Abstract
Granulysin and NK-lysin are homologous bactericidal proteins with a moderate residue identity (35%), both of which have antimycobacterial activity. Short loop peptides derived from the antimycobacterial domains of granulysin, NK-lysin, and a putative chicken NK-lysin were examined and shown to have comparable antimycobacterial but variable Escherichia coli activities. The known structure of the NK-lysin loop peptide was used to predict the structure of the equivalent peptides of granulysin and chicken NK-lysin by homology modeling. The last two adopted a secondary structure almost identical to that of NK-lysin. All three peptides form very similar three-dimensional (3-D) architectures in which the important basic residues assume the same positions in space. The basic residues in granulysin are arginine, while those in NK-lysin and chicken NK-lysin are a mixture of arginine and lysine. We altered the ratio of arginine to lysine in the granulysin fragment to examine the importance of basic residues for antimycobacterial activity. The alteration of the amino acids reduced the activity against E. coli to a larger extent than that against Mycobacterium smegmatis. In granulysin, the arginines in the loop structure are not crucial for antimycobacterial activity but are important for cytotoxicity. We suggest that the antibacterial domains of the related proteins granulysin, NK-lysin, and chicken NK-lysin have conserved their 3-D structure and their function against mycobacteria.
Granulysin and NK-lysin are two similar 9-kDa cationic proteins produced by human and pig cytolytic lymphocytes, respectively (4, 27). They have a broad spectrum of activity and lyse a variety of bacterial, fungal, and tumor cells (4, 5, 7, 33). Both proteins are activated/synthesized upon antigen and cytokine stimulation and are stored in granules (4, 19, 37). Their most important function may be that of killing intracellular pathogens, including Mycobacterium tuberculosis (33), the pathogen responsible for human tuberculosis.
Granulysin and NK-lysin are members of the saposin-like protein family SAPLIP (3). The members of this family share several structural properties, including six similarly located cysteines forming three disulfide bridges with the same cysteine pairings (3). The structural homology and the conserved disulfide bridges suggest a common fold, called a saposin fold, for all SAPLIPs. The protein structure has been established for NK-lysin and granulysin (2, 22). Both granulysin and NK-lysin consist of a precise fold, comprising five α-helices, spaced by three loops in the protein molecule. In previous work, we have shown that shorter peptide fragments or loop peptides derived from granulysin and NK-lysin have similar activities against M. tuberculosis to those of their parent molecules (7). Other groups have confirmed this and, likewise, determined this region to be the antibacterial and membranolytic domain of granulysin and NK-lysin (6, 16, 36). These granulysin and NK-lysin fragments span the second and third α-helices of their parent proteins, which define a helix-loop-helix motif (NK-lysin amino acids [aa] 34 to 55). This domain is believed to be of importance in initial membrane docking and subsequent membrane-disturbing activities (2, 22, 24).
The genus Mycobacterium comprises an ample variety of species with a worldwide distribution that are of medically, veterinary, and environmental importance. Some of these have a wide host spectrum (e.g., Mycobacterium bovis), while others (e.g., Mycobacterium tuberculosis) principally encompass one natural host. Comparative genomic analyses have shown that organisms belonging to the M. tuberculosis complex (M. tuberculosis, M. africanum, M. canettii, M. microti, and M. bovis) have evolved from a progenitor of the present M. tuberculosis organism and adapted to new hosts (11).
Even though there are striking variations in environmental adaptation, there are indeed many parallels between certain pathogenic mycobacteria. Mycobacterium marinum, the phylogenetically closest relative to M. tuberculosis, is a natural pathogen of ectotherms, including fish and amphibians, that occasionally gives rise to infections in humans described as “fish tank granuloma.” The granulomas caused by an M. marinum dermal infection are similar to those caused by M. tuberculosis in the lungs of humans (32). The pathogenic mechanisms of infection by M. marinum and M. tuberculosis are clearly analogous, as are the host immune response against these two organisms.
Over the years, or rather millennia, the parasite-host relationship has changed as environmental changes have taken place. Peptide-mediated defense is an evolutionarily old mechanism of fighting bacteria; therefore, it is likely that different species have evolved similar molecules to fight mycobacterial infections. Granulysin and NK-lysin are principal antibacterial peptides in cytolytic cells, and cell-mediated immunity is an important arm of protection against tuberculosis. It has been shown that granulysin, which is produced by cytolytic T cells (CTLs) or Vγ9/Vδ2 T cells, in synergy with perforin can kill the intracellular tubercle bacillus M. tuberculosis without simultaneous destruction of macrophages (13, 33).
Using the rapidly growing species Mycobacterium smegmatis and M. marinum, we examined if an identified sequence from activated chicken splenocytes (35) that resembles granulysin and NK-lysin would also possess antimycobacterial activity. We suggest that nature has conserved the antimycobacterial three-dimensional (3-D) structure and function in the granulysin/NK-lysin family of peptides rather than residue identity.
MATERIALS AND METHODS
Peptides.
