Abstract
Metformin is the most commonly prescribed antidiabetes drug, yet its precise mechanism of action remains controversial. Previous studies have suggested that metformin acts peripherally by reducing hepatic glucose output and altering gut functions. Here, we report a neural mechanism via the small guanosine triphosphatase Ras-related protein 1 (Rap1). Mice with forebrain-specific Rap1 knockout exhibited resistance to the antidiabetic effects of low-dose metformin while remaining sensitive to other antidiabetic agents. Centrally administered metformin inhibited brain Rap1 and reduced hyperglycemia. Conversely, forced activation of brain Rap1 increased glycemia and abolished the glycemic effect of metformin. Metformin activated a specific subset of neurons in the ventromedial hypothalamic nucleus (VMH) that requires Rap1. Both loss-of-function and gain-of-function studies suggest that VMH Rap1 is indispensable for the antidiabetic effects of metformin. These findings highlight the VMH Rap1 pathway as a critical mediator of metformin action.
Metformin lowers blood glucose by inhibiting Rap1 in ventromedial hypothalamic neurons that regulate glucose balance.
INTRODUCTION
Metformin is a biguanide derivative that has potent glucose-lowering activity (1, 2). Metformin also has multiple metabolic effects that lead to modest weight loss, improved lipid profile, and enhanced insulin sensitivity, all of which contribute to its antidiabetic effect (3–7). Because of these excellent metabolic effects, low cost, and long-term safety, metformin has been the first-line therapy for type 2 diabetes for decades (8–14). Mechanistically, it has been widely accepted that metformin lowers blood glucose primarily by reducing glucose output in the liver (15–18). This has been proposed to be achieved through activation of adenosine 5′-monophosphate (AMP)–activated protein kinase (AMPK) (19, 20). However, accumulating evidence suggests that there are AMPK-independent pathways (21), such as the inhibition of mitochondrial respiratory chain complexes (22–24), cyclic AMP (cAMP) signaling (25), mitochondrial glycerophosphate dehydrogenase (26), and fructose 1,6-bisphosphatase (27). Furthermore, recent studies have identified the gut as a nonhepatic site that mediates the therapeutic effects of metformin by altering microbiome dynamics, increasing glucose uptake in the gut, and enhancing hormonal secretion of growth and differentiation factor 15 and glucagon-like peptide 1 (GLP-1) (6, 28–33). Thus, decades of research have gradually revealed a much more complex picture of metformin’s action, expanding the initial perspective of AMPK-dependent hepatic glycemic improvement to multiple modes of action across peripheral organs (9, 10, 34).
The brain is now widely recognized as a key regulator of whole-body glucose metabolism and a potential therapeutic target for the treatment of diabetes (35–39). However, whether and how the brain contributes to the antidiabetic effects of metformin have not been thoroughly explored. We have previously demonstrated that Ras-related protein 1 (Rap1), a small guanosine triphosphatase in the hypothalamus, is a key regulator of whole-body glucose metabolism (40, 41). Specifically, activating hypothalamic Rap1 induces a diabetic-like phenotype (40), while reducing its activity ameliorates hyperglycemia in mice (40). Here, we report that brain-specific Rap1 knockout mice are unresponsive to metformin but sensitive to other glucose-lowering agents (fig. S1), implying a neural mechanism for the antidiabetic effect of metformin. By further exploring this possibility through genetic, pharmacological, and electrophysiological studies, we uncover a previously unrecognized neural pathway mediating the therapeutic effects of metformin via Rap1.
RESULTS
Selective defect in the therapeutic effects of metformin in mice lacking brain Rap1
Therapeutically achievable circulating concentrations of metformin are typically in the range of ~10 to 40 μM in metformin-treated humans (8, 10, 11, 42). However, higher doses of metformin are often used in experimental settings, and some peripheral mechanisms are observed only at suprapharmacologic levels that are irrelevant in the clinical setting (10, 11). Therefore, it is important to determine whether the Rap1-dependent neural mechanism specifically mediates therapeutic effects at the lower, clinically relevant doses. In this study, we administered low doses of metformin (50 to 150 mg/kg), which are known to produce therapeutic serum metformin concentrations (4, 10). Our measurements confirmed that intraperitoneal (ip) administration of 150 mg/kg of metformin resulted in serum levels of ~23 μM 4 hours after injection [serum; wild type (WT) in fig. S2A].
