Abstract
Chronic lung infection by Pseudomonas aeruginosa causes significant morbidity in cystic fibrosis patients initiated by the failure of innate immune responses. We used microarray analysis and real-time PCR to detect transcriptional changes associated with cytokine production in isogenic bronchial epithelial cell lines with either wild-type (WT) or mutant cystic fibrosis transmembrane conductance regulator (CFTR) in response to P. aeruginosa infection. The transcription of four NF-κB-regulated cytokine genes was maximal in the presence of WT CFTR: the interleukin-8 (IL-8), IL-6, CXCL1, and intracellular adhesion molecule 1 (ICAM-1) genes. Analysis of protein expression in two cell lines paired for wild-type and mutant CFTR with three P. aeruginosa strains showed IL-6 and IL-8 expressions were consistently enhanced by the presence of WT CFTR in both cell lines with all three strains of P. aeruginosa, although some strains gave small IL-8 increases in cells with mutant CFTR. CXCL1 production showed consistent enhancement in cells with WT CFTR using all three bacterial strains in one cell line, whereas in the other cell line, CXCL1 showed a significant increase in cells with either WT or mutant CFTR. ICAM-1 was unchanged at the protein level in one of the cell lines but did show mild enhancement with WT CFTR in the other cell pair. Inhibitions of NF-κB prior to infection indicated differing degrees of dependence on NF-κB for production of the cytokines, contingent on the cell line. Cytokine effectors of innate immunity to P. aeruginosa were found to be positively influenced by the presence of WT CFTR, indicating a role in resistance to P. aeruginosa infection.
Cystic fibrosis (CF) is a common genetic disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is a cyclic AMP-dependent chloride channel (2) and has been shown to regulate other secretory channels (32). In addition to these functions, CFTR acts as a specific receptor for Pseudomonas aeruginosa (27), a process involved in the recognition and clearance of bacteria from the respiratory mucosa of individuals with wild-type (WT) CFTR. The lack of a functional CFTR protein leads to chronic P. aeruginosa lung infections in CF patients, causing significant morbidity and mortality. The most common mutation in cystic fibrosis, the ΔF508 mutation, causes a misfolding of CFTR which results in the degradation of the protein before it reaches the surface of the cell (34). The reduced amount of CFTR at the cell membrane changes the interaction of P. aeruginosa with the epithelial cell, preventing the internalization of P. aeruginosa by CF cells and preventing NF-κB nuclear translocation, both of which are involved in mediating innate immunity (28, 31).
NF-κB has been proposed as a critical link between the interaction of P. aeruginosa with airway epithelial cells and the innate immune response that ensues. The activation of NF-κB has been shown to occur in response to P. aeruginosa in the airway epithelial cells of mice expressing WT CFTR but not in mice lacking lung epithelial cell CFTR (31). Schroeder et al. (31) also measured NF-κB translocation in human bronchial epithelial cell lines, CFT1-LCFSN cells, which express WT CFTR, and CFT1-ΔF508 cells, which express only ΔF508 CFTR. NF-κB translocation was observed in response to P. aeruginosa only in the cells expressing wild-type CFTR. When the ΔF508 misfolding phenotype was rescued by the addition of glycerol to the medium, NF-κB translocation was observed in the ΔF508 CFTR cells, emphasizing that the presence of CFTR at the cell membrane is required for NF-κB activation in response to P. aeruginosa in this system.
Because of the important role of the innate immune response in CF lung infection, we were interested in what transcriptional and protein-level differences related to this response existed between cells with wild-type CFTR and cells with ΔF508 CFTR in response to infection by P. aeruginosa. In this study, we first performed microarray experiments to measure changes in transcription in response to P. aeruginosa infection in WT CFTR LCFSN cells and ΔF508 CFTR cells and conducted confirmatory real-time PCR (RT-PCR) analysis on genes with expressions that were increased more than twofold. We chose 3 h postinfection as the time point to look for transcriptional changes that are likely to be involved in the inflammatory response because the NF-κB nuclear translocation measured by Schroeder et al. in WT CFTR cells in response to P. aeruginosa was rapid, peaking at 15 min after bacterial exposure and no longer detectable at 45 min (31). We have also shown that in the same bronchial epithelial cells, the induction of apoptosis in 90% of cells in response to P. aeruginosa was achieved by 5 h (6). Consequently, 3 h postinfection would be indicated as a time point of maximal epithelial cell transcriptional responses but before the initiation of significant apoptosis, which also requires shutdown of transcriptional responses to bacterial pathogens of NF-κB (39). We also measured the protein level of each gene product in cell lysates for the CFT1 cell pair as well as for a second pair of human bronchial epithelial cells either expressing mutant CFTR (CF-IB3-1 cells, ΔF508/1282X CFTR alleles) or corrected to express WT CFTR (S9 cells). Analyses were conducted with multiple strains of P. aeruginosa, including clinical isolates from CF patients. Overall, we found restricted CFTR enhancements and NF-κB dependences in transcriptional and translational innate immune responses to P. aeruginosa which were mostly but not totally consistent across the two cell lines and among different P. aeruginosa strains.
MATERIALS AND METHODS
Cell lines.
CFT1-ΔF508 (ΔF508 CFTR) cells and CFT1-LCFSN (WT CFTR LCFSN) cells are both human bronchial epithelial cell lines derived from a CF patient homozygous for the ΔF508 allele of CFTR (25). CFT1-ΔF508 has a third allele of ΔF508 CFTR, whereas CFT1-LCFSN has a WT allele for human CFTR expressed in the homozygous ΔF508 background. CFT1 cells were grown in F-12 medium supplemented with insulin (10 mg/liter), hydrocortisone (10−6 M), epithelial cell growth supplement (3.75 mg/liter), epidermal growth factor (25 μg/liter), 3,3′,5-triiodo-l-thyronine sodium salt (3 × 10−8 M), human holotransferrin (5 mg/liter), cholera toxin (10 μg/liter), and neomycin sulfate (150 mg/liter). The same medium without neomycin sulfate was used for the infection experiments. IB3-1 (CF-IB3-1) and S9 (WT CFTR S9) are both human bronchial epithelial cell lines with the compound heterozygous background of ΔF508/W1282X alleles of CFTR (12). The S9 cell line is transfected with a WT CFTR gene to produce WT CFTR protein. S9 and IB3-1 cells were grown in collagen-coated flasks in LHC-8 serum-free medium with glutamine supplemented with penicillin (100 U/ml) and without gentamicin. The same medium without penicillin was used for the infection experiments. All epithelial cell lines were grown at 37°C in 5% CO2.
Bacterial infections and RNA isolation for microarray experiments.
Bacteria were grown on tryptic soy agar plates overnight at 37°C, resuspended in antibiotic-free cell medium to an optical density of 650 nm of 0.4, and then diluted to a ratio of 1:15 in the same medium. WT CFTR LCFSN and ΔF508 CFTR cells were infected with P. aeruginosa strain PAO1-V (originally obtained from M. Vasil [University of Colorado, Denver]) at a multiplicity of infection (MOI) determined by plate counts of the infecting inoculum to be between 30:1 and 45:1 for 3 h at 37°C in 5% CO2. The infected cells and uninfected controls were then lysed using Buffer RLT (QIAGEN) and homogenized using an OMNI homogenizer. Total RNA was isolated from homogenates (RNeasy Midi kit; QIAGEN), and mRNA was isolated from the total RNA (Oligotex mRNA Midi kit; QIAGEN). The quantity and quality of mRNA were analyzed by spectrophotometry and electrophoresis in glyoxal gels (Ambion). The mRNA was concentrated to ∼1 μg/ml by ethanol precipitation.