All peptides were synthesized by Innovagen (Lund, Sweden), except PR-39 (Macromolecular Resources Research Core Laboratory, Colorado State University) and Cecropin P1 (21). The peptides were purified as reduced peptides and controlled by mass spectrometry and matrix-assisted laser desorption ionization-time of flight analysis (ReflexIII; BrukerDaltronics, Germany). Oxidation was performed by dissolving the peptide at 1 mg/ml in 50 mM Tris, pH 8.0, and leaving it at room temperature overnight. Oxidized peptides were purified by reversed-phase high-performance liquid chromatography (VydacC18; Separation Group), and purity was controlled by matrix-assisted laser desorption ionization-time of flight analysis. Peptides were reduced, carboxymethylated with iodoacetoamide, and desalted on Sep-Pak C18 columns (Waters, United Kingdom). Stock solutions at 2 μg/μl were prepared in polypropylene microtubes (Eppendorf AG, Germany) and stored in water at −20°C. The hydrolysis of peptides and analyses of amino acid contents were performed to determine the molar concentrations of peptide test solutions.
Structure prediction.
The sequences of chicken NK-lysin (cNK-lysin), granulysin, modified granulysin, bovine NK-lysin, and equine NK-lysin were aligned with that of pig NK-lysin using ClustalW (34). Homology modeling was carried out in the ICM software package (1), using 1NKL (22) as the template. Hydrophobic scores were calculated with the Protscale program (http://www.expasy.org/cgi-bin/protscale.pl) according to the method of Kyte and Doolittle (20), using a seven-residue window.
CD analysis.
Peptide secondary structures were examined by circular dichroism (CD) spectroscopy (Aviv 62DS spectropolarimeter; Aviv Associates Inc., N.J.). The peptide concentration was 40 μM, and analysis was done with 15 mM sodium carbonate, pH 7.2. All spectra were recorded at 25°C at a 1-nm/point resolution. The average contents of helical conformations were calculated from the experimental molar ellipticities at 208 and 222 nm (17, 26).
Antimycobacterial assay.
Mycobacterium smegmatis mc2155 was grown on mycobacterium 7H11 agar (Difco, Mich.). Bacteria were inoculated into 20 ml of Middlebrook 7H9 broth (Difco) containing 0.05% Tween 80 (Sigma-Aldrich, Germany). Cultures were incubated on a rotary shaker at 180 rpm and 37°C until they reached an optical density at 600 nm (OD600) of 0.15. The cultures were then diluted 1:10 to obtain 106 to 107 CFU/ml. Antimycobacterial activity testing was performed using the alamarBlue assay (Serotec, United Kingdom) with clear flat-bottomed 96-well polystyrene plates (Falcon, Becton-Dickinson, France). Peptide dilutions were prepared in sterile milli-Q water from stock solutions (2 μg/μl). Ten microliters of peptide solution was added to each of triplicate test wells to reach the final test dilutions. Next, 170 μl of the 1:10 bacterial suspension was added to each test well. Additional control wells consisted of bacteria plus solvent (water) and medium plus solvent. Plates were incubated at 37°C overnight. The next day, 20 μl of alamarBlue solution was added to each well, and the plates were reincubated at 37°C. Wells were read at 4 h to 6 h to determine the OD600. The percentage of growth inhibition was calculated according to the manufacturer's recommendation. The MIC of the peptide was defined as 50% or 90% growth inhibition.
Mycobacterium marinum ATCC 2275 was grown on mycobacterium 7H11 agar (Difco, Mich.) containing oleic acid-albumin-dextrose-catalase enrichment (Becton Dickinson, MD). Bacteria were inoculated into 20 ml of Middlebrook 7H9 broth containing 0.05% Tween 80. Cultures were incubated on a rotary shaker at 180 rpm and 30°C until they reached an OD600 of 0.1. The cultures were then washed and filtered with a 5-μm Millipore filter to prevent clumping before being diluted 1:1,000 to obtain 105 to 106 CFU/ml.
Standard colony count assay.
Gram-positive and gram-negative bacteria were grown in LB broth until the OD600 reached 0.6. Various concentrations of peptides with 90 μl of 107 bacteria/ml were incubated in clear flat-bottomed 96-well polystyrene plates (Falcon, Becton-Dickinson, France) at 37°C for 3 h. After incubation, the bacteria were placed on ice. Tenfold dilutions were prepared and plated on LB agar plates. CFU were counted after overnight incubation at 37°C. The peptide activity in minimal medium was determined by culturing bacteria as described previously. The bacteria were then incubated with peptides in a 1:100 dilution of LB-sodium phosphate at pH 7.4 for 3 h before CFU determination.
Antimycobacterial activity was assessed by CFU determination using M. marinum. Mycobacterial test suspensions were obtained as described above. Ten microliters of each peptide test solution was added in duplicate to each test well containing bacteria in a polystyrene 96-well flat-bottomed microtiter plate to reach a final volume of 100 μl, and the plate was incubated at 30°C for 4 days. After incubation, serial dilutions were plated in duplicate on Middlebrook 7H11 agar plates containing oleic acid-albumin-dextrose-catalase. The plates were incubated at 30°C, and CFU were determined after 5 days.
Inhibition-zone assay.
An inhibition-zone assay in thin agarose plates (1 mm) was used to determine the antibacterial activities of gram-positive and -negative bacteria (18). The test plates were incubated at 30°C overnight. The inhibition zones were recorded, and lethal concentrations were calculated from the zones observed for serial dilutions of peptide.
Cytotoxicity assays.