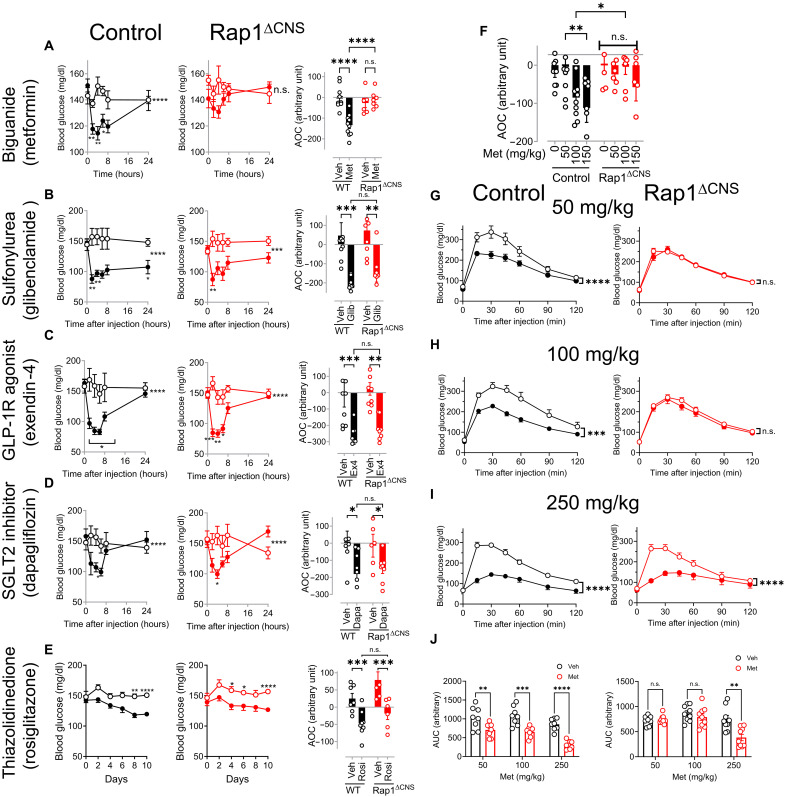
We first explored the role of neural Rap1 in mediating the effects of antidiabetic agents. To do this, we used brain-specific Rap1–deficient mice, where both Rap1a and Rap1b are selectively deleted in forebrain neurons (Rap1ΔCNS; Rap1afl/fl, Rap1bfl/fl, and CaMKIIαCre) (41). These mice and their littermate controls were fed a high-fat diet (HFD) to induce moderate hyperglycemia. There were no statistically significant differences in basal blood glucose levels between Rap1ΔCNS mice and their littermate controls before the treatment with antidiabetic agents (fig. S1, A and K). After administering different classes of antidiabetic drugs, we observed a significant glucose lowering in littermate controls following peripheral injections of antihyperglycemic drugs, including a biguanide (metformin), thiazolidinedione (rosiglitazone), GLP-1 receptor agonist (exendin-4), sulfonylurea (glibenclamide), sodium-glucose transport protein 2 (SGLT2) inhibitor (dapagliflozin), and insulin (Humulin R) (fig. S1, A to F). However, in Rap1ΔCNS mice, metformin failed to significantly lower blood glucose levels (fig. S1A), whereas the other antihyperglycemic agents exhibited similar glucose-lowering effects in both control and Rap1ΔCNS mice (fig. S1, B to F). Similarly, HFD-fed Rap1ΔCNS mice did not show decreased blood glucose levels when treated chronically with metformin (100 mg/kg) daily for 4 days, which resulted in lower blood glucose levels in littermate controls (fig. S1, G to J). A time-course study further confirmed that Rap1ΔCNS mice had an impaired ability to lower blood glucose in response to metformin, despite receiving the same dose as the control mice (Fig. 1A). Notably, Rap1ΔCNS mice exhibited nearly identical time courses and magnitudes of response to all other antihyperglycemic agents tested, comparable to those observed in control mice (Fig. 1, A to E), suggesting that the genetic deletion of brain Rap1 did not significantly alter the response to the other glucose-lowering agents. Collectively, these results suggest that Rap1ΔCNS mice do not have inherent defects in glucose lowering in general but have a selective impairment in metformin’s glucose-lowering effect.
Fig. 1. Rap1ΔCNS mice are resistant to the glycemic effect of low-dose metformin.
(A to E) Blood glucose levels in control (left; control; n = 8 to 10) and Rap1ΔCNS (middle; Rap1ΔCNS; n = 6 to 7) mice after a single injection of US Food and Drug Administration (FDA)–approved antidiabetic agents. The area of the curve (AOC) of metformin-induced glucose lowering (right). Metformin (A) (100 mg/kg, ip; 39 weeks of HFD) lowered blood glucose in control mice but not in Rap1ΔCNS mice. Acute glucose lowering was observed in both mice receiving glibenclamide (B) (1 mg/kg, ip; 39 weeks of HFD), exendin-4 (C) (0.1 mg/kg, ip; 40 weeks of HFD), dapagliflozin (D) [20 mg/kg, per os (p.o.); 45 weeks of HFD], or rosiglitazone (E) (10 mg/kg, ip; 40 weeks of HFD; note: glucose lowering was observed only after chronic treatment in mice). Open circles: vehicle-treated mice; closed circles: drug-treated mice. Glib, glibenclamide; GLP-1R, GLP-1 receptor; Ex4, exendin-4; Dapa, dapagliflozin; Rosi, rosiglitazone. (F) Dose-dependent glucose lowering by metformin in control and Rap1ΔCNS mice (n = 6 to 10; HFD for 39 to 42 weeks). The data show the AOC of metformin-induced glucose lowering. Metformin was administered at various doses (0 to 150 mg/kg, ip), and blood glucose levels were measured over 24 hours as shown in (A) to (E). The AOC was calculated to determine the overall glucose-lowering effect of metformin. (G to J) Glucose tolerance was assessed in control and Rap1ΔCNS mice by administering a bolus of glucose (1.5 g/kg), 30 min after an injection of metformin (0 to 250 mg/kg, ip; n = 8 to 9; HFD for 59 weeks). The area under the curve (AUC) is the area under the glucose tolerance test (GTT) curve (J). *P < 0.05, **P < 0.01, ***P < 0.001, and ***P < 0.0001 by two-way analysis of variance (ANOVA) followed by Šídák’s multiple-comparison test or uncorrected Fisher’s least significant difference (LSD) test.
