Abstract
Leptospira interrogans serovar Copenhageni strain RJ16441, a blood isolate from humans with the severe pulmonary form of leptospirosis, has previously been shown to cause fatal pulmonary hemorrhage in guinea pigs and asymptomatic chronic renal tubular colonization with urinary shedding in rats. In this study, RJ16441 caused lethal infection of both C3H/HeJ and C3H/SCID mice, but no hemorrhagic phenomena were observed.
Leptospirosis is the most geographically widespread zoonotic disease in the world. Chronically infected mammalian hosts harbor pathogenic Leptospira species in renal tubules of the kidney, from which they are shed via urine into the environment and survive in suitable moist conditions (2, 6, 7). Humans are infected via broken skin and mucosal surfaces during contact with contaminated environs. Clinical manifestations of acute leptospirosis reflect the systemic dissemination of the spirochete, ranging from a mild febrile illness to the more severe icteric Weil's disease, characterized by renal and liver failure. Additionally, a severe pulmonary form of leptospirosis (SPFL) is being recognized with increased frequency, with cases from Brazil, Argentina, Nicaragua, India, Thailand, Korea, and Australia being reported (3, 13, 14, 16-21).
Leptospira interrogans serovar Copenhageni strain RJ16441, originally isolated from blood of a patient with SPFL, has been used to develop models of acute and chronic disease in guinea pigs and rats, respectively (11, 12). Guinea pigs infected with strain RJ16441 develop fatal pulmonary hemorrhages like human SPFL patients (11), while experimental infection of rats is clinically asymptomatic and results in chronic renal tubular carriage with excretion of infectious leptospires into urine (4). In this study, we addressed the outcomes of infection of C3H/HeJ and C3H/SCID mice with RJ16441.
Like the rat, the common house mouse is a significant reservoir of leptospirosis (9). Most studies exploring the pathogenicity of Leptospira have employed hamsters or guinea pigs. The ability of mice to harbor experimental chronic carriage has long been established (5). Lethal infection of mice, noted in few reports, depends on strain, age, and Leptospira serovar (5). Mice resistant to infection by L. interrogans serovar Pomona can be rendered susceptible by immunosuppression (1). C3H/HeJ mice, up to 3 weeks of age, were highly susceptible to lethal infection with L. interrogans serovar Icterohaemorrhagiae (15). A more recent report noted the susceptibility of C3H/HeJ mice to lethal infection with serovars Manilae and Icterohaemorrhagiae (6).
Three- or 6-week-old C3H/HeJ mice (stock number 659; The Jackson Laboratory, Bar Harbor, ME) and 3- or 6-week-old C3H/SCID mice (four mice per group, except where indicated) (C3Smn.CB17-prkdcscid/J, stock number 1131) were experimentally infected by intraperitoneal injection with 107 organisms of a virulent, low-passage (<2) isolate of L. interrogans serovar Copenhageni RJ16441. In addition, a high-passage (>5) isolate of RJ16441, which was avirulent for guinea pigs, as determined by no weight loss or loss of mobility after infection, was used to experimentally infect four additional mice from each group. Control mice included 6-week-old C3H/HeJ and C3H/SCID mice injected with medium alone. Animals were monitored daily for clinical signs of illness, including weight loss, loss of mobility, and general unkemptness, and in the case of mice infected with low-passage virulent RJ16441, were euthanized when they appeared moribund (Table 1). Tissues were collected at the time of euthanasia and processed for routine light microscopy and immunohistochemistry as previously described (11, 12). Spirochetemia was determined by enumeration of leptospires in plasma using dark-field microscopy as previously described (8, 10). All animal research was approved by the Animal Research Committee of the University of California—Los Angeles.
TABLE 1.
Levels of spirochetemia in infected C3H/HeJ and C3H/SCID mice
| Mouse age (wks), strain, and infecting isolate (n = 4)a | No. of days postinfection before euthanasiab | Spirochetemia (Leptospira isolates/ ml of plasma)c |
|---|---|---|
| 3, C3H/HeJ, Vir | 5 | 0-6 × 105 (2) |
| 6, C3H/HeJ, Vir | 7 | 3 × 105-5 × 105 (3) |
| 3, C3H/SCID, Vir | 4 | 3.5 × 106 (1) |
| 6, C3H/SCID, Vir (n = 8) | 5 (2), 6 (4) | 1.3 × 106-5 × 106 (6) |
| 3, C3H/HeJ, Avir | 10 (2), 15 (2) | 0 |
| 6, C3H/HeJ, Avir | 10 (2), 15 (2) | 0 |
| 3, C3H/SCID, Avir (n = 3) | 10 (1), 17 (2) | 1 × 105-1 × 107 (2) |
| 6, C3H/SCID, Avir | 10 (2), 17 (2) | 1 × 105-2 × 105 (4) |
| 6, C3H/HeJ, negative control | 15 (2), 17 (2) | 0 |
| 6, C3H/SCID, negative control | 15 (2), 17 (2) | 0 |
Groups of mice (n = 4, except where indicated) were infected with either virulent low-passage (Vir) or avirulent high-passage (Avir) L. interrogans serovar Copenhageni RJ16441.
Numbers of days postinfection at time of euthanasia are indicated, with the numbers of mice euthanized on each day indicated in parentheses.
The ranges of spirochetemia as numbers of leptospires/ml of plasma are provided, with the numbers of mice in each group that were sampled for spirochetemia indicated in parentheses.
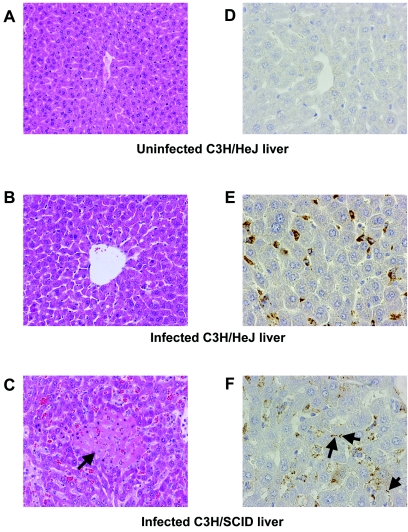
All mice were susceptible to acute lethal infection with low-passage virulent SPFL isolate L. interrogans serovar Copenhageni RJ16441. Additionally, all mice sampled for spirochetemia tested positive, with numbers of organisms ranging from >105 leptospires/ml of plasma in C3H/HeJ mice to >106 leptospires/ml in C3H/SCID mice; thus, infected C3H/SCID mice produced spirochetemia an order of magnitude greater than that observed in C3H/HeJ mice. Livers of C3H/HeJ mice infected with low-passage virulent RJ16441 showed discohesion, some necrosis, and increased numbers of Kuppfer cells and macrophages (Fig. 1B), as did livers of infected C3H/SCID mice (Fig. 1C). In C3H/HeJ mice, granular deposits of leptospiral antigen in Kupffer cells and macrophages but not intact leptospires were detected by immunohistochemistry (11, 12) (Fig. 1B). In the case of the C3H/SCID mice, intact leptospires were visualized along hepatocyte membranes by immunohistochemistry (Fig. 1C). There were minimal signs of inflammation in the livers of all infected mice, despite the presence of large numbers of leptospires and/or leptospiral antigen.
FIG. 1.
Mouse livers infected with Leptospira. A hematoxylin and eosin stain of a normal C3H/HeJ liver (A), compared to an infected C3H/HeJ (B) and infected C3H/SCID (C) liver, is shown (original magnification, ×200). (B) Discohesion is evident in infected livers of C3H/HeJ mice. (C) An area of necrosis (indicated by arrow) in an infected C3H/SCID liver is shown. The immunohistochemistry of a negative-control liver (D), compared to an infected C3H/HeJ liver (E) and infected C3H/SCID liver (F), is shown (original magnification, ×200). Intact leptospires in livers of infected C3H/SCID mice are indicated by arrows. By comparison, infected C3H/HeJ liver (E) shows only reactive granular debris in Kupffer cells.
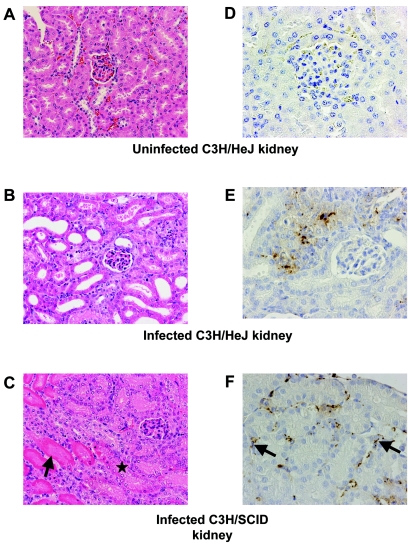
Examination of kidneys by immunohistochemistry revealed large numbers of intact leptospires in the interstitium, as well as reactive granular debris, in C3H/SCID mice (Fig. 2C). Only reactive granular leptospiral debris was noted in kidneys of C3H/HeJ mice (Fig. 2B). All infected kidneys of C3H/SCID and C3H/HeJ mice showed evidence of tubular injury (Fig. 2B and C). The absence of inflammatory cells despite the presence of numerous leptospires or leptospiral antigen was striking.
FIG. 2.
Mouse kidney tissue infected with Leptospira. A hematoxylin and eosin stain of a normal C3H/HeJ kidney (A), compared to an infected C3H/HeJ kidney (B) and an infected C3H/SCID kidney (C), is shown (original magnification, ×200). Infected kidneys show evidence of tubular injury. Frankly necrotic renal tubules are indicated by arrows compared to a renal tubule with early necrotic changes (asterisk). The immunohistochemistry of a negative-control kidney (D), compared to an infected C3H/HeJ kidney with granular debris (E) and infected C3H/SCID kidney with intact leptospires (arrows) (F), is shown (original magnification, ×200).
Examination of the lungs of infected mice was of particular interest, since RJ16441 had caused fatal pulmonary hemorrhages in guinea pigs in the setting of deposition of antibodies and complement component C3 along alveolar septa (12). At necropsy, there was no gross pulmonary hemorrhage. Microscopic examination of the lungs also revealed no pulmonary hemorrhage. Rare leptospiral antigen in the form of granular debris in phagocytic cells in the lungs of both C3H/SCID and C3H/HeJ mice was noted (data not shown).
The renal functions of six C3H/HeJ mice infected with RJ16441 showed elevations of blood urea nitrogen (range, 13 to 197 mg/dl; mean, 106.5 mg/dl) and creatinine (range, 0.5 to 1.4 mg/dl; mean, 1.08 mg/dl) compared to five uninfected controls (for blood urea nitrogen, the range was 21 to 24 mg/dl and the mean was 23 mg/dl; for creatinine, the range was 0.2 to 0.3 mg/dl and the mean was 0.28 mg/dl) consistent with the microscopic evidence indicative of acute tubular necrosis in the infected mice. Liver function tests of these infected and uninfected C3H mice showed no elevations of total bilirubin, gammaglutamyl transferase, aspartate transaminase, or alanine aminotransferase (data not shown). Taken together, our findings suggest that the cause of death in RJ16441-infected mice was acute renal failure.
All mice infected with high-passage avirulent RJ16441 appeared clinically normal at the time of euthanasia on day 10 or day 17. However, spirochetemia was found to be as high as 3 × 106 leptospires/ml in C3H/SCID mice but was not detectable by dark-field microscopy with C3H/HeJ mice (Table 1). Both liver and kidney tissues from C3H/SCID mice infected with high-passage avirulent RJ16441 were normal histologically; small amounts of antigenic debris were detected by immunohistochemistry in these tissues (data not shown).
Low-passage RJ16441 causes acute lethal infection in guinea pigs due to pulmonary hemorrhage (12). The outcomes of infection of other rodents with this serovar Copenhageni strain have been distinct. Rats developed chronic renal tubular colonization and urinary shedding of leptospires (4-12). In this study, infection of C3H and C3H/SCID mice was lethal, but pulmonary hemorrhage was not found.
Acknowledgments
These studies were supported by National Institutes of Health grant AI056258 to M.A.L. and Ruth L. Kirschenstein National Research Service Award AI055235 to J.E.N. from the United States Public Health Service National Institute of Allergy and Infectious Diseases. M.C.F. is funded in part by an endowment from the Piansky Family Trust.
We thank Longsheng Hong for technical assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adler, B., and S. Faine. 1976. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans serovar pomona. Infect. Immun. 14:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho, C. R., and E. P. Bethlem. 2002. Pulmonary complications of leptospirosis. Clin. Chest Med. 23:469-478. [DOI] [PubMed] [Google Scholar]
- 4.Chow, E., J. E. Nally, X. Y. Wu, M. C. Fishbein, D. R. Blanco, and M. A. Lovett. Unpublished data.
- 5.Faine, S. 1962. Factors affecting the development of the carrier state in leptospirosis. J. Hyg. (Cambridge) 60:427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Melbourne, Australia.
- 7.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnuson, H. J., H. Eagle, and R. Fleischman. 1948. The minimal infectious inoculum of Spirochaeta pallida (Nichols strain), and a consideration of its rate of multiplication in vivo. Am. J. Syph. Gonorrhea Vener. Dis. 32:1-18. [PubMed] [Google Scholar]
- 9.Matthias, M. A., and P. N. Levett. 2002. Leptospiral carriage by mice and mongooses on the island of Barbados. West Indian Med. J. 51:10-13. [PubMed] [Google Scholar]
- 10.Miller, J. N., V. H. Falcone, B. Golden, C. W. Israel, U. S. G. Kuhn III, and R. M. Smibert. 1971. Spirochetes in body fluids and tissues. Manual of investigative methods. Charles C. Thomas, Springfield, Ill.
- 11.Nally, J. E., C. Chantranuwat, X. Y. Wu, M. C. Fishbein, M. M. Pereira, J. J. Da Silva, D. R. Blanco, and M. A. Lovett. 2004. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am. J. Pathol. 164:1115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nally, J. E., E. Chow, M. C. Fishbein, D. R. Blanco, and M. A. Lovett. 2005. Changes in lipopolysaccharide O antigen distinguish acute versus chronic Leptospira interrogans infections. Infect. Immun. 73:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niwattayakul, K., J. Homvijitkul, O. Khow, and V. Sitprija. 2002. Leptospirosis in northeastern Thailand: hypotension and complications. Southeast Asian J. Trop. Med. Public Health 33:155-160. [PubMed] [Google Scholar]
- 14.Park, S. K., S. H. Lee, Y. K. Rhee, S. K. Kang, K. J. Kim, M. C. Kim, K. W. Kim, and W. H. Chang. 1989. Leptospirosis in Chonbuk Province of Korea in 1987: a study of 93 patients. Am. J. Trop. Med. Hyg. 41:345-351. [PubMed] [Google Scholar]
- 15.Pereira, M. M., J. Andrade, R. S. Marchevsky, and R. Ribeiro dos Santos. 1998. Morphological characterization of lung and kidney lesions in C3H/HeJ mice infected with Leptospira interrogans serovar icterohaemorrhagiae: defect of CD4+ and CD8+ T-cells are prognosticators of the disease progression. Exp. Toxicol. Pathol. 50:191-198. [DOI] [PubMed] [Google Scholar]
- 16.Seijo, A., H. Coto, J. San Juan, J. Videla, B. Deodato, B. Cernigoi, O. G. Messina, O. Collia, D. de Bassadoni, R. Schtirbu, A. Olenchuk, G. D. de Mazzonelli, and A. Parma. 2002. Lethal leptospiral pulmonary hemorrhage: an emerging disease in Buenos Aires, Argentina. Emerg. Infect. Dis. 8:1004-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva, J. J., M. O. Dalston, J. E. Carvalho, S. Setubal, J. M. Oliveira, and M. M. Pereira. 2002. Clinicopathological and immunohistochemical features of the severe pulmonary form of leptospirosis. Rev. Soc. Bras. Med. Trop. 35:395-399. [DOI] [PubMed] [Google Scholar]
- 18.Simpson, F. G., K. A. Green, G. J. Haug, and D. L. Brookes. 1998. Leptospirosis associated with severe pulmonary haemorrhage in Far North Queensland. Med. J. Aust. 169:151-153. [DOI] [PubMed] [Google Scholar]
- 19.Trevejo, R. T., J. G. Rigau-Perez, D. A. Ashford, E. M. McClure, C. Jarquin-Gonzalez, J. J. Amador, J. O. de los Reyes, A. Gonzalez, S. R. Zaki, W. J. Shieh, R. G. McLean, R. S. Nasci, R. S. Weyant, C. A. Bolin, S. L. Bragg, B. A. Perkins, and R. A. Spiegel. 1998. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J. Infect. Dis. 178:1457-1463. [DOI] [PubMed] [Google Scholar]
- 20.Vijayachari, P., S. C. Sehgal, M. G. Goris, W. J. Terpstra, and R. A. Hartskeerl. 2003. Leptospira interrogans serovar Valbuzzi: a cause of severe pulmonary haemorrhages in the Andaman Islands. J. Med. Microbiol. 52:913-918. [DOI] [PubMed] [Google Scholar]
- 21.Yersin, C., P. Bovet, F. Merien, J. Clement, M. Laille, M. Van Ranst, and P. Perolat. 2000. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Trans. R. Soc. Trop. Med. Hyg. 94:71-76. [DOI] [PubMed] [Google Scholar]