Cytotoxicity assays with the human U937 monocyte cell line were performed with a CytoTox96 assay kit (Promega, WI), which quantitatively measures lactate dehydrogenase release upon cell lysis. The cells were grown in RPMI 1640 culture medium (Sigma-Aldrich, Germany) supplemented with 10% heat-inactivated fetal calf serum (Sigma-Aldrich) and penicillin-streptomycin solution (Sigma-Aldrich). The cells were adjusted to 2 × 104/ml in a final volume of 100 μl in clear-bottomed 96-well polystyrene plates (Falcon, Becton-Dickinson, France). Monocytes were differentiated using phorbol myristate acetate (PMA) at a final concentration of 100 nM and incubated at 37°C for 3 days. The cells were washed, and 90 μl of medium plus 10 μl of peptide solution was added to each test well, giving final concentrations of 50 to 200 μg/ml. The plate was incubated at 37°C in 5% CO2 for 4 h. Due to the experimental setup, the serum concentration was reduced to 2% to minimize the background absorbance of endogenous lactate dehydrogenase in calf serum when performing the assay. Preparations of primary monocytes/macrophages were performed as follows. Buffy coats were obtained from healthy volunteers at the Karolinska University Hospital Huddinge, Stockholm, Sweden. Peripheral blood mononuclear cells were obtained through Ficoll (Lymphoprep; Axis-Shield, Oslo, Norway) centrifugation of buffy coat blood, and monocytes were thereafter prepared from peripheral blood mononuclear cells by using a RosetteSep kit (StemCell Technologies) according to the manufacturer's instructions. Primary macrophages were differentiated from blood monocytes through culturing for 7 to 10 days in cell culture medium (RPMI; 1% sodium pyruvate, 1% HEPES buffer, 1% glutamine, and 1% streptomycin; all from GIBCO, Invitrogen) supplemented with 20% human AB serum. Monocytes or macrophages were seeded at a concentration of 2 × 104 cells/well in semitransparent polystyrene 96-well plates (Corning LabDesign, Sweden). The indicated concentrations of antimicrobial peptides were incubated with the cells for 4 h at 37°C in the presence of RPMI plus 2% fetal calf serum. Cytotoxicity was measured with CytoTox-ONE (Promega, WI) according to the manufacturer's instructions. The viability of cells was established by staining with trypan blue (Sigma Chemical, MO) and counting of live cells.
RESULTS
Similarities among granulysin/NK-lysin peptides.
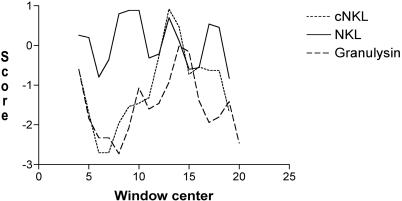
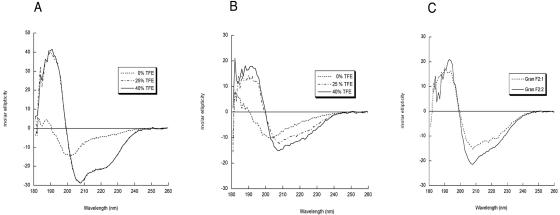
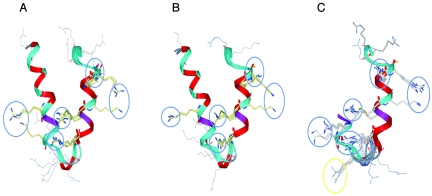
Although granulysin and NK-lysin differ in their amino acid compositions (35% identity), their structural elements (basic residues and saposin fold) have been preserved. This low amino acid identity was also noted for what has been determined to be the antimycobacterial domain (GRANF2 and NKLF2) (Table 1). The deduced chicken NK-lysin sequence (GenBank accession no. AI980505) shows 35% identity to NK-lysin and maintains one proline and six cysteine residues, which are a hallmark of the saposin family (3). When a chicken NK-lysin fragment, cNKLF2, was aligned with the corresponding granulysin/NK-lysin loop peptides (Table 1), a similar spacing of basic residues was maintained, suggesting an amphipathic structure. The basic residues in GRANF2 are arginines, while lysines are predominant in the N-terminal part of NKLF2 and the C-terminal end of cNKLF2. Overall, the “hydrophobic moment” of cNKLF2 is very similar to that of GRANF2, while the NKLF2 peptide is clearly more hydrophobic (Fig. 1). The hydrophobic moments of the bovine and equine homologous peptides resemble that of NKLF2 (not shown). The solution conformations of the peptides were examined by CD analysis in both aqueous and organic solvent environments. In water, CD spectra of GRANF2 and cNKLF2 displayed large minima at 203 nm, indicating a high degree of aperiodic conformation (Fig. 2). Both peptides adopted a typical α-helical conformation with increasing trifluoroethanol (TFE) concentrations. The calculated α-helical contents of GRANF2 and cNKLF2 in 25% TFE solution (36% and 75%, respectively) fit well with the values derived from the nuclear magnetic resonance (NMR) structure (22) and suggest that cNKLF2 has a more extended α-helix than GRANF2. When the granulysin and cNKLF2 fragments were placed in the NK-lysin NMR coordinates and allowed to stabilize, they adopted a secondary structure almost identical to that of NK-lysin (Fig. 3A). The only difference noticed was an extended loop in granulysin as a result of one additional residue. The space coordinates of positively charged residues fell into defined areas (Fig. 3A, blue circles).