Fluorescent microarray probe labeling and hybridization to microarray.
Fluorescent-labeled cDNA probes were synthesized by reverse transcription of the mRNA samples in the presence of cyanine 3-dCTP and cyanine 5-dCTP dyes (NEN) as described by Ichikawa and coworkers (19). The probes were hybridized to the microarrays as described previously (14). Probes made from uninfected samples and those made from infected samples were labeled with different dyes and hybridized together on a single array. Duplicate probes where the dyes were switched with respect to the cDNA being labeled were hybridized on a second array to control for differences in signal intensities due to differences in levels of dye incorporation.
Microarray construction and analysis.
The construction, characteristics, and quality of the microarrays used were previously described, as were the protocols followed for their use (13, 14, 19). Each microarray contained 4,608 genes and expressed sequence tags (ESTs) spotted in duplicate. For each experiment, four measurements of relative gene expression were generated for each gene due to the duplicate spots on each slide and the use of two microarray slides to control for dye incorporation as described above. The ratios of fluorescent signals for uninfected versus infected samples were quantified and analyzed using Spot-on software developed at the University of Washington (13). A gene was considered to be differentially expressed if the background-subtracted intensity was greater than 1,000 (which is at least three times the standard deviation of the background) and the ratio of the signals of the cohybridized samples was at least two to one. Four replicate experiments for each cell line were performed, providing 16 data points per gene. The reported error estimate of the n-fold changes is given as the standard deviation of the data from the four replicate experiments.
Real-time PCR analysis. (i) cDNA template preparation for real-time PCR.
mRNA was isolated from WT CFTR LCFSN and ΔF508 CFTR cells as described above, DNase treated (Invitrogen) according to the manufacturer's instructions, and made into cDNA by use of an Omniscript reverse transcriptase kit (QIAGEN) according to the manufacturer's instructions.
(ii) PCR primers and clones to generate standards.
Oligonucleotide primers were designed to amplify each gene of interest (Operon Technologies). Sequence-verified clones for the genes were ordered from Research Genetics (IMAGE ID numbers: interleukin 8 [IL-8] gene, 549933; IL-6 gene, 310406; CXCL1 gene [GRO1 oncogene], 323238; intracellular adhesion molecule 1 (ICAM-1) gene, 145112; ICAM-3 gene, 754080; plasminogen activator inhibitor type II gene, 70692; fibroblast growth factor 7 gene, 365515; and EGLN-3 gene [previously known as EST; highly similar to growth factor-responsive protein-producing gene], 122982). DNA was isolated from each clone (QIAprep Spin Miniprep kit; QIAGEN), and the concentrations of DNA were determined by spectrophotometry. Standard curves to be used for real-time PCR were generated with serial dilutions of the sequence-verified cloned DNA.
(iii) Real-time PCR using an iCycler.
PCR amplification and product detection were carried out on an iCycler machine (Bio-Rad) with QuantiTect SYBR green PCR master mix (QIAGEN). Amplification for each gene consisted of 1 cycle at 95°C for 15 min followed by 40 cycles of 95°C for 30 s, 58.2°C for 30 s, and 72°C for 30 s. The specificities of primer pairs were determined by the detection of single bands on ethidium bromide-stained agarose gels and by the detection of single peaks when performing a melt curve procedure between 55°C and 80°C on an iCycler machine according to the manufacturer's instructions.
Cytokine analysis.
Lysates were made of each cell line, either uninfected or infected for 3 h with one of three strains of P. aeruginosa (PAO1, N13, or 324), at an MOI of between 30:1 and 50:1, by use of chilled lysis buffer (20 mM Tris, 1 mM EDTA, and 0.05% Triton X-100, pH 7.4) containing Complete Mini protease inhibitor cocktail (Roche Diagnostics GmbH). The protein concentration in each sample was measured by the Bradford assay.
Cytokine protein array membranes (RayBiotech, Inc.) were processed according to the manufacturer's instructions. In brief, membranes were blocked with 5% bovine serum albumin/Tris-buffered saline, and then each was incubated with 300 μg of total protein from cell lysates for 2 h. Cytokines were detected with biotin-conjugated antibodies (included in the kit) and finally with horseradish peroxidase-streptavidin and detection buffer. Membranes were scanned and spots were quantified using NIH image analysis software. Membranes were compared by normalizing the positive control readings. Background measurements for each membrane were subtracted from each measurement. A list and map of the cytokines contained on the arrays can be found at the website of RayBiotech, Inc.
CXCL1 and ICAM-1 enzyme-linked immunosorbent assay (ELISA) protocols.
CXCL1 (also known as GRO1 and GROα) and ICAM-1 were detected using a human GROα Quantikine immunoassay and a Parameter human sICAM-1 immunoassay, respectively (R&D Systems). Cells were uninfected or infected for 3 h (MOIs of 50:1 to 66:1), and cell lysates were made as described above. The manufacturer's instructions for testing cell culture supernatants were followed.
NF-κB inhibition.
WT CFTR LCFSN cells and WT CFTR S9 cells were pretreated with 15 μM Bay 11-7082 (Sigma) for 15 min at 37°C. The cells were washed twice with phosphate-buffered saline before being infected and lysed as described above. The exposure of cells to 15 μM Bay 11-7082 for 15 min was found in a preliminary analysis to produce a maximal inhibition of NF-κB nuclear translocation in response to P. aeruginosa infection, as determined with a TransAM NF-κB assay kit (Active Motif), which measures nuclear NF-κB levels.
Statistics.
Unpaired, two-tailed t tests were used to make two-group comparisons. t tests were used in some multiple comparison cases when the number of comparisons was small and the specific comparisons of interest were defined in advance, as recommend by Rosner (30).
RESULTS
Microarray analysis of the effect of CFTR on the transcriptional response of bronchial epithelial cells to infection by P. aeruginosa.
Four independent infections of the paired ΔF508 CFTR cells and WT CFTR LCFSN cell lines with P. aeruginosa strain PAO1 were performed to create four replicate microarray data sets per cell line. We define infection in this model as the exposure of the cells to P. aeruginosa in the medium for 3 h. Normalized log ratios for all replicates for all 4,608 spots can be accessed at http://www.ncbi.nlm.nih.gov/geo/ under accession number GSE2357. Table 1 lists the genes and gene products that had more-than-twofold changes in expression levels in response to infection with P. aeruginosa in the two cell lines studied that are isogenic for WT or ΔF508 CFTR. mRNA levels of 16 genes were increased by more than twofold in response to infection in the WT CFTR LCFSN cells, and 6 of these were also increased in response to infection in the ΔF508 CFTR cells. mRNA levels for only one gene were increased by more than twofold in response to infection in the ΔF508 CFTR cells but not the WT CFTR LCFSN cells. No decreases of more than twofold were observed in either cell line. Four of the 10 genes that had increases in expression with infection only in the WT CFTR LCFSN cell line, the IL-8, IL-6, CXCL1, and ICAM-1 genes, are reported to be NF-κB regulated (3). No NF-κB-regulated genes had changed expression levels upon infection in the ΔF508 CFTR cell line.