Next, we examined the dose-response effect of metformin in Rap1ΔCNS and littermate controls by intraperitoneal injection of increasing doses of metformin and measuring blood glucose levels. As shown in Fig. 1F, analysis of the area of the curve (AOC) of a metformin tolerance test revealed that metformin significantly reduced blood glucose levels in a dose-dependent manner in control mice. However, this effect was absent in Rap1ΔCNS mice receiving the same doses (Fig. 1F), although circulating concentrations of metformin were similar in both groups at 150 mg/kg (fig. S2A). To further determine the dose-dependent effect of metformin, we performed a glucose tolerance test (GTT) in Rap1ΔCNS mice and their controls that received metformin or vehicle. Given that metformin affects glucose absorption in the intestine (43), we administered glucose intraperitoneally to perform GTT. While metformin (50 to 250 mg/kg) improved glucose tolerance in control mice (Fig. 1, G to J; control), this ability of metformin was completely abolished in Rap1ΔCNS mice at lower doses (50 and 100 mg/kg; Fig. 1, G and H; Rap1ΔCNS). A suprapharmacological dose of metformin (200 and 250 mg/kg) enhanced the ability to lower glycemia and dispose of the glucose load during the GTT in Rap1ΔCNS mice, comparable to control mice (Fig. 1, I and J, and fig. S3), suggesting that the higher dose (≥ 200 mg/kg), which is likely to result in circulating metformin concentrations of ~200 to 400 μM (10, 44–46), bypasses the requirement of brain Rap1 for the action of metformin. Similarly, orally administered metformin (150 mg/kg) failed to significantly improve glucose tolerance in Rap1ΔCNS mice while inducing a significant improvement in controls (fig. S4), confirming that the Rap1-dependent mechanism also plays a role in the standard clinical oral route. These findings collectively support a critical role for brain Rap1 in responding to and mediating the effects of clinically relevant low doses of metformin, but the requirement for brain Rap1 is bypassed at higher suprapharmacological doses.
Glucose-lowering action of centrally delivered metformin
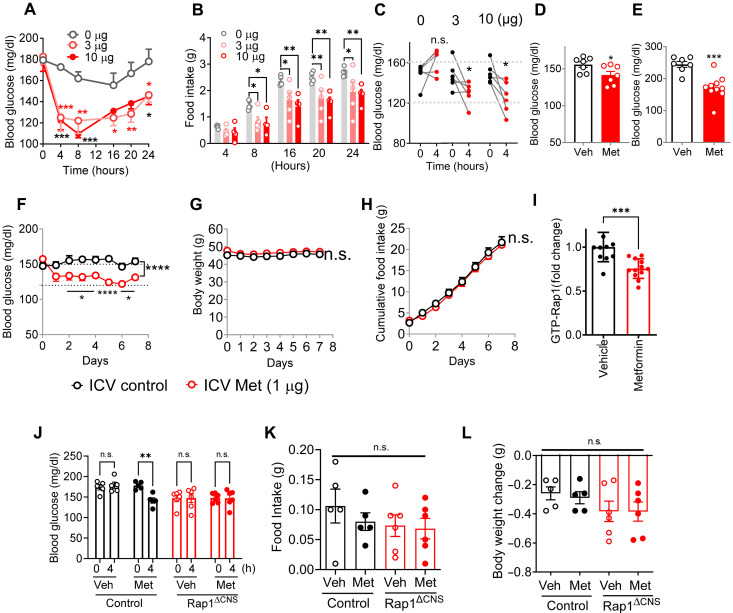
Our genetic studies of Rap1ΔCNS mice suggest that the antihyperglycemic effects of metformin may be due, in part, to its actions in the brain. Therefore, we sought to determine whether intracerebroventricular (ICV) administration of a low dose of metformin could effectively lower blood glucose levels. Metformin (3 to 10 μg per brain) was directly injected into the brains of diet-induced obese (DIO) C57BL/6J mice with moderate hyperglycemia. We found that ICV injection of metformin significantly reduced blood glucose levels (at 3 to 10 μg for 4 to 24 hours) (Fig. 2A). Consistent with previous studies (47, 48), we observed that metformin reduced food intake (Fig. 2B). However, the reduction in blood glucose cannot be accounted for by reduced food intake because the glucose-lowering effect was observed before the suppression of food intake and because ICV metformin was able to lower blood glucose even in the absence of food (Fig. 2C). In addition, this effect was not due to leakage into the bloodstream, as no glucose-lowering effect was observed when the centrally effective dose (30 μg) was administered peripherally (fig. S5A). We repeated the same experiments in other mouse models of type 2 diabetes, leptin-deficient ob/ob mice (a monogenic mouse model of type 2 diabetes) and DIO/streptozotocin (STZ) mice, a well-established animal model of type 2 diabetes mimicking both insulin resistance and partial β cell failure in human type 2 diabetes. Glucose lowering induced by ICV metformin was observed in both ob/ob and DIO/STZ mouse models (Fig. 2, D and E) and was independent of food intake and body weight (fig. S5, B and C). We also demonstrated that chronic metformin treatment reduced blood glucose levels in DIO mice without affecting food intake and body weight (Fig. 2, F to H). The effect was seen with as little as 1 μg of metformin, which is several thousand times lower than the doses required for oral or peripheral administration in rodent studies [typically 50 to 500 mg/kg of body weight (10, 11, 21, 25, 26)], suggesting that the brain is highly sensitive to metformin.
Fig. 2. Centrally administered metformin reduces blood glucose levels without affecting food intake or body weight.
(A) Acute ICV injection of metformin (3 and 10 μg) reduced blood glucose levels in DIO mice (n = 4 to 5). (B) Food intake of mice as in (A). (C) ICV metformin–induced glucose lowering in the absence of food (n = 6). (D and E) Glucose lowering by ICV metformin (3 μg) in STZ/DIO mice (D) (16 hours; n = 7) or ob/ob mice (E) (4 hours; n = 7 to 10). (F to H) Low dose of metformin reduced blood glucose levels (F) but did not change body weights (G) and cumulative food intake (H) when infused into the brain of DIO C57BL/6J mice (1 μg; n = 8 to 9; 18 weeks of HFD) over 7 days. (I) Hypothalamic Rap1 activity is reduced in C57BL/6J mice receiving ICV metformin (3 μg for 4 hours; n = 12). (J to L) Centrally administered metformin did not further lower blood glucose levels in brain-specific Rap1–deficient mice. Blood glucose levels (A), food intake (B), and body weight change (C) of control and Rap1ΔCNS mice before or 4 hours after a single ICV injection of metformin (3 μg) or vehicle (1 μl; n = 4 to 6). h, hours. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by t test [(C) to (E) and (I)] and two-way ANOVA followed by Tukey’s multiple-comparison test [(A), (B), (F) to (H), and (J) to (L)].
