.05) (Figure 3F), suggesting a link between these secreted proteins and an overall decline in physical function. For instance, VTN, a multifunctional glycoprotein involved in cell adhesion and tissue remodeling, has reduced gene expression in murine skeletal muscle during post-injury regeneration [41]. Conversely, increased VTN expression has been observed in bioengineered human skeletal muscle models for muscular dystrophy [42], supporting the inverse relationship between VTN and physical function. In addition to the positive correlation with walking speed, lipoprotein lipase (LPL) was associated with better memory performance and contrast sensitivity (both p < 0.05) (Figure 3F), aligning with previous findings that linked circulating [43,44] and brain-specific [45] LPL expression with cognitive function. Among the secreted proteins that were positively associated with executive function (p < 0.05, Figure 3F), APOA4, LUM, and CLU are reported to be expressed in the brain [46,47], and lower circulating abundance of APOA4 and CLU in circulation has been observed in individuals with mild cognitive impairment and Alzheimer's Disease [48,49]. Some secreted proteins were associated with poorer physical function but better executive function (e.g., C1S, CLU, A1BG) or memory (e.g., C5), indicating a possibility of the multifaceted role of ASAT-secreted proteins (p < 0.05, Figure 3F). We also examined the relationships between CM proteins and clinical traits highlighted in the sex-adjusted correlation analysis and found that these significant associations were largely retained (Fig. S3). Together, our characterization of ASAT-secreted proteins derived from ex vivo conditioned media provides new insights into the relationship between ASAT and physical and cognitive function in older adults.
2.4. Integration of WGCNA and secreted proteins reveals immunoglobulins as a signature of aging-related functional declines
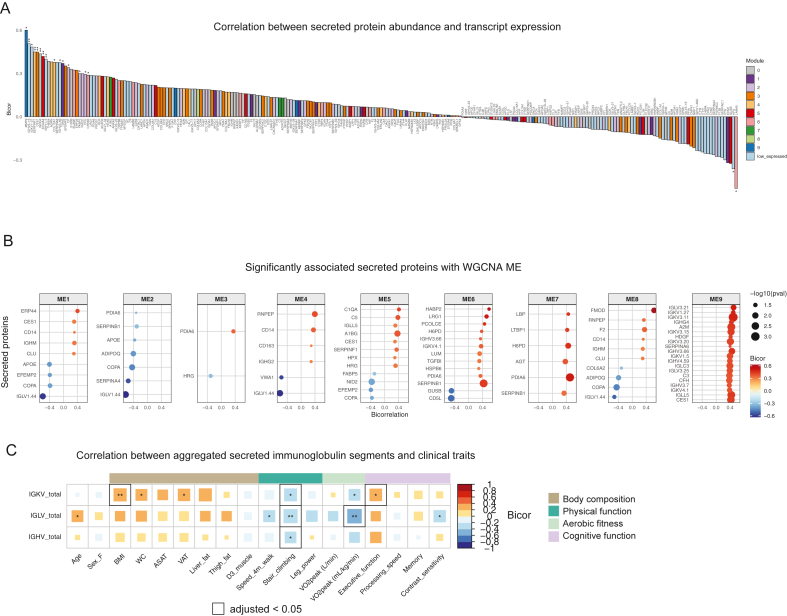
To explore the relationship between ASAT transcripts and secreted proteins, we correlated the expression of 254 genes (matching genes for secreted proteins) with the abundance of secreted proteins and overlaid their module memberships (Figure 4A, S4A). MMP9, a member gene for module 9 and mapped as a marker for cellular senescence from the curated senescence list (Figure 3E), exhibited the highest correlation (bicor = 0.6, p < 0.05) between gene expression and protein abundance (Figure 4A). The abundance of secreted MMP9 was associated with age and slower walking speed (Figure 3E,F). We also found several other significant associations between ASAT gene expression and secreted protein abundance, such as SERPINF1, IGKV1-5, and EFEMP2 (all p < 0.05, Figure 4A). The associations between transcript expression and secreted protein abundance of HPX and LAMA5 exhibited significant inverse relationships, implying complex post-transcriptional/translational modifications (both p < 0.05, Figure 4A). We further analyzed correlations between the abundance of secreted proteins and MEs to understand relationships between gene clusters and protein secretion. Notably, the secreted abundance of numerous variable regions of immunoglobulins (e.g., IGLVs, IGKVs, IGHVs), primarily produced by plasmacytes, were significantly associated with ME9 (Figure 4B), suggesting that excessive immunoglobulin production from ASAT may be linked to poorer physical and cognitive functions. The overall correlation trends were similar across aggregated variable regions (IGKV, IGLV, and IGHV); however, differences in significance were observed (Figure 4C). Notably, IGKV abundance was significantly associated with higher adiposity and better executive function (Figure 4C). While all variable regions correlated with poorer stair climbing, IGLV abundance was also significantly correlated with slower walking speed, lower aerobic fitness, and poorer contrast sensitivity (Figure 4C). Correlation analysis of aggregated IGLV abundance and ASAT genes revealed both positive and negative associations (p < 0.05) (Fig. S4B), with enriched pathways in positively associated genes indicating immune/inflammatory upregulation, while pathways in negatively associated genes suggested organic acid and lipid metabolic processes (FDR<0.05, Fig. S4C).
Figure 4.
Integration of ASAT transcripts and secreted proteins.
A) Correlation between secreted protein abundances and their protein-coding transcript expressions. ‘Low expressed’ refers to genes that were excluded before WGCNA analysis due to lower expression. ∗p < 0.05, ∗∗p < 0.01. B) Secreted proteins that are significantly associated with module eigengenes. (p < 0.05). Top 20 secreted proteins are shown. C) Correlation matrix between mean abundance of immunoglobulin chains and clinical traits. ∗p < 0.05, ∗∗p < 0.01. The black outline indicates Benjamini-Hochberg adjusted p < 0.05. Sample N = 48.
2.5. Systems genetics analysis suggests potential ASAT-mediated endocrine effects on multiple tissues
Discovering inter-organ signaling networks through gene–gene correlations across tissues has become increasingly useful in tissue crosstalk research due to its robustness and versatility [[50], [51], [52], [53], [54]]. Gene-derived correlations across tissues (GD-CAT) [55] is a platform we developed that leverages transcriptome data from 135 “Older” (age:60–79) and 175 “Younger” (age:20–59) participants in the post-mortem GTEx cohorts [56] to identify candidates of target tissues for a given gene of origin tissue and further examine enriched biological pathways in the target tissues that may explain the action of crosstalk (Figure 5A). Leveraging the relationship between ASAT genes/secreted proteins and our clinical traits, we highlight the potential endocrine effects of selected ASAT-secreted proteins on secondary target tissues in silico.
Figure 5.
Validation of endocrine effects of ASAT-secreted proteins via systems genetics.
A) Workflow of GD-CAT analysis. B) Pie chart and bar plots show significantly associated genes (adjusted p < 0.001) in all tissues with ASAT-derived protein candidates using GTEx cohorts (“Younger”, 20–59 years; “Older”, 60–79 years). Enrichment plot shows overrepresented pathways in target tissues from genes significantly associated with ASAT-derived protein candidates using GTEx cohorts. B) Pan tissue correlation with SAT-SERPINF1. C) Enrichment plots from target tissue genes (heart, skeletal muscle) that correlated with SAT-SERPINF1. D) Pan tissue correlation with SAT-NID2. D) Enrichment plots from target tissue genes (VAT, skeletal muscle) that correlated with SAT-NID2. F) Pan tissue correlation with SAT-APOA4. C) Enrichment plots from target tissue genes (pituitary) that correlated with SAT-APOA4. Gene ontology databases (Biological process, BP; cellular component, CC; molecular function, MF) were used for enrichment analysis. GD-CAT, Gene-Derived Correlations Across Tissues; GTEx, The Genotype-Tissue Expression; SAT, Subcutaneous Adipose Tissue; VAT, Visceral Adipose Tissue; NES, Normalized Enrichment Score.
SERPINF1 is linked to adiposity and adverse metabolic health [57,58]. SERPINF1 gene expression strongly correlated with its secreted protein abundance (p < 0.05, Figure 4A), and SERPINF1 protein abundance was negatively associated with walking speed, stair climbing, leg power, and aerobic fitness (all p < 0.05, Figure 3F). Additionally, by using BioGPS, a panel that provides multi-tissue expression of genes [59,60], we found that subcutaneous adipose tissue (SAT) is a primary secretory tissue for SERPINF1 (Fig. S5). In the Older GTEx cohort, SAT-SERPINF1 correlated most strongly with genes in the heart and skeletal muscle, while its correlations were weaker in the Younger GTEx cohort (Figure 5B). Enrichment analysis of correlating genes in the heart with SAT-SERPINF1 suggested upregulated cytokine production and inflammatory responses in both Older and Younger GTEx cohorts, while mitochondrial translation was downregulated only in the Older GTEx cohort (Figure 5C). Moreover, enrichment analysis of genes in skeletal muscle correlating with SAT-SERPINF1 revealed a pronounced downregulation of muscle contractility components, accompanied by upregulation of inflammatory pathways and catabolic processes in both age groups (Figure 5C).
We also inspected NID2, which encodes cell adhesion protein Nidogen2, given its positive associations with muscle mass and walking speed (Figure 3E,F). Secreted abundance of NID2 was also negatively associated with module 5, which is a module that is negatively associated with walking speed and aerobic fitness in both females and males (p < 0.05, Figure 2B). Abundantly expressed in SAT (Fig. S5), GD-CAT analysis identified VAT and skeletal muscle as the top secondary tissues associated with SAT-NID2 in both age groups (Figure 5D). Enrichment analysis of genes in VAT correlating with SAT-NID2 revealed substantial downregulation of inflammatory responses, lipid metabolism, and ribosome biogenesis (Figure 5E). In skeletal muscle, SAT-NID2 was associated with enhanced muscle contraction- and component-related terms, while protein catabolism, ER stress, autophagy, and ribosome biogenesis were downregulated (Figure 5E).
Although SAT is not a primary origin for APOA4 (Fig. S5), the secreted abundance of APOA4 displayed positive associations with executive function and memory performance (p < 0.05, Figure 3F), suggesting a link between ASAT and cognitive function. GD-CAT revealed distinct secondary tissue gene correlations between Older vs. Younger GTEx cohorts, where more than 5-fold genes were correlated with SAT-APOA4 in the pituitary and hypothalamus in the Younger GTEx cohort compared with the Older GTEx cohort (Figure 5F). In the Younger GTEx cohort, enriched pathways from pituitary genes that were associated with SAT-APOA4 included upregulation of anti-inflammatory responses. Conversely, pathways related to organelle structure were downregulated in both cohorts (Figure 5G).
2.6. Molecular characteristics of ASAT in older adults with high physical fitness
As an exploratory analysis, we next investigated whether ASAT-secreted proteins differed across participants with varying physical function. To quantify physical function, we computed a composite ‘physical fitness’ measure by averaging z-scores of five key traits: walking speed, stair climbing, leg power, relative VO2peak (ml/kg/min), and muscle mass (see ‘Physical fitness composite measure’ in Methods). Principal component analysis and k-means clustering of the protein–trait correlation coefficients supported that these five traits clustered tightly (Figure 6A). Based on the composite measure, participants were stratified into sex-specific tertiles representing low, moderate, and high fitness. For downstream analyses, we focused on comparing the lowest (‘LOW’) and highest (‘HIGH’) tertiles (Figure 6B). By design, the five physical fitness component traits were significantly lower in both LOW men and women compared to their HIGH counterparts (Table 2). In addition, although participants were stratified by physical fitness measures, the HIGH group exhibited lower adiposity and higher cognitive function (Table 2). Between HIGH and LOW females (n = 9 each), 10 secreted proteins were differentially expressed: 1 upregulated and 9 downregulated in the HIGH group (p < 0.05) (Figure 6C). Many of the downregulated secreted proteins in HIGH females were immune cell-related proteins (e.g., IGLV3-21, CD163, CD14, CD5L, ERAP1), suggesting suppressed immune activity in ASAT of older females with high physical fitness, low adiposity, and high cognitive function. Conversely, secreted LPL was more abundant in HIGH females compared with LOW females (p < 0.05) (Figure 6C). In HIGH vs. LOW men (n = 12 each), 30 out of 31 differentially expressed secreted proteins were downregulated, including complement proteins (C3, C5, C4a, CFH), immunoglobulin variables (IGHG1, IGKV3-20, IGKV3-15, IGKV3-11, IGHV3-66, IGLC3), and iron carrier proteins (HPX, TF) (Figure 6D). The abundance of secreted LPL and S100A9 was upregulated in HIGH in females and males, respectively (p < 0.05) (Figure 6D). We also stratified participants with available RNAseq data and compared ME between LOW and HIGH females (n = 32 each) and males (n = 33 each) (Table S1, Figure 6E,F). For both females and males, ME for modules 1 (enriched for ion transport; Figure 2SB), 5 (enriched for ribosome biogenesis and amide metabolism; Figure 2SB), and 9 (enriched for inflammation; Figure 2SB) were lower in HIGH vs. LOW (all p < 0.05) (Figure 6E,F). ME for module 8 was lower in HIGH females (p < 0.01) (Figure 6E).
Figure 6.
Older adults with higher ‘physical fitness’ have distinct ASAT transcripts and secreted protein profiles.
A) PCA analysis of the correlation between clinical traits and secreted proteins. K-means clustering was used to group clinical traits into clusters. The number of centers was set to four. Sample N = 61. B) Flow of participants stratification by ‘physical fitness’. C) Volcano plot of differentially expressed secreted proteins in LOW vs. HIGH females (p < 0.05). LOW, n = 9; HIGH, n = 9. D) Volcano plot of differentially expressed secreted proteins in LOW vs. HIGH males (p < 0.05). LOW, n = 12; HIGH, n = 12. E) Comparison of WGCNA ME between LOW vs. HIGH females. LOW, n = 33; HIGH, n = 33. F) Comparison of WGCNA ME between LOW vs. HIGH males. LOW, n = 32; HIGH, n = 32. Rel.VO2peak, Relative VO2peak (ml/kg/min). PCA, Principal component analysis; ME, Module eigengene.
Table 2.
Participant characteristics of LOW (tertile 1) and HIGH (tertile 3) of ‘Physical fitness’ whose ASAT CM was analyzed.
| Female |
Male |
|||
|---|---|---|---|---|
| LOW |
HIGH |
LOW |
HIGH |
|
| (N = 9) | (N = 9) | (N = 12) | (N = 12) | |
| Age, y | 78.8 (5.1) | 74.7 (3.6) | 78.0 (4.5) | 73.3 (2.0)∗∗ |
| Weight, kg | 72.7 (9.45) | 76.0 (14.3) | 94.2 (14.3) | 82.5 (12.4)∗ |
| BMI, kg/m2 | 29.8 (3.9) | 29.4 (5.0)∗ | 31.2 (4.0) | 26.5 (2.6)∗∗ |
| Waist circumference, cm | 92.4 (10.3) | 90.1 (10.1)∗ | 105 (16.6) | 96.4 (13.9) |
| ASAT, L | 9.09 (2.08) | 9.50 (3.23) | 8.27 (2.98) | 5.88 (1.84)∗ |
| VAT, L | 3.81 (1.19) | 3.75 (1.87) | 7.94 (2.19) | 4.90 (2.08)∗∗ |
| Liver fat, % | 6.38 (4.66) | 10.1 (13.1) | 5.99 (3.22) | 3.14 (1.64) |
| Thigh muscle fat infiltration, % | 8.30 (1.82) | 6.28 (0.61) | 7.17 (1.26) | 5.42 (1.00)∗∗ |
| D3CR muscle Mass, kg/weight | 26.5 (3.1) | 29.4 (3.6)∗ | 29.6 (4.8) | 36.6 (5.2)∗∗ |
| VO2peak, L/min | 1.22 (0.18) | 1.54 (0.20) | 1.86 (0.31) | 2.25 (0.29)∗∗ |
| VO2peak, ml/kg/min | 16.8 (1.94) | 20.6 (3.74)∗ | 19.9 (2.85) | 27.5 (3.18)∗∗∗∗ |
| Walking speed (4 m), m/s | 0.99(0.17) | 1.20 (0.26)∗∗ | 1.05 (0.09) | 1.22 (0.10)∗∗∗ |
| Stair climbing, stairs/s | 1.40 (0.61) | 1.78 (0.43)∗∗ | 1.53 (0.17) | 1.91 (0.25)∗∗∗ |
| Leg power (1RM), Watt/kg | 2.79 (1.20) | 5.03 (1.08)∗∗∗ | 4.69 (1.36) | 7.56 (0.91)∗∗∗∗ |
| Executive function#, sec | 130.0 (82.3) | 93.3 (28.5) | 120.0 (51.1) | 80.8 (22.1)∗ |
| Processing speed, score | 49.7 (20.8) | 58.4 (16.3)∗∗∗ | 53.3 (9.7) | 59.1 (8.3) |
| Memory, score | 24.0 (4.8) | 27.2 (2.4) | 23.3 (4.5) | 27.6 (5.1) |
| Contrast sensitivity, score | 1.47 (0.14) | 1.59 (0.11)∗∗ | 1.55 (0.15) | 1.63 (0.15) |
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 within sex group. #Executive function is shown in original values (time to complete all tasks), thus higher value indicates slower speed.
3. Discussion
Our multi-omic analysis, integrating in vivo RNAseq and ex vivo conditioned media (CM) proteomics of ASAT collected from older adults, combined with detailed measurements of physical and cognitive performance, provided a comprehensive characterization of ASAT genes and secreted proteins that may contribute to functional health. A key finding was the novel association of ASAT-secreted proteins – including SERPINF1, NID2, and APOA4 – with physical and cognitive functions in older adults. We also identified a distinct gene cluster, primarily immune cell-derived, that was strongly associated with poor physical and cognitive function. Integration of transcriptomics and CM proteomics highlighted potential aging-related molecular signatures, such as MMP9 and immunoglobulins, linked to functional decline. Our study demonstrates the utility of systems genetics analysis in examining the endocrine roles of ASAT-secreted proteins in secondary tissues in silico. Stratification of older adults by physical fitness levels enabled further insights into ASAT gene expression and secreted protein profiles associated with physical fitness, as well as cognitive function. This unique approach, integrating ASAT gene expression, secreted protein characterization, and estimation of ASAT endocrine roles, advances our understanding of ASAT that may impact physical performance and cognitive function in aging populations.
Our approach to the relatively short-term (i.e., 3-hour) ASAT ‘organ culture’, which preserves intact ASAT fragments in a complete culture medium [61], captured a pool of secreted proteins that reflect the in vivo transcriptomic profile of ASAT. The observed associations of secreted adiponectin and leptin abundances with adiposity align with prior literature [[62], [63], [64], [65]], supporting the reliability of our CM experimental model. Interestingly, the positive correlation of secreted adiponectin abundance with walking speed and executive function contrasts with the ‘adiponectin paradox’, where higher circulating adiponectin is linked to frailty and poor physical and cognitive performances in older adults [[66], [67], [68]]. This discrepancy suggests that the ‘secretory capacity’ of ASAT for adiponectin per se may not underlie the negative association between circulating adiponectin and health outcomes in aged populations. While leptin promotes skeletal muscle growth and function during development [69], our findings align with studies indicating its association with reduced skeletal muscle function in older populations [70,71], possibly due to aging-specific mechanisms. For example, leptin has been shown to drive IL6-mediated inflammation in the skeletal muscle of aged mice [72], indicating its complex and context-dependent role in aging-related skeletal muscle health.
In this study, we aimed to identify ASAT-secreted proteins associated with physical and cognitive function and investigate the potential endocrine roles of secreted proteins in secondary tissues using GD-CAT. PEDF (encoded by SERPINF1), a known adipokine, has been implicated in the pathophysiology of type 2 diabetes due to its strong association with cardiometabolic risk factors such as obesity-associated inflammation and insulin resistance [73,74]. Treatment of human-derived skeletal muscle cells with PEDF inhibits Akt phosphorylation and activates NFκB and p38 MAPK pathways, inducing insulin resistance and inflammation [75]. Our finding that the protein abundance of secreted SERPINF1 was associated with higher adiposity and reduced muscle mass, walking speed, stair climbing, and leg power supports this link, as insulin resistance and elevated inflammation are often associated with aging, sarcopenic skeletal muscle [10]. Additionally, the adipose SERPINF1 and skeletal muscle gene correlation observed in GD-CAT analysis – showing suppressed muscle contraction and enhanced inflammatory responses – aligns with existing literature and highlights the utility of in silico systems genetics analysis in studying tissue crosstalk. Interestingly, our finding suggests that the heart could be another major tissue linked with ASAT-derived SERPINF1, particularly in older adults, where it may interfere with mitochondrial protein translation while inciting inflammatory responses. Conversely, nidogen-2 (encoded by NID2) was associated with faster walking speed and negatively correlated with module 5, a cluster linked to higher adiposity and slower walking and stair climbing speeds. GD-CAT analysis further supported this, showing upregulation of muscle contraction and development in skeletal muscle genes correlated with adipose NID2, suggesting its potential role in promoting favorable adaptations in aging muscle. Our finding also suggests adipose nidogen-2 as a negative regulator of VAT inflammation and lipid uptake, indicating a potential favorable role of ASAT-secreted nidogen-2 in cardiometabolic health. Although circulating apolipoprotein A4 (encoded by APOA4) is largely produced by the intestine [76], the positive associations between ASAT-secreted apolipoprotein A4 abundance and memory and executive function suggest ASAT-secreted apolipoprotein A4 may play a role in adipose-brain crosstalk. This relationship may weaken in older adults, as indicated by GD-CAT analysis. In addition to aiding lipid metabolism, apolipoprotein A4 possesses anti-inflammatory and antioxidant properties [77,78], which are processes known to support cognitive health. Moreover, deficiency of APOA4 promotes β-amyloid formation and results in cognitive deficits, suggesting that a higher abundance of apolipoprotein A4 may be favored for preserving cognitive functions.
Our findings reveal a strong correlation between module 9, a unique cluster of immune cell-derived genes, and reduced physical and cognitive functions, supporting the connection of ASAT inflammation and senescence in age-related functional impairments [79,80]. Proinflammatory and senescence-associated secretory phenotype (SASP) cytokines, such as TNF, are known to inhibit myogenesis and induce insulin resistance in skeletal muscle [81,82]. Peripheral immune processes, including chronic pro-inflammatory signaling, have also been implicated in cognitive decline, neurodegeneration, and dementia-related pathology, though the specific pathways are not yet understood. Using plasma proteomics, recent studies have revealed multiple immune-related proteins associated with brain aging and increased dementia risk [83,84]. Our findings extend this work by identifying ASAT as a potent source of immunologically relevant proteins that may contribute to cognitive decline in older adulthood. Additionally, recent reports suggested immunoglobulin-associated senescence as a hallmark of aging, with accumulation of immunoglobulin G (IgG) observed in adipose tissue [85] and multiple tissues [86] in aged mice. Integrating WGCNA and CM proteomics, we identified ASAT-secreted immunoglobulins were strongly associated with reduced walking speed, stair climbing, aerobic fitness, and contrast sensitivity, suggesting they could be additional signatures of aging-associated ASAT abnormalities that may contribute to functional declines in older adults. While immunoglobulins are primarily released by plasmacytes, other cells may also contribute [86]. The exact role of ASAT-specific immunoglobulin accumulation in driving inflammation and senescence remains unclear, though increased immunoglobulin release may prime ASAT macrophages to secrete proinflammatory cytokine or systemically accelerate cellular senescence [[85], [86], [87]]. Our findings suggest that the biological role of immunoglobulins may vary by gene segment. For example, while many secreted immunoglobulin gene segments were positively associated with module 9, IGLV1-44 abundance was negatively associated with modules 1, 2, 4, and 8, suggesting a linkage with lower adiposity and faster walking speed. ASAT-secreted MMP9, a gelatinase involved in extracellular matrix protein turnover, also emerged as a signature of aging due to its strong correlation between transcript expression and secreted protein abundance, as well as its association with age. While MMP9 can promote collagen degradation and angiogenesis, counteracting fibrosis [88], elevated MMP9 levels in ASAT or plasma have been linked to insulin resistance and cardiovascular disease risk [89,90]. Lifestyle interventions, such as exercise or weight loss, have been shown to reduce MMP9 protein abundance or gene expression in ASAT, at least in young or middle-aged adults, indicating that excessive ASAT MMP9 may be unfavorable [91,92]. Although the precise mechanism remains unclear, MMP9 overexpression has been linked to increased inflammation [93], while MMP9 deletion has been shown to shift macrophage polarization toward a more anti-inflammatory M2 phenotype in the mouse heart [94]. Given that both exercise training and diet-induced weight loss attenuate ASAT inflammation [95,96], it is possible that reduced MMP9 expression may contribute to this anti-inflammatory effect.
Our exploratory analysis comparing participants stratified by the composite physical fitness score revealed ASAT characteristics that may be particularly relevant to older adults with reduced physical function. In both female and male tertile comparisons, lower physical fitness was associated with a higher abundance of inflammatory factors and immunoglobulin segment, including JCHAIN – a polypeptide that facilitates IgA and IgM polymer formation and exocrine secretion [97] – suggestive of ASAT as a source of excessive secretion of various immunoglobulin classes in older adults with poor physical fitness. Conversely, older adults with higher physical fitness exhibited elevated abundance of LPL and S100A9 (a neutrophil marker gene that encodes calprotectin) in females and males, respectively. S100A9 has been shown to enhance insulin-independent glucose uptake in skeletal muscle via TLR4 and attenuate systemic inflammation [98], potentially benefiting muscle function and mobility. While stratification was based on a composite physical fitness measure, differences in executive function and processing speed between tertiles suggest that the identified secreted proteins and transcriptomes could also be linked to cognitive function.
While our study design enabled comprehensive profiling of ASAT-secreted proteins associated with physical and cognitive functions in older adults, it is important to acknowledge some of the limitations inherent to observational studies. Although our findings of ASAT-secreted proteins that were significantly associated with physical or cognitive function are intriguing, the direct causal link remains unclear, and the definitive mechanistic validation requires further exploration. However, in silico GD-CAT analysis provided insight into the expected endocrine roles of secreted proteins, which aligned well with established literature (e.g., SERPINF1), lending credibility to our findings. Also, it is possible that our relatively short incubation of ASAT explants (i.e., 3 h) may not have fully captured the secreted proteins in the conditioned media. While we acknowledge that incubation time may differentially impact protein secretion, our approach enabled integrative analysis of transcripts and secreted proteins, which led to the identification of secreted immunoglobulins as potential mediators of ASAT-driven physical and cognitive function impairments and aligned with established literature of classic adipokine secretion of leptin and adiponectin associations with adiposity and other clinical outcomes. Our exclusion criteria (e.g., inability to complete a 400 m walk in 15min, BMI >40 kg/m2) may limit broader applicability, as ASAT characteristics could differ in populations with very slow walking speed or severe obesity, both strong predictors of cardiovascular mortality [99,100]. These criteria were selected for our study to enable participants to complete detailed mobility and body composition assessments, which strengthened the observed correlations between clinical traits and ASAT-secreted proteins. Future studies should include higher-risk populations to expand the scope of tissue crosstalk research. Additionally, while stratifying participants by a composite physical fitness score enabled us to identify novel ASAT-secreted proteins potentially linked to better or poorer functional health in older adults, it is important to note that this fitness score has not been rigorously validated or operationalized. The SOMMA study has recently developed an operationalized ‘Frailty Score’ based on six health traits spanning multiple domains [101], which may offer a more comprehensive framework. Our approach provides a useful starting point but warrants further refinement and validation in future studies.
In summary, our findings indicate that transcriptional and secretome alterations in ASAT are highly associated with, or even underlie, physical and cognitive performance in older adults. Notably, ASAT inflammation and immunoglobulin production may be key factors linked to reduced physical and cognitive functions. Using global proteomics from ASAT-conditioned media, we identified numerous secreted proteins with significant associations with clinical functionalities. Furthermore, we present a workflow that integrates multi-omics analyses with systems genetics analysis, offering a robust approach to enhance the translatability of clinical insights in tissue crosstalk research. Overall, our study underscores the central role of ASAT in regulating physical and cognitive functions in aging adults, providing a valuable platform for future investigations into tissue crosstalk.
4. Methods
4.1. Participant recruitment
A total of 241 participants aged 70 and older were recruited as part of the SOMMA (https://www.sommastudy.com/) from April 2019 to December 2021 across two clinical sites; the University of Pittsburgh and Wake Forest University School of Medicine [24]. Detailed descriptions of the parent SOMMA study cohorts and clinical measurements are available here [28]. Individuals who reported being unable to walk ¼ mile or climb a flight of stairs, had an active malignancy, suffered from advanced chronic conditions (e.g., severe heart or lung disease preventing them from walking ¼ mile, severe kidney disease requiring dialysis, Parkinson's disease, or dementia), or had body mass index (BMI) ≥ 40 kg/m2 were excluded from the study. Additionally, individuals with medical contraindications to biopsy or MRI, such as chronic anticoagulation or incompatible metal implants, were also excluded. In-person assessments at enrollment ensured that all participants were capable of walking 400 m. All participants provided written informed consent, and the study was approved by the Western Institutional Review Board (20180764) for all participating sites.
4.2. Body size and composition measurements
The SOMMA baseline evaluations were conducted over several days and included self-reported information on sex, race, ethnicity, health history, medication use, diet, and physical activity. Height was measured using stadiometers, and weight was recorded on digital scales. VO2 peak was assessed via cardiopulmonary exercise testing (CPET) [102]. Body composition was measured using magnetic resonance imaging (MRI). Whole-body MR scans were processed with Dixon water-fat imaging using AMRA Researcher (AMRA Medical AB, Linköping, Sweden) to estimate the volumes of abdominal subcutaneous adipose tissue (ASAT), visceral adipose tissue (VAT), thigh fat infiltration, and liver fat percentage (%). Total skeletal muscle mass (D3C muscle) was assessed by validated D3C dilution method [103].
4.3. Fitness and physical performance
Walking speed. 4 m walking speed was measured during the Short Physical Performance Battery (SPPB), which is a well-established tool in clinical research for evaluating lower extremity function. Walking speed (m/sec) was assessed over a 4-meter walking course.
Stair climbing. Participants climbed up and down four standard steps (10 inches deep, 6 inches tall) three consecutive times without stopping, and the total time (in seconds) was recorded. ‘Stair climbing’ was calculated as the inverse of the total time recorded (stairs per second). Handrails were available if needed.
CPET. A standardized CPET, using a modified Balke or manual protocol, was administered to participants to measure aerobic fitness [102]. Two slow 5-minute walking tests were conducted before and after the maximal effort test to assess walking energetics at a preferred walking speed and a slow fixed speed of 1.5 mph. Participants who were excluded from the maximal effort symptom-limited peak test had acute electrocardiogram (ECG) abnormalities, uncontrolled blood pressure, or a history of myocardial infarction, unstable angina, or angioplasty in the preceding 6 months. Testing continued until the respiratory exchange ratio, ratio between VCO2 and VO2, was ≥1.05 and self-reported Borg Rating of Perceived Exertion was ≥17. Blood pressure, pulse oximetry, and ECG were monitored throughout exercise. VO2 peak was determined in the BREEZESUITE software as the highest 30-second average of VO2 (ml/min) achieved. Relative VO2 (ml/kg/min) was calculated by normalizing average VO2 (ml/min) by body weight (kg).
Leg power. Knee extensor leg power was measured using a Keiser Air 420 exercise machine, primarily on the right leg unless otherwise indicated. Participants with recent strokes, aneurysms, cerebral hemorrhages, or abnormal blood pressure were excluded. Resistance was set at 40%, 50%, 60%, and 70% of the participant's 1 repetition maximum leg strength to assess power output.
Executive function. The Trail Making Test, Part B, is a timed test that measures executive function, and in particular set-shifting, by evaluating participants’ abilities to connect a sequence of alternating numbers and letters. A faster time to completion (in seconds) represents better task performance. The Trails B exam was stopped when the participant completed the exam successfully, 5 min (300 s) elapsed or 5 errors were made. The time taken (seconds) to complete the test was recorded. Performance on this executive measure was calculated as the inverse of this time, so that higher values reflect better task performance.
Processing speed. The Digit Symbol Substitution Test (DSST) from the WAIS-III was used to assess processing speed, motor speed, visuoperceptual functions, attention, and manual dexterity. Scores range from 0 to 133, with higher scores indicating better cognitive performance.
Memory function. The California Verbal Learning Test (CVLT)-II (Short Form) SF was used to assess verbal learning ability and memory. Participants were read a list of nine nouns (from three categories: fruits, tools, and clothing) and were asked to recall as many as possible after each repetition. The process was repeated four times. After a 10-minute delay, participants were asked to recall the list again. Correct answers and intrusions were noted. Immediate recall was used to measure memory performance, and was calculated as the sum of items correctly recalled across the four learning trials (range 0–36).
Contrast sensitivity. Contrast sensitivity is a fundamental component of visual processing involved in supporting various higher-order cognitive processes. Contrast sensitivity was included as a variable of interest given evidence of age-related contrast deficits, which have been linked to increased risk of cognitive decline, dementia, and mobility limitations. Contrast sensitivity was measured using the Pelli-Robson eye chart. Correct answers were converted to log contrast sensitivity (LCS) scores. Lower LCS values indicate worse contrast sensitivity.
4.4. ASAT biopsy collection and processing
A detailed description of biopsy sample collection and processing is available here [104]. Briefly, ASAT biopsies were performed via tumescent liposuction (with a maximum of three needle passes) between the flank and the abdominal midline, below the umbilical scar. ASAT samples were flash-frozen in liquid nitrogen for subsequent bulk RNA sequencing. A portion of ASAT sample was immediately transferred to M199 for conditioned media experiments (see details below).
4.5. Analytical procedures
ASAT explant conditioned media. Transferred ASAT samples were rinsed with warm Hank's Balanced Salt Solution (HBSS) (ThermoFisher Scientific) to remove visible blood clots and connective tissue. Pre-warmed M199 containing 25 mM Hepes was used at a ratio of 100 mg of ASAT to 2 mL media. ASAT sample was t transferred to a 50 mL conical tube containing media which was gassed then with 95% O2 for 30 s and tightly sealed. ASAT explants were incubated in a shaking water bath at 37 °C and 100 rpm for 3 h. After incubation, the ASAT and CM were poured through a 40 μm cell strainer (Fisher Scientific) into fresh 50 mL conical tubes. The CM was centrifuged at 500×g at room temperature for 5 min, then aliquoted into multiple vials for storage at −80 °C at the biorepository.
Targeted assessment of secreted proteins. Enzyme-linked immunosorbent assay (ELISA) was used to measure the abundance of adiponectin and leptin in the CM (N = 168), following the manufacturer's protocols. The measured abundance was normalized to ASAT explant mass.
LC-MS/MS-based proteomics. Protein from ASAT explant conditioned media samples was isolated and processed for mass spectrometry-based analysis using S-Trap micro sample processing columns (Protifi, Fairport NY) with a protocol adapted from manufacturer recommendations. Briefly, 200 μL CM aliquots were concentrated to 90 μL in a vacuum centrifuge, mixed 1:1 with lysis buffer (10% SDS, 100 mM TEAB pH 8.5), reduced with dithiothreitol (DTT, final concentration 20 mM) at 55 °C for 10 min, and alkylated with iodoacetamide (IAA, final concentration 40 mM) in the dark at room temperature for 30 min. Samples were acidified to pH < 1, mixed with binding buffer (100 mM TEAB in 90% methanol), and applied to S-Trap micro columns via centrifugation. Captured proteins were digested with 2.5 μg of sequencing-grade trypsin per sample for 2 h at 47 °C, and digested peptides were eluted from columns according to manufacturer's protocol. Digested peptides were concentrated in a vacuum centrifuge, measured by BCA assay, and resuspended to a final concentration of 0.1 μg/μL in 3% acetonitrile/0.1% formic acid solution. Peptides (0.5 μg per sample) were separated using a Vanquish Neo UHPCL system (ThermoFisher Scientific) equipped with a homemade 75 μm I.D. x 30 cm length separation column packed with Acquity 130 A, 1.7 μm BEH-C18 (Waters) and a homemade 150 μm I.D. x 5 cm length trapping column packed with Jupiter 300 A, 5 μm C18 (Phenomenex). A 120-minute gradient of 100% mobile phase A (0.1% formic acid in water) to 75% mobile phase B (0.1% formic acid in acetonitrile) was applied to each sample, and the separation was coupled to an Orbitrap Exploris 240 mass spectrometer (ThermoFisher Scientific) for MS/MS analysis. MS spectra were collected from 300 to 1800 m/z at an MS1 resolution setting of 60,000. Ions were selected with an isolation width of 0.7 m/z for higher energy collision dissociation (HCD, 30%); +1 charged species were excluded, and a dynamic exclusion window of 45 s was applied. MS2 spectra were acquired at a mass resolution of 30,000 and a normalized AGC target of 250%. Raw mass spectrometry data files were analyzed with MaxQuant software (v2.2.0.0) against a human UniProt database (downloaded in March 2023) with the following parameters: LFQ and match-between-run enabled, fixed modifications of cysteine carbamidomethylation, variable modifications of methionine oxidation and n-terminal acetylation, 20 ppm mass tolerance, and a maximum of 4 missed cleavages. Protein-level LFQ intensity data (from the resulting proteinGroups.txt file from MaxQuant analysis) was log2-transformed and median-centered within each sample. Batch correction was applied using limma::removeBatchEffect to account for ten samples that were processed and analyzed in a separate batch, and this dataset (log2-transformed, median-centered, batch-corrected) was exported for use in downstream analysis.
ASAT bulk RNA sequencing. Frozen ASAT samples were homogenized using pellet pestle cordless motor. RNA was subsequently extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen #74804). Nanodrop Spectrophotometer was used to measure RNA concentration and ratio. Library preparation was conducted using Mini Amp Plus Thermal Cycle (Thermo Fisher) and magnetic racks (Zymo Research) with a total of 500 ng of RNA. Library concentration was measured using Qubit dsDNA HS Assay Kit (Invitrogen Q32851) and the Qubit 4 Fluorometer device (Invitrogen). Individual libraries were pooled and sequenced using a NovaSeq 6000 (Novagene) following in-house established protocols which also run quality control checking. Raw RNAseq reads were inspected for quality using FastQC v0.11.9 (Barbraham Institute, Barbraham, England). Reads were then aligned to the human reference transcriptome (Homo_sapiens.GRCh38.cdna v94) using kallisto [105]. Count matrix was produced by averaging kallisto TPM at the gene-level per sample.
4.6. Bioinformatics
Proteomics. Among 2366 proteins that were identified, 1276 proteins were detected by at least 25% of participants. Proteins were subsequently filtered for secreted proteins, using the secreted protein list downloaded from The Human Protein Atlas (Supporting information 1), retaining 275 secreted proteins. Only these 275 secreted proteins were used for downstream analyses. For comparison of secreted proteins between stratified tertiles (Figure 6C,D), limma R package (version 3.62.1) was used.
Weighted gene co-expression network analysis (WGCNA). WGCNA [106] (version 1.73) was used to identify non-overlapping clusters (modules) of ASAT transcripts. Connectivity for each transcript was determined by summing its connection strengths with all other transcripts. A scale-free signed network was built using an optimal soft-threshold power (β = 5). From this network, 10 distinct modules (including Module 0) were identified using a dynamic tree-cutting algorithm and a merging threshold of 0.3. Module 0, often referred to as the ‘grey’ module, contains transcripts that failed to cluster into other modules due to weak correlation patterns. Top hub genes per module were extracted by their kME (module eigengene-based connectivity). MEs, the principal eigenvector of each module, were extracted and biweight midcorrelation was used to assess the relationship between modules and clinical traits/secreted protein abundances.
Gene set analysis. Overrepresentation analysis (ORA) was conducted using hypeR R package (version 2.4.0). Gene Ontology Biological Process (Figure 2B, S2B) and HALLMARK (Fig. S2B) were used as reference databases. Enrichplot R package was used to conduct rank-based gene set enrichment analysis. The top 10 terms (FDR <0.05) for each module were shown (Fig. S2B).
Cell-type gene markers. Cell-type gene markers were identified from ‘Markers for human clusters and subclusters’ obtained from Emont et al., 2022 [29]. A threshold of log2FC > 1 was further applied to retain gene markers that are specifically expressed.
Senescence gene markers. A custom senescence gene set (n = 1369) was curated by combining previously reported datasets for cellular senescence: GO term (GO:0090398, cellular senescence), CellAge [31], SenMayo [32], and AgingAtlas [33].
Gene Derived Correlation Across Tissues (GD-CAT). GTEx data (n = 310) was adopted from GD-CAT pipeline [55], with samples filtered by age into younger (n = 175) and older (n = 135) cohorts [56], based on the provided age ranges. Transcriptomics correlations were calculated using biweight midcorrelation (bicor) via the bicorAndPvalue() function in the WGCNA package. Correlation values (bicor) and corresponding p-values were processed and adjusted for multiple testing using the Benjamini-Hochberg (BH) method to control the false discovery rate. For visualizing significant correlations, bar plots were generated for each gene to depict the tissue distribution of significantly correlated genes, stratified by age group. Gene set enrichment analysis (GSEA) was performed downstream using highly correlated genes. Genes were ranked by bicor values, and the gseGO() function from the clusterProfiler R package was used to perform GSEA. The analysis utilized the “org.Hs.eg.db” annotation database for human genes, and pathways were evaluated across Gene Ontology (GO) categories (“Biological Process,” “Molecular Function,” and “Cellular Component”). Adjusted p-values (BH correction) were used to identify significant pathways. Pathway enrichment results were stratified by age and displayed for both positive and negative correlation subsets.
Physical fitness composite measure. To stratify participants by physical function and examine associated features of ASAT-secreted proteins, we derived a physical fitness composite measure. We hypothesized that muscle mass, aerobic fitness (ml/kg/min), walking speed, stair climbing, and leg power would be interrelated and collectively reflect physical fitness status relevant to ASAT-derived signals. To test this, we performed principal component analysis and k-means clustering (k = 4) on the biweight midcorrelation (bicor) matrix between ASAT-secreted protein abundance and clinical traits. This analysis supported that five traits—muscle mass, aerobic fitness, walking speed, stair climbing, and leg power—clustered closely. These traits were z-scaled and averaged to generate a composite physical fitness measure. Participants were then stratified into sex-specific tertiles (low, moderate, and high fitness) based on this measure.
4.7. Statistics
Biweight midcorrelation (R package: WGCNA) was used for correlation analysis (Figures 1C, 2(A,B), 3(B,C,E,F), 4(A–C), S1, S2C, and S3(B,D)). For sex-adjusted protein-trait correlations (Fig. S3), the residual adjustment approach was used, where clinical traits and protein abundances were separately regressed on the covariate (sex) using linear regression models. The residuals from these models, representing the variation in clinical traits and protein abundances after accounting for the effects of the covariate, were extracted. Biweight midcorrelation was then performed on the residuals to assess the sex-adjusted association. Spearman's R correlation was used for non-normalized variables (Figs. S2A and S4B). To assess statistical differences between stratified tertiles in females and males (Figure 6E,F), two-tailed independent Student's t-tests were used within each group. Benjamini-Hochberg was used to correct for multiple testing. Statistical computations were performed using R (version: 4.4.2, Vienna, Austria). All experiments were performed once.
CRediT authorship contribution statement
Cheehoon Ahn: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization. Ian Tamburini: Writing – review & editing, Methodology, Formal analysis. James A. Sanford: Writing – review & editing, Supervision, Methodology, Formal analysis, Data curation. Mingqi Zhou: Writing – review & editing, Visualization, Methodology, Formal analysis. Farah Gamie: Writing – review & editing, Visualization, Methodology, Formal analysis. Reichelle X. Yeo: Writing – review & editing, Data curation. Carlos H. Viesi: Writing – review & editing, Data curation. Maria Pino: Writing – review & editing, Methodology, Formal analysis, Data curation. Katie L. Whytock: Writing – review & editing. Lauren E. Oberlin: Writing – review & editing. Theresa Mau: Writing – review & editing, Data curation. Joshua N. Adkins: Writing – review & editing, Supervision. Jamie N. Justice: Writing – review & editing, Supervision. Ashlee N. Wood: Writing – review & editing, Methodology, Data curation. Zana M. Ross: Writing – review & editing, Methodology, Data curation. Paul.D. Piehowski: Writing – review & editing, Methodology. Chelsea M. Hutchinson Bunch: Writing – review & editing, Methodology. Kirk I. Erickson: Writing – review & editing. Frederico G.S. Toledo: Writing – review & editing, Methodology. Nancy E. Lane: Writing – review & editing. Peggy M. Cawthon: Writing – review & editing, Supervision, Project administration, Data curation. Anne B. Newman: Writing – review & editing, Funding acquisition. Stephen B. Kritchevsky: Writing – review & editing, Supervision, Project administration, Funding acquisition. Steven R. Cummings: Writing – review & editing, Supervision, Project administration, Funding acquisition. Bret H. Goodpaster: Writing – review & editing, Supervision, Project administration, Funding acquisition. Erin E. Kershaw: Writing – review & editing, Project administration, Methodology, Funding acquisition, Conceptualization. Marcus M. Seldin: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Lauren M. Sparks: Writing – review & editing, Project administration, Investigation, Funding acquisition, Conceptualization.
Code availability
No new code was generated in this study.
Declaration of competing interest
PMC reports consulting income and stock ownership in Myocorps.
Acknowledgements
We are grateful to acknowledge the contributions of the SOMMA-AT participants. We also acknowledge the assistance from SOMMA clinical, research, and administrative staff. This study was supported by the National Institute on Aging (R01AG059416 and R01AG066474), the National Center for Advancing Translational Science at Wake Forest University (UL1TR001420), and NIA Claude D. Pepper Older American Independence Centers at the University of Pittsburgh (P30AG024827) and Wake Forest University School of Medicine (P30AG021332). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2025.102213.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Nair K.S. Aging muscle. Am J Clin Nutr. 2005;81(5):953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 2.Yankner B.A., Lu T., Loerch P. The aging brain. Annu Rev Pathol. 2008;3(1):41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 3.Jackson A.S., Sui X., Hébert J.R. Church TS, and blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169(19):1781–1787. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mau T., Barnes H.N., Blackwell T.L., Kramer P.A., Bauer S.R., Marcinek D.J., et al. Lower muscle mitochondrial energetics is associated with greater phenotypic frailty in older women and men: The study of muscle, mobility and aging. Geroscience. 2024;46(2):2409–2424. doi: 10.1007/s11357-023-01002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frontera W.R., Hughes V.A., Fielding R.A., Fiatarone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 6.Poon H.F., Calabrese V., Scapagnini G., Butterfield D.A. Free radicals and brain aging. Clin Geriatr Med. 2004;20(2):329–359. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Mattson M.P., Arumugam T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018;27(6):1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Vaca-Dempere M., Mortimer T., Deryagin O., Smith J.G., Petrus P., et al. Brain-muscle communication prevents muscle aging by maintaining daily physiology. Science. 2024;384(6695):563–572. doi: 10.1126/science.adj8533. [DOI] [PubMed] [Google Scholar]
- 9.Lauretani F., Meschi T., Ticinesi A., Maggio M. "Brain-muscle loop" in the fragility of older persons: From pathophysiology to new organizing models. Aging Clin Exp Res. 2017;29(6):1305–1311. doi: 10.1007/s40520-017-0729-4. [DOI] [PubMed] [Google Scholar]
- 10.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Oliveras-Cañellas N., Castells-Nobau A., de la Vega-Correa L., Latorre-Luque J., Motger-Albertí A., Arnoriaga-Rodriguez M., et al. Adipose tissue coregulates cognitive function. Sci Adv. 2023;9(32) doi: 10.1126/sciadv.adg4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer A.K., Jensen M.D. Metabolic changes in aging humans: Current evidence and therapeutic strategies. J Clin Investig. 2022;132(16) doi: 10.1172/JCI158451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Carvalho F.G., Justice J.N., Freitas E.C., Kershaw E.E., Sparks L.M. Adipose tissue quality in aging: How structural and functional aspects of adipose tissue impact skeletal muscle quality. Nutrients. 2019;11(11) doi: 10.3390/nu11112553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourassa K., Sbarra D.A. Body mass and cognitive decline are indirectly associated via inflammation among aging adults. Brain Behav Immun. 2017;60:63–70. doi: 10.1016/j.bbi.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Tchkonia T., Morbeck D.E., Von Zglinicki T., Van Deursen J., Lustgarten J., Scrable H., et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whytock K.L., Divoux A., Sun Y., Pino M.F., Yu G., Jin C.A., et al. Aging human abdominal subcutaneous white adipose tissue at single cell resolution. Aging Cell. 2024 doi: 10.1111/acel.14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronti T., Lupattelli G., Mannarino E. The endocrine function of adipose tissue: An update. Clin Endocrinol. 2006;64(4):355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaess B.M., Pedley A., Massaro J.M., Murabito J., Hoffmann U., Fox C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn D., Macias E., Zarini S., Garfield A., Zemski Berry K., MacLean P., et al. Exploring visceral and subcutaneous adipose tissue secretomes in human obesity: Implications for metabolic disease. Endocrinology. 2022;163(11) doi: 10.1210/endocr/bqac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerschow E., Anwar S., Barzilai N., Rosenstreich D. Macrophages accumulation in visceral and subcutaneous adipose tissue correlates with age. J Allergy Clin Immunol. 2007;119(1) [Google Scholar]
- 21.Lakowa N., Trieu N., Flehmig G., Lohmann T., Schon M.R., Dietrich A., et al. Telomere length differences between subcutaneous and visceral adipose tissue in humans. Biochem Biophys Res Commun. 2015;457(3):426–432. doi: 10.1016/j.bbrc.2014.12.122. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G., Shvetsov Y.B., Wong M.C., Garber A., Monroe K., Ernst T.M., et al. Subcutaneous and visceral fat assessment by DXA and MRI in older adults and children. Obesity. 2022;30(4):920–930. doi: 10.1002/oby.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimokata H., Andres R., Coon P., Elahi D., Muller D., Tobin J. Studies in the distribution of body fat. II. Longitudinal effects of change in weight. Int J Obes. 1989;13(4):455–464. [PubMed] [Google Scholar]
- 24.Yeo R.X., Mau T., Ross Z.M., Edenhoffer N.P., Liu J., Barnes H.N., et al. Investigating the role of adipose tissue in mobility and aging: design and methods of the adipose tissue ancillary to the study of muscle, mobility and aging (SOMMA-AT) J Gerontol A Biol Sci Med Sci. 2025 doi: 10.1093/gerona/glaf015. [DOI] [PubMed] [Google Scholar]
- 25.Swenor B.K., Wang J., Varadaraj V., Rosano C., Yaffe K., Albert M., et al. Vision impairment and cognitive outcomes in older adults: The health ABC study. J Gerontol A Biol Sci Med Sci. 2019;74(9):1454–1460. doi: 10.1093/gerona/gly244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter M.M., Vandervoort A.A., Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 27.Glisky E.L. Changes in cognitive function in human aging. Brain Aging. 2007:3–20. [PubMed] [Google Scholar]
- 28.Cummings S.R., Newman A.B., Coen P.M., Hepple R.T., Collins R., Kennedy Ms K., et al. The study of muscle, mobility and aging (SOMMA): A unique cohort study about the cellular biology of aging and age-related loss of mobility. J Gerontol A Biol Sci Med Sci. 2023;78(11):2083–2093. doi: 10.1093/gerona/glad052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emont M.P., Jacobs C., Essene A.L., Pant D., Tenen D., Colleluori G., et al. A single-cell atlas of human and mouse white adipose tissue. Nature. 2022;603(7903):926–933. doi: 10.1038/s41586-022-04518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freund A., Orjalo A.V., Desprez P.-Y., Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avelar R.A., Ortega J.G., Tacutu R., Tyler E.J., Bennett D., Binetti P., et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 2020;21(1):91. doi: 10.1186/s13059-020-01990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saul D., Kosinsky R.L., Atkinson E.J., Doolittle M.L., Zhang X., LeBrasseur N.K., et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun. 2022;13(1):4827. doi: 10.1038/s41467-022-32552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aging atlas: A multi-omics database for aging biology. Nucleic Acids Res. 2021;49(D1):D825–D830. doi: 10.1093/nar/gkaa894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combs T.P., Pajvani U.B., Berg A.H., Lin Y., Jelicks L.A., Laplante M., et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145(1):367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 35.Kim J.-Y., Van De Wall E., Laplante M., Azzara A., Trujillo M.E., Hofmann S.M., et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Investig. 2007;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantzoros C.S. The role of leptin in human obesity and disease: A review of current evidence. Ann Intern Med. 1999;130(8):671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 37.Phung M.W., Dass C.R. In-vitro and in-vivo assays for angiogenesis-modulating drug discovery and development. J Pharm Pharmacol. 2006;58(2):153–160. doi: 10.1211/jpp.58.2.0001. [DOI] [PubMed] [Google Scholar]
- 38.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D.M., et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Investig. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unamuno X., Gomez-Ambrosi J., Ramirez B., Rodriguez A., Becerril S., Valenti V., et al. Dermatopontin, A novel adipokine promoting adipose tissue extracellular matrix remodelling and inflammation in obesity. J Clin Med. 2020;9(4) doi: 10.3390/jcm9041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto O., Fujiwara S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect Tissue Res. 2006;47(4):177–189. doi: 10.1080/03008200600846564. [DOI] [PubMed] [Google Scholar]
- 41.Ceafalan L.C., Dobre M., Milanesi E., Niculae A.M., Manole E., Gherghiceanu M., et al. Gene expression profile of adhesion and extracellular matrix molecules during early stages of skeletal muscle regeneration. J Cell Mol Med. 2020;24(17):10140–10150. doi: 10.1111/jcmm.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.In 't Groen S.L.M., Franken M., Bock T., Kruger M., de Greef J.C., Pijnappel W. A knock down strategy for rapid, generic, and versatile modelling of muscular dystrophies in 3D-tissue-engineered-skeletal muscle. Skeletal Muscle. 2024;14(1):3. doi: 10.1186/s13395-024-00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong H., Dong W., Rostad S.W., Marcovina S.M., Albers J.J., Brunzell J.D., et al. Lipoprotein lipase (LPL) is associated with neurite pathology and its levels are markedly reduced in the dentate gyrus of Alzheimer's disease brains. J Histochem Cytochem. 2013;61(12):857–868. doi: 10.1369/0022155413505601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An K., Guo P., Zhang H., Zhu W., Cao W., Shi J., et al. Decreased plasma level of lipoprotein lipase predicted verbal disfluency in Chinese type 2 diabetes mellitus patients with early cognitive deficits. Curr Alzheimer Res. 2021;18(8):656–666. doi: 10.2174/1567205018666210922105850. [DOI] [PubMed] [Google Scholar]
- 45.Hu W., Liu J., Hu Y., Xu Q., Deng T., Wei M., et al. Transcriptome-wide association study reveals cholesterol metabolism gene Lpl is a key regulator of cognitive dysfunction. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1044022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott D.A., Weickert C.S., Garner B. Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clin Lipidol. 2010;51(4):555–573. doi: 10.2217/CLP.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itoh Y., Sahni V., Shnider S.J., McKee H., Macklis J.D. Inter-axonal molecular crosstalk via Lumican proteoglycan sculpts murine cervical corticospinal innervation by distinct subpopulations. Cell Rep. 2023;42(3) doi: 10.1016/j.celrep.2023.112182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Q., Cao Y., Gao J. Decreased expression of the APOA1-APOC3-APOA4 gene cluster is associated with risk of Alzheimer's disease. Drug Des Dev Ther. 2015;9:5421–5431. doi: 10.2147/DDDT.S89279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song F., Poljak A., Kochan N.A., Raftery M., Brodaty H., Smythe G.A., et al. Plasma protein profiling of mild cognitive impairment and Alzheimer's disease using iTRAQ quantitative proteomics. Proteome Sci. 2014;12:1–13. doi: 10.1186/1477-5956-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koplev S., Seldin M., Sukhavasi K., Ermel R., Pang S., Zeng L., et al. A mechanistic framework for cardiometabolic and coronary artery diseases. Nat Cardiovasc Res. 2022;1(1):85–100. doi: 10.1038/s44161-021-00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seldin M.M., Lusis A.J. Systems-based approaches for investigation of inter-tissue communication. J Lipid Res. 2019;60(3):450–455. doi: 10.1194/jlr.S090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seldin M.M., Koplev S., Rajbhandari P., Vergnes L., Rosenberg G.M., Meng Y., et al. A strategy for discovery of endocrine interactions with application to whole-body metabolism. Cell Metab. 2018;27(5):1138–11355 e6. doi: 10.1016/j.cmet.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Y., Wang Y., Zhou Z., Pan C., Jiang L., Zhou Z., et al. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science. 2022;377(6613):1399–1406. doi: 10.1126/science.abn0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Velez L.M., Van C., Moore T., Zhou Z., Johnson C., Hevener A.L., et al. Genetic variation of putative myokine signaling is dominated by biological sex and sex hormones. eLife. 2022;11 doi: 10.7554/eLife.76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou M., Tamburini I., Van C., Molendijk J., Nguyen C.M., Chang I.Y., et al. Leveraging inter-individual transcriptional correlation structure to infer discrete signaling mechanisms across metabolic tissues. eLife. 2024;12 doi: 10.7554/eLife.88863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Consortium GT, Laboratory DA, Coordinating Center -Analysis Working G, Statistical Methods groups-Analysis Working G, Enhancing Gg, Fund NIHC Genetic effects on gene expression across human tissues. Nature. 2017;550(7675):204–213. doi: 10.1038/nature24277. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang P., Smit E., Brouwers M.C., Goossens G.H., van der Kallen C.J., van Greevenbroek M.M., et al. Plasma pigment epithelium-derived factor is positively associated with obesity in Caucasian subjects, in particular with the visceral fat depot. Eur J Endocrinol. 2008;159(6):713–718. doi: 10.1530/EJE-08-0521. [DOI] [PubMed] [Google Scholar]
- 58.Yang B., Lu L., Zhou D., Fan W., Barbier-Torres L., Steggerda J., et al. Regulatory network and interplay of hepatokines, stellakines, myokines and adipokines in nonalcoholic fatty liver diseases and nonalcoholic steatohepatitis. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su A.I., Cooke M.P., Ching K.A., Hakak Y., Walker J.R., Wiltshire T., et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci. 2002;99(7):4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu C., Orozco C., Boyer J., Leglise M., Goodale J., Batalov S., et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10(11) doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried S.K., Moustaid-Moussa N. Adipose tissue protocols. 2001. Culture of adipose tissue and isolated adipocytes; pp. 197–212. [DOI] [PubMed] [Google Scholar]
- 62.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 63.Scherer P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 64.Ryan A.S., Berman D.M., Nicklas B.J., Sinha M., Gingerich R.L., Meneilly G.S., et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26(8):2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 65.Tabara Y., Okada Y., Ochi M., Ohyagi Y., Igase M. Associations between adiponectin and leptin levels and skeletal muscle mass and myosteatosis in older adults: The Shimanami health promoting program study. Geriatr Gerontol Int. 2023;23(6):444–449. doi: 10.1111/ggi.14582. [DOI] [PubMed] [Google Scholar]
- 66.Baker J.F., Newman A.B., Kanaya A., Leonard M.B., Zemel B., Miljkovic I., et al. The adiponectin paradox in the elderly: Associations with body composition, physical functioning, and mortality. J Gerontol A Biol Sci Med Sci. 2019;74(2):247–253. doi: 10.1093/gerona/gly017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang C., Tomata Y., Kakizaki M., Sugawara Y., Hozawa A., Momma H., et al. High circulating adiponectin levels predict decreased muscle strength among older adults aged 70 years and over: A prospective cohort study. Nutr Metabol Cardiovasc Dis. 2015;25(6):594–601. doi: 10.1016/j.numecd.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Wennberg A.M., Gustafson D., Hagen C.E., Roberts R.O., Knopman D., Jack C., et al. Serum adiponectin levels, neuroimaging, and cognition in the Mayo clinic study of aging. J Alzheimers Dis. 2016;53(2):573–581. doi: 10.3233/JAD-151201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins K.H., Gui C., Ely E.V., Lenz K.L., Harris C.A., Guilak F., et al. Leptin mediates the regulation of muscle mass and strength by adipose tissue. J Physiol. 2022;600(16):3795–3817. doi: 10.1113/JP283034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karvonen-Gutierrez C.A., Zheng H., Mancuso P., Harlow S.D. Higher leptin and adiponectin concentrations predict poorer performance-based physical functioning in midlife women: The Michigan study of Women's health across the nation. J Gerontol A Biol Sci Med Sci. 2016;71(4):508–514. doi: 10.1093/gerona/glv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lana A., Struijk E., Guallar-Castillon P., Martin-Moreno J.M., Rodriguez Artalejo F., Lopez-Garcia E. Leptin concentration and risk of impaired physical function in older adults: The Seniors-ENRICA cohort. Age Ageing. 2016;45(6):819–826. doi: 10.1093/ageing/afw142. [DOI] [PubMed] [Google Scholar]
- 72.Tazawa R., Uchida K., Fujimaki H., Miyagi M., Inoue G., Sekiguchi H., et al. Elevated leptin levels induce inflammation through IL-6 in skeletal muscle of aged female rats. BMC Muscoskelet Disord. 2019;20(1):199. doi: 10.1186/s12891-019-2581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins A.J., Fu D., Azar M., Stoner J.A., Kaufman D.G., Zhang S., et al. Clinical correlates of serum pigment epithelium-derived factor in type 2 diabetes patients. J Diabet Complicat. 2014;28(3):353–359. doi: 10.1016/j.jdiacomp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui E., Yeung C.-Y., Lee P.C., Woo Y.-C., Fong C.H., Chow W.-S., et al. Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metabol. 2014;99(11):E2169–E2177. doi: 10.1210/jc.2014-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Famulla S., Lamers D., Hartwig S., Passlack W., Horrighs A., Cramer A., et al. Pigment epithelium-derived factor (PEDF) is one of the Most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes. 2011;35(6):762–772. doi: 10.1038/ijo.2010.212. [DOI] [PubMed] [Google Scholar]
- 76.Karathanasis S.K., Yunis I., Zannis V.I. Structure, evolution, and tissue-specific synthesis of human apolipoprotein AIV. Biochemistry. 1986;25(13):3962–3970. doi: 10.1021/bi00361a034. [DOI] [PubMed] [Google Scholar]
- 77.Kohan A.B., Wang F., Lo C.M., Liu M., Tso P. ApoA-IV: current and emerging roles in intestinal lipid metabolism, glucose homeostasis, and satiety. Am J Physiol Gastrointest Liver Physiol. 2015;308(6):G472–G481. doi: 10.1152/ajpgi.00098.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Recalde D., Ostos M.A., Badell E., Garcia-Otin A.L., Pidoux J., Castro G., et al. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24(4):756–761. doi: 10.1161/01.ATV.0000119353.03690.22. [DOI] [PubMed] [Google Scholar]
- 79.Stout M.B., Justice J.N., Nicklas B.J., Kirkland J.L. Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32(1):9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spyridaki E.C., Avgoustinaki P.D., Margioris A.N. Obesity, inflammation and cognition. Curr Opin Behav Sci. 2016;9:169–175. [Google Scholar]
- 81.Szalay K., Rázga Z., Duda E. TNF inhibits myogenesis and downregulates the expression of myogenic regulatory factors myoD and myogenin. Eur J Cell Biol. 1997;74(4):391–398. [PubMed] [Google Scholar]
- 82.Steinberg G.R., Michell B.J., van Denderen B.J., Watt M.J., Carey A.L., Fam B.C., et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4(6):465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Cui Y., Huang M., He Y., Zhang S., Luo Y. Genetic ablation of apolipoprotein A-IV accelerates Alzheimer's disease pathogenesis in a mouse model. Am J Pathol. 2011;178(3):1298–1308. doi: 10.1016/j.ajpath.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W.S., You J., Chen S.D., Zhang Y., Feng J.F., Xu Y.M., et al. Plasma proteomics identify biomarkers and undulating changes of brain aging. Nat Aging. 2024;5(1):99–112. doi: 10.1038/s43587-024-00753-6. [DOI] [PubMed] [Google Scholar]
- 85.Yu L., Wan Q., Liu Q., Fan Y., Zhou Q., Skowronski A.A., et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 2024;36(4):793–807 e5. doi: 10.1016/j.cmet.2024.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma S., Ji Z., Zhang B., Geng L., Cai Y., Nie C., et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell. 2024;187(24):7025–7044. doi: 10.1016/j.cell.2024.10.019. [DOI] [PubMed] [Google Scholar]
- 87.Yan J., Yan F., Li Z., Sinnott B., Cappell K.M., Yu Y., et al. The 3M complex maintains microtubule and genome integrity. Mol Cell. 2014;54(5):791–804. doi: 10.1016/j.molcel.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun K., Li X., Scherer P.E. Extracellular matrix (ECM) and fibrosis in adipose tissue: Overview and perspectives. Compr Physiol. 2023;13(1):4387–4407. doi: 10.1002/cphy.c220020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tinahones F.J., Coín-Aragüez L., Mayas M.D., Garcia-Fuentes E., Hurtado-del-Pozo C., Vendrell J., et al. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12(1):1–8. doi: 10.1186/1472-6793-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ritter A.M., de Faria A.P., Barbaro N., Sabbatini A.R., Correa N.B., Brunelli V., et al. Crosstalk between obesity and MMP-9 in cardiac remodelling -a cross-sectional study in apparent treatment-resistant hypertension. Blood Press. 2017;26(2):122–129. doi: 10.1080/08037051.2016.1249336. [DOI] [PubMed] [Google Scholar]
- 91.Ahn C., Ryan B.J., Schleh M.W., Varshney P., Ludzki A.C., Gillen J.B., et al. Exercise training remodels subcutaneous adipose tissue in adults with obesity even without weight loss. J Physiol. 2022;600(9):2127–2146. doi: 10.1113/JP282371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imbert A., Vialaneix N., Marquis J., Vion J., Charpagne A., Metairon S., et al. Network analyses reveal negative link between changes in adipose tissue GDF15 and BMI during dietary-induced weight loss. J Clin Endocrinol Metab. 2022;107(1):e130–e142. doi: 10.1210/clinem/dgab621. [DOI] [PubMed] [Google Scholar]
- 93.Toba H., Cannon P.L., Yabluchanskiy A., Iyer R.P., D'Armiento J., Lindsey M.L. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol. 2017;312(3):H375–H383. doi: 10.1152/ajpheart.00633.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yabluchanskiy A., Ma Y., DeLeon-Pennell K.Y., Altara R., Halade G.V., Voorhees A.P., et al. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol: Series A. 2015;71(4):475–483. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahn C., Zhang T., Yang G., Rode T., Varshney P., Ghayur S.J., et al. Years of endurance exercise training remodel abdominal subcutaneous adipose tissue in adults with overweight or obesity. Nat Metab. 2024;6(9):1819–1836. doi: 10.1038/s42255-024-01103-x. [DOI] [PubMed] [Google Scholar]
- 96.Magkos F., Fraterrigo G., Yoshino J., Luecking C., Kirbach K., Kelly S.C., et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johansen F.E., Braathen R., Brandtzaeg P. Role of J chain in secretory immunoglobulin formation. Scand J Immunol. 2000;52(3):240–248. doi: 10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 98.Ursino G., Lucibello G., Teixeira P.D., Höfler A., Veyrat-Durebex C., Odouard S., et al. S100A9 exerts insulin-independent antidiabetic and anti-inflammatory effects. Sci Adv. 2024;10(1) doi: 10.1126/sciadv.adj4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dumurgier J., Elbaz A., Ducimetiere P., Tavernier B., Alperovitch A., Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339 doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdelaal M., le Roux C.W., Docherty N.G. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Newman A.B., Blackwell T.L., Mau T., Cawthon P.M., Coen P.M., Cummings S.R., et al. Vigor to frailty as a Continuum-A new approach in the study of muscle, mobility, and aging cohort. J Gerontol A Biol Sci Med Sci. 2024;79(1) doi: 10.1093/gerona/glad244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolf C., Blackwell T.L., Johnson E., Glynn N.W., Nicklas B., Kritchevsky S.B., et al. Cardiopulmonary exercise testing in a prospective multicenter cohort of older adults. Med Sci Sports Exerc. 2024;56(9):1574–1584. doi: 10.1249/MSS.0000000000003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark R.V., Walker A.C., O'Connor-Semmes R.L., Leonard M.S., Miller R.R., Stimpson S.A., et al. Total body skeletal muscle mass: Estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol. 2014;116(12):1605–1613. doi: 10.1152/japplphysiol.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zamora Z., Lui L.Y., Sparks L.M., Justice J., Lyles M., Gentle L., et al. Percutaneous biopsies of skeletal muscle and adipose tissue in individuals older than 70: methods and outcomes in the study of muscle, mobility and aging (SOMMA) Geroscience. 2024;46(3):3419–3428. doi: 10.1007/s11357-024-01087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 106.Langfelder P., Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.