TABLE 1.
Primary sequences of granulysin and NK-lysin peptides
| Peptide | Mr | Sequencea | pI |
|---|---|---|---|
| GRANF2 | 2,989 | VCRTGRSRWRDVCRNFMRRYQSR | 11.8 |
| GRANF2:1 | 2,876 | VCKTGKSKWKDVCRNFMRRYQSR | 10.6 |
| GRANF2:2 | 2,764 | VCKTGKSKWKDVCKNFMKKYQSK | 9.9 |
| GRANF3 | 1,994 | GRSRWRDVCRNFMRR | 12.0 |
| NKLF2 | 2,692 | VCDKMKI-LRGVCKKIMRSFLRR | 11.0 |
| cNKLF2 | 2,629 | VCSTGRRQ-RSICKQLLKKLRQQ | 11.6 |
| bNKL | 2,584 | VCSKMR-LLKGLCKSIMKKFLRT | 10.6 |
| eNKL | 2,518 | VCRNLG-LLRGACKKIMRTCLRL | 10.9 |
Bold letters indicate arginine residues, and italics indicate lysine residues.
FIG. 1.
Hydrophobic moments of granulysin/NK-lysin peptides. Hydrophobic scores were calculated with the ProtScale program according to the method of Kyte and Doolittle (20), using a seven-residue window.
FIG. 2.
TFE concentration-dependent conformations of granulysin/NK-lysin peptides. The graphs show comparisons of circular dichroism spectra of GRANF2 (A), cNKLF2 (B), and GRANF2:1 and GRANF2:2 (C). For panel C, the spectra of modified peptides were obtained in 25% TFE solution.
FIG. 3.
3-D structures of granulysin/NK-lysin peptides. (A) Human, porcine, and avian structures. (B) Bovine, equine, and modified GRANF2:2 peptides. (C) All peptides in panels A and B are superimposed. Strictly conserved residues between the species variants are shown in magenta. Residues with conserved properties are shown in cyan. Cationic residues encircled in blue originated from different peptides and are located nearby in space. Residues encircled in yellow are conserved basic residues in all peptides except for the equine peptide.
We also modeled the recently characterized proteins found in other species, bovine NK-lysin (GenBank accession no. AY245799) and equine NK-lysin (AF538056) (12, 14). Both peptides formed a secondary structure identical to that of NK-lysin, with the positively charged residues occupying the defined areas in blue shown in Fig. 3B. In this figure, we also included the structure of GRANF2:2. Likewise, the lysines in GRANF2:2 projected into the space within the blue circles (Fig. 3B). All peptides showed similar spatial distributions of arginines and lysines (blue circles), except in the loop structure (Fig. 3C). In this case, all peptides but equine NK-lysin formed a cluster of positively charged residues in a confined area (yellow circle).
Antimycobacterial activity.
When tested against M. smegmatis, all three loop fragments in their oxidized state showed similar MIC90 values of 7.5 to 13 μM (Table 2). Reducing the length of GRANF2 by removing the N-terminal loop structure, including one cysteine residue (GRANF3), lowered the activity almost twofold.
TABLE 2.
Antibacterial activities of peptides
| Peptide | Antibacterial activity for indicated organismc
|
|||||
|---|---|---|---|---|---|---|
| M. smegmatis mc2 155 (MIC90a [μM]) | M. marinum ATCC 2275 (MIC99b [μM]) |
E. coli D21
|
B. megaterium 11
|
|||
| LC (μM) | MIC99 (μM) | LC (μM) | MIC99 (μM) | |||
| Gran F2 | 10.3 (9) | <∼2 | 23 (26) | >35 | 1.3 (1.1) | <5 |
| Gran F2:1 | 13 (16) | NA (53) | 0.9 (3.2) | |||
| Gran F2:2 | 23 (32) | <∼2 | NA (>210) | 0.5 (13.7) | ||
| Gran F3 | 18 | 19.4 | 0.4 | |||
| NKL F2 | 7.5 | <∼2 | 3.4 | 7.4 | 0.3 | <5 |
| cNKL F2 | 13 (15) | <∼2 | >124 (>160) | >35 | 3.3 (6.1) | <5 |
| Cecropin P1 | NA | 0.3d | 5.3d | |||
| L-C(1-13)M(1-13) | 7.6 | 2.4 | ||||
The antimicrobial activity assay was alamarBlue.
The antimicrobial activity assay was standard colony count (CFU).
Data for carboxymethylated peptides are in parentheses. NA, not active.
Data from reference 28.
To test if the nature of basic residues is crucial for antimycobacterial activity, we altered the ratio of arginine to lysine residues in GRANF2 and examined its activity against M. smegmatis. This alteration did not change the position of the basic residues or the overall charge. The peptides maintained a typical α-helical conformation in the presence of TFE, with a higher α-helical content in GRANF2:1 than in GRANF2:2 (Fig. 2). Table 2 shows that GRANF2 with all arginine residues changed to lysine (GRANF2:2) had a twofold decreased activity. We found that changing the arginine residues to lysine in the N-terminal region (GRANF2:1) moderately decreased the activity compared to that of GRANF2. Thus, the three loop peptides with intact disulfide bonds exhibit similar 3-D structures and antimycobacterial activities.
It has been noticed that an intact disulfide bond may be of importance for antibacterial activity (5). The peptides in Table 1 were thus tested in their carboxymethylated forms. As shown in Table 2, there was no major difference in activity between the oxidized and reduced forms of GRANF2 and its modified versions. We also tested GRANF2 peptides with either an amidated C-terminal end that increased the net positive charge of the peptide or a peptide synthesized with all d-type amino acids. Both peptides were somewhat more potent (MIC90, 7.4 and 5.9 μM, respectively) against M. smegmatis than the parent peptide GRANF2 (data not shown).
We showed earlier that GRANF2 and NKLF2 are active against M. tuberculosis. It was therefore relevant to evaluate if the antimycobacterial pattern of chicken NKLF2 would be similar to those of the human and porcine peptides against pathogenic mycobacteria. All three peptides reduced the CFU of M. marinum by 4 to 5 log (99% inhibition) after 4 days of incubation at 5 μg/ml (1.7 to 2.4 μM). The granulysin peptide GRANF2:2 was as potent as the native peptide GRANF2 against M. marinum. Hence, the avian and modified peptides with similar 3-D structures uphold the antimicrobial activity against pathogenic mycobacteria.
Activity against other bacteria.
We also tested the various peptides against gram-positive and gram-negative bacteria. All peptides tested had high activities against Bacillus megaterium (0.3 to 3.3 μM) and various activities against Escherichia coli (Table 2). NKLF2 was active against E. coli, while GRANF2 showed moderate activity and cNKLF2 was inactive.
The modified GRANF2 peptides lost more of the potency of their parent peptide against E. coli than that against M. smegmatis and B. megaterium. None of the peptides showed activity at the highest concentration tested against Pseudomonas aeruginosa or Staphylococcus aureus, suggesting lethal concentration values above 100 μM. For comparison, we determined the activity spectra of inactive cecropin P1 and L-C(1-13)M (1-13), which have high activities against M. smegmatis.
The effect of peptides (GRANF2, NKLF2, and cNKLF2) was also examined by a standard colony count assay in LB medium. All three homologous peptides were active (reduced the CFU >3 log) against B. megaterium at 5 μM but displayed various potencies against E. coli. At 7.4 μM, NKLF2 reduced viable E. coli by 2 log (99% inhibition), in contrast to GRANF2 and cNKLF2, which showed no activity against E. coli at 35 μM. None of the three homologous peptides were potent against the S. aureus strain at concentrations up to 35 μM. The peptide activity increased drastically in minimal medium (1:100 LB to sodium phosphate, pH 7.4), where all three peptides showed a 5-log inhibition of S. aureus growth at <5 μM.
Cytotoxicity.
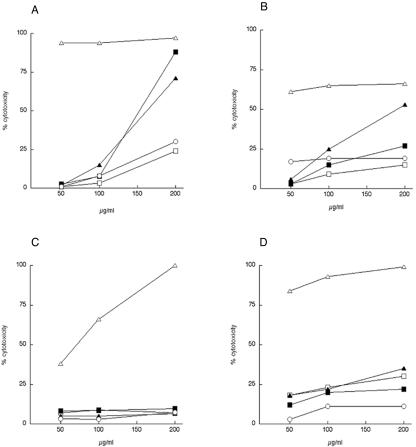
Mycobacteria use macrophages as normal host cells in mammals. A current hypothesis suggests the site-directed release of granulysin from cytotoxic lymphocytes into infected macrophages. Hence, it was of interest to see if monocytes or macrophages were sensitive to granulysin peptides. First, we assayed their lytic activity against U937 cells. These cells were either grown in medium, representing monocytes, or differentiated for 3 days with PMA to become macrophages attached to the bottoms of the wells. The different cell populations were then incubated with various concentrations of GRANF2 (reduced, oxidized, or all in d-form), C(1-13)M(1-13), and PR39 (1-20). The last two peptides have activities against M. smegmatis comparable to that of GRANF2 and were added to evaluate the cytolytic effects of antimycobacterial peptides (23). Except for C(1-13)M(1-13), no peptide was toxic against U937 macrophages up to 200 μg/ml (67 to 100 μM, depending on the peptide) (Fig. 4C). The U937 monocytes were sensitive to peptides in the following order of toxicity: C(1-13)M(1-13) > reduced GRANF2 = all-d GRANF2 > oxidized GRANF2 > PR39 (1-20) (Fig. 4A).
FIG. 4.
Cytotoxic activities of synthetic peptides against U937 monocytes (A), primary monocytes (B), PMA-differentiated U937 cells (C), and primary macrophages (D). Peptides GRANF2 S-S (□), GRANF2-SH (▪), d-GRANF2-SH (▴), C(1-13)M(1-13) (▵), and PR39 (1-20) (○) were incubated for 4 h at different concentrations with cells. Data are presented as the means of two or three experiments.
Since U937 monocytes represent a human lymphoma cell line, we also examined the cytolytic effect on human primary monocytes and macrophages. These two cell types were similarly treated with different peptides up to 200 μg/ml. As shown with U937 cells, no peptide but C(1-13)M(1-13) was toxic to primary macrophages (Fig. 4D). The primary monocytes were sensitive to C(1-13)M(1-13) and all-d GRANF2 (Fig. 4B). A small difference in toxicity was noticed between oxidized and reduced GRANF2 at 67 μM. Additionally, the shorter, 15-residue GRANF3 peptide was not toxic to differentiated U937 cells, but its cytolytic potency gradually increased against U937 monocytes up to 200 μg/ml (100 μM), when it lysed 100% of the cells. Neither the reduced nor oxidized forms of modified GRANF2:1 and GRANF2:2 exhibited any toxic activity against undifferentiated U937 cells (not shown). Reduced GRANF2:2 was nonlytic against primary cells (not shown).
DISCUSSION
Both granulysin and NK-lysin are bactericidal against M. tuberculosis as well as a number of other bacteria (4, 7, 33). However, their antibacterial spectra do not overlap; one clear difference is the activities against Salmonella, where granulysin is potent and NK-lysin is inactive. The mechanism of action is not clear, but the initial contact between granulysin or NK-lysin and a membrane is proposed/calculated to involve the basic residues in helix 2-3 (2, 22, 24). This portion of granulysin and NK-lysin also retains significant antimicrobial activity, as identified by testing of synthetic fragments (7, 16, 36). The fragments used here are based on these observations. The chicken NK-lysin preprosequence (35) is similar in length to NK-lysin/granulysin, and the postulated active peptide includes the hallmark of saposin peptides, i.e., six cysteines and one proline. Additional homologous proteins in cows and horses have been characterized, thus further expanding the peptide family (12, 14). The bovine NK-lysin exhibits antimycobacterial activity (14), whereas the equine NK-lysin activities remain to be determined.
Our results suggest that the 3-D structure and spatial distribution of basic amino acids are preserved in the helix 2-3 regions of granulysin/NK-lysin/cNK-lysin, although the sequence identity is not very high, at about 35%. Granulysin and NK-lysin are polymorphic in nature, with several sequence variations found in porcine (4, 10) and bovine (14) NK-lysins, whereas granulysin bears a single polymorphic site (15). The topology similarity in antibacterial peptide families is exemplified in both the α- and β-defensins (29). Each species expresses several defensin genes, and as a consequence this diversification generates several peptides that together encompass a broad antibacterial spectra. The granulysin/NK-lysin family, in contrast, has so far only one gene in each species, which suggests that the overall activity would be somewhat more restricted, possibly directed against bacteria relevant for CTL-dependent killing. In this context, it was interesting to find that GRANF2, NKLF2, and cNKLF2 had similar activities against M. marinum and M. smegmatis but various potencies against E. coli and no activity against growing P. aeruginosa or S. aureus. We compared this to two potent antimicrobial peptides, cecropin P1 (21) and L-C(1-13)M(1-13) (9), and found that cecropin P1 was not active against mycobacteria. This indicates that a net positive charge by itself is not sufficient for activity (23).
Both the GRANF2 and NKLF2 peptides mimic the activity of the full-length protein (7, 16, 36), and it is reasonable to assume that cNKLF2 will also have potent antimicrobial activity. We have previously shown that a seven-residue-longer NKLF2 fragment, NKLF1, is more active against P. aeruginosa and S. aureus. We cannot exclude that the activities of cNKLF2 and cNKL will differ against certain bacteria. This may also depend on the choice of media and experimental design as well as on differences in salt sensitivity between cNKLF2 and cNKL. We noticed a clear difference in the sensitivities of S. aureus towards GRANF2, NKLF2, and cNKLF2 in dissimilar media. It is not clear whether this reflects altered ion strengths in the media or the fact that the bacteria are less metabolically active and therefore less capable of protecting themselves in minimal media.
Ernst et al. showed that granulysin activity is dependent on free nitrogen groups on arginine side chains (16). We showed here that arginine is readily exchanged with lysine, although at a cost of twofold lower activity with an all-lysine fragment (GRANF2:2) against M. smegmatis. However, this peptide reduced the growth of M. marinum as efficiently as its parent peptide. The arginine residues in the loop structure are thus important against certain bacteria (16) but are not crucial for antimycobacterial activity. The activity difference between oxidized and reduced forms of GRANF2:2 may well be related to the fragments' capacity to form a stable helix-loop-helix fold. This is illustrated with both a lowered activity of carboxymethylated GRANF2:2 and the CD profile. The fact that GRANF2 peptides made of either d- or l-type amino acids have similar activities suggests that the target is not chirally dependent. This is in line with the carpet-like bacteriolytic mechanism that was recently proposed (2, 16, 31). Although the action of antibacterial peptides is rapid, it usually needs the time of bacterial cell division to act. This is also true for mycobacteria (23, 25).
Whether these peptides are secreted at physiologically relevant concentrations is currently unclear. An in vitro determination of the NK-lysin content in interleukin-2-stimulated lymphocytes by an enzyme-linked immunosorbent assay (10) gives about 6 ng per 106 cells. With a rough calculation based on a cell size of 20 μm and an estimate that granules occupy 10% of the cell volume, the NK-lysin granule concentration then reaches about 1.5 mM. If the protein is diluted 10 times during the delivery process to a target cell, we could expect concentrations around 150 μM. This would be a relevant concentration for mycobacterial killing but would also put a strain on host cell toxicity. Our results in Fig. 4 suggest that granulysin may indeed have evolved in part to minimize toxicity, as the reduced peptides are clearly more cytolytic to human U937 cells. Full-length granulysin is also more toxic in its reduced form (36).
It has been suggested that although granulysin fragments from both helixes 2 and 3 are bactericidal, only helix 3 fragments are toxic (36). We found a low toxicity of granulysin peptides towards differentiated U937 cells as well as primary macrophages. Undifferentiated cells were more sensitive to reduced GRANF2 than to the oxidized form. Cells from two additional cancer cell lines also showed this sensitivity (8). This observation differs from the results of Wang and colleagues, who showed a diminished cytolytic activity against Jurkat cells of a peptide, G8, in part overlapping the sequence of GRANF2, when it was reduced (36). The reason for this is not clear, but it is possible that cell type-specific activities can occur. The shorter GRANF3 peptide with only one cysteine was also lytic against undifferentiated U937 cells but lost its toxicity against differentiated cells. This suggests that disulfide formation is more important for avoiding host cell toxicity than for improving antibacterial activity, although this was less evident in primary monocytes. The replacement of arginine residues with lysines in GRANF2:1 and GRANF2:2 diminishes the toxicity of the reduced forms against undifferentiated U937 cells, which suggests a crucial role for loop arginines in host cell cytotoxicity. The importance of charge in antibacterial peptides is well established and explains our model of “molecular electroporation” (24). Therefore, charge compensation provides a natural way to avoid lysis by bacteria (28) and may also be a prominent factor in different eukaryotic cell susceptibilities. Studies with cationic ferritin suggest that surface charges can differ between normal and malignant cells (30), and this may be one factor responsible for differences between U937 and differentiated U937 cells and primary monocytes. Disulfide formation, charge compensation, and vector-induced delivery are therefore natural ways of restricting self-cytotoxicity of host cells to granulysin/NK-lysin.
In summary, human cytolytic cells produce an antibacterial peptide, granulysin, which is a key player in the granule-release pathway directed toward killing intracellular mycobacteria. Homologous peptides exist in other species, e.g., pigs and chickens. Although they have moderate residue identity, their antibacterial domains have very similar 3-D architectures, spacing of important basic residues, and activities against M. marinum and M. smegmatis.
Acknowledgments
We thank Anna Päiviö and Waltteri Hosia for valuable assistance with CD analysis and Tuija Koivula at Smittskyddsinstitutet, Sweden, for providing us with the M. marinum ATCC 2275 strain.
This investigation was supported by grants from the Swedish Research Council, Magnus Bergvall's Stiftelse, The King Oscar II Jubilee Foundation, Karolinska Institutet, and the European Union (5th framework program, contract QLK2-2000-01761).
Editor: J. D. Clements
REFERENCES
- 1.Abagyan, R., S. Batalov, T. Cardozo, M. Totrov, J. Webber, and Y. Zhou. 1997. Homology modeling with internal coordinate mechanics: deformation zone mapping and improvements of models via conformational search. Proteins 1(Suppl.):29-37. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. H., M. R. Sawaya, D. Cascio, W. Ernst, R. Modlin, A. Krensky, and D. Eisenberg. 2003. Granulysin crystal structure and a structure-derived lytic mechanism. J. Mol. Biol. 325:355-365. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, M., T. Curstedt, H. Jornvall, and J. Johansson. 1995. An amphipathic helical motif common to tumourolytic polypeptide NK-lysin and pulmonary surfactant polypeptide SP-B. FEBS Lett. 362:328-332. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, M., H. Gunne, B. Agerberth, A. Boman, T. Bergman, R. Sillard, H. Jornvall, V. Mutt, B. Olsson, H. Wigzell, et al. 1995. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 14:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson, M., A. Holmgren, and G. Spyrou. 1996. NK-lysin, a disulfide-containing effector peptide of T-lymphocytes, is reduced and inactivated by human thioredoxin reductase. Implication for a protective mechanism against NK-lysin cytotoxicity. J. Biol. Chem. 271:10116-10120. [DOI] [PubMed] [Google Scholar]
- 6.Andra, J., and M. Leippe. 1999. Candidacidal activity of shortened synthetic analogs of amoebapores and NK-lysin. Med. Microbiol. Immunol. (Berlin) 188:117-124. [DOI] [PubMed] [Google Scholar]
- 7.Andreu, D., C. Carreno, C. Linde, H. G. Boman, and M. Andersson. 1999. Identification of an anti-mycobacterial domain in NK-lysin and granulysin. Biochem. J. 344:845-849. [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorkhem-Bergman, L., K. Jonsson-Videsater, C. Paul, M. Bjornstedt, and M. Andersson. 2004. Mammalian thioredoxin reductase alters cytolytic activity of an antibacterial peptide. Peptides 25:1849-1855. [DOI] [PubMed] [Google Scholar]
- 9.Boman, H. G., D. Wade, I. A. Boman, B. Wahlin, and R. B. Merrifield. 1989. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 259:103-106. [DOI] [PubMed] [Google Scholar]
- 10.Bonetto, V., M. Andersson, T. Bergman, R. Sillard, Å. Norberg, V. Mutt, and H. Jörnvall. 1999. Spleen antibacterial peptides: high levels of PR-39 and presence of two forms of NK-lysin. Cell Mol. Life Sci. 56:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brosch, R., A. S. Pym, S. V. Gordon, and S. T. Cole. 2001. The evolution of mycobacterial pathogenicity: clues from comparative genomics. Trends Microbiol. 9:452-458. [DOI] [PubMed] [Google Scholar]
- 12.Davis, E. G., Y. Sang, B. Rush, G. Zhang, and F. Blecha. 2005. Molecular cloning and characterization of equine NK-lysin. Vet. Immunol. Immunopathol. 105:163-169. [DOI] [PubMed] [Google Scholar]
- 13.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, A. M. Krensky, M. Bonneville, M. A. Peyrat, N. Caccamo, G. Sireci, and A. Salerno. 2001. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J. Infect. Dis. 184:1082-1085. [DOI] [PubMed] [Google Scholar]
- 14.Endsley, J. J., J. L. Furrer, M. A. Endsley, M. A. McIntosh, A. C. Maue, W. R. Waters, D. R. Lee, and D. M. Estes. 2004. Characterization of bovine homologues of granulysin and NK-lysin. J. Immunol. 173:2607-2614. [DOI] [PubMed] [Google Scholar]
- 15.Ericson, K. G., B. Fadeel, M. Andersson, G. H. Gudmundsson, A. Gurgey, N. Yalman, G. Janka, M. Nordenskjold, and J. I. Henter. 2003. Sequence analysis of the granulysin and granzyme B genes in familial hemophagocytic lymphohistiocytosis. Hum. Genet. 112:98-99. [DOI] [PubMed] [Google Scholar]
- 16.Ernst, W. A., S. Thoma-Uszynski, R. Teitelbaum, C. Ko, D. A. Hanson, C. Clayberger, A. M. Krensky, M. Leippe, B. R. Bloom, T. Ganz, and R. L. Modlin. 2000. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol. 165:7102-7108. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield, N., and G. D. Fasman. 1969. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8:4108-4116. [DOI] [PubMed] [Google Scholar]
- 18.Hultmark, D., Å. Engström, K. Andersson, H. Steiner, H. Bennich, and H. G. Boman. 1983. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 2:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jongstra, J., T. J. Schall, B. J. Dyer, C. Clayberger, J. Jorgensen, M. M. Davis, and A. M. Krensky. 1987. The isolation and sequence of a novel gene from a human functional T cell line. J. Exp. Med. 165:601-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J. Y., A. Boman, C. X. Sun, M. Andersson, H. Jornvall, V. Mutt, and H. G. Boman. 1989. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc. Natl. Acad. Sci. USA 86:9159-9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liepinsh, E., M. Andersson, J. M. Ruysschaert, and G. Otting. 1997. Saposin fold revealed by the NMR structure of NK-lysin. Nat. Struct. Biol. 4:793-795. (Letter.) [DOI] [PubMed] [Google Scholar]
- 23.Linde, C. M., S. E. Hoffner, E. Refai, and M. Andersson. 2001. In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and multi-drug-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 47:575-580. [DOI] [PubMed] [Google Scholar]
- 24.Miteva, M., M. Andersson, A. Karshikoff, and G. Otting. 1999. Molecular electroporation: a unifying concept for the description of membrane pore formation by antibacterial peptides, exemplified with NK-lysin. FEBS Lett. 462:155-158. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa, Y., P. Ratnakar, A. Gururaj Rao, M. L. Costello, O. Mathieu-Costello, R. I. Lehrer, and A. Catanzaro. 1996. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect. Immun. 64:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrisett, J. D., J. S. David, H. J. Pownall, and A. M. J. Gotto. 1973. Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry 12:1290-1299. [DOI] [PubMed] [Google Scholar]
- 27.Pena, S. V., D. A. Hanson, B. A. Carr, T. J. Goralski, and A. M. Krensky. 1997. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 158:2680-2688. [PubMed] [Google Scholar]
- 28.Peschel, A., M. Otto, R. W. Jack, H. Kallbacher, G. Jung, and F. Götz. 1999. Inactivation of the dtl operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 29.Raj, A. R., and A. R. Dentino. 2002. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 206:9-18. [DOI] [PubMed] [Google Scholar]
- 30.Resnitzky, P., A. Bustan, A. Peled, and Y. Marikovsky. 1988. Variations in surface charge distributions of leukemic and non-leukemic transformed cells. Leukoc. Res. 12:315-320. [DOI] [PubMed] [Google Scholar]
- 31.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 32.Stamm, L. M., and E. J. Brown. 2004. Mycobacterium marinum: the generalization and specialization of a pathogenic mycobacterium. Microbes Infect. 6:1418-1428. [DOI] [PubMed] [Google Scholar]
- 33.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 34.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirunagaru, V. G., L. Sofer, J. Cui, and J. Burnside. 2000. An expressed sequence tag database of T-cell-enriched activated chicken splenocytes: sequence analysis of 5251 clones. Genomics 66:144-151. [DOI] [PubMed] [Google Scholar]
- 36.Wang, Z., E. Choice, A. Kaspar, D. Hanson, S. Okada, S. C. Lyu, A. M. Krensky, and C. Clayberger. 2000. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J. Immunol. 165:1486-1490. [DOI] [PubMed] [Google Scholar]
- 37.Yabe, T., C. McSherry, F. H. Bach, and J. P. Houchins. 1990. A cDNA clone expressed in natural killer and T cells that likely encodes a secreted protein. J. Exp. Med. 172:1159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]