We next asked whether ICV metformin alters hypothalamic Rap1 activity. To test this, we delivered metformin directly into the brains of DIO mice. Four hours postadministration, we observed a significant reduction in the amount of guanosine 5′-triphosphate (GTP)–bound active Rap1 in the hypothalamus of the metformin-treated mice (Fig. 2I), indicating that metformin inhibits hypothalamic Rap1 activity. Last, to determine whether brain Rap1 mediates the central action of metformin, we administered ICV metformin into HFD-fed Rap1ΔCNS mice and their littermate controls. As shown in Fig. 2 (J to L), Rap1ΔCNS mice displayed lower baseline blood glucose than controls, and metformin failed to further reduce their already lowered glycemia. Therefore, these results suggest that centrally administered metformin inhibits Rap1 and reduces blood glucose levels, probably through brain Rap1 signaling.
Constitutive activation of Rap1 in the brain blocks metformin’s glycemic effect
If metformin acts by inhibiting the brain Rap1 pathway, then expressing a constitutively active Rap1 in the brain, one that cannot be inhibited, should block metformin’s antidiabetic effects. To test this, we created a gain-of-function Rap1 mouse model expressing a Cre-dependent, constitutively active human Rap1a mutant (Rosa26-LSL-Rap1V12 mice) (Fig. 3A and fig. S6A). We targeted Rap1V12 expression to forebrain neurons by crossing Rosa26-LSL-Rap1V12 mice with the CaMKIIα Cre line, which expresses Cre recombinase specifically in postnatal forebrain neurons (49), creating Rap1CNSV12 mice. We confirmed CRISPR-mediated Rap1V12 knock-in at Rosa26 (fig. S6, A and B), central nervous system (CNS)–specific recombination of the Rap1V12 alleles (fig. S6C), forebrain expression of Rap1V12 mRNAs (fig. S6D), elevated hypothalamic Rap1 protein (fig. S6, E and F), and increased active GTP-bound Rap1 in the brain (Fig. 3B). While HFD-fed Rap1CNSV12 mice maintained similar body weight to littermate controls, they exhibited higher fasting glucose levels and impaired glucose tolerance relative to HFD-fed controls (Fig. 3, D and E). During GTT, metformin (150 mg/kg, ip) failed to improve glucose excursions in the Rap1CNSV12 mice, while it did in control mice, as confirmed by area under the curve (AUC) analysis (Fig. 3, E and F). These findings suggest that neural activation of Rap1 sufficiently impairs glucose balance and nullifies metformin’s ability to improve it.
Fig. 3. Neuronal expression of a constitutively active form of Rap1 (Rap1V12) diminishes the glycemic effects of metformin.
(A) Strategy for generating conditional Rap1V12 overexpression mice (Rosa26-LSL-Rap1V12 mice). The construct containing “CAG promoter-loxP-Transcription blocker-loxP-RAP1V12-IRES-eGFP” was inserted into the Rosa26 locus. Upon Cre-mediated recombination, the transcription blocker is removed, enabling Rap1V12 expression. (B) Increased Rap1 activity in the hypothalamus of Rap1CNSV12 (Rosa26-LSL-Rap1V12; CaMKCre) mice. Rap1 activity was measured as the amount of active GTP-bound Rap1 using a Rap1 pull-down assay. (C) Body weight comparison between control and Rap1CNSV12 mice showing no significant difference (n = 8). (D) Fasting blood glucose levels in control and Rap1CNSV12 mice. (E) GTT in control and Rap1CNSV12 mice after vehicle (Veh) or metformin (Met) treatment (150 mg/kg, ip) 30 min before glucose administration (1.5 g/kg). Metformin significantly improved glucose tolerance in control mice but not in Rap1CNSV12 mice (n = 4). (F) AUC analysis of the GTT data shown in (E). Littermate controls were used in all experiments. Statistical analysis was performed using one-tailed Student’s t test (B); two-tailed Student’s t test [(C) and (D)]; three-way repeated-measures ANOVA (time × treatment × genotype) followed by uncorrected Fisher’s LSD test (E); and two-way ANOVA followed by Šídák’s multiple-comparison test (F). *P < 0.05, ***P < 0.001, and n.s. = not significant. Asterisks indicate metformin versus its paired vehicle within the same genotype at each time point in (E).
Ventromedial hypothalamic nucleus steroidogenic factor 1 neurons that respond to metformin in a Rap1-dependent manner
We next sought to determine the brain site(s) that may potentially mediate the therapeutic effects of metformin in the brain. Because the hypothalamus is the site for Rap1-mediated glycemic control (40), we systematically analyzed the expression of c-Fos, a surrogate marker of neuronal activity, in the hypothalamus of mice receiving ICV metformin. We found that c-Fos was robustly increased in the ventromedial nucleus of the hypothalamus (VMH), in animals treated with ICV metformin at concentrations as low as 1 μg (Fig. 4A), which is consistent with a previous study showing metformin-stimulated c-Fos induction in the VMH (47). This effect was not observed in the adjacent hypothalamic nuclei that are known to regulate metabolism, including the dorsomedial hypothalamus (DMH) or the arcuate nucleus (ARC) (Fig. 4B), indicating that metformin selectively activates neurons in the VMH, where neurons expressing steroidogenic factor 1 (SF1) reside (50). These neurons could be a potential target for metformin action because they are well established for glycemic regulation and because Rap1 also determines blood glucose levels via its action in VMH SF1 neurons (40). Supporting this in vivo observation, electrophysiological recordings of VMH SF1 neurons in brain slices demonstrate that metformin (1 to 100 μM) depolarized 36 of 47 (76.6%) SF1 neurons (Fig. 4, C to E). Metformin dose-dependently depolarized the resting membrane potential of VMH SF1 neurons and modestly increased their spontaneous action potential firing frequency (2.8 ± 2.1 Hz) (Fig. 4, C to E) starting at 1 μM. Furthermore, we found that metformin-induced neuronal activation of VMH SF1 neurons was abrogated in most SF1 neurons lacking Rap1 (Fig. 4, F to H). Notably, the effective metformin concentrations are within the range of concentrations found in the cerebrospinal fluid and brain (~0.5 to 10 μM) (51–55) and hypothalamus (4.4 μM; fig. S2C) of animals and humans with clinically relevant serum metformin levels. Collectively, these results implicate VMH SF1 neurons as the site of central Rap1-mediated metformin action.