TABLE 1.
Change (n-fold) in fluorescence upon infection with P. aeruginosa as measured by cDNA-spotted microarrays in quadruplicate
| Gene or gene producta | WT CFTR | ΔF508 CFTRb | I.M.A.G.E. no.c |
|---|---|---|---|
| IL-6* | 6.61 (±5.50) | NC | 310406 |
| ICAM-3 (CD50) | 6.20 (±1.88) | NC | 754080 |
| Low-affinity immunoglobulin | 4.52 (±4.31) | NC | 51447 |
| Gamma Fc receptor III-1 precursor | |||
| Endothelin 3 | 4.32 (±4.78) | NC | 66532 |
| IL-8* | 4.00 (±2.14) | NC | 549933 |
| IL-8* | 3.57 (±1.63) | NC | 549933 |
| Fibroblast growth stimulatory factor 7 (keratinocyte growth factor) | 3.45 (±2.44) | NC | 365515 |
| Intercellular adhesion molecule 1 (CD54) | 3.13 (±1.85) | NC | 145112 |
| IL-6* | 3.05 (±1.31) | NC | 310406 |
| EGLN3 | 2.69 (±1.58) | NC | 122982 |
| CXCL1 (GRO1 oncogene) | 2.44 (±0.66) | NC | 323238 |
| Plasminogen activator inhibitor type II (arginine-serpin) | 2.23 (±0.60) | NC | 70692 |
| Cytochrome P450 IB1 (dioxin inducible) | 3.70 (±1.07) | 3.44 (±0.82) | 782760 |
| Transforming growth factor beta 1 precursor | 3.67 (±1.60) | 3.04 (±1.10) | 136821 |
| Human c-jun proto-oncogene (JUN), clone hCJ-1 | 3.59 (±1.32) | 2.75 (±0.81) | 358531 |
| Transforming protein RHOB | 3.40 (±0.82) | 3.71 (±1.56) | 768370 |
| Complement component 8, beta polypeptide | 3.11 (±0.78) | 3.20 (±0.59) | 83210 |
| Activating transcription factor 3 | 2.42 (±0.97) | 2.32 (±0.69) | 51448 |
| Stanninocalcin 2 | NC | 2.19 (±0.51) | 130057 |
*, For both IL-6 and IL-8, the same cDNA clone was represented twice on the array. Both sets of results are given for each gene product.
NC, no change (i.e., change of less than twofold detected).
I.M.A.G.E., Integrated Molecular Analysis of Genomes and Expression consortium.
Real-time PCR confirmation of microarray analyses.
Real-time PCR analysis was performed for 8 of the 10 genes identified by microarray analysis as having greater-than-twofold increases in transcription in WT CFTR LCFSN cells with infection and no change in transcription in ΔF508 CFTR cells with infection. Real-time PCR was not performed on endothelin 3 and low-affinity immunoglobulin gamma Fc receptor III-1 precursor because primers failed to amplify these genes from reverse transcription reactions, although the same primers could amplify the correct PCR product from genomic DNA. Levels of glyceraldehyde-3-phosphate dehydrogenase and beta-2-microglobulin were used to normalize amounts of PCR product in different samples. Of the eight remaining genes increased more than twofold only in the cell line with WT CFTR as measured by microarray, greater-than-twofold transcriptional changes were confirmed by RT-PCR for all genes except the ICAM-3 gene (Table 2). In the ΔF508 CFTR cells, the expressions of the following four genes that were increased by more than twofold in WT CFTR cells were unchanged (<2×) with infection: the fibroblast growth factor 7 gene, the IL-8 gene, the ICAM-1 gene, and the CXCL1 gene. Although more-than-twofold changes were measured in mRNA for IL-6 and plasminogen activator inhibitor type 2 in P. aeruginosa-infected ΔF508 CFTR cells, the change detected in WT CFTR LCFSN cells was much greater. To show which cell line had the more robust response to P. aeruginosa, Table 2 indicates whether there was a twofold-or-greater difference in the mean n-fold change in specific mRNA detected in the WT CFTR LCFSN compared with the ΔF508 CFTR cell line for each transcript quantified by real-time PCR. Overall, IL-6, IL-8, ICAM-1, CXCL1, and plasminogen activator inhibitor genes were transcribed to greater degrees in infected WT CFTR LCFSN cells than in mutant ΔF508 CFTR cells.
TABLE 2.
Real-time PCR analysis of transcriptional changes of WT CFTR LCFSN and ΔF508 CFTR cells in response to 3 h of infection with P. aeruginosa
| Gene product | ≥2× increase in mRNA with infection ina:
|
Ratio of LCFSN and ΔF508 mean changes ≥ twofold | |
|---|---|---|---|
| WT CFTR LCFSN cells | ΔF508 CFTR cells | ||
| IL-6 | + | + | + |
| ICAM-3 (CD50) | − | − | − |
| IL-8 | + | − | + |
| Fibroblast growth stimulatory factor 7 (keratinocyte growth factor) | +/− | − | + |
| Intercellular adhesion molecule 1 (CD54) | + | +/− | + |
| EGLN3 | + | + | − |
| CXCL1 (GRO1 oncogene) | +/− | − | + |
| Plasminogen activator inhibitor, type II (arginine-serpin) | + | + | + |
+ indicates a change of more than twofold, − indicates a change of less than twofold, and +/− is entered when only some samples tested had increases above twofold.
Protein level measurements of IL-6, IL-8, CXCL1, and ICAM-1 in response to P. aeruginosa strain PAO1.
Preliminary analysis of cell lysates and supernatants showed a much higher concentration of the proteins of interest in the lysates, indicating that a significant intracellular pool was produced and/or retained in response to P. aeruginosa infection. We thus chose to measure the protein concentrations in lysates in an attempt to confirm that the changes we measured at the mRNA level were reflected by changes in protein production. Cytokine antibody arrays were used to measure IL-6 and IL-8 protein levels in cell lysates from uninfected and infected WT CFTR LCFSN and ΔF508 CFTR cells after 3 h of exposure to P. aeruginosa strain PAO1. Both IL-6 and IL-8 protein levels were significantly increased with infection in the WT CFTR LCFSN cell line (P < 0.05) and were not significantly changed with infection in the ΔF508 CFTR cell line (Fig. 1A). These same results were obtained for each of two replicates. Of importance is the fact that the array contained capture antibodies for detecting 20 additional cytokines, including CXCL1. On several membranes, the increases in CXCL1 in WT cells could be seen, but spots were usually too faint for accurate quantitation. However, none of the other 19 cytokines potentially detectable with the array (see the RayBiotech website for the list) were found to be consistently expressed by either uninfected or infected ΔF508 CFTR or WT CFTR LCFSN cells. Using protein arrays that can detect several cytokines allowed us to ascertain that none of these other cytokines had accumulated in the cells to reach detectable quantities in response to P. aeruginosa infection. This does not rule out the possibility of the production and accompanying degradation of these other cytokines prior to 3 hours or production at levels not detectable by the protein arrays.
FIG. 1.
Role of CFTR in IL-6, IL-8, CXCL1, and ICAM-1 protein production in WT CFTR LCFSN or ΔF508 CFTR cells in response to P. aeruginosa after 3 h of infection. (A) Relative levels of IL-6 and IL-8 cytokines in lysates from uninfected and infected cells as measured by NIH image analysis software from RayBiotech cytokine array images. (B) CXCL1 levels measured by ELISA in lysates from uninfected and infected cells. (C) ICAM-1 levels measured by ELISA in lysates from uninfected and infected cells.
The levels of CXCL1 produced by the WT CFTR LCFSN and ΔF508 CFTR cell lines were readily measured using a specific ELISA kit. Using this method, there was significantly more CXCL1 present in the lysates from the infected WT CFTR LCFSN cells (P < 0.001) than in the uninfected lysate of the same cell line. The levels of CXCL1 did not change significantly following infection in the ΔF508 CFTR cell line (Fig. 1B).
ICAM-1 protein levels were also measured in cell lysates by ELISA. There was no significant change in ICAM-1 following the infection of WT CFTR LCFSN cells, whereas a small but significant increase in ICAM-1 was seen in ΔF508 CFTR cells (P < 0.005) (Fig. 1C). However, the basal levels of ICAM-1 noted in the WT CFTR LCFSN cells were higher than those in the ΔF508 CFTR cells before and after infection.
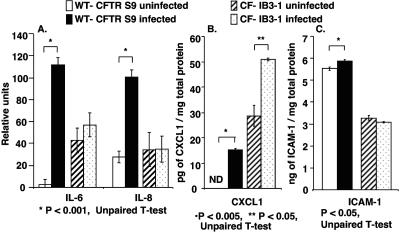
A second pair of cell lines isogenic for wild-type and mutant CFTR genes was used to investigate whether the protein level changes found in WT CFTR LCFSN and ΔF508 CFTR cell lines were also found in airway epithelial cells generated from other CF genotypes. Cell lysates from WT CFTR S9 and CF-IB3-1 (ΔF508/W1282X CFTR) cells both uninfected and infected with P. aeruginosa strain PAO1 for 3 h were assayed to determine their levels of IL-6, IL-8, CXCL1, and ICAM-1. Levels of both IL-6 and IL-8 were significantly increased with infection by P. aeruginosa strain PAO1 in the WT CFTR S9 cell line (P < 0.001) and were not significantly changed with infection in the CF-IB3-1 cell line (Fig. 2A). This is consistent with our findings for WT CFTR LCFSN and ΔF508 CFTR cells.
FIG. 2.
Role of CFTR in IL-6, IL-8, CXCL1, and ICAM-1 protein production in WT CFTR S9 or CF-IB3-1 cells in response to P. aeruginosa after 3 h of infection. (A) Relative levels of IL-6 and IL-8 cytokines in lysates from uninfected and infected cells as measured by NIH image analysis software from RayBiotech cytokine array images. (B) CXCL1 levels measured by ELISA in lysates from uninfected and infected cells. ND, none detected. (C) ICAM-1 levels measured by ELISA in lysates from uninfected and infected cells.
CXCL1 production was found to increase in both WT CFTR S9 and CF-IB3-1 cells with infection; however, basal levels of CXCL1 were elevated in CF-IB3-1 cells compared with those in WT CFTR S9 cells (Fig. 2B). The release of CXCL1 in response to P. aeruginosa in WT CFTR S9 cells is consistent with our findings for the WT CFTR LCFSN cells.
ICAM-1 was increased by a very small but nonetheless significant amount with infection in WT CFTR S9 (P < 0.05) but was unchanged in CF-IB3-1 cells with infection (Fig. 2C). Given the small magnitude of the increase in ICAM-1 in the WT CFTR S9 cells, it appears that changes in ICAM-1 protein levels in cells with WT CFTR are likely of little biological consequence. Overall, microarray and RT-PCR analyses of WT CFTR and ΔF508 CFTR cells indicate transcriptional changes in ICAM-1 in cells with WT CFTR but without mutant CFTR, but we observed little overall effect on protein levels when they were analyzed in two different pairs of cells lines with WT or mutant CFTR genotypes.
IL-6, IL-8, CXCL1, and ICAM-1 levels after infection by Pseudomonas aeruginosa strains N13 and 324.
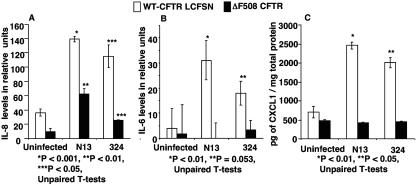
We infected WT CFTR LCFSN and ΔF508 CFTR cells with two clinical isolates of P. aeruginosa, strains N13 and 324, which are nonmucoid, smooth-lipopolysaccharide (LPS) strains obtained early in the course of infection from two different CF patients, and measured the IL-6 and IL-8 levels using cytokine membranes as described above. IL-8 was significantly increased with infection by strains N13 and 324 in WT CFTR LCFSN cells (P < 0.05) (Fig. 3A). Interestingly, IL-8 levels were modestly, but significantly, increased in ΔF508 CFTR cells following infection with both N13 and 324 (P < 0.01), but not to the same degree as in the cells with WT CFTR.
FIG. 3.
IL-8 levels (A), IL-6 levels (B), and CXCL1 levels (C) in response to two clinical isolates of P. aeruginosa. Lysates from WT CFTR-LCFSN or ΔF508 CFTR cells, uninfected or infected by P. aeruginosa strain N13 or 324 for 3 h, were tested for the presence of IL-8 and IL-6 by cytokine arrays. Relative levels were measured using NIH image analysis software. CXCL1 levels in lysates were measured by ELISA. Asterisks indicate comparisons of the infected samples to the uninfected samples of the same cell line.
Figure 3B shows the relative levels of IL-6 detected in the WT CFTR LCFSN and ΔF508 CFTR cell lysates in response to P. aeruginosa strains N13 and 324. IL-6 was significantly increased only in WT CFTR LCFSN cell lysates infected with these two strains (P < 0.01, P = 0.053) and not in infected ΔF508 CFTR cell lysates.
Figure 3C indicates the levels of CXCL1 measured by ELISA in the cell lysates following infection with P. aeruginosa strains N13 and 324. CXCL1 was significantly increased in WT CFTR LCFSN cell lysates following infection with N13 and 324 (P < 0.05). No increase was observed in ΔF508 cell lysates with infection by either N13 or 324.
No change in ICAM-1 levels was seen after infection with P. aeruginosa strains N13 or 324 in ΔF508 CFTR cells (data not shown). In WT CFTR LCFSN cells, strain N13 caused no change in ICAM-1 levels, whereas cells infected with strain 324 had an unexpected and significant decrease in ICAM-1 levels (data not shown). While the microarray and RT-PCR data indicated increased transcription of the ICAM-1 gene, we could not confirm increased production of ICAM-1 protein with any P. aeruginosa strain except for the biologically inconsequential change seen in WT CFTR S9 cells in response to strain PAO1 (Fig. 2C).
The role of NF-κB in the expression of IL-6, IL-8, CXCL1, and ICAM-1 in response to infection.
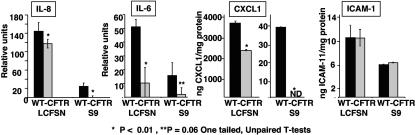
Transcription of the genes encoding IL-6, IL-8, CXCL1, and ICAM-1 is all reported to be regulated by NF-κB (3). We investigated the contribution of NF-κB to the production of cytokines following infection of WT CFTR LCFSN cells and WT CFTR S9 cells with P. aeruginosa strain PAO1. NF-κB translocation was inhibited by the addition of 15 μM of Bay 11-7082 to the media for 15 min prior to infection with PAO1. Following 3 h of bacterial infection, cell lysates were made, and IL-6, IL-8, CXCL1, and ICAM-1 protein levels were measured (Fig. 4). In WT CFTR LCFSN cells, IL-6 was decreased by 80%, IL-8 was decreased by only 19%, and CXCL1 was decreased by 37%. In WT CFTR S9 cells, IL-6 levels were reduced by 87%, and IL-8 and CXCL1 levels were reduced to basal levels by the inhibitor; however, the starting quantities of each of these cytokines was less than in WT CFTR LCFSN cells. ICAM-1 levels were not significantly affected by NF-κB inhibition in either cell line.
FIG. 4.
Role of NF-κB in regulation of cytokines made in response to P. aeruginosa. WT CFTR-LCFSN or WT CFTR S9 cells were infected with P. aeruginosa strain PAO1 following a 15-min exposure to Bay-11-7082 (SIGMA), an NF-κB inhibitor (gray bars). Black bars represent infected samples not exposed to inhibitor. Results for CXCL1 for WT CFTR-LCFSN and WT CFTR S9 cells are indicated using different scales due to the difference in the quantity of cytokines produced by the different cell lines. Inhibited samples were compared to uninhibited samples of the same cell line using one-tailed unpaired t tests to detect significant decreases. ND, none detected.
DISCUSSION
Microarray analysis is a powerful tool for investigating transcriptional differences between host cells during their responses to microbial pathogens, but the results are restricted by the conditions chosen for analysis. The number of potential variables for measuring both mRNA and protein changes in the context of infection, including but not limited to MOI, kinetics, bacterial strains used, cell lines used, and growth conditions for both prokaryotic and eukaryotic cells, makes it nearly impossible to obtain a comprehensive set of changes encompassing all possible variables. The short-term survival of cells exposed in vitro to pathogens producing toxic factors or inducing apoptosis also limits the degree to which microarray experiments can provide useful data. However, using prior observations and some preliminary analysis, it is possible to form testable hypotheses and devise experimental conditions to refute or support the hypotheses. In this case, prior observations that WT CFTR was a receptor for P. aeruginosa, resulting in the rapid nuclear translocation of NF-κB (31), lead us to hypothesize that a subsequent step in the innate immunity to P. aeruginosa that would be defective in CF patients would be the transcriptional activation and protein synthesis of NF-κB-dependent inflammatory cytokines. Additional prior observations that airway cells in a culture exposed to P. aeruginosa start to undergo significant apoptosis 3 h postinfection, reaching a level of 90% apoptotic cells 5 h postinfection (6), indicated that the production and accumulation of inflammatory mediators of immunity would likely be maximal around 3 h postinfection. Because of the heterogeneity in the abilities of the epithelial cells within a single monolayer to ingest P. aeruginosa (16), transcriptional changes detected at the 3-hour time point will also include responses made by cells not initially infected when bacteria were added.
To account for bacterial strain differences and host cell line differences, we conducted some of the analyses with different P. aeruginosa strains, including clinical isolates, and compared the effects in two cell lines commonly used in CF research. We chose an MOI of between 30 and 45, since preliminary experiments using lower bacterial amounts resulted in no detectable changes in gene transcription in the host cells, and much higher doses lead to earlier cytotoxic and apoptotic inductions of cellular death. While admittedly not completely comprehensive, these conditions allowed us to test the hypothesis that CFTR-dependent activation of NF-κB nuclear translocation in response to P. aeruginosa binding to CFTR would elicit host-inflammatory mediators that possibly could be effective at eliminating this pathogen from WT lung mucosa, while a reduction or complete lack of these factors being produced rapidly on the CF respiratory mucosa would result in a delay in bacterial clearance that might contribute to the initiation of chronic infection in this setting. The current study is focused on the earliest interactions of lung epithelial cells with P. aeruginosa, with the premise that these early interactions may impact the establishment of chronic colonization of the lung by this bacterium.
We were surprised to find that only 16 of the 4,608 genes on the microarray changed by more than twofold in the WT CFTR LCFSN cells. This may have been related to our choice of an MOI of between 30 and 45, which, as mentioned above, was the minimal level needed to consistently detect reproducible changes in the transcription of genes. Using a much higher MOI (∼300) and two different statistical filters, Perez and Davis detected 114 or 413 significant changes out of a possible 12,625 genes following P. aeruginosa infection of cells with WT CFTR (26). This represents changes in 1.1 to 3.3% of the total number of genes tested, whereas in our study using ∼10× fewer bacteria, we detected ∼6× fewer changes. The higher MOI used by Perez and Davis likely activated cellular responses that are usually more refractory to bacterial products, but in this case there was a sufficient stimulus for these additional pathways to be affected. Indeed, the relatively modest number of genes transcriptionally activated by an MOI of 30 to 50 P. aeruginosa cells likely is closer to what cells encounter in vivo, where ratios of bacterial cells to epithelial cells in the earliest stages of infection are quite low.
Because prior results showed a rapid nuclear translocation of NF-κB in response to P. aeruginosa infection in cells and mice with WT CFTR but without mutant CFTR, we particularly wanted to evaluate whether genes known to respond to NF-κB were differentially expressed by cells with WT but not ΔF508 CFTR. Microarray analysis and real-time PCR identified four likely participants in this process: the IL-8, IL-6, CXCL1, and ICAM-1 genes. All four of these genes are known to be regulated by NF-κB, providing a possible link between the regulation of these genes and the presence of functional CFTR. In addition, the role of the proteins encoded by these genes in rapid recruitment of neutrophils, important effectors of innate immunity to P. aeruginosa, could be key to the effective immunity in individuals with WT CFTR. As it is well known that neutropenic mice and humans are highly susceptible to P. aeruginosa infection (5, 9, 15, 24, 33) it is likely that a rapid recruitment of polymorphonuclear leukocytes (PMNs) to the lung mucosa is key to the elimination of P. aeruginosa. In contrast, a delayed recruitment of PMNs to the CF mucosa could allow for bacterial persistence, subsequent to which non-CFTR-dependent inflammation ensues in the CF lung, leading to the classic clinical picture seen in the chronic infection of an ineffective PMN-based inflammatory response.
After determining that the genes for IL-8, IL-6, CXCL1, and ICAM-1 were transcriptionally enhanced by WT CFTR, we set out to confirm whether WT CFTR produced the proteins encoded by these genes, since cells with ΔF508 did not. We used two isogenic pairs of CF cell lines, three P. aeruginosa strains, and several analytic methods for these confirmations. For three of the four proteins, i.e., IL-6, IL-8, and CXCL1, there was a reasonably consistent pattern indicating that WT CFTR enhanced production in response to P. aeruginosa, whereas changes in ICAM-1 levels could not be consistently confirmed. It is possible that this was due to rapid protein turnover, with a balance of new protein production and old protein degradation occurring in response to infection. We did note, however, that cells with WT CFTR had basal levels of ICAM-1 that were higher than that of ΔF508 CFTR cells. The basis for this lower basal level of ICAM-1 protein in the ΔF508 CFTR cells is unknown, but other factors, in addition to a direct effect from the mutant CFTR, could include differences in cell growth, the effect of contact inhibition, or cellular differentiation in vitro.
