Abstract
Host parasitism by Trichomonas vaginalis is complex, and the adhesion to vaginal epithelial cells (VECs) by trichomonads is preparatory to colonization of the vagina. Since we showed increased synthesis of adhesins after contact with VECs (A. F. Garcia, et al., Mol. Microbiol. 47:1207-1224, 2003) and more recently demonstrated up-regulated gene expression in VECs after parasite attachment (A. S. Kucknoor, et al., Cell. Microbiol. 7:887-897, 2005), we hypothesized that enhanced expression of adhesin and other genes would result from signaling of trichomonads following adherence. In order to identify the genes that are up-regulated, we constructed a subtraction cDNA library enriched for differentially expressed genes from the parasites that were in contact with the host cells. Thirty randomly selected cDNA clones representing the differentially regulated genes upon initial contact of parasites with host cells were sequenced. Several genes encoded functional proteins with specific functions known to be associated with colonization, such as adherence, change in morphology, and gene transcription and translation. Interestingly, genes unique to trichomonads with unknown functions were also up-regulated. Semiquantitative reverse transcription-PCR (RT-PCR) confirmed expression of select genes. An increased amount of protein was demonstrated by immunoblotting with monoclonal antibody. Finally, we showed the transcriptional regulation of some genes by iron by using RT-PCR. To our knowledge, this is the first report addressing the differential regulation of T. vaginalis genes immediately upon contact with VECs.
Trichomonas vaginalis colonizes the urogenital tract of humans, causing trichomonosis, the number one nonviral sexually transmitted disease worldwide. Despite an estimated 8 million new cases per year in the United States alone (53), this health disparities disease (48) remains poorly studied. T. vaginalis infection is associated with adverse health consequences to both men and women, including infertility (20, 46), atypical pelvic inflammatory disease (35), and increased human immunodeficiency virus transmission (24, 49). Trichomonosis is also associated with preterm birth, low-birth-weight infants (16), predisposition to development of cervical neoplasia in women (51), and nongonococcal urethritis (9) and chronic prostatitis (10) in men. The complex interplay of trichomonad responses and that of the host cells during infection have not been investigated so far. Of the few reports concerning host-parasite interactions, one study showed that after short contact of trichomonads with host vaginal epithelial cells (VECs), but not HeLa cells, the parasite morphology is transformed from an ellipsoid to an amoeboid form (8), suggesting host-specific signaling of parasites. In addition, synthesis of all four adhesin proteins in the amoeboid forms bound to VECs was enhanced (8, 22). In a separate study using different forms of parasites grown in culture flasks, α-actinin was shown to be overexpressed in amoeboid parasites compared to batch-cultured ellipsoid trichomonads (1).
The transcriptional regulation of parasite genes in response to interactions with host cells using in vitro models has been studied in Neisseria meningitides (23), Porphyromonas gingivalis (25, 41), and Helicobacter pylori (26). Due to the lack of a good animal model system to study T. vaginalis pathogenesis, we have used an in vitro model of immortalized human VECs (22) in our present study. Because of the unavailability of genome sequence data at the time this study was initiated, we used the subtraction cDNA library approach to identify the transcriptional changes in gene expression during the initial step of T. vaginalis attachment to VECs. Differentially expressed gene profiling using cDNA subtraction has been an ideal tool in identifying novel genes and transcripts of low abundance (13). Our data identify numerous T. vaginalis genes that are up-regulated upon contact, which was confirmed by semiquantitative reverse transcription-PCR (RT-PCR) and protein immunoblot analyses. We believe that functional analyses of up-regulated genes of both VECs, as done recently (29), and of the organisms after the adherence event will contribute to our understanding of the host-pathogen interrelationship and the elucidation of the mechanisms of pathogenesis.
MATERIALS AND METHODS
Parasites and host cells.
T. vaginalis isolate T016 was grown in trypticase-yeast extract-maltose (TYM) medium supplemented with 10% serum at 37°C (19). For iron-replete parasites, TYM serum was supplemented with 200 μM ferrous ammonium sulfate, and iron-depleted parasites were obtained by cultivation in medium depleted of iron with 50 μM 2,2-dipyridyl (32). Immortalized MS-74 human VECs (22, 27, 29, 36) used for adherence experiments were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum at 37°C in the presence of 5% CO2.
Isolation of total RNA.
The MS-74 VECs were used for adherence as recently detailed (22). Briefly, 6 × 105 VECs were seeded onto T75 culture flasks and allowed to form a monolayer for 2 days. VECs were then washed with a medium mixture of Dulbecco's modified Eagle's medium-TYM (2:1, vol/vol) without serum. Parasites at the mid-logarithmic phase of growth were added to the MS-74 monolayer at a parasite-VEC ratio of 10:1 and incubated at 37°C to allow for parasite adherence. Parasites were incubated with the VECs for 30 min, and nonadherent parasites were removed by aspiration. The monolayer with adherent parasites was further incubated for 2 h until the monolayer was completely lysed. The parasites after interaction with the monolayer are referred to as primed parasites. Total RNA from control and primed parasites after contact with VECs was isolated using Trizol reagent (Invitrogen).
Construction of subtraction cDNA library.
The cDNA from primed (tester cDNA) and control (driver cDNA) parasites was prepared using a cDNA synthesis system (Roche Diagnostics Inc.). Briefly, 2 μg of total RNA was reverse transcribed using an oligo(dT)15 primer and avian myeloblastosis virus reverse transcriptase according to the manufacturer's protocol. The subtractive cDNA library was constructed according to the standard protocol (52). Briefly, each set of cDNA was digested with AluI and RsaI to generate shorter, blunt-ended cDNA fragments. The tester DNA and driver DNA were ligated with different sets of adaptors. Ligated cDNA was amplified by PCR using the adaptor primers to obtain large amounts of cDNA. The tester cDNA was labeled with [32P]dCTP, and the driver cDNA was biotinylated using 0.5 mM bio-11-dUTP (Biotium Inc.). A first hybridization between an excess of driver and tester was carried out to enrich the differentially expressed sequences. Tester/driver and driver/driver hybrids and biotinylated single-stranded driver cDNA were removed by addition of streptavidin and extraction with phenol-chloroform. The percentage of tester cDNA removed was determined by the radioactive counts remaining after phenol-chloroform extraction. Further rounds of subtraction were performed using the cDNAs from the previous round as a template for PCR synthesis of tester and driver cDNAs. After 10 rounds of alternating long and short hybridizations, the subtracted cDNA fraction was cloned into a TA vector and transformed into Escherichia coli to create a primed T. vaginalis (pTv) cDNA library.
PCR analysis of the subtraction efficiency.
PCR was performed on subtracted and unsubtracted cDNA with α-tubulin primers (Table 1) as an internal control. Each reaction was amplified for 30 cycles, and 5-μl aliquots were removed after 15, 20, and 25 cycles. The PCR products were electrophoresed on 1% agarose gels followed by staining with ethidium bromide (EtBr). The efficiency of subtraction was reflected by the difference in the number of cycles required for equal amplification of the PCR product in subtracted and unsubtracted samples.
TABLE 1.
Primers used for RT-PCR analyses
| Gene | Primer sequence (5′-3′) | Annealing temp (°C) | Amplicon size (bp) |
|---|---|---|---|
| α-Actinin | AAGTTCGGACGTGTTGTTCGTCTGGGCCTGCTGGAGGTAGTTG | 48 | 400 |
| α-Tubulin | GAAATGACTGGTGCATAAGAGCACTCTGCTGCCTCGAGCACGGTA | 48 | 700 |
| AP33 | CTCATTTTCGTCC CAGCTCCAAACAATACCGATCTTACCG | 45 | 300 |
| AP65 | CAGTTAGATATGGGTACAGACCGCTCGCAGTTAGCGCATGTAG | 48 | 650 |
| GTP-BP | ACACTT ACACCAGTTCCTGGCTTGTTCAACACTAGAAGTAATTG | 46 | 300 |
| PDI | TACTCCTTCACAGAAATCACTATGAAGAACA CCGAATTTGTA | 42 | 300 |
Sequencing and analysis.
Colonies were randomly selected, and plasmids were prepared using a Miniprep kit (QIAGEN, Valencia, CA). The cDNA inserts were verified by restriction digestion, and the clones were sequenced in our institutional DNA-sequencing facility. First, the sequence data were compared with data in GenBank using a BLAST program. Since there were not many matches, we then used the TIGR database, which consisted of partially annotated T. vaginalis genome sequences (http://tigrblast.tigr.org/er-BLAST/index.cgi?project=tvg).
RT-PCR analysis of selected genes.
Differential expression of a subset of cloned genes was confirmed by semiquantitative RT-PCR. Total RNA from control nonprimed and primed parasites was reverse transcribed with the oligo(dT)15 primer using Superscript II reverse transcriptase (Invitrogen), according to the manufacturer's protocol. PCR amplification of cDNA was carried out using gene-specific primers (Table 1). The trichomonad α-tubulin gene was used as an internal control. RT-PCR from iron-depleted and iron-replete organisms was also performed as explained above. Twenty-five cycles were used for amplification of specific genes (see Fig. 2), except for the protein disulfide isomerase (PDI) gene, which was amplified for 35 cycles because of its low levels of expression. As a control, all reactions were spiked with a known amount of human DNA, and RT-PCR was performed using α-tubulin primers. This assured the abundance of PCR product detected under these experimental conditions with the known primers. In addition, all RNA samples without reverse transcription were also used for PCR to detect genomic DNA contamination, if any. PCR products were visualized on EtBr-stained agarose gels, and the band intensity was quantitated using the Scion image beta program. The PCRs were carried out at four different times to verify the reproducibility of results. The result from one of the experiments is used for the data presentation.
FIG. 2.
Representative experiment showing confirmation of gene expression patterns in primed (pTv) and control (T. vaginalis [Tv]) parasites by semiquantitative RT-PCR analyses. Total RNA from pTv and T. vaginalis was isolated as detailed in Materials and Methods. RNA was reverse transcribed using oligo(dT) primer, and PCR was performed using gene-specific primers (Table 1). (A) RT-PCR products separated on EtBr-stained gels after electrophoresis in 2% agarose. (B) Gene expression pattern relative to the housekeeping trichomonad α-tubulin gene used as a control. The values were obtained by scanning the intensities of bands from pictures of the gels in A using the Scion image beta program. The expression for each gene was relative to baseline density for α-tubulin plotted on the graph. This experiment was repeated on four separate occasions with similar results.
Production of MAb HA423 against α-actinin.
Hybridomas producing monoclonal antibodies (MAbs) against the α-actinin protein were obtained using standard protocols, as we have described previously (4). The MAb HA423 was identified by screening supernatants of antibody-producing hybridomas with different preparations of high-Mr protein antigens eluted from acrylamide gels after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and using anti-mouse immunoglobulin G followed by alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G. After subsequent single-cell cloning, the hybridomas were then reacted with E. coli expressing the recombinant protein from the full-length α-actinin gene (1).
SDS-PAGE and immunoblotting.
Lysates from identical numbers of control and primed parasites were separated by 10% SDS-PAGE, and the proteins were blotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA). The nitrocellulose blots were then incubated in a solution containing 0.1% Tween 20 and 5% bovine serum albumin prior to probing with the MAbs F5, DM116, and HA423, specific for adhesin protein 33 (AP33), AP65, and anti-α-actinin, respectively, at a dilution of hybridoma supernatant of 1:1,000. As a protein loading control, an identical blot was probed with human α-tubulin MAb clone B512 (Sigma, St. Louis, MO) at a dilution of 1:50. The blots were further incubated with goat anti-mouse secondary antibody (1:1,500 final dilution) conjugated with horseradish peroxidase (Bio-Rad). The blots were washed well and incubated in horseradish peroxidase substrate (Bio-Rad) to visualize the reactive band.
RESULTS
PCR-based subtraction cDNA library.
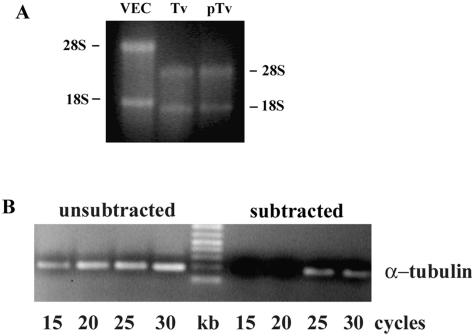
We used a subtractive cDNA library to obtain T. vaginalis genes that were up-regulated in response to contact with VECs. Total RNA from the primed parasites free of host cell RNA was isolated as detailed in Materials and Methods. Figure 1A shows the RNA without any apparent degradation on an agarose gel. Importantly, there was no contamination of host RNA in the trichomonad RNA preparations. Following reverse transcription, a cDNA library was constructed by subtracting the cDNA of T. vaginalis parasites incubated with VECs (referred to as primed T. vaginalis, or pTv) from control cDNA of T. vaginalis handled identically. The subtraction methodology involved suppression of PCR amplification of the common sequences. The amplified cDNAs were ligated to the TA vector to create the library. About 250 clones were obtained in the first round of plating from the transformed cDNA library. The average size of cDNA inserts was ∼200 bp. The subtraction efficiency was estimated by comparing the abundance of a known gene prior to and after subtraction. We amplified the T. vaginalis α-tubulin housekeeping gene as a control from subtracted and unsubtracted cDNA. Figure 1B shows that the PCR product for α-tubulin is detectable only after ≥25 cycles in the subtracted sample, while it is readily detectable after ≤15 cycles in the unsubtracted sample, indicating successful subtraction efficiency.
FIG. 1.
RNA isolation and PCR analysis of subtraction efficiency. (A) Total RNA was isolated from primed parasites after contact with VECs (pTv) and control organisms handled identically (T. vaginalis [Tv]). Total RNA (3 μg) was separated on a 1.2% agarose gel followed by staining with EtBr to visualize the purity and assess degradation of RNA. 28S and 18S refer to the rRNA bands of the VECs and the parasites. (B) PCR using α-tubulin primers was performed on unsubtracted and subtracted cDNAs. Aliquots (5 μl) were removed at a predetermined number of cycles and analyzed by EtBr-stained gels after electrophoresis in 1.2% agarose. The α-tubulin product appeared only after 25 cycles in the subtracted sample compared to 15 cycles in the unsubtracted sample.
T. vaginalis gene expression regulated by attachment to VECs.
A total of 30 rescued plasmid clones selected at random were sequenced. The sequences were used to perform BLAST searches of the GenBank database and the incomplete TIGR T. vaginalis genome database. The list of genes that were identified is shown in Table 2. The up-regulated genes exhibited homologies with the genomic sequences or expressed sequence tags encoding various functional classes of proteins. Interestingly, only 33% of the genes were previously documented in GenBank, and these included the adhesins AP65 and AP33, α-actinin, and enolase. A putative PDI gene, a phospoglucomutase family protein involved in glucose metabolism, and a conserved GTP-binding protein (GTP-BP) were up-regulated. Genes involved in transcription and protein translation in addition to six genes with unknown functions were also elevated in expression. The identification of adhesin genes in the subtraction library validates the increased amounts of adhesins after adherence (8, 22).
TABLE 2.
Genes upregulated in T. vaginalis upon early stages of cytoadherence to MS-74 VECs
| Gene product | Accession no.b | Description and/or putative function |
|---|---|---|
| Genes with known function | ||
| AP65 (2)a | U35243 | Adhesin, decarboxylating malic enzyme |
| AP33 (3) | U87098 | Adhesin, α-subunit of succinyl coenzyme A synthetase |
| α-actinin (3) | AF072678 | Cell structure maintenance |
| Enolase (2) | AF159525 | Glycolytic enzyme |
| PDI (1) | 74220.m00007 | Disulfide formation, isomerization, chaperone |
| (GTP-BP) (2) | 40053.m00110 | Protein sorting, endocytosis |
| Phosphoglucomutase family protein (1) | 43341.m00129 | Glucose metabolism |
| Transcription | ||
| ATP-dependant RNA helicase (Dbp5) (1) | 61898.m00047 | Gene transcription |
| Protein kinase domain protein (2) | 52196.m00022 | Gene transcription/cell signalling |
| Translation | ||
| Acidic ribosomal protein p2-β | 39445.m00044 | Protein synthesis |
| Glutamyl tRNA synthetase (1) | 40147.m00031 | Protein synthesis |
| Ribosomal protein L-16, cytosolic (1) | 38379.m00089 | Protein synthesis |
| Translation elongation factor EF-1 (3) | 43366.m00104 | Protein synthesis |
| Unknown | ||
| Conserved hypothetical protein (1) | 55466.m00002 | Unknown |
| Hypothetical protein (1) | 56189.m00008 | Unknown |
| Hypothetical protein (2) | 40839.m00207 | Unknown |
| Large supernatant protein 1 (1) | 37893.m00027 | Unknown |
| Pollen-specific glycine-rich protein (1) | 52891.m00042 | Unknown |
| Unnamed protein product (1) | 41853.m00030 | Unknown |
Numbers of copies of the clone picked up out of total 30 clones sequenced are shown in parentheses.
Accession numbers listed are from GenBank and the TIGR database.
RT-PCR confirms increased gene expression.
Relative levels of transcription of specific genes were analyzed by semiquantitative RT-PCR. As mentioned above, AP65 and AP33 were selected because of increased synthesis of adhesins upon contact with host cells (7, 22). In addition, we chose α-actinin, GTP-BP, and PDI genes based on a possible role in adherence and the functional role played by these enzymes in other organisms. RT-PCR products of the selected genes were separated and visualized on ethidium bromide-stained gels, and as shown in Fig. 2A, band intensities were greater in PCR products from primed parasites compared to those of controls. Furthermore, the bar graph in Fig. 2B illustrates the higher levels of increased expression for these genes relative to α-tubulin, as quantitated by the Scion image beta program. Compared to unprimed control RT-PCR products, the range of increased expression varied from twofold (α-actinin) to sevenfold (PDI). These data reaffirm the up-regulation of genes identified by the subtraction library.
Verification of protein expression of up-regulated genes.
We felt that it was important to verify elevated levels of transcription with amounts of those proteins where MAbs were available. Figure 3 shows that MAbs F5, DM116, and HA423 against AP33, AP65, and α-actinin, respectively, readily detected each corresponding protein on nitrocellulose blots containing total proteins of control unprimed versus primed T. vaginalis parasites. Increased amounts of proteins based on the intensities of the bands for identical numbers of parasites loaded onto gels were obtained for the primed trichomonads. As an additional control, MAb that was reactive to the trichomonad α-tubulin was used similarly, and identical intensities of protein bands were obtained for control and primed parasites, indicating specific upregulation of expression of AP33, AP65, and α-actinin upon contact. Negative control experiments were performed on duplicate nitrocellulose blots using secondary antibody alone and were unreactive with any trichomonad proteins. These data suggest that signaling of parasites upon contact with VECs is evidenced by increased transcription of genes and corresponding translation of transcripts to protein.
FIG. 3.
Detection of protein expression in T. vaginalis upon adherence to VECs. Cell lysates were prepared from equivalent numbers of primed (pTv) and control (T. vaginalis [Tv]) parasites prior to SDS-PAGE and blotting onto nitrocellulose membranes, as described in Materials and Methods. Quadruplicate blots were probed with the MAbs F5, DM116, HA423, and B512, specific for AP33, AP65, α-actinin, and α-tubulin, respectively. The numbers indicated on the left-hand side are the molecular mass standards. A negative control without MAb and with only secondary antibody was always nonreactive in duplicate blots (data not shown).
Effect of iron on gene regulation.
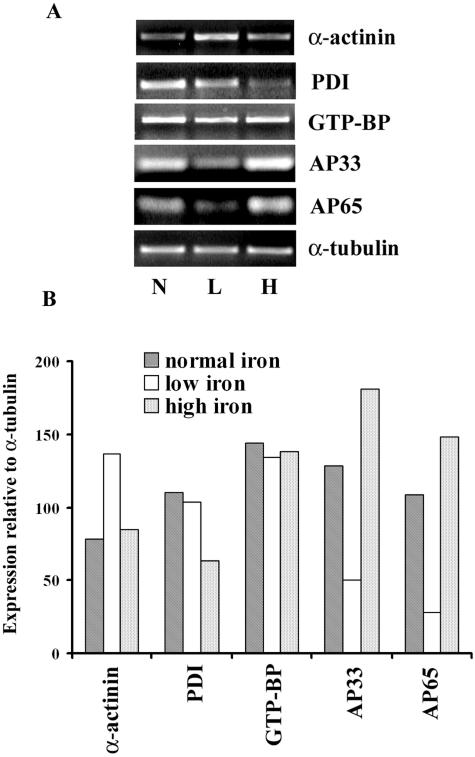
The genes for the AP65 and AP33 adhesins were up-regulated upon contact with the host cells, and both also are known to be transcriptionally regulated by iron (22, 31). We therefore wanted to examine whether other genes that were increased in expression upon contact were equally modulated by iron. For trichomonads grown in medium limited in or supplemented with iron, we performed RT-PCR on the four other genes with the AP65 and AP33 genes as controls. Only the adhesin genes for AP65 and AP33 gave decreased and increased amounts of transcript for parasites grown in the presence of low- compared to high-iron medium, respectively (Fig. 4A). The intensity of RT-PCR products for the internal control α-tubulin gene and the GTP-binding protein remained unaltered, although the gene for GTP-binding protein was expressed at higher levels above α-tubulin. Interestingly, the α-actinin gene had a 60% higher level of expression based on the amount of transcript in low-iron-grown parasites than those of normal or high-iron organisms (Fig. 4B). Last, the amounts of transcript for PDI were decreased when trichomonads were grown in high-iron medium. These results reinforce the idea of multiple signaling and/or regulatory pathways influencing the expression of genes induced by contact of organisms with host cells.
FIG. 4.
Representative experiment showing the effect of iron on regulation of selected genes. Total RNA from parasites grown in normal TYM medium (N), iron-depleted medium (L), and iron-replete medium (H) was used in RT-PCR. (A) PCR products were separated on 2% agarose gels for visualization after EtBr staining. (B) Bar graph plotted using the relative values of band intensity obtained by Scion image beta scanning. Again, all values were normalized to the band intensity of α-tubulin as a control. This experiment was repeated on four separate occasions with similar results.
DISCUSSION
Specific adherence by Trichomonas vaginalis to epithelial cells of the vagina is mediated by surface proteins (7). We believe that dissection of the cross talk between the host cells and the adhering parasites is important to understand the mechanisms of pathogenesis, and this knowledge is prerequisite for intervention strategies. Recently, it was demonstrated that numerous genes of VECs are up-regulated in response to contact by parasites (29). We now describe the use of subtraction cDNA hybridization to evaluate the signaling and subsequent transcriptional responses of the parasite after adherence to host cells, similar to what we reported recently for VEC genes (29). We compared gene expression in T. vaginalis adhering to human VECs with that of nonadherent parasites that were handled identically. Interestingly, the up-regulated genes with known functions appear to be related to events known to result from adherence. The fact that adhesin genes accounted for 5 of 30 randomly selected clones reinforces the important role of these proteins in infection and increased synthesis and placement of adhesins on the parasite surface after contact with VECs (8, 22). Indeed, these results strongly support past (5, 7, 21-23) and recent genetic studies using antisense RNA silencing of AP65 gene expression (36) and heterologous expression in Tritrichomonas fetus of AP65 to show a prominent role of this protein in adherence (30). Furthermore, 3 out of 30 clones were identified as α-actinin, which is known to have enhanced expression in the amoeboid forms of trichomonads (1).
We also picked up two clones of the poorly characterized T. vaginalis enolase gene. Importantly, recent studies have shown that enolase has functional diversity and is a plasminogen-binding protein in other pathogenic organisms, including Fasciola hepatica (12), Streptococcus spp. (11, 39), and Staphylococcus aureus (34). In addition, enolase acts as a spreading factor during tissue invasion of group A streptococci (40). Of particular interest to us will be future localization and functional analyses of the trichomonad enolase to determine whether there is an alternative, nonenzymatic role, if any, in surface expression and parasite adherence. The fact that the gene for GTP-binding protein was also up-regulated indicates an important role for this gene product during early events of infection. GTP-binding protein is mainly involved in the endocytic and vacuolar protein-sorting pathways (47) in higher eukaryotes and also is involved in signal transduction pathways (15). As both signaling and compartmentalization of adhesins are mediated by contact and iron (8, 22, 32), it is conceivable that this GTP-binding protein may likewise play an essential role in adherence.
Moreover, the finding that PDI was up-regulated in expression may be noteworthy, given the important function of these proteins in the complex processes of protein folding for biological functions (28). Little is known about protein folding in T. vaginalis, and, although speculative, it is likely that trafficking, compartmentalization, and surface placement of adhesins may require proteins such as PDI. It has been proposed that PDIs confer protection as an important survival mechanism against oxidative stress and a denaturing environment for F. hepatica (45) and Giardia lamblia (28). Equally noteworthy are the chaperone and nonchaperone activities of PDIs in addition to the disulfide bond isomerization. Our finding, therefore, highlights the possibly critical role of these multifunctional PDI proteins in initial steps of colonization. Finally, the fact that genes with unknown functions specific for T. vaginalis respond to parasite contact with VECs perhaps highlights the importance of these proteins both in the property of adherence and in pathogenesis. These findings make evident the need for structure-function characterization of these novel virulence factors heretofore not identified previously.
The finding that the genes for AP65, AP33, α-actinin, GTP-BP, and PDI were up-regulated in expression was consistent with the relative abundance of the clones in the subtraction library, and indeed, AP65, AP33, and α-actinin also had increased amounts of protein. The coordinated up-regulation of α-actinin with the adhesin genes is not surprising given the involvement of α-actinin in cytoskeletal rearrangements for transformation from ellipsoid to amoeboid forms and for recruitment and surface placement of adhesins following contact (1).
In the vagina, the menstrual cycle likely plays a role in modulating the availability of iron sources and other nutrients (14) essential for optimal parasite viability and colonization (5, 33, 42-44). Despite the fact that both high- and low-iron environments are found in the vagina (31), most trichomonads during infection are high iron (2, 3). Accordingly and not surprisingly, an iron-responsive promoter element regulating the expression of the prominent AP65 adhesin gene of T. vaginalis has been characterized previously (50). While the AP65 and AP33 genes had increased amounts of mRNA for parasites grown in high-iron medium (6, 21, 37), it was previously hypothesized that the increased synthesis of adhesins by parasites after attachment may be due to utilization of intracellular iron pools (7). Alternatively, the data may indicate the possibility of two distinct signaling pathways for up-regulation of expression of genes, i.e., iron and contact. The fact that the α-actinin gene had elevated amounts of transcript for low-iron trichomonads is also consistent with the notion of at least two signaling pathways modulating α-actinin gene expression (1, 7). Given the role of α-actinin in maintenance of the amoeboid form during adherence to VECs, it is conceivable that a dual signaling system for increased expression of α-actinin permits sustained adherence when organisms are in a low-iron niche. In this scenario, trichomonads are unable to synthesize new adhesins because of low iron yet retain the amoeboid morphology for maximum surface interaction and utilize existing adhesins for optimal attachment to VECs. In contrast, PDI had decreased amounts of transcript when organisms were grown in high-iron medium. To our knowledge, there are no reports of such regulation of PDI in other systems (28, 45). It is possible that this reduction in amounts of transcript may be a posttranscriptional event regulated in part by iron. Furthermore, since PDI is a highly stable protein with a half-life of approximately 7 days in rat liver (38), the decreased transcript amounts may not be problematic for PDI-mediated protein folding and other functions. The fact that GTP-binding protein remained unaltered under all the conditions tested may indicate a need for steady-state levels of this protein at all times to ensure the proper sorting and compartmentalization of proteins (47) regardless of environmental cues.
Collectively, these results indicate that there are different mechanisms of gene regulation for parasites in the complex, constantly changing environment of the vagina (14). That the gene regulation patterns are altered to achieve optimal host parasitism regardless of external stimuli represents a theme of the T. vaginalis organisms (2, 3, 5, 8, 17, 18, 31, 32). In conclusion, we have identified a set of known and unknown parasite genes that are up-regulated after contact with host cells. Although the functional role of some of these gene products in terms of parasite adherence and pathogenesis remains to be determined, we feel that identification of both parasite and VEC-induced genes (29) after a crucial step preparatory for successful infection will help us elucidate and understand the complex host-parasite interrelationship.
Acknowledgments
This work was supported by Public Health Service grants AI43940 and AI45429 from the National Institutes of Health.
Members of the laboratory are acknowledged for their suggestions and discussion of our work. The excellent assistance by Leo Chang is acknowledged for work involving the monoclonal antibodies, especially the new MAb against α-actinin.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Addis, M. F., P. Rappelli, G. Delogu, F. Carta, P. Cappuccinelli, and P. L. Fiori. 1998. Cloning and molecular characterization of a cDNA clone coding for Trichomonas vaginalis alpha-actinin and intracellular localization of the protein. Infect. Immun. 66:4924-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete, J. F. 1999. Iron modulates phenotypic variation and phosphorylation of P270 in double-stranded RNA virus-infected Trichomonas vaginalis. Infect. Immun. 67:4298-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderete, J. F., P. Demes, A. Gombosova, M. Valent, A. Yanoska, H. Fabusova, L. Kasmala, G. E. Garza, and E. C. Metcalfe. 1987. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect. Immun. 55:1037-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderete, J. F., and L. Kasmala. 1986. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect. Immun. 53:697-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderete, J. F., J. Nguyen, V. Mundodi, and M. W. Lehker. 2004. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263-271. [DOI] [PubMed] [Google Scholar]
- 6.Alderete, J. F., J. L. O'Brien, R. Arroyo, J. A. Engbring, O. Musatovova, O. Lopez, C. Lauriano, and J. Nguyen. 1995. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 17:69-83. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo, R., J. Engbring, and J. F. Alderete. 1992. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol. Microbiol. 6:853-862. [DOI] [PubMed] [Google Scholar]
- 8.Arroyo, R., A. Gonzalez-Robles, A. Martinez-Palomo, and J. F. Alderete. 1993. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol. Microbiol. 7:299-309. [DOI] [PubMed] [Google Scholar]
- 9.Bakare, R. A., J. O. Ashiru, F. A. Adeyemi-Doro, C. C. Ekweozor, A. A. Oni, A. O. Okesola, and J. A. Adebayo. 1999. Non-gonococcal urethritis (NGU) due to Trichomonas vaginalis in Ibadan. West Afr. J. Med. 18:64-68. [PubMed] [Google Scholar]
- 10.Bennett, J. R., W. G. Barnes, and S. Coffman. 1989. The emergency department diagnosis of Trichomonas vaginalis. Ann. Emerg. Med. 18:564-566. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273-1287. [DOI] [PubMed] [Google Scholar]
- 12.Bernal, D., J. E. de la Rubia, A. M. Carrasco-Abad, R. Toledo, S. Mas-Coma, and A. Marcilla. 2004. Identification of enolase as a plasminogen-binding protein in excretory-secretory products of Fasciola hepatica. FEBS Lett. 563:203-206. [DOI] [PubMed] [Google Scholar]
- 13.Cao, W., C. Epstein, H. Liu, C. DeLoughery, N. Ge, J. Lin, R. Diao, H. Cao, F. Long, X. Zhang, Y. Chen, P. S. Wright, S. Busch, M. Wenck, K. Wong, A. G. Saltzman, Z. Tang, L. Liu, and A. Zilberstein. 2004. Comparing gene discovery from Affymetrix GeneChip microarrays and Clontech PCR-select cDNA subtraction: a case study. BMC Genomics 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, M. S., B. E. Britigan, M. French, and K. Bean. 1987. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am. J. Obstet. Gynecol. 157:1122-1125. [DOI] [PubMed] [Google Scholar]
- 15.Colombo, M. I., J. Inglese, C. D'Souza-Schorey, W. Beron, and P. D. Stahl. 1995. Heterotrimeric G proteins interact with the small GTPase ARF. Possibilities for the regulation of vesicular traffic. J. Biol. Chem. 270:24564-24571. [DOI] [PubMed] [Google Scholar]
- 16.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, G. G. Rhoads, et al. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 17.Crouch, M. L., M. Benchimol, and J. F. Alderete. 2001. Binding of fibronectin by Trichomonas vaginalis is influenced by iron and calcium. Microb. Pathog. 31:131-144. [DOI] [PubMed] [Google Scholar]
- 18.Crouch, M. V., and J. F. Alderete. 2001. Trichomonas vaginalis has two fibronectin-like iron-regulated genes. Arch. Med. Res. 32:102-107. [DOI] [PubMed] [Google Scholar]
- 19.Diamond, L. S. 1957. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43:488-490. [PubMed] [Google Scholar]
- 20.El-Shazly, A. M., H. M. El-Naggar, M. Soliman, M. El-Negeri, H. E. El-Nemr, A. E. Handousa, and T. A. Morsy. 2001. A study on Trichomonas vaginalis and female infertility. J. Egypt. Soc. Parasitol. 31:545-553. [PubMed] [Google Scholar]
- 21.Engbring, J. A., and J. F. Alderete. 1998. Three genes encode distinct AP33 proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 28:305-313. [DOI] [PubMed] [Google Scholar]
- 22.Garcia, A. F., T. H. Chang, M. Benchimol, D. J. Klumpp, M. W. Lehker, and J. F. Alderete. 2003. Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis. Mol. Microbiol. 47:1207-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J. Berger, F. Randazzo, and G. Grandi. 2002. Gene expression profile in Neisseria meningitidis and Neisseria lactamica upon host-cell contact: from basic research to vaccine development. Ann. N. Y. Acad. Sci. 975:202-216. [DOI] [PubMed] [Google Scholar]
- 24.Hobbs, M. M., P. Kazembe, A. W. Reed, W. C. Miller, E. Nkata, D. Zimba, C. C. Daly, H. Chakraborty, M. S. Cohen, and I. Hoffman. 1999. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex. Transm. Dis. 26:381-387. [DOI] [PubMed] [Google Scholar]
- 25.Hosogi, Y., and M. J. Duncan. 2005. Gene expression in Porphyromonas gingivalis after contact with human epithelial cells. Infect. Immun. 73:2327-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, N., E. A. Marcus, Y. Wen, D. L. Weeks, D. R. Scott, H. C. Jung, I. S. Song, and G. Sachs. 2004. Genes of Helicobacter pylori regulated by attachment to AGS cells. Infect. Immun. 72:2358-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klumpp, D. J., S. G. Forrestal, J. E. Karr, C. S. Mudge, B. E. Anderson, and A. J. Schaeffer. 2002. Epithelial differentiation promotes the adherence of type 1-piliated Escherichia coli to human vaginal cells. J. Infect. Dis. 186:1631-1638. [DOI] [PubMed] [Google Scholar]
- 28.Knodler, L. A., R. Noiva, K. Mehta, J. M. McCaffery, S. B. Aley, S. G. Svard, T. G. Nystul, D. S. Reiner, J. D. Silberman, and F. D. Gillin. 1999. Novel protein-disulfide isomerases from the early-diverging protist Giardia lamblia. J. Biol. Chem. 274:29805-29811. [DOI] [PubMed] [Google Scholar]
- 29.Kucknoor, A. S., V. Mundodi, and J. F. Alderete. 2005. Trichomonas vaginalis adherence mediates differential gene expression in human vaginal epithelial cells. Cell. Microbiol. 7:887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucknoor, A. S., V. Mundodi, and J. F. Alderete. 2005. Heterologous expression in Tritrichomonas foetus of functional Trichomonas vaginalis AP65 adhesin. BMC Mol. Biol. 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehker, M. W., and J. F. Alderete. 1992. Iron regulates growth of Trichomonas vaginalis and the expression of immunogenic trichomonad proteins. Mol. Microbiol. 6:123-132. [DOI] [PubMed] [Google Scholar]
- 32.Lehker, M. W., R. Arroyo, and J. F. Alderete. 1991. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J. Exp. Med. 174:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehker, M. W., T. H. Chang, D. C. Dailey, and J. F. Alderete. 1990. Specific erythrocyte binding is an additional nutrient acquisition system for Trichomonas vaginalis. J. Exp. Med. 171:2165-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molkanen, T., J. Tyynela, J. Helin, N. Kalkkinen, and P. Kuusela. 2002. Enhanced activation of bound plasminogen on Staphylococcus aureus by staphylokinase. FEBS Lett. 517:72-78. [DOI] [PubMed] [Google Scholar]
- 35.Moodley, P., D. Wilkinson, C. Connolly, J. Moodley, and A. W. Sturm. 2002. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin. Infect. Dis. 34:519-522. [DOI] [PubMed] [Google Scholar]
- 36.Mundodi, V., A. S. Kucknoor, D. J. Klumpp, T. H. Chang, and J. F. Alderete. 2004. Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis. Mol. Microbiol. 53:1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien, J. L., C. M. Lauriano, and J. F. Alderete. 1996. Molecular characterization of a third malic enzyme-like AP65 adhesin gene of Trichomonas vaginalis. Microb. Pathog. 20:335-349. [DOI] [PubMed] [Google Scholar]
- 38.Ohba, H., T. Harano, and T. Omura. 1981. Intracellular and intramembranous localization of a protein disulfide isomerase in rat liver. J. Biochem. (Tokyo) 89:889-900. [DOI] [PubMed] [Google Scholar]
- 39.Pancholi, V., and V. A. Fischetti. 1998. Alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273:14503-14515. [DOI] [PubMed] [Google Scholar]
- 40.Pancholi, V., P. Fontan, and H. Jin. 2003. Plasminogen-mediated group A streptococcal adherence to and pericellular invasion of human pharyngeal cells. Microb. Pathog. 35:293-303. [DOI] [PubMed] [Google Scholar]
- 41.Park, Y., O. Yilmaz, I. Y. Jung, and R. J. Lamont. 2004. Identification of Porphyromonas gingivalis genes specifically expressed in human gingival epithelial cells by using differential display reverse transcription-PCR. Infect. Immun. 72:3752-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson, K. M., and J. F. Alderete. 1984. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J. Exp. Med. 160:398-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson, K. M., and J. F. Alderete. 1984. Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement for this species. Mol. Biochem. Parasitol. 12:37-48. [DOI] [PubMed] [Google Scholar]
- 44.Peterson, K. M., and J. F. Alderete. 1984. Trichomonas vaginalis is dependent on uptake and degradation of human low density lipoproteins. J. Exp. Med. 160:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quan, H., G. Fan, and C. C. Wang. 1995. Independence of the chaperone activity of protein disulfide isomerase from its thioredoxin-like active site. J. Biol. Chem. 270:17078-17080. [DOI] [PubMed] [Google Scholar]
- 46.Sherman, K. J., W. H. Chow, J. R. Daling, and N. S. Weiss. 1988. Sexually transmitted diseases and the risk of tubal pregnancy. J. Reprod. Med. 33:30-34. [PubMed] [Google Scholar]
- 47.Singer-Kruger, B., H. Stenmark, A. Dusterhoft, P. Philippsen, J. S. Yoo, D. Gallwitz, and M. Zerial. 1994. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 125:283-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorvillo, F., A. Kovacs, P. Kerndt, A. Stek, L. Muderspach, and L. Sanchez-Keeland. 1998. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am. J. Trop. Med. Hyg. 58:495-500. [DOI] [PubMed] [Google Scholar]
- 49.Sorvillo, F., L. Smith, P. Kerndt, and L. Ash. 2001. Trichomonas vaginalis, HIV, and African-Americans. Emerg. Infect. Dis. 7:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai, C. D., H. W. Liu, and J. H. Tai. 2002. Characterization of an iron-responsive promoter in the protozoan pathogen Trichomonas vaginalis. J. Biol. Chem. 277:5153-5162. [DOI] [PubMed] [Google Scholar]
- 51.Viikki, M., E. Pukkala, P. Nieminen, and M. Hakama. 2000. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 39:71-75. [DOI] [PubMed] [Google Scholar]
- 52.Wang, Z., and D. D. Brown. 1991. A gene expression screen. Proc. Natl. Acad. Sci. USA 88:11505-11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstock, H., S. Berman, and W. Cates, Jr. 2004. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect. Sex. Reprod. Health 36:6-10. [DOI] [PubMed] [Google Scholar]