Fig. 4. Metformin activated VMH SF1 neurons in a Rap1-dependent manner.
(A and B) c-Fos induction by ICV metformin. HFD-fed C57BL/6 mice (13 weeks of HFD) received daily ICV injections of metformin (1 μg) or vehicle (1 μl) for 3 days. Four hours after the last injection, brains were immunostained for c-Fos expression (A). 3V, third ventricle. (B) Quantification of the number of c-Fos–positive neurons (n = 2 to 3) in (A). (C to E) Metformin increases action potential frequency and depolarizes the resting membrane potential of VMH SF1 neurons. (C) Representative action potential firing traces after treatment with metformin (100 μM) in VMH SF1–positive neurons of the hypothalamic slice from tdTomato/SF1 Cre mice. Quantification of changes in resting membrane potential (D) and spontaneous action potential firing frequency (E) of VMH SF1 neurons (n = 15 to 17) after treatment with increasing doses of metformin. (F to H) Rap1 is required for the metformin response. (F) Representative action potential firing traces of VMH SF1 neurons in hypothalamic slices from control and SF1-specific Rap1-knockout slices with or without metformin (1 μM). Rap1 deletion abolishes metformin-induced increases in firing frequency (G) and membrane depolarization (H). *P < 0.05, **P < 0.01, ***P < 0.001, and ***P < 0.0001 by t test [(B), (G), and (H)] and one-way ANOVA followed by Dunnett’s multiple-comparison test [(D) and (E)].
The VMH neurons mediate the Rap1-dependent therapeutic effects of metformin
Having observed that metformin affects VMH SF1 neurons and inhibits Rap1, we hypothesized that the antidiabetic effects of metformin may arise from Rap1 inhibition in VMH neurons. To test this hypothesis, we used both loss-of-function and gain-of-function approaches. First, we tested whether the loss of Rap1 in SF1 cells would recapitulate the glucose-lowering effect of metformin and negate the ability of metformin to further reduce blood glucose levels. To achieve this, we generated and used SF1 cell–specific Rap1 knockout mice [Rap1aflox/flox/Rap1bflox/flox mice/SF1-Cre (Rap1∆SF1) (40)]. In agreement with our previous findings (40), Rap1∆SF1 mice exhibited significantly reduced blood glucose levels compared to littermate controls (Fig. 5, A and B). The magnitude of glucose reduction in Rap1∆SF1 mice was comparable to that achieved by either acute (50 mg/kg, ip, for 4 hours; Fig. 5A) or chronic (50 mg/kg per day, ip, for 4 weeks; Fig. 5B) metformin treatment in control mice. Notably, the absence of Rap1 in SF1 cells abolished the acute glucose-lowering ability of metformin (Fig. 5A), and chronic metformin treatment failed to further decrease blood glucose concentrations in these mice (Fig. 5B). These results suggest that Rap1 deficiency in SF1 neurons phenocopies the glucose-lowering effects of metformin, supporting the notion that Rap1 inhibition in VMH SF1 neurons is a key mechanism underlying the antidiabetic effects of metformin.
Fig. 5. Rap1 in SF1 neurons mediates metformin’s antidiabetic effects.
(A and B) Rap1∆SF1 mice exhibited reduced blood glucose levels comparable to those of metformin-treated mice, with no additional reduction following acute or chronic treatment. (A) Blood glucose levels in response to acute metformin treatment (50 mg/kg, ip) or vehicle in Rap1∆SF1 and control mice (n = 5 to 8; 22 weeks of HFD) over 4 hours. (B) Blood glucose levels in Rap1∆SF1 or control mice (n = 8; 40 weeks of HFD) receiving vehicle (saline; intraperitoneally, for 3 days) or metformin (50 mg/kg, ip, for 4 days). (C to G) VMH expression of constitutively active Rap1 significantly attenuated metformin’s glycemic effects. (C) Schematic representation of adeno-associated virus injection into the VMH. (D) Representative image of AAV-Rap1V12 viral injection into the VMH. (E) Mice expressing Rap1V12 in the VMH showed an attenuated glucose-lowering response to chronic metformin treatment (150 mg/kg, orally, for 4 days; n = 6 to 7; 13 weeks of HFD). (F) AUC of the data in (E). (G) Metformin (150 mg/kg, ip, for 24 hours; n = 6 to 7; 13 weeks of HFD) acutely reduced blood glucose in controls but not in VMH Rap1V12 mice. (H) Metformin (150 mg/kg, ip; n = 9 to 11; 37 weeks of HFD) improved glucose tolerance in HFD-fed controls, but this effect was significantly attenuated in Rap1V12 mice. (I) AUC of GTT in (H). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by two-way ANOVA followed by Tukey’s multiple-comparison test [(A) and (B)]; two-way ANOVA followed by uncorrected Fisher’s LSD test in (E); unpaired two-tailed t tests in (F); two-way ANOVA followed by uncorrected Fisher’s LSD test (G); and three-way repeated-measures ANOVA (time × treatment × genotype) followed by Šídák’s post hoc test (H). Asterisks indicate significant differences between metformin and vehicle within the same genotype in (H).