Several recent papers have investigated the transcriptional profiles of P. aeruginosa-infected cells. Cobb et al. (8) studied the role of flagellin and alginate in gene transcription in lung carcinoma cells, which presumably have wild-type CFTR. Using a flagellated, nonmucoid strain of P. aeruginosa, they measured increases in IL-8, CXCL1, and ICAM-1 such as those we have found with a similar strain in wild-type bronchial epithelial cells. Our study extends these findings by identifying the transcription of these genes as misregulated in cells homozygous for the ΔF508 CFTR mutation.
Perez and Davis (26) used Affymetrix gene chips to study the transcriptional differences between normal and CF cell lines in response to P. aeruginosa. They reported elevated levels of IL-8 and IL-6 in the CF lines relative to wild-type levels. A number of differences between our study and theirs could account for their results, including the aforementioned use of a much larger MOI (at least 300 bacteria per cell) and the use of two different cell lines that were not derived from the same individual. Although the cells differed in their expressions of WT or mutant CFTR, they also must have had differences in numerous other genetic loci. Also, Perez and Davis (26) reportedly used strain P. aeruginosa PAO1 in their studies, but their report indicates that their version of this strain is sensitive to the bactericidal effects of serum, which is not a property of the smooth-LPS PAO1 strain widely used by us and other investigators. Indeed, extensive studies of several P. aeruginosa PAO1 variants in our laboratory have not turned up a single strain that is killed by serum used in cell culture (G. B. Pier, unpublished observation). Thus, the serum sensitivity of the PAO1 variant they used does not appear to be representative of either strain PAO1 or the smooth-LPS strains of P. aeruginosa that initially infect CF patients.
Because it is well known that different CF cell lines along with their WT CFTR-corrected versions often show discrepant responses in a variety of biologic assays (1), we confirmed our findings in the matched CFT1 cells (WT CFTR LCFSN and ΔF508 CFTR cells) with a second matched cell line to determine the variability in the dependences of innate immune responses to P. aeruginosa upon CFTR. While we did find reasonable consistency across cell lines and P. aeruginosa strains for most of the observations, our findings were not necessarily in accord with others who have conducted similar investigations. As an example, Virella-Lowell et al. (35) reported that IL-6 and IL-8 showed exaggerated activation in response to strain PAO1 in CF-IB3-1 cells when compared to results for WT CFTR S9 cells. However, when we tested the responses of CF-IB3-1 and WT CFTR S9 cells to P. aeruginosa infection, our results for IL-6 and IL-8 showed a significant increase in the WT CFTR S9 cells and no change in the CF-IB3-1 cells, which is consistent with the findings with WT CFTR LCFSN and ΔF508 CFTR cells. Several differences could account for the discrepancy between our findings and those of Virella-Lowell et al. (35). One important difference is that Virella-Lowell et al. exposed CF-IB3-1 cells to the aminoglycoside amikacin before harvesting the RNA. Aminoglycosides are known to cause read-through of stop mutations, such as the W1282X mutation present in CF-IB3-1 cells (4, 18). Read-through could lead to a level of functional CFTR being made in the CF-IB3-1 cell line that is sufficient to transduce responses to P. aeruginosa and thus obscure differences with the WT CFTR-corrected S9 cells. The level of CFTR expression needed to transduce responses to P. aeruginosa is not known. This effective read-through of stop mutations in CFTR mRNA by aminoglycosides is currently being pursued as a therapy in humans (7, 37). Another difference in the methods used by us and those of Virella-Lowell et al. is that they had a 24-h lag period between infection and RNA extraction, which could lead to a very different transcriptional profile that perhaps would be more representative of the chronically infected CF lung than of the initially infected WT or CF lung. Importantly, although Virella-Lowell et al. treated their infected cells with 50 μg amikacin/ml after 3 h of infection to kill residual P. aeruginosa cells, they did not report on the efficacy of this treatment. We have found that it usually takes 200 μg/ml of an aminoglycoside to kill all residual P. aeruginosa cells in infected cell cultures (11), raising the possibility that the results of Virella-Lowell et al. were based on 24 h of continuous infection with an unknown quantity of live P. aeruginosa cells. Under these circumstances, it is not surprising that the early and rapid NF-κB-dependent, CFTR-enhanced transcriptional responses seen in WT cells were over and that the delayed, non-CFTR-dependent cytokine response was activated in these experiments.
CXCL1 production in WT CFTR S9 and CF-IB3-1 cells showed a profile different from that seen in the CFT1 cell pair. The spontaneous release of cytokines, which may affect the regulation of CXCL1, has been previously reported for CF-IB3-1 cells (10). Additionally, the weak CXCL1 response in these cells compared to that in the WT CFTR LCFSN cells indicates that the mechanism inducing CXCL1 is not functioning at the same level in these cells and may need to be measured at a later time point or in response to a greater number of bacteria to get a more representative result.
Many studies of P. aeruginosa pathogenesis have used a single strain, such as PAO1. However, this strain often does not give responses representative of clinical isolates of this organism. Notably, 85% of P. aeruginosa strains contain a 40-kb segment of DNA referred to as PAGI-1, which is missing in strain PAO1 (22). In addition, strain PAO1 lacks two pathogenicity islands identified in the genome of another clinical isolate of P. aeruginosa, strain PA14 (17). To determine the variability in cellular responses to different P. aeruginosa strains, we used two nonmucoid clinical isolates: strains N13 and 324. Both strains induced enhanced production of IL-6 and CXCL1 in cells with WT CFTR, consistent with the response to strain PAO1. However, infections with strains N13 and 324 triggered IL-8 production in both WT CFTR LCFSN and ΔF508 CFTR cell lines, but in both cases much more IL-8 was made by cells with WT CFTR, again consistent with the finding with strain PAO1. This indicates that under some circumstances CFTR is partially dispensable for IL-8 production during 3 h of cell infection. Other factors present in strains N13 and 324 may trigger IL-8 through a CFTR-independent mechanism; however, the magnitude of the effect is much less than that in the presence of functional CFTR. It is possible that a muted IL-8 response may be induced by these clinical isolates by a CFTR-independent mechanism in the cells with ΔF508 CFTR and, as such, may be insufficient to mediate rapid, innate immunity to P. aeruginosa such as that seen in cells with WT CFTR. Also, without other factors, such as IL-6 and CXCL1, the low levels of IL-8 made by CF cells would likely not mediate the same innate response as that which occurs with WT CFTR-expressing cells. Overall, while the generation of IL-8 by cells with functional CFTR was consistent across all strains tested, there were small amounts of CFTR-independent production of IL-8 with two of three P. aeruginosa strains tested.
The IL-6, IL-8, CXCL1, and ICAM-1 genes are reported in the literature to be NF-κB dependent; however, other transcriptional factors can regulate activation of these genes. In this study, we used Bay 11-7082, which prevents the translocation of NF-κB by irreversibly phosphorylating IκB-α, to determine what role NF-κB plays in regulating the transcription and translation of the four genes that are activated in response to P. aeruginosa infection of bronchial epithelial cells. Interestingly, in the WT CFTR LCFSN cells, the four genes showed differing degrees of dependence on NF-κB. IL-6 production was almost completely dependent on NF-κB (80%), whereas CXCL1 was much less dependent on NF-κB (37%). ICAM-1 levels were unaffected by the NF-κB inhibitor. Surprisingly, IL-8 showed only a very modest but statistically significant decrease in production (19%) in the presence of the NF-κB inhibitor in the WT CFTR LCFSN cells. This finding is interesting because there is a large body of literature on the IL-8 response in CF, and it is often assumed that NF-κB is responsible for its regulation in this system. It is quite possible that IL-8 production in response to P. aeruginosa infection may be regulated by other transcriptional factors, such as β-catenin. β-Catenin has been shown to be capable of activating IL-8 and has been proposed as another possible mechanism of IL-8 activation in response to P. aeruginosa, based on its physical association with Muc-1 and the colocalization of Muc-1 and β-catenin in the nucleus (21, 23, 36, 38). Further study is required to determine whether other transcription factors are responsible for the increase in IL-8 due to P. aeruginosa infection. In WT CFTR S9 cells, the reduction of IL-6 levels in response to the inhibitor was 87%, whereas the reduction in IL-8 and CXCL1 levels was complete; however, the starting quantity of each of these cytokines was much less than that in WT CFTR LCFSN cells. This may indicate that NF-κB plays a slightly different role in cells with different CFTR genotypes or that other genetic loci affect basal levels of cytokines in the cells. ICAM-1 was not reduced by the NF-κB inhibitor in WT CFTR S9 cells, as was seen in the WT CFTR LCFSN cells.
A recent study of primary cultures of CF and non-CF human airway epithelia by Joseph et al. (20) reported NF-κB activation and IL-8 mRNA expression in CF cells in response to P. aeruginosa higher than those measured in non-CF cells. The primary cells from CF patients studied by Joseph et al. (20) presumably had previous exposure to P. aeruginosa, which could alter their later responsiveness. Indeed, Ribeiro et al. (29) recently showed that primary ΔF508 CFTR bronchial epithelial cells in culture were hyperinflammatory during short-term (6- to 11-day) culturing but lost this property with longer-term (30- to 40-day) culturing, indicating that inflammation was independent of the presence of ΔF508 CFTR. Another difference between our study and that of Joseph et al. (20) is that the responses they measured from primary cells were obtained at time points much later (up to 29 h after infection) than those in our study. Joseph et al. (20) reported that IL-8 mRNA levels did not differ between CF and non-CF primary cultures at their earliest time point of 4 h. This could be due to the induction of IL-8 mRNA by the P. aeruginosa infection of the WT CFTR cells, but infection-independent IL-8 production from the CF cells due to prior infection, as Ribeiro et al. showed, occurs with primary CF cells (29). To confirm whether the mRNA levels are biologically relevant, IL-8 protein levels would need to be measured in the primary cell cultures, as there can be discrepancies between the mRNA levels and the levels of protein produced. It is possible that our findings of an early elevated IL-8 protein response in cells with WT CFTR that is lacking in CF cells may be due to a delayed response in CF cells; however, at later time points following infection, high IL-8 levels may be produced due to a combination of a lack of WT CFTR and a prolonged exposure to P. aeruginosa.
In conclusion, the transcriptional and translational responses we have measured in cells of differing CF genotypes to three different P. aeruginosa strains support the idea that the innate immune response triggered in the normal lung is misregulated in the CF lung. Our results suggest that the reduced production of IL-6, IL-8, and CXCL1 in the CF lung, partially due to lack of the normally CFTR-dependent NF-κB translocation in response to P. aeruginosa infection, may lead to a dampened innate immune response leading in turn to a reduced early clearance of P. aeruginosa from the lung. The roles of these three cytokines, along with that of ICAM-1, whose transcriptional levels, but not protein levels, were found to be increased only in the presence of functional CFTR in response to P. aeruginosa infection, point to a possible role of early PMN recruitment in clearing the normal lung of P. aeruginosa. In the absence of WT CFTR, this recruitment could be delayed sufficiently to allow the bacteria to establish foci of infection which are not amenable to clearance once the later influx of PMNs occurs in a CFTR-independent fashion in the CF lung. The chronic infection eventually leads to an increased triggering of inflammation, typified by PMN recruitment, but at this point it is too late to effectively clear the foci of infection established within the dehydrated mucus. The ultimate consequences are lung destruction over time due to chronic P. aeruginosa infection and chronic inflammation, with early mortality occurring in over 90% of CF patients, usually by 25 to 35 years of age.
Acknowledgments
This work was supported by NIH grant AI HL58398 and the Albert J. Ryan Foundation.
We thank Stephen Lory for advice, reagents, use of the microarray scanner, and analysis equipment; Sean Colgan and his laboratory for the use of their Bio-Rad iCycler; Ellen Cahir-McFarland for the NF-κB inhibitor and advice; Rebekah Jackson for help with microarray technique; Lea Medeiros and Angela Ross for advice on optimizing real-time PCR; and Marcia Goldberg, David Golan, Jeff Lyczak, and Carolyn Cannon for advice and support.
Editor: J. T. Barbieri
REFERENCES
- 1.Aldallal, N., E. E. McNaughton, L. J. Manzel, A. M. Richards, J. Zabner, T. W. Ferkol, and D. C. Look. 2002. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 166:1248-1256. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, M. P., D. P. Rich, R. J. Gregory, A. E. Smith, and M. J. Welsh. 1991. Generation of cAMP-activated chloride currents by expression of CFTR. Science 251:679-682. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 4.Bedwell, D. M., A. Kaenjak, D. J. Benos, Z. Bebok, J. K. Bubien, J. Hong, A. Tousson, J. P. Clancy, and E. J. Sorscher. 1997. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat. Med. 3:1280-1284. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez, L. E., J. C. Martinelli, R. Gascon, M. Wu, and L. S. Young. 1990. Protection against gram-negative bacteremia in neutropenic mice with recombinant granulocyte-macrophage colony-stimulating factor. Cytokine 2:287-293. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, C. L., M. P. Kowalski, K. S. Stopak, and G. B. Pier. 2003. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Cell Mol. Biol. 29:188-197. [DOI] [PubMed] [Google Scholar]
- 7.Clancy, J. P., Z. Bebok, F. Ruiz, C. King, J. Jones, L. Walker, H. Greer, J. Hong, L. Wing, M. Macaluso, R. Lyrene, E. J. Sorscher, and D. M. Bedwell. 2001. Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 163:1683-1692. [DOI] [PubMed] [Google Scholar]
- 8.Cobb, L. M., J. C. Mychaleckyj, D. J. Wozniak, and Y. S. Lopez-Boado. 2004. Pseudomonas aeruginosa flagellin and alginate elicit very distinct gene expression patterns in airway epithelial cells: implications for cystic fibrosis disease. J. Immunol. 173:5659-5670. [DOI] [PubMed] [Google Scholar]
- 9.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1983. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect. Immun. 39:1067-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eidelman, O., M. Srivastava, J. Zhang, X. Leighton, J. Murtie, C. Jozwik, K. Jacobson, D. L. Weinstein, E. L. Metcalf, and H. B. Pollard. 2001. Control of the proinflammatory state in cystic fibrosis lung epithelial cells by genes from the TNF-alphaR/NFkappaB pathway. Mol. Med. 7:523-534. [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig, S. M., T. S. Zaidi, and G. B. Pier. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flotte, T. R., R. Solow, R. A. Owens, S. Afione, P. L. Zeitlin, and B. J. Carter. 1992. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 7:349-356. [DOI] [PubMed] [Google Scholar]
- 13.Geiss, G. K., M. C. An, R. E. Bumgarner, E. Hammersmark, D. Cunningham, and M. G. Katze. 2001. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and -independent events. J. Virol. 75:4321-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiss, G. K., R. E. Bumgarner, M. C. An, M. B. Agy, A. B. van 't Wout, E. Hammersmark, V. S. Carter, D. Upchurch, J. I. Mullins, and M. G. Katze. 2000. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology 266:8-16. [DOI] [PubMed] [Google Scholar]
- 15.Gencer, S., T. Salepci, and S. Ozer. 2003. Evaluation of infectious etiology and prognostic risk factors of febrile episodes in neutropenic cancer patients. J. Infect. 47:65-72. [DOI] [PubMed] [Google Scholar]
- 16.Gerceker, A. A., T. Zaidi, P. Marks, D. E. Golan, and G. B. Pier. 2000. Impact of heterogeneity within cultured cells on bacterial invasion: analysis of Pseudomonas aeruginosa and Salmonella enterica serovar Typhi entry into MDCK cells by using a green fluorescent protein-labeled cystic fibrosis transmembrane conductance regulator receptor. Infect. Immun. 68:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, J., R. L. Baldini, E. Deziel, M. Saucier, Q. Zhang, N. T. Liberati, D. Lee, J. Urbach, H. M. Goodman, and L. G. Rahme. 2004. The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc. Natl. Acad. Sci. USA 101:2530-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard, M., R. A. Frizzell, and D. M. Bedwell. 1996. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat. Med. 2:467-469. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa, J. K., A. Norris, M. G. Bangera, G. K. Geiss, A. B. van 't Wout, R. E. Bumgarner, and S. Lory. 2000. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. USA 97:9659-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph, T., D. Look, and T. Ferkol. 2005. NF-{kappa}B activation and sustained IL-8 gene expression in primary cultures of cystic fibrosis airway epithelial cells stimulated with Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L471-L479. [DOI] [PubMed] [Google Scholar]
- 21.Levy, L., C. Neuveut, C. A. Renard, P. Charneau, S. Branchereau, F. Gauthier, J. T. Van Nhieu, D. Cherqui, A. F. Petit-Bertron, D. Mathieu, and M. A. Buendia. 2002. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J. Biol. Chem. 277:42386-42393. [DOI] [PubMed] [Google Scholar]
- 22.Liang, X., X. Q. Pham, M. V. Olson, and S. Lory. 2001. Identification of a genomic island present in the majority of pathogenic isolates of Pseudomonas aeruginosa. J. Bacteriol. 183:843-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lillehoj, E. P., H. Kim, E. Y. Chun, and K. C. Kim. 2004. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L809-L815. [DOI] [PubMed] [Google Scholar]
- 24.Mayer, P., E. Schutze, C. Lam, F. Kricek, and E. Liehl. 1991. Recombinant murine granulocyte-macrophage colony-stimulating factor augments neutrophil recovery and enhances resistance to infections in myelosuppressed mice. J. Infect. Dis. 163:584-590. [DOI] [PubMed] [Google Scholar]
- 25.Olsen, J. C., L. G. Johnson, M. J. Stutts, B. Sarkadi, J. R. Yankaskas, R. Swanstrom, and R. C. Boucher. 1992. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum. Gene Ther. 3:253-266. [DOI] [PubMed] [Google Scholar]
- 26.Perez, A., and P. B. Davis. 2004. Gene profile changes after Pseudomonas aeruginosa exposure in immortalized airway epithelial cells. J. Struct. Funct. Genomics 5:179-194. [DOI] [PubMed] [Google Scholar]
- 27.Pier, G. B., M. Grout, and T. S. Zaidi. 1997. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl. Acad. Sci. USA 94:12088-12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pier, G. B., M. Grout, T. S. Zaidi, J. C. Olsen, L. G. Johnson, J. R. Yankaskas, and J. B. Goldberg. 1996. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro, C. M., A. M. Paradiso, U. Schwab, J. Perez-Vilar, L. Jones, W. O'Neal, and R. C. Boucher. 2005. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 280:17798-17806. [DOI] [PubMed] [Google Scholar]
- 30.Rosner, B. 1990. Fundamentals of biostatistics, 3rd ed. PWS-Kent Publishing Company, Boston, Mass.
- 31.Schroeder, T. H., M. M. Lee, P. W. Yacono, C. L. Cannon, A. A. Gerceker, D. E. Golan, and G. B. Pier. 2002. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc. Natl. Acad. Sci. USA 99:6907-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwiebert, E. M., M. E. Egan, T. H. Hwang, S. B. Fulmer, S. S. Allen, G. R. Cutting, and W. B. Guggino. 1995. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81:1063-1073. [DOI] [PubMed] [Google Scholar]
- 33.Siripassorn, K., S. Santiprasitkul, S. Udompanthurak, and V. Thamlikitkul. 2002. Risk factors for Pseudomonas aeruginosa bacteremia in Thai patients. J. Med. Assoc. Thail. 85:1095-1099. [PubMed] [Google Scholar]
- 34.Thomas, P. J., and P. L. Pedersen. 1993. Effects of the delta F508 mutation on the structure, function, and folding of the first nucleotide-binding domain of CFTR. J. Bioenerg. Biomembr. 25:11-19. [DOI] [PubMed] [Google Scholar]
- 35.Virella-Lowell, I., J. D. Herlihy, B. Liu, C. Lopez, P. Cruz, C. Muller, H. V. Baker, and T. R. Flotte. 2004. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell line. Mol. Ther. 10:562-573. [DOI] [PubMed] [Google Scholar]
- 36.Wen, Y., T. C. Caffrey, M. J. Wheelock, K. R. Johnson, and M. A. Hollingsworth. 2003. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J. Biol. Chem. 278:38029-38039. [DOI] [PubMed] [Google Scholar]
- 37.Wilschanski, M., Y. Yahav, Y. Yaacov, H. Blau, L. Bentur, J. Rivlin, M. Aviram, T. Bdolah-Abram, Z. Bebok, L. Shushi, B. Kerem, and E. Kerem. 2003. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N. Engl. J. Med. 349:1433-1441. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto, M., A. Bharti, Y. Li, and D. Kufe. 1997. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J. Biol. Chem. 272:12492-12494. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Y., A. T. Ting, K. B. Marcu, and J. B. Bliska. 2005. Inhibition of MAPK and NF-{kappa}B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J. Immunol. 174:7939-7949. [DOI] [PubMed] [Google Scholar]