Abstract
Immune defense at an interface with the external environment reflects the functions of physical and chemical barriers provided by epithelial and immune cells. Resident epithelial cells, such as keratinocytes, produce numerous peptides with direct antimicrobial activity but also provide a physical barrier against invading pathogens and signal the recruitment of circulating immune cells, such as neutrophils. Antimicrobial peptides such as cathelicidin are produced constitutively by neutrophils and are inducible in keratinocytes in response to infection. The multiplicity of antimicrobial peptides and their cellular sources has resulted in an incomplete understanding of the role of cathelicidin production by epithelial cells in cutaneous immune defense. Therefore, this study sought to evaluate keratinocyte antimicrobial activity and the potential contribution of keratinocyte cathelicidin to host protection against two leading human skin pathogens. Wild-type mice and those with a targeted deletion of the cathelicidin gene, Cnlp, were rendered neutropenic prior to cutaneous infection. Interestingly, Cnlp-deficient mice remained more susceptible to group A streptococcus infection than mice with Cnlp intact, suggesting the involvement of epithelial cell-derived cathelicidin in host immune defense. Keratinocytes were then isolated in culture and found to inhibit the growth of Staphylococcus aureus, an effect that was partially dependent on their ability to synthesize and activate cathelicidin. Further, lentivirus-mediated delivery of activated human cathelicidin enhanced keratinocyte antimicrobial activity. Combined, these data illustrate the potential contribution of keratinocyte cathelicidin to the innate immune defense of skin against bacterial pathogens and highlight the need to consider epithelial antimicrobial function in the diagnosis and therapy of skin infection.
The skin functions both as a barrier and as an immune organ capable of alerting the body of the danger of microbial attack. Keratinocytes (KC), the major cell type of the epidermis, have traditionally been thought to participate in cutaneous immune defense in two ways: by providing a physical barrier to invasion and through release of inflammatory mediators that recruit phagocytic leukocytes to sites of infection (3, 65). However, it is increasingly recognized that keratinocytes produce molecules similar to the antimicrobial peptides (AMPs) used by neutrophils and macrophages to kill potential pathogens (8, 14, 16, 18, 43, 49). These AMPs are generally small, cationic, amphipathic molecules whose modes of action involve disruption of microbial membranes and modification of host cell inflammatory events (30, 48). The production of AMPs by epithelial cells may provide an additional immune defense barrier, but the specific biological relevance of this phenomenon remains unproven.
Several families of AMPs have been well studied in keratinocytes, including cathelicidins and β-defensins. Human cathelicidin (hCAP18/LL-37) and human β-defensin-2 and -3 (hBD-2 and -3) are induced in keratinocytes during inflammation, while hBD-1 is constitutively synthesized (18, 51). In addition to keratinocytes, cathelicidin is derived from other cells present in the skin, including neutrophils (57), mast cells (15), and eccrine glands (39). The multiplicity of cutaneous AMPs and cellular sources has contributed to an incomplete understanding of the relative contribution of keratinocyte cathelicidin to cutaneous barrier defense.
Common inflammatory skin diseases such as psoriasis and atopic dermatitis (AD) have phenotypes suggesting that differences in AMP production may directly affect susceptibility to infection (11, 33, 46). Expression levels of cathelicidin, hBD-2, and hBD-3 are elevated in keratinocytes of psoriatic lesions, and secondary infection is extremely rare (18, 24, 25, 27, 44). In contrast, AD patients are relatively deficient in cathelicidin and hBD-2 and demonstrate increased susceptibility to bacterial and viral superinfection of the involved skin (28, 33, 34, 46). Interestingly, there is no apparent neutrophil defect in these inflammatory diseases, suggesting that expression of AMPs by epithelial cells may be responsible for the observed differences in susceptibility to infection. The dual functionality of these AMPs in antimicrobial and immunomodulatory activities highlights the importance of proper AMP expression for optimal skin immune defense.
A critical function of cathelicidin AMPs in skin defense against bacterial infection has been shown in cathelicidin-deficient mice (28, 43). Neutrophils store abundant cathelicidin in its precursor form, and their importance to skin immunity has also been well documented. However, the contribution made by keratinocytes, which produce low constitutive levels of cathelicidin compared to neutrophils and upregulate expression upon wounding or bacterial challenge, is unclear (16). This study set out to evaluate the antimicrobial activity and cutaneous immune defense potential of keratinocyte cathelicidin against two leading human pathogens that infect both normal and immunocompromised skin, group A streptococcus (GAS) and Staphylococcus aureus. Findings presented here suggest that keratinocyte synthesis and processing of cathelicidin contribute to skin innate immunity by forming a direct antimicrobial defense barrier that supplements circulating immune cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
GAS strain NZ131, a well-characterized wild-type (WT) serotype M49 human skin isolate that is susceptible to cathelicidin AMPs (43), was propagated in tryptic soy broth (TSB). S. aureus WT strain SA113 (ATCC 35556) and its isogenic lysophosphatidylglycerol synthase-deficient mutant ΔmprF (47) were also propagated in TSB. Bacteria were grown to logarithmic phase (optical density at 600 nm [OD600], 0.9 [∼108 CFU/ml]) and diluted in the appropriate medium for use in mouse infectious challenges or keratinocyte antimicrobial assays.
Neutrophil depletions.
Age- and sex-matched adult Cnlp+/+ and Cnlp−/− 129SVJ mice were administered an intraperitoneal (i.p.) dose (200 μl) of either cyclophosphamide (250 mg/kg of body weight) or monoclonal antibody RB6-8C5 (450 μg/mouse) to deplete circulating neutrophils (55, 59). Control littermates were administered 200 μl phosphate-buffered saline (PBS) i.p. To evaluate the duration of neutropenia, peripheral blood samples from control and neutrophil-depleted mice were collected daily for 7 days from the saphenous vein into Microtainer tubes with EDTA (Becton Dickinson, Franklin Lakes, NJ). Hematologic analyses were performed at the UCSD Hematology Core Facility using the Hemavet analysis system (CDC Technologies).
Subcutaneous GAS infections.
Mouse infections with GAS were performed based on modifications to a previously described model (43). Twenty-four hours following neutrophil depletion, the backs of neutrophil-depleted and control mice were shaved and hair was removed by chemical depilation (Nair). Mice were infected subcutaneously with 200 μl of a sublethal dose of log-phase GAS (1 × 105 to 5 × 105 CFU) complexed to Cytodex beads as a carrier. Digital photographs of skin lesions were taken daily, and lesion size was measured using NIH Imager software. Lesions were biopsied on day 7 postinfection for cyclophosphamide-treated mice and on day 3 postinfection for antibody RB6-8C5-treated mice. Excised lesions were homogenized in PBS using a PowerGen 125 handheld homogenizer (Fisher Scientific, Pittsburgh, PA) to recover bacteria. Dilutions were plated on blood agar and cultured overnight at 37°C for enumeration of CFU. To confirm that neutrophil populations remained suppressed throughout the infection period, cutaneous lesions from control and neutrophil-depleted mice were biopsied daily and fixed in formalin for histologic analysis. Hematoxylin-and-eosin (H&E) staining was performed by the UCSD Histology Core Facility. Images were obtained using an Olympus BX41 microscope.
MIC assays.
To evaluate the potential antimicrobial activity of cathelicidin in cell culture medium, MIC assays were performed based on modifications to a previously described method (39). Briefly, S. aureus WT and ΔmprF strains were grown to log phase and diluted in TSB or antibiotic-free EpiLife cell culture medium (total volume, 50 μl) in the presence or absence of synthetic human cathelicidin LL-37 peptide in 96-well round-bottom tissue culture plates (Corning Inc., NY). LL-37 was used at concentrations of 2 μM, 4 μM, 8 μM, and 16 μM. Following incubation at 37°C for 4 h or overnight, absorbance was read as OD600 using a spectrophotometer (SpectraMax Plus 384; Molecular Devices, CA) and dilutions were plated on tryptic soy agar (TSA) and incubated overnight at 37°C. CFU were quantified, and the percent survival of live bacteria was calculated as (cell survival after incubation with peptide)/(cell survival after incubation without peptide) × 100.
Keratinocyte isolation and culture.
Human KC derived from neonatal foreskin were grown in serum-free EpiLife cell culture medium (Cascade Biologics, Portland, OR) containing 0.06 mM Ca2+, 1× EpiLife defined growth supplement, 50 U/ml penicillin, and 50 μg/ml streptomycin under standard tissue culture conditions. HaCaT cells were cultured in Dulbecco's modified Eagle's medium with 4.5 g/liter glucose (BioWhittaker; Walkersville, MD) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. To isolate Cnlp+/+ and Cnlp−/− mouse KC, neonatal 129SVJ mice were sacrificed within 4 days of birth, prior to the onset of hair growth. Skin was removed and sterilized in Hibiclens antiseptic/antimicrobial cleanser (Zeneca Pharmaceuticals, Wilmington, DE). Following overnight dispase treatment to separate the epidermis and dermis, the epidermis was trypsinized for 12 min at 37°C to disaggregate the KC. Trypsin was neutralized with Hanks' buffered saline solution containing 5% Chelex-treated calcium, and the KC suspension was centrifuged for 10 min at 1,000 × g to pellet the cells. Mouse KC were resuspended and grown in serum-free 154CF keratinocyte culture medium (Cascade Biologics, Portland, OR) containing 0.05 mM Ca2+, 1× human keratinocyte growth supplement, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Cathelicidin lentivirus production.
Human cathelicidin lentivirus constructs were generated as previously described for the pig cathelicidin PR-39 (32). Briefly, human cathelicidin cDNA (23) corresponding to either the full-length hCAP18 precursor (signal peptide plus cathelin plus LL-37), cathelin protein, or the mature LL-37 peptide was amplified by PCR and inserted into the pLenti6/V5/TOPO vector (Invitrogen, Carlsbad, CA) to generate the following constructs under the control of a cytomegalovirus promoter: pLV/hCAP18, pLV/Cathelin, and pLV/LL-37. Insertion and orientation of cathelicidin genes were confirmed by plasmid sequencing. Constructs were cotransfected with a mixture of packaging plasmids (pLP1, pLP2, and pLP/VSV-G) into human embryonic kidney 293T cells. Transfections of 293T cells (1.5 × 106) were carried out in serum-free medium using Lipofectamine (Gibco, Carlsbad, CA). After 12 h, the transfection medium was replaced with complete medium and cells were further incubated for 72 h. Viral supernatant was collected and filtered (0.45-μm-pore-size filter). pLV/β-gal was used as a positive control. Expression of β-galactosidase was scored by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining. Titers were calculated by counting the foci of blue cells per well and dividing by the dilution factor. In HaCaT cells, the control pLV/LacZ titer was approximately 5 × 107 transducing units/ml. pLV/hCAP18, pLV/Cathelin, and pLV/LL-37 were similarly produced by transient transfection of 293T cells. Viral stocks were stored at −80°C.
HaCaT cell transduction.
Transduction of HaCaT cells with lentivirus stock was performed as previously described (32). Briefly, HaCaT cells were grown in 6-well plates to approximately 30 to 50% confluence. Prior to infection, the culture medium was replaced by serum-free medium supplemented with 8 μg/ml polybrene. After addition of medium (300 μl) from 293T cells infected with hCAP18, cathelin, LL-37, or LacZ, cells were incubated at 37°C overnight. Twenty-four hours later, the medium was changed to growth medium without polybrene. After expansion in culture for 48 to 72 h, HaCaT cells were collected and analyzed for cathelicidin expression by reverse transcription-PCR and Western blotting. Transduction efficiency was analyzed by visualization of β-galactosidase expression in controls.
PI assays.
Human KC were pretreated in the presence or absence of protease inhibitors (PI) diluted in antibiotic-free cell culture medium for 24 h prior to assessment of antimicrobial activity against S. aureus ΔmprF. The EDTA-free PI cocktail used inhibits various serine and cysteine proteases (Roche, Indianapolis, IN).
Radial diffusion assays.
Log-phase S. aureus ΔmprF (10 μl) was inoculated into sterile tryptone top agar (10 ml) at 50°C (1% agarose-0.5% tryptone). The bacterial suspension, containing approximately 105 CFU/ml bacteria, was poured into plates and allowed to solidify at room temperature. Wells were punched (2 mm) into the plates using a Pasteur pipette. Human KC (with or without a 24-h PI pretreatment) were extracted in 1 M HCl-1% trifluoroacetic acid, lyophilized, and resuspended in sterile water. Bicinchoninic acid assays were performed to confirm equivalent protein loading, and 2 μl of each KC extract or control was loaded in triplicate wells. Synthetic LL-37 peptide served as a positive control, and KC extraction buffer served as a negative control. Plates were incubated at 37°C overnight, and photographs were taken using ChemiImager 4400. The diameter of each zone of bacterial growth inhibition was measured using NIH Imager software.
Keratinocyte CFU assays.
KC were passaged in the absence of antibiotics and cultured to confluence in 24-well tissue culture plates. To evaluate the ability of KC to kill bacteria, 104 CFU/ml log-phase S. aureus ΔmprF was added to cell culture wells containing antibiotic-free medium. Bacteria were centrifuged onto cells at 350 × g for 10 min and incubated at 37°C in humidified 5% CO2 incubators for various times. At each time point, total well contents were collected, plated on TSA, and incubated overnight at 37°C for enumeration of CFU. Control wells without KC contained bacteria grown under identical conditions.
Gentamicin protection assay.
Human and mouse KC were grown to confluence and incubated with 104 or 108 CFU/ml log-phase S. aureus WT or ΔmprF. Gentamicin protection assays were performed based on previously described methods (22, 36, 42, 66). Briefly, bacteria were centrifuged onto cells at 350 × g for 10 min and incubated for 2 h at 37°C. KC were then washed with PBS and treated with 100 μg/ml gentamicin for 1 h to kill extracellular bacteria. To collect intracellular bacteria, KC were washed, trypsinized, and lysed in 0.025% Triton X-100-H2O. Lysates were plated on TSA and incubated overnight at 37°C for enumeration of CFU.
Statistical analysis.
Results are generally expressed as means ± standard errors from three independent experiments. The paired Student t test was used to test for significance, and P values less than 0.05 were considered significant.
RESULTS
Cathelicidin production confers resistance to cutaneous bacterial infection in neutrophil-depleted mice.
Neutrophils produce abundant cathelicidin in the active LL-37 peptide form and migrate to the skin in response to injury or infection (57). Mast cells and macrophages also synthesize cathelicidin, which has been shown to confer antimicrobial activity in vitro (15, 49). Epidermal cells, namely, keratinocytes, demonstrate inducible cathelicidin expression during inflammation (16, 18), but their role in skin antimicrobial activity relative to that of recruited immune cells is not well understood. In order to evaluate the contribution of cathelicidin made by keratinocytes to skin antimicrobial activity, WT (Cnlp+/+) and cathelicidin-deficient (Cnlp−/−) mice were first depleted of circulating immune cells by intraperitoneal injection with cyclophosphamide and then infected subcutaneously with GAS. Cyclophosphamide treatment has been reported to effectively suppress the numbers and function of neutrophils, mast cells, and macrophages (2, 13, 45, 54). Daily blood sampling following cyclophosphamide treatment demonstrated that neutrophil populations were reduced by 96%, lymphocytes by 85%, and monocytes by 82% in comparison with control mice (data not shown). These cell populations remained suppressed for 7 days after cyclophosphamide treatment; therefore, this time point was chosen as the termination point for experiments evaluating resistance to GAS infection. Histological analysis of lesion biopsy specimens from GAS-infected control and depleted mice confirmed the absence of cellular inflammatory infiltrates at lesion sites (Fig. 1A). In parallel, quantitation of bacteria from skin lesion biopsy specimens showed that, as expected, depleted mice harbored more bacteria than control mice at sites of infection—in this case, more than 3,000-fold (Fig. 1B).
FIG. 1.
Neutrophil depletion increases susceptibility to GAS skin infection. (A) H&E staining of excised skin lesions from representative control and depleted Cnlp+/+ mice infected with subcutaneous GAS (inoculum, 4.5 × 105 CFU). Arrows indicate infiltrating leukocytes. Bars, 50 μm. (B) Bacterial counts from excised skin lesions of control and depleted Cnlp+/+ mice infected with GAS (n = 3). Skin lesions were biopsied, homogenized, and plated on TSA to recover GAS.
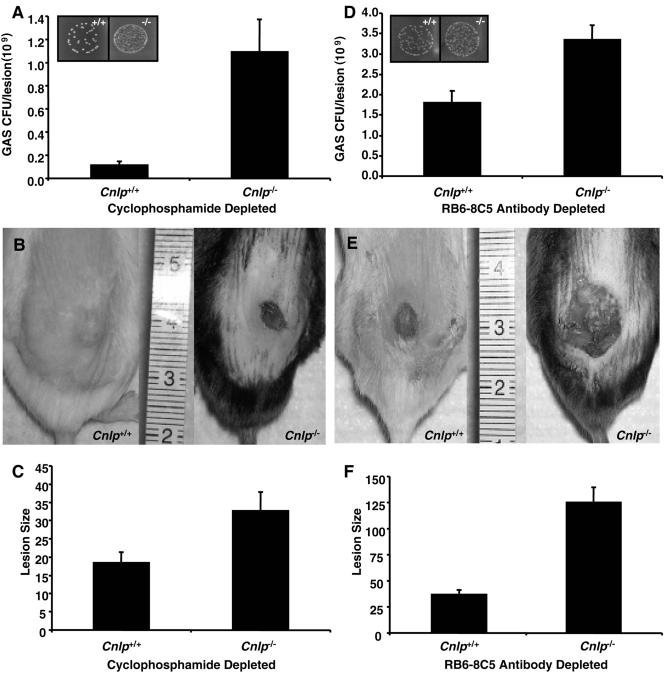
When cyclophosphamide-treated Cnlp+/+ and Cnlp−/− mice were infected with a sublethal dose of GAS and compared, Cnlp−/− mice demonstrated an increased susceptibility to infection in comparison with Cnlp+/+ mice, as evidenced by significantly higher bacterial loads and larger skin lesions (P < 0.01). In fact, Cnlp−/− mice harbored an average of nearly 10-fold more bacteria at sites of infection (Fig. 2A) and developed necrotic lesions that were twofold larger than those of Cnlp+/+ mice (Fig. 2B and C). Several cyclophosphamide-treated Cnlp−/− mice did not survive for the duration of infection period and were therefore not included in the analysis (data not shown). These results indicated that even in the absence of infiltrating immune cells such as neutrophils, cathelicidin contributes significantly to skin resistance to bacterial infection.
FIG. 2.
Epidermal cathelicidin confers resistance to GAS skin infection in neutropenic mice. (A) Bacterial counts from excised skin lesions of cyclophosphamide-treated Cnlp+/+ and Cnlp−/− mice (n = 13) infected with GAS. Mice were administered 250 mg/kg cyclophosphamide i.p. and infected 24 h postdepletion with subcutaneous GAS (inoculum, 4.5 × 105 CFU). Depletion was confirmed by hematology analysis. Inset depicts GAS CFU recovered from skin biopsy specimens on day 7 postinfection. (B) Digital photographs of skin lesions from representative cyclophosphamide-treated Cnlp+/+ and Cnlp−/− mice. (C) Sizes of lesions measured from digital photographs of cyclophosphamide-treated mice Cnlp+/+ and Cnlp−/− by using NIH Imager. (D) Bacterial counts from excised skin lesions of antibody RB6-8C5-treated Cnlp+/+ and Cnlp−/− mice (n = 9) infected with GAS. Mice were administered 450 μg antibody RB6-8C5 i.p. and were infected subcutaneously with GAS (inoculum, 1.5 × 105 CFU) 24 h postdepletion. Depletion was confirmed by hematology analysis. Inset depicts GAS CFU recovered on day 3 postinfection. (E) Digital photographs of skin lesions from representative antibody RB6-8C5-treated Cnlp+/+ and Cnlp−/− mice. (F) Sizes of lesions measured from digital photographs of antibody RB6-8C5-treated Cnlp+/+ and Cnlp−/− mice by using NIH Imager. All data are means ± standard errors from at least three independent experiments. Statistical analysis was performed using the paired Student t test; P < 0.01 for each set of experiments.
To control for the potential cytotoxic effects of cyclophosphamide on keratinocyte function, further experiments were performed in which Cnlp+/+ and Cnlp−/− mice were depleted of neutrophils by intraperitoneal injection with the neutrophil-specific monoclonal antibody RB6-8C5 prior to subcutaneous GAS challenge. Hematology analysis showed that treatment with antibody RB6-8C5 depleted 65% of neutrophils, without significantly affecting the numbers of other circulating immune cells, in comparison with control mice (data not shown). Treatment with antibody RB6-8C5 rendered mice neutropenic for at least 3 days, and this time point was chosen as the termination point for experiments evaluating resistance to GAS infection. The trends observed following neutrophil depletion by RB6-8C5 were similar to those seen with cyclophosphamide treatment. Skin lesions from antibody RB6-8C5-treated Cnlp+/+ mice contained fewer bacteria and were significantly smaller than those biopsied from the corresponding Cnlp−/− mice (P < 0.01). Cnlp−/− mice developed necrotic lesions that harbored an average of nearly twofold more bacteria (Fig. 2D) and were more than threefold larger than those of Cnlp+/+ mice (Fig. 2E and 2F). These data offer further confirmation that cathelicidin-producing cells in addition to neutrophils contribute to resistance to skin infection.
Keratinocyte antimicrobial activity requires proteolytic processing.
Since the results of leukocyte depletion experiments suggested that production of cathelicidin by the epidermis may provide an additional skin defense system, experiments were next designed to directly investigate the antimicrobial activity of keratinocytes (18). Proteolytic processing is essential for cathelicidin activation, and keratinocytes have recently been shown to process endogenous cathelicidin (8). Therefore, keratinocytes are equipped with the proteolytic machinery required to process the cathelicidin precursor protein into its two functional components, cathelin and LL-37 (64).
To determine whether cathelicidin processing is required for the generation of keratinocyte antimicrobial activity, HaCaT cells, a transformed human keratinocyte cell line, were transduced with lentivirus constructs engineered to express precursor hCAP18, cathelin protein, or mature LL-37 peptide. One recent study showed that overexpression of LL-37 enhances keratinocyte antimicrobial activity (9). Following lentivirus infection, expression of mRNA and protein encoding each of the expected products was verified by reverse transcription-PCR and Western blotting, respectively (data not shown). Cathelicidin expression was found to be approximately fivefold greater in cathelicidin-transduced keratinocytes than in LacZ-transduced control cells. S. aureus ΔmprF was chosen for keratinocyte antimicrobial assays based on its increased susceptibility to cathelicidin in keratinocyte cell culture medium (data not shown). Consistent with the requirement for cathelicidin processing to generate antimicrobial activity, keratinocytes engineered to overexpress either cathelin protein or mature LL-37 peptide significantly inhibited the growth of S. aureus ΔmprF in comparison to keratinocytes overexpressing either the hCAP18 precursor or the LacZ control (P < 0.001) (Fig. 3A).
FIG. 3.
Keratinocytes demonstrate antimicrobial activity against S. aureus that is dependent on proteolytic activation. (A) Bacterial recovery at 2 h following incubation of log-phase S. aureus ΔmprF (inoculum, 104 CFU/ml) with HaCaT cells overexpressing either hCAP18, cathelin, LL-37, or LacZ. Asterisks, P < 0.001 in comparison with hCAP18. (B) Zones of inhibition (ZOI) measured from radial diffusion assays against S. aureus ΔmprF. Isolated human KC were treated in the presence or absence of PI for 24 h prior to extraction. ZOI were measured using NIH Imager. P < 0.01 for KC versus KC plus PI. (C) Bacterial recovery at 3, 4, and 5 h following incubation of log-phase S. aureus ΔmprF (inoculum, 104 CFU/ml) with isolated human KC treated in the presence or absence of PI for 24 h. “No KC” control wells were incubated with bacteria grown under identical conditions. P < 0.001 for KC versus No KC at 3, 4, and 5 h; P < 0.02 for KC versus KC plus PI at 5 h. All data are means ± standard errors from at least three independent experiments performed in triplicate. Statistical analysis was performed using the paired Student t test, and P values are given for each set of experiments.
The enhanced antimicrobial activity of keratinocytes overexpressing processed cathelicidin forms suggested that inhibition of proteolysis could serve to further confirm the contribution of cathelicidin to keratinocyte antimicrobial activity. Both radial diffusion assays and CFU assays were undertaken to evaluate keratinocyte antimicrobial activity in the presence and absence of protease inhibitors. The inhibitory activity of keratinocyte extracts on the growth of S. aureus ΔmprF was reversed by pretreatment of keratinocytes with protease inhibitors (P < 0.01) (Fig. 3B). In addition, keratinocytes cultured in a monolayer demonstrated antimicrobial activity against S. aureus ΔmprF (P < 0.001 at all time points tested) that was partially blocked by protease inhibitor treatment (P < 0.02 at 5 h) (Fig. 3C). The presence of protease inhibitors alone in these assays did not negatively impact the growth of either the bacteria or the keratinocytes (data not shown). Therefore, proteolytic processing contributes to keratinocyte antimicrobial activity, supporting a role for AMPs such as cathelicidin in keratinocyte immune defense.
Cathelicidin production contributes to keratinocyte antimicrobial activity.
To directly study the contribution of cathelicidin to keratinocyte antimicrobial activity, keratinocytes isolated and cultured from Cnlp+/+ and Cnlp−/− mice were incubated with log-phase S. aureus ΔmprF and subsequent bacterial growth was measured. Mouse keratinocytes demonstrated significant antimicrobial activity compared to the cell-free control (P < 0.001 at all time points tested) (Fig. 4). Keratinocyte antimicrobial activity was partially dependent on cathelicidin production, as evidenced by the fact that bacterial growth in culture was significantly reduced in cathelicidin-expressing keratinocytes in comparison to those derived from cathelicidin-deficient mice (P < 0.01 at 3 and 4 h; P < 0.02 at 5 h) (Fig. 4). Thus, cathelicidin expression contributes to, but is not solely responsible for, the antimicrobial activity of cultured keratinocytes.
FIG. 4.
Keratinocytes demonstrate antimicrobial activity against S. aureus that is partially dependent on cathelicidin expression. Bacterial recovery at 3, 4, and 5 h following incubation of log-phase S. aureus ΔmprF (inoculum, 104 CFU/ml) with isolated Cnlp+/+ and Cnlp−/− mouse KC is shown. “No KC” control wells were incubated with bacteria grown under identical conditions. Data are means ± standard errors from at least three independent experiments performed in triplicate. Statistical analysis was performed using the paired Student t test. P < 0.001 for Cnlp+/+ KC versus No KC at 3, 4, and 5 h; P < 0.02 for Cnlp+/+ KC versus Cnlp−/− KC at 3, 4, and 5 h.
Keratinocytes internalize bacteria and demonstrate cathelicidin-dependent intracellular antimicrobial activity.
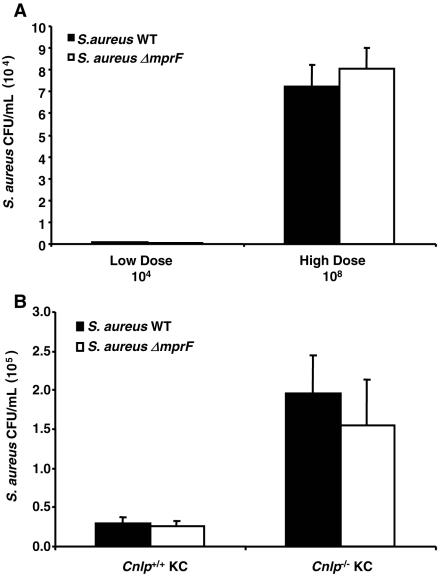
The experiments described above largely reflected the ability of keratinocytes to kill S. aureus ΔmprF extracellularly, since in separate experiments it was determined that the number of bacteria used in these assays did not permit significant intracellular invasion. Keratinocytes have previously been shown to internalize bacteria, which then colocalize with cathelicidin stored in intracellular granules (8). To confirm the internalization of S. aureus, keratinocytes were incubated with tetramethyl rhodamine isothiocyanate (TRITC)-labeled S. aureus for various times, extracellular fluorescence was then quenched with trypan blue, and intracellular fluorescence was measured. Keratinocytes internalized equivalent numbers of TRITC-labeled S. aureus WT and ΔmprF cells in a time- and dose-dependent manner (data not shown). Next, a dose-response experiment was carried out to determine the optimal S. aureus inoculum for assessment of intracellular bacterial survival. Human keratinocytes were found to internalize and maintain live S. aureus WT and ΔmprF in a dose-dependent manner (Fig. 5A), thereby allowing further studies of keratinocyte intracellular antimicrobial activity at the higher inoculum of 108 CFU. Intracellular bacteria also accumulated in a time-dependent manner (data not shown).
FIG. 5.
Keratinocytes internalize S. aureus and demonstrate cathelicidin-dependent intracellular antimicrobial activity. (A) Intracellular bacterial recovery at 2 h following incubation of log-phase S. aureus WT or ΔmprF (low-dose inoculum, 104 CFU; high-dose inoculum, 108 CFU) with isolated human KC. KC were washed and treated with 100 μg/ml gentamicin for 1 h to kill extracellular and cell surface-bound bacteria prior to quantification of intracellular bacteria. (B) Intracellular bacterial recovery at 2 h following incubation of log-phase S. aureus WT or ΔmprF (inoculum, 108 CFU) with isolated Cnlp+/+ and Cnlp−/− mouse KC. KC were washed and treated with 100 μg/ml gentamicin for 1 h to kill extracellular and cell surface-bound bacteria prior to quantification of intracellular bacteria. Data are means ± standard errors from three independent experiments performed in triplicate. Statistical analysis was performed using the paired Student t test. P < 0.01 for Cnlp+/+ KC versus Cnlp−/− KC with WT S. aureus; P < 0.04 for Cnlp+/+ KC versus Cnlp−/− KC with S. aureus ΔmprF.
After the antimicrobial capacity of cultured keratinocytes as well as their ability to internalize bacteria had been established, it became possible to directly evaluate the participation of cathelicidin in this process. Therefore, gentamicin protection assays comparing Cnlp+/+ and Cnlp−/− mouse keratinocytes were performed. By comparison of S. aureus WT and ΔmprF, it was found that both strains were killed to a greater extent (seven- and sixfold, respectively) in the intracellular compartment of Cnlp+/+ keratinocytes than in that of Cnlp−/− keratinocytes (P < 0.01 for S. aureus WT; P < 0.04 for S. aureus ΔmprF) (Fig. 5B). Cnlp+/+ and Cnlp−/− keratinocytes showed no statistical differences in bacterial internalization rates of TRITC-labeled S. aureus (data not shown). These results indicate that cathelicidin is essential for keratinocyte intracellular antimicrobial activity against S. aureus.
DISCUSSION
Suppression of neutrophil function by exposure to immunosuppressive drugs during cancer treatment and bone marrow transplantation often leads to increased susceptibility to bacterial, fungal, and viral infections (7, 38, 58, 61). Life-threatening necrotizing skin and soft-tissue infections can develop in neutropenic patients (29, 40). Animal models of neutropenia induced by cyclophosphamide have also demonstrated an increased incidence of bacterial infection (13, 59). These studies clearly illuminate the importance of neutrophil recruitment in cutaneous defense against bacterial infection. Conversely, numerous clinical examples exist in which individuals with increased frequency of skin and other epithelial surface infections have no demonstrable defect in leukocyte recruitment or function. Recent advances in understanding of innate immune defense systems have suggested that these ancient evolutionary immune mechanisms may be important to human disease yet previously underappreciated. This study sought to evaluate whether keratinocytes participate in skin resistance to infection by providing a functional antimicrobial barrier that may supplement circulating immune cells.
Resident skin cells such as keratinocytes demonstrate constitutive expression of AMPs such as hBD-1 and inducible expression of LL-37, hBD-2, and hBD-3 (18, 19, 24, 25). The role of AMP production by epithelia is unclear, as both cathelicidin and the β-defensins have effects on the host that are independent of their antibiotic activity. These functions include altering proteoglycan synthesis in the dermis and recruiting lymphoid cells to the epidermis (1, 20, 62, 63). To determine whether AMPs made by skin epithelial cells provide an additional component of the direct antimicrobial shield, the two leading causes of superficial and invasive bacterial infection in normal and immunocompromised human skin, GAS and S. aureus, were utilized.
To eliminate the contribution of neutrophil cathelicidin to skin defense, mice were rendered neutropenic by treatment with either cyclophosphamide or antibody RB6-8C5. Cyclophosphamide is an alkylating agent; therefore, it targets all actively dividing cells, potentially including basal keratinocytes. Previous studies have suggested that epidermal dysmaturation or dysplasia, defined by alteration of keratinocyte architecture and maturation, may be linked to the direct cytotoxic actions of chemotherapeutic drugs such as cyclophosphamide (10, 26). Although these effects on keratinocytes are rare, additional experiments were performed to eliminate this potential cytotoxicity, using antibody RB6-8C5 to specifically deplete neutrophils from circulation. As expected, neutropenic mice were significantly more susceptible to GAS skin infection than untreated control mice, as assessed by CFU recovered from lesions. Additionally, in comparison with neutropenic Cnlp+/+ mice, neutropenic Cnlp−/− mice maintained larger lesions that were colonized by higher numbers of bacteria.
Due to the widespread immunosuppressive effects of cyclophosphamide, it was expected that cyclophosphamide-treated mice would demonstrate greater susceptibility to GAS infection than antibody RB6-8C5-treated mice. Interestingly, however, the differences in both bacterial CFU recovered and lesion size between the two depletion techniques were striking and unexpected, with antibody RB6-8C5-treated mice harboring greater bacterial loads and larger necrotic lesions. There may be several potential explanations for these differences. First, following cyclophosphamide treatment, several mice became ill and succumbed to infection. In these mice, the infection clearly spread systemically rather than remaining localized at the lesion site. Therefore, these mice, which presumably harbored high numbers of bacteria, were not present at the end of the infection period for bacterial recovery and lesion size measurements. Further, due to the duration of depletion by antibody RB6-8C5 treatment in the 129SVJ mouse background, the end points of these experiments fell at different times: 7 days for depletion by cyclophosphamide and 3 days for depletion by antibody RB6-8C5. The additional several days during the course of infection are likely important for bacterial clearance from the lesion site. Finally, the disparity in lesion size between the two depletion techniques suggests that the superficial nature of the lesions of antibody RB6-8C5-treated mice clearly led to larger lesion surface area measurements. In addition, these superficial lesions may have been more effective at trapping bacteria near the skin surface rather than permitting systemic spread and toxicity. Taken together, these two independent depletion techniques suggest that neutrophils are not the only important contributors to skin antimicrobial defense in vivo and that skin resistance to bacterial infection in the absence of neutrophils remains largely due to cathelicidin production, potentially by resident keratinocytes.
As discussed earlier, keratinocytes were prime suspects for delivery of non-neutrophil-derived cathelicidin in skin defense. Therefore, to evaluate their specific contribution, and to eliminate the role of AMPs as paracrine stimulators of cellular immune defense, it was of interest to develop an isolated keratinocyte infection model. GAS did not grow well under keratinocyte culture conditions, so S. aureus, an equally prominent human skin pathogen, was chosen for keratinocyte antimicrobial activity studies (35, 56). Initially, assays were performed to verify cathelicidin antimicrobial activity against S. aureus in keratinocyte culture medium, since elevated concentrations of NaCl and serum have been reported to diminish the antimicrobial activity of several AMPs when assayed in vitro (4, 5, 21, 53). Therefore, the antimicrobial activity of synthetic LL-37 peptide against S. aureus strains grown in either bacterial growth medium (TSB) or keratinocyte cell culture medium (EpiLife) was assessed. Surprisingly, S. aureus WT and ΔmprF strains (31, 47) were both inhibited in keratinocyte medium containing physiological (130 mM) NaCl. The MIC for S. aureus ΔmprF was 8 μM, while the MIC for S. aureus WT was 32 μM. S. aureus ΔmprF lacks the ability to modify membrane phosphatidylglycerol with l-lysine and is unable to reduce its negative surface charge, thus providing a more sensitive microbial indicator of susceptibility to cationic AMPs (47). Our results demonstrated that cathelicidin maintained antimicrobial activity against both S. aureus strains under keratinocyte growth conditions, suggesting that either the conditions of the mammalian culture environment, the local concentration of cathelicidin, or a combined activity with other keratinocyte AMPs may facilitate activity against bacterial growth. Since the MIC of cathelicidin in culture for S. aureus ΔmprF was comparable to that for the GAS strain used for in vivo experiments, these bacteria were chosen for the in vitro antimicrobial assays.
It has been shown previously that keratinocytes synthesize and process cathelicidin, the expression of which is upregulated in response to injury or infection (8, 16, 18). Therefore, a lentivirus delivery system was employed to overexpress precursor hCAP18, cathelin protein, or mature LL-37 peptide in the HaCaT keratinocyte cell line. The upregulated cathelicidin expression in transduced keratinocytes is representative of levels of cathelicidin production and activation in vivo during infection, while control cells reflect unstimulated keratinocytes (16, 18). Therefore, the cathelicidin-transduced keratinocytes provide an appropriate model for studying the contribution of keratinocyte cathelicidin synthesis and processing to host defense against bacterial infection. In CFU assays, it was clearly shown that keratinocytes overexpressing either the processed cathelin protein or the LL-37 peptide demonstrated enhanced ability to inhibit the growth of S. aureus ΔmprF, while those expressing the precursor form lacked enhanced antimicrobial activity relative to control keratinocytes. This is consistent with previous reports that cathelicidin requires processing for activation and that cleavage of hCAP18 into the cathelin protein and mature LL-37 peptide generates two AMPs with distinct, but overlapping, activities (64). The lack of detectable antimicrobial activity in keratinocytes overexpressing the hCAP18 precursor suggested that the processing capacity of keratinocytes may be rate-limiting due to a lack of sufficient proteases, since hCAP18 expression in transduced cells was more than fivefold greater than in control cells. Alternatively, hCAP18 may bind to various proteins, such that proteases cannot gain access to the cleavage site. Further, overexpressed hCAP18 may be targeted to an intracellular compartment in keratinocytes such that processing enzymes are inaccessible. In another recent study, a similar cutaneous gene therapy approach was undertaken to address the observation of high bacterial loads and loss of epidermal AMPs in burn wound beds. This study showed that keratinocytes overexpressing LL-37, hBD-2, or hBD-3 alone or in combination demonstrated increased capacity to inhibit bacterial growth (9).
Consistent with the observations presented here showing that keratinocytes engineered to overexpress processed cathelicidin peptides demonstrate enhanced antimicrobial activity, it was further observed that keratinocytes treated with protease inhibitors displayed diminished antimicrobial activity in both cell extracts and monolayer cell cultures. The differences in microbial growth between protease inhibitor-treated keratinocytes and cell-free controls likely reflect an incomplete inhibition of AMP processing, since the protease inhibitors used for these assays targeted serine and cysteine proteases, which have been shown to process and activate cathelicidin in other cell types (6, 12, 52). In addition to cathelicidin, keratinocytes produce other AMPs, such as defensins, which are activated by metalloproteinases rather than serine proteases (60). Alternatively, keratinocyte AMPs may be separated intracellularly from their processing enzymes, thereby minimizing the effects of the protease inhibitors. Taken together, these findings suggest that hCAP18 and similar peptides requiring proteolytic processing contribute to keratinocyte antimicrobial activity following proteolytic activation.
Cathelicidin was specifically implicated in the antimicrobial action of epithelial cells through studies using keratinocytes obtained from mice lacking expression of functional cathelicidin. Compared to Cnlp+/+ keratinocytes, the Cnlp−/− keratinocytes exhibited significantly decreased ability to inhibit the growth of S. aureus in both the extracellular and intracellular compartments. It was noteworthy that the magnitude of inhibition of keratinocyte antimicrobial activity by protease inhibitor treatment closely resembled the difference in antimicrobial activity between Cnlp+/+ and Cnlp−/− keratinocytes. Similar results showing residual antimicrobial activity in the absence of cathelicidin have been published from studies with neutrophils, mast cells, and macrophages isolated from Cnlp+/+ and Cnlp−/− mice (15, 43, 49). The partial inhibition of S. aureus ΔmprF growth by Cnlp−/− keratinocytes, as well as the incomplete inhibition of keratinocyte antimicrobial activity by protease inhibitor treatment, likely reflects important interactions among various cathelicidin-producing cell types during in vivo infection, the expression of multiple immune defense molecules by keratinocytes (18, 19, 24, 25, 51), and the synergistic activity observed between cathelicidin and other antimicrobial peptides (3, 50). Further, differences in intracellular antimicrobial activity observed between Cnlp+/+ and Cnlp−/− keratinocytes were not due to decreased uptake of bacteria by Cnlp−/− cells, as evidenced by the fact that internalization of fluorescent S. aureus WT and ΔmprF strains by both Cnlp+/+ and Cnlp−/− keratinocytes was equivalent. These results extend the in vivo data with GAS and support a functional role for keratinocyte cathelicidin in cutaneous defense against bacterial infections (17, 22, 36, 37).
In this study, cathelicidin expression by keratinocytes was shown to be a significant contributor to cutaneous antimicrobial defense against important human bacterial skin pathogens. Expression and proteolytic processing of cathelicidin appear to be equally important for inhibiting bacterial growth. These observations confirm a role for the epidermis as a direct immune barrier to bacterial skin infection, which is consistent with observations from several studies. Both psoriasis and AD patients have altered skin barrier function and drastically different levels of cathelicidin and other AMPs in the skin yet exhibit fairly normal neutrophil recruitment and function (28, 44, 46). Interestingly, approximately 30% of patients with AD develop secondary bacterial or viral skin infections, compared with only 7% of psoriasis patients (11, 33). It is possible that sublethal concentrations of cutaneous AMP in AD patients may contribute to acquisition of resistance to host killing mechanisms by colonizing bacteria, thereby contributing to the frequency of skin infection. Therefore, differences in endogenous cathelicidin expression by epithelial cells may contribute to the disparity in susceptibility to secondary infection among patients with these inflammatory skin disorders, both by decreasing the level of AMP available for direct antimicrobial activity and by decreasing the susceptibility of colonizing bacteria to AMP.
This work supplies direct evidence that the immune barrier provided by epithelial cells of skin comprises more than a physical barrier or leukocyte recruitment vehicle. The observations presented here suggest that other epithelial tissues in which cathelicidin is made, such as the lung and gut, may also depend on expression and processing of cathelicidins for normal immune defense against susceptible microbes. Epithelial cells can therefore act as the “first responders” by providing essential immune defense functions in the absence of neutrophil recruitment. This system is consistent with a recent report of the ability of keratinocytes to kill GAS by autophagy (41) in a compartment that may contain cathelicidin in its active processed form. In this way, the mammalian epithelium has maintained an immune defense mechanism similar to that of other, more ancient organisms that lack adaptive immune systems, such as plants and invertebrates. Neutrophil-mediated antimicrobial defense comes into play later in response to invasion by a foreign organism or in cases of severe barrier compromise, but this cellular defense system remains partially dependent on the innate barrier functions provided by cathelicidin. Detecting, modifying, or supplementing this potent peptide shield could therefore provide a useful diagnostic and therapeutic approach for treatment or prevention of infectious diseases.
Acknowledgments
Financial assistance was provided by a VA Merit Award, NIH AI052453, and NIH AR45676 (R.L.G.), and NIH Training Grant DK07202 (M.H.B.).
We thank Andreas Peschel for the generous gift of S. aureus ΔmprF. We also acknowledge the UCSD Hematology Core for mouse blood sample analysis and the UCSD Histology Core Facility for staining of mouse skin lesion biopsy specimens.
Editor: A. D. O'Brien
REFERENCES
- 1.Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jornvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. [PubMed] [Google Scholar]
- 2.Anderson, C. 1985. The effect of selected immunomodulating agents on experimental contact reactions. Acta Derm. Venereol. Suppl. (Stockholm) 116:1-48. [PubMed] [Google Scholar]
- 3.Bals, R. 2000. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 1:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., M. J. Goldman, and J. M. Wilson. 1998. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 66:1225-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belaaouaj, A., R. McCarthy, M. Baumann, Z. Gao, T. J. Ley, S. N. Abraham, and S. D. Shapiro. 1998. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4:615-618. [DOI] [PubMed] [Google Scholar]
- 7.Bodey, G. P., M. Buckley, Y. S. Sathe, and E. J. Freireich. 1966. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 64:328-340. [DOI] [PubMed] [Google Scholar]
- 8.Braff, M. H., A. Di Nardo, and R. L. Gallo. 2005. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Investig. Dermatol. 124:394-400. [DOI] [PubMed] [Google Scholar]
- 9.Carretero, M., M. Del Rio, M. Garcia, M. J. Escamez, I. Mirones, L. Rivas, C. Balague, J. L. Jorcano, and F. Larcher. 2004. A cutaneous gene therapy approach to treat infection through keratinocyte-targeted overexpression of antimicrobial peptides. FASEB J. 18:1931-1933. [DOI] [PubMed] [Google Scholar]
- 10.Castano, E., J. L. Rodriguez-Peralto, F. Lopez-Rios, C. Gomez, M. Zimmermann, and L. Iglesias Diez. 2002. Keratinocyte dysplasia: an usual finding after transplantation or chemotherapy. J. Cutan. Pathol. 29:579-584. [DOI] [PubMed] [Google Scholar]
- 11.Christophers, E., and T. Henseler. 1987. Contrasting disease patterns in psoriasis and atopic dermatitis. Arch. Dermatol. Res. 279(Suppl.):S48-S51. [DOI] [PubMed] [Google Scholar]
- 12.Cole, A. M., J. Shi, A. Ceccarelli, Y. H. Kim, A. Park, and T. Ganz. 2001. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 97:297-304. [DOI] [PubMed] [Google Scholar]
- 13.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowland, J. B., A. H. Johnsen, and N. Borregaard. 1995. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 368:173-176. [DOI] [PubMed] [Google Scholar]
- 15.Di Nardo, A., A. Vitiello, and R. L. Gallo. 2003. Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 170:2274-2278. [DOI] [PubMed] [Google Scholar]
- 16.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 17.Drevets, D. A., T. A. Jelinek, and N. E. Freitag. 2001. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect. Immun. 69:1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 19.Fulton, C., G. M. Anderson, M. Zasloff, R. Bull, and A. G. Quinn. 1997. Expression of natural peptide antibiotics in human skin. Lancet 350:1750-1751. [DOI] [PubMed] [Google Scholar]
- 20.Gallo, R. L., M. Ono, T. Povsic, C. Page, E. Eriksson, M. Klagsbrun, and M. Bernfield. 1994. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA 91:11035-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman, M. J., G. M. Anderson, E. D. Stolzenberg, U. P. Kari, M. Zasloff, and J. M. Wilson. 1997. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553-560. [DOI] [PubMed] [Google Scholar]
- 22.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 23.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 24.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 25.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 26.Henry, L. B., and T. D. Horn. 2002. Chemotherapy and keratinocytes. J. Cutan. Pathol. 29:575-578. [DOI] [PubMed] [Google Scholar]
- 27.Henseler, T., and E. Christophers. 1995. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 32:982-986. [DOI] [PubMed] [Google Scholar]
- 28.Howell, M. D., J. F. Jones, K. O. Kisich, J. E. Streib, R. L. Gallo, and D. Y. Leung. 2004. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 172:1763-1767. [DOI] [PubMed] [Google Scholar]
- 29.Johnston, D. L., J. H. Waldhausen, and J. R. Park. 2001. Deep soft tissue infections in the neutropenic pediatric oncology patient. J. Pediatr. Hematol. Oncol. 23:443-447. [DOI] [PubMed] [Google Scholar]
- 30.Kamysz, W., and M. Okroj. 2003. Novel properties of antimicrobial peptides. Acta Biochim. Pol. 50:461-469. [PubMed] [Google Scholar]
- 31.Kristian, S. A., M. Durr, J. A. Van Strijp, B. Neumeister, and A. Peschel. 2003. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect. Immun. 71:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, P. H., T. Ohtake, M. Zaiou, M. Murakami, J. A. Rudisill, K. H. Lin, and R. L. Gallo. 2005. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc. Natl. Acad. Sci. USA 102:3750-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung, D. Y. 2003. Infection in atopic dermatitis. Curr. Opin. Pediatr. 15:399-404. [DOI] [PubMed] [Google Scholar]
- 34.Leung, D. Y., M. Boguniewicz, M. D. Howell, I. Nomura, and Q. A. Hamid. 2004. New insights into atopic dermatitis. J. Clin. Investig. 113:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 36.Medina, E., O. Goldmann, A. W. Toppel, and G. S. Chhatwal. 2003. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 187:597-603. [DOI] [PubMed] [Google Scholar]
- 37.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Weidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943-951. [DOI] [PubMed] [Google Scholar]
- 38.Morrison, V. A., R. J. Haake, and D. J. Weisdorf. 1993. The spectrum of non-Candida fungal infections following bone marrow transplantation. Medicine (Baltimore) 72:78-89. [DOI] [PubMed] [Google Scholar]
- 39.Murakami, M., T. Ohtake, R. A. Dorschner, B. Schittek, C. Garbe, and R. L. Gallo. 2002. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Investig. Dermatol. 119:1090-1095. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, J. J., R. Granger, G. K. Blair, G. G. Miller, G. C. Fraser, and J. F. Magee. 1995. Necrotizing fasciitis in childhood. J. Pediatr. Surg. 30:1131-1134. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa, I., A. Amano, N. Mizushima, A. Yamamoto, H. Yamaguchi, T. Kamimoto, A. Nara, J. Funao, M. Nakata, K. Tsuda, S. Hamada, and T. Yoshimori. 2004. Autophagy defends cells against invading group A Streptococcus. Science 306:1037-1040. [DOI] [PubMed] [Google Scholar]
- 42.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 44.Nomura, I., E. Goleva, M. D. Howell, Q. A. Hamid, P. Y. Ong, C. F. Hall, M. A. Darst, B. Gao, M. Boguniewicz, J. B. Travers, and D. Y. Leung. 2003. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J. Immunol. 171:3262-3269. [DOI] [PubMed] [Google Scholar]
- 45.Novak, M., L. G. Buchannan, and H. Howlader. 1990. Effects of cyclophosphamide and dexamethasone on mast cell populations in Hymenolepis microstoma-infected mice. Parasitology 100:337-343. [DOI] [PubMed] [Google Scholar]
- 46.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 47.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. van Kessel, and J. A. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramanathan, B., E. G. Davis, C. R. Ross, and F. Blecha. 2002. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 4:361-372. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 101:2422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh, P. K., B. F. Tack, P. B. McCray, Jr., and M. J. Welsh. 2000. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L799-L805. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen, O. E., J. B. Cowland, K. Theilgaard-Monch, L. Liu, T. Ganz, and N. Borregaard. 2003. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 170:5583-5589. [DOI] [PubMed] [Google Scholar]
- 52.Sorensen, O. E., P. Follin, A. H. Johnsen, J. Calafat, G. S. Tjabringa, P. S. Hiemstra, and N. Borregaard. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951-3959. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka, D., K. T. Miyasaki, and R. I. Lehrer. 2000. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: a cathelicidin found in human leukocytes and epithelium. Oral Microbiol. Immunol. 15:226-231. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, H., S. Miyazaki, Y. Sumiyama, and T. Kakiuchi. 2004. Role of macrophages in a mouse model of postoperative MRSA enteritis. J. Surg. Res. 118:114-121. [DOI] [PubMed] [Google Scholar]
- 55.Tepper, R. I., R. L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257:548-551. [DOI] [PubMed] [Google Scholar]
- 56.Travers, J. B., D. A. Norris, and D. Y. Leung. 2001. The keratinocyte as a target for staphylococcal bacterial toxins. J. Investig. Dermatol. Symp. Proc. 6:225-230. [DOI] [PubMed] [Google Scholar]
- 57.Turner, J., Y. Cho, N. N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tutschka, P. J. 1988. Infections and immunodeficiency in bone marrow transplantation. Pediatr. Infect. Dis. J. 7:S22-S29. [PubMed] [Google Scholar]
- 59.Vassiloyanakopoulos, A. P., S. Okamoto, and J. Fierer. 1998. The crucial role of polymorphonuclear leukocytes in resistance to Salmonella dublin infections in genetically susceptible and resistant mice. Proc. Natl. Acad. Sci. USA 95:7676-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 61.Wood, M. J. 1998. Viral infections in neutropenia—current problems and chemotherapeutic control. J. Antimicrob. Chemother. 41(Suppl. D):81-93. [DOI] [PubMed] [Google Scholar]
- 62.Yang, D., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]
- 64.Zaiou, M., V. Nizet, and R. L. Gallo. 2003. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Investig. Dermatol. 120:810-816. [DOI] [PubMed] [Google Scholar]
- 65.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 66.Zaretzky, F. R., and T. H. Kawula. 1999. Examination of early interactions between Haemophilus ducreyi and host cells by using cocultured HaCaT keratinocytes and foreskin fibroblasts. Infect. Immun. 67:5352-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]