To further substantiate our model, we performed a gain-of-function study by expressing a constitutively active Rap1 mutant, Rap1V12, bilaterally in the VMH via AAV-mediated delivery (Fig. 5, C and D). We reasoned that if metformin exerts its effects by inhibiting Rap1 in the VMH, then the glucose-lowering effects of metformin should be abolished in VMH Rap1V12–expressing mice because Rap1V12 is locked in its GTP-bound state (56), rendering it resistant to inactivation by metformin. We observed that metformin’s glucose-lowering effect was blunted in the VMH Rap1V12–expressing mice compared to littermate controls during both acute and chronic studies (Fig. 5, E to G) and that its ability to enhance glucose tolerance was markedly impaired in these animals (Fig. 5, H and I). Notably, the AAV-Rap1V12 mice were not hyperglycemic during the metformin experiments, although they developed hyperglycemia over a 2-month period (fig. S7), likely due to daily glucose fluctuations and the timing of the metformin experiments (fig. S7).
Collectively, these loss-of-function and gain-of-function studies provide compelling evidence that the therapeutic effects of metformin in lowering blood glucose are mediated by the inhibition of Rap1 in VMH SF1 neurons. These results highlight the VMH as a pivotal neural substrate for the therapeutic effects of metformin.
DISCUSSION
Metformin’s molecular mechanism remains elusive even after 60 years of successful clinical use. While many models have been proposed for its glucoregulatory effects (8–12), comparatively less attention has been paid to its role in the brain. Our findings offer compelling evidence for the neural aspect of metformin’s action.
Our findings illustrate a neural model of metformin action whereby metformin inhibits Rap1 in the brain, specifically in VMH SF1 neurons, which, in turn, leads to reduced hyperglycemia. Several lines of evidence support this model. First, we observed that mice with Rap1 deficiency in the forebrain do not respond to metformin. These mice did not lower blood glucose levels or improve glucose tolerance when treated with metformin, but they did respond to other US Food and Drug Administration (FDA)–approved antidiabetic drugs. In addition, this neural mechanism is necessary for low-dose clinically relevant doses but is bypassed by suprapharmacological high doses of metformin that are less therapeutically relevant, highlighting the pharmacological significance of the mechanism. Low-dose metformin, when administered centrally, inhibits hypothalamic Rap1 and lowers blood glucose levels. Metformin activates VMH SF1 neurons that require Rap1. In line with this, manipulation of Rap1 activity in VMH SF1 neurons, such as genetic deletion or forced expression of active Rap1, results in corresponding changes in blood glucose levels and abolishes the ability of metformin to correct hyperglycemia, as both manipulations are likely to prevent Rap1 activity from being altered by metformin treatment. This neural model offers a different perspective compared to previous mechanisms, which focus mainly on peripheral actions.
If the therapeutic effect of metformin is indeed mediated by the Rap1-dependent mechanism in the brain, then peripheral administration of metformin should achieve sufficient brain concentrations to trigger this pathway. The literature demonstrates the ability of metformin to cross the blood-brain barrier and reach concentrations of ~0.5 to 10 μM in cerebrospinal fluid and brain tissue in both humans and animals with clinically relevant circulating metformin concentrations of 10 to 40 μM (45, 46, 51–55). In our study, a dose of 150 mg/kg of metformin led to a serum concentration of 22 and 4.6 μM in the hypothalamus 4 hours after injection. These findings suggest that the brain is exposed to approximately 1/10 the concentration of metformin present in the bloodstream. Corroborating these observations, our data indicate that as little as 1 μg of metformin in the brain is sufficient to correct hyperglycemia, a dose that is 3 to 4 orders of magnitude lower than that required in peripheral tissues to achieve a comparable glycemic effect. Moreover, very low concentrations of metformin (<1 to 5 μM) were observed to activate SF1 neurons and decrease Rap1 activity. These findings collectively suggest that while the brain is exposed to markedly lower concentrations of metformin than the systemic circulation, the Rap1-dependent neural mechanism exhibits greater sensitivity, thereby enabling an effective response to low doses of metformin in the brain.
Our study confirmed and extended previous studies by demonstrating that centrally administered metformin can effectively lower glucose levels in mice. Prior studies have shown that metformin, when administered directly to the brain, can alter glucose balance (48, 57, 58). However, the results of these studies have been inconsistent (48, 57), likely, in part, due to the use of high doses of metformin (20 to 100 μg per brain), which led to adverse effects such as anorexia, weight loss, and lethal neurotoxic effects (47, 48), all of which are likely to affect glucose balance. Using low doses of metformin, we were able to avoid these negative effects and observed that ICV administration effectively lowered blood glucose levels without causing anorexia or neurotoxicity. These findings further support the notion that the brain plays a key role in the antidiabetic effects of metformin.
We emphasize that our findings do not exclude the possibility that metformin directly affects peripheral tissues such as the liver and intestine. Our data indicate that a Rap1-mediated mechanism in the brain is necessary for the effects of low-dose metformin but not for higher doses (>200 mg/kg in mice). This differential response is likely due to the significantly higher concentrations of metformin in the liver and intestine (liver: >100 μM; intestine: >1 mM) compared to the brain (~1 to 10 μM) (8, 10, 11). These higher concentrations may activate different mechanisms in peripheral tissues that require higher doses to elicit their effects (11). At higher exposures (≥ 250 mg/kg), metformin triggers classic hepatic mechanisms, such as AMP-mediated inhibition of adenylate cyclase/cAMP signaling (25) and direct inhibition of fructose 1,6-bisphosphatase (FBP1) (27). When hepatic metformin concentrations reach the hundreds of micromolar ranges, AMP levels rise markedly and directly inhibit both hepatic adenylate cyclase and FBP1 (10, 34), likely bypassing the CNS Rap1 pathway. Our findings imply that clinically relevant, low-dose metformin acts mainly through the VMH Rap1 pathway, whereas supratherapeutic doses activate additional peripheral mechanisms.
While this study focuses on the central mechanisms underlying the glucose-lowering effects of metformin, clarifying the downstream peripheral pathways is a crucial next step. Our data suggest that these effects are mediated by Rap1 signaling in the brain, primarily through VMH SF1 neurons. Prior work from our laboratory demonstrated that deleting VMH Rap1 enhances skeletal muscle glucose uptake, reduces hepatic glucose output, and improves peripheral insulin sensitivity (40). Together, these findings indicate that central Rap1 inhibition, triggered by ICV metformin, orchestrates multitissue glucose-lowering effects through mechanisms that are distinct from the canonical peripheral actions of metformin. Thus, probing tissue-specific mediators is key to linking central metformin action to peripheral glycemic control, including hepatic glucose production. Future studies will offer definitive insights into the peripheral effects of central metformin. To our knowledge, our current study provides the first evidence that metformin’s glucose-lowering effects originate in the brain and are dependent on Rap1.
A key limitation of our study is that we rely on blood glucose as the primary measure for metformin efficacy in neural Rap1–deficient mice. These mice frequently show reduced baseline glycaemia (40, 41). This creates a possible floor effect: Already low glucose levels leave little room for metformin to act, masking any need for brain Rap1. While Rap1ΔCNS mice (fig. S1K) and Rap1ΔSF1 mice (Fig. 5, A and B) did exhibit higher glucose levels than controls, multiple Rap1ΔCNS cohorts (Fig. 1, A and F, and fig. S1, A, G, and H) had glucose levels indistinguishable from littermates (fig. S1K). However, even in these glycaemia-matched groups, metformin failed to lower glucose in Rap1ΔCNS mice but remained effective in controls (e.g., Fig. 1A and fig. S1A). Variability in baseline glycaemia likely reflects differences in HFD onset or study length (fig. S7). One could argue that Rap1ΔCNS mice still respond to insulin or GLP-1 merely because those drugs are more potent glucose-lowering agents than metformin. However, rosiglitazone, whose glucose-lowering effect is comparable to metformin’s (fig. S1E and table S1), reduced glycemia equally in Rap1ΔCNS and control mice. Thus, loss of neural Rap1 specifically impairs metformin’s mode of action, regardless of baseline glycemia or drug potency.
Among the direct regulators of Rap1, EPAC2 (exchange protein activated by cAMP 2) is a major GTP–guanosine diphosphate exchange factor that activates Rap1 in the brain (41, 59). In our previous work, ICV delivery of an EPAC2 inhibitor lowered blood glucose and improved glucose tolerance through Rap1-dependent mechanisms (40). Recent single-cell RNA sequencing profiles reveal EPAC2 transcripts in SF1-positive VMH neurons. This raises the intriguing possibility of EPAC2 acting as a direct Rap1 activator in these cells. Further studies are needed to experimentally confirm whether EPAC2 directly activates Rap1 or mediates metformin’s effects in vivo. Nevertheless, current evidence shows that perturbations in CNS EPAC-Rap1 signaling significantly affect whole-body glucose metabolism, and inhibition of this pathway with metformin improves glycemic control.
The low-dose dependency of the Rap1-mediated neural mechanism is intriguing in light of a recent report by Ma et al. (60) identifying a lysosomal AMPK pathway that is exclusively responsive to and mediated by low-dose metformin. They showed that low-dose metformin activates a lysosomal signaling complex (PEN2-vATPase-AMPK) in the liver and intestine to produce therapeutic effects (60). Rap1 is also known to localize to the lysosomal membrane (61, 62) where the complex resides. These observations may raise the intriguing possibility that Rap1 signaling may coordinate with the lysosomal complex to mediate antidiabetic effects via the hypothalamus, which is only exposed to low concentrations of metformin. Future studies are warranted to explore this potential link in the hypothalamus to learn more about the central glucoregulatory mechanisms targeted by metformin. In summary, our findings provide a previously unknown neural mechanism mediating the antidiabetic effect of metformin.
MATERIALS AND METHODS
Animals
All animal care and procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press; 2011) and were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine (AN-6076). Mice were used for all experiments. C57BL/6 mice, C56BL/6J (JAX: 000664), SF1Cre (JAX: 012462), Ai9 (JAX: 007905), and ob/ob (JAX: 000632) were obtained from the Jackson Laboratory. Rap1aflox/flox/Rap1bflox/flox mice were provided by A. Morozov (63). Rap1ΔCNS mice were generated by crossing male Rap1aflox/flox; Rap1bflox/flox mice with female Rap1aflox/flox; Rap1bflox/flox; CaMKIIα Cre driver (line 159) (49). Rap1ΔSF1 mice were produced by breeding Rap1 double-floxed males to SF1Cre females. This breeding scheme produced 50% mice with deletion of Rap1a and Rap1b and 50% control mice with floxed Rap1a and Rap1b genes. To identify SF1 Cre-expressing neurons in the brain, the SF1-Cre line was mated with a Cre-dependent tdTomato expression reporter line, Ai9 mice. We used only female CaMKIICre and SF1Cre mice to obtain cohorts because both express Cre in the testis, and the male germline can produce offspring that carry the Cre allele in all tissues. For Rap1 knockout mouse studies, littermate controls were used. All mice were maintained under a 12-hour light/12-hour dark cycle condition (lights on 6 a.m. to 6 p.m.) and in a temperature-controlled environment at 22° to 24°C with ad libitum access to water and normal diet (LabDiet, Pico Lab 5V5R) or HFD (60% kcal fat; Research Diets, D12492). Before each experimental treatment, mice were subjected to daily handling and mock injections for 2 to 5 days to minimize stress induced by the procedures.
Generation of Rosa26-Rap1V12-LSL mice
Rap1V12 knock-in (Rosa26-LSL-Rap1V12) mice were generated by knocking in a constitutively active form of the Rap1 (Rap1V12) gene at the Rosa26 locus of C57BL/6 mice using CRISPR-Cas–mediated genome engineering at Cyagen Biosciences. Briefly, a donor vector containing a CAG-loxP-PGK-Neo-STOP-loxP-Rap1V12-IRES-EGFP-polyA cassette flanked by 1.1-kb/1.2-kb Rosa26 homology arms was constructed. The donor plasmid (50 ng/μl) was coinjected with Cas9 mRNA (100 ng/μl) and a single-guide RNA targeting Rosa26 (5′-CTCCAGTCTTTCTAGAAGATGGG-3′; 100 ng/μl) into fertilized C57BL/6J zygotes. Founder (F0) animals were screened by long-range polymerase chain reaction (PCR) across both junctions, confirmed by DNA sequencing of the G12V mutation, and validated with Southern blotting analysis using 5′ and 3′ probes. Germ-line–positive F1 mice were intercrossed to obtain homozygous Rosa26-Rap1V12-LSL animals, routinely genotyped with a 249–base pair knock-in PCR. Conditional expression of Rap1V12 was achieved by crossing Rosa26-LSL-Rap1V12 mice with the CaMKIIα-Cre driver, thereby excising the loxP-flanked STOP cassette and generating Rap1V12 gain-of-function (Rap1CNSV12) animals.
Stereotaxic surgery
Cannula implantation: Mice were anesthetized with isoflurane and positioned in a stereotaxic frame. For cannula implantation, the skull was exposed, and a single 26-gauge stainless steel guide cannula (PlasticsOne, C315GS-5-SPC) was implanted in the lateral ventricle (LV; −0.45 mm from bregma, ±0.9 mm lateral, and −2.5 mm from the skull). The cannula was secured to the skull with screws and dental cement. After ICV cannulation, the mice were housed singly and allowed to recover for at least 1 week.
AAV injection
To express human Rap1V12 in the hypothalamus, C57BL/6J male mice that were fed on HFD for 4 months received stereotaxic injections of AAV-DJ-Rap1V12–GFP (green fluorescent protein) (AAV-Rap1V12) or AAV-DJ-GFP (control AAV) in both sides of the hypothalamus [200 ng per side, −1.7 mm from bregma, ± 0.33 mm lateral, and −5.7 mm from the skull based on Franklin and Paxinos’ Mouse Brain Atlas (64)]. Three months after the AAV-RapV12 injection, we performed metformin experiments to ensure Rap1V12-induced hyperglycemia during HFD feeding. This timeline is based on our previous study (40), which showed that AAV-mediated expression of Rap1V12 takes ~12 weeks to significantly increase HFD-induced hyperglycemia. After completing the physiological studies, the viral injection sites were confirmed by capturing images of viral GFP fluorescence using a Leica 5500 OptiGrid fluorescence microscope.
Metformin treatment of mice
Acute glucose-lowering assay
HFD-fed Rap1ΔCNS male mice and their controls received a single dose of an antidiabetic drug (n = 6 to 10): metformin (150 mg/kg, ip; Sigma-Aldrich, PHR1084), exendin-4 (0.1 mg/kg, ip; Sigma-Aldrich, E7144-0.5MG), glibenclamide (1 mg/kg, ip; Sigma-Aldrich, PHR1287-1G), dapagliflozin [20 mg/kg, per os (p.o.); Selleck Chemicals, S1548], insulin (1 U/kg, ip; Eli Lilly, Humulin R), or a matched volume of vehicle [saline, Dulbecco’s phosphate buffered saline (dPBS), or dimethyl sulfoxide] corresponding to the drugs. Age- and weight-matched cohorts were used. Blood glucose was measured at the indicated time points after the metformin administration through tail vein bleeding using a OneTouch Glucometer. In the dose-response study, HFD-fed Rap1ΔCNS mice and their control group received intraperitoneal bolus injections of metformin at doses of 50, 100, or 150 mg/kg or vehicle. Blood glucose levels were measured at 0, 2, 4, 6, 8, and 24 hours after metformin administration using tail vein bleeding and a OneTouch Glucometer. To calculate the AOC, we subtracted the area below or above the baseline value to provide a more accurate measure of the glucose-lowering activity of the antidiabetic agents, independent of differences in baseline glucose levels, as recommended by Virtue and Vidal-Puig (65). The AOC was determined using GraphPad Prism 10.3.0. For the Rap1ΔSF1 mouse study, Rap1ΔSF1 mice were fed on HFD for 5 months to induce DIO. They received a single intraperitoneal injection of metformin (50 mg/kg). Blood glucose was measured at 4 hours after the metformin administration through tail vein bleeding using a OneTouch Glucometer.
Chronic glucose-lowering assay
HFD-fed Rap1ΔCNS female mice and their controls received daily single intraperitoneal injections of metformin (100 mg/kg, ip), rosiglitazone (10 mg/kg, ip), or an equal amount of vehicle corresponding to the drugs. Daily blood glucose was measured via tail vein sampling using a OneTouch Glucometer before daily drug injection. Note: Glucose lowering by rosiglitazone was observed only after chronic treatment in mice. For the Rap1ΔSF1 mouse study, mice maintained on an HFD for 10 months were randomized into two groups. One group received a daily injection of a vehicle control (water) for 7 days. The other group received the vehicle for the first 3 days and then switched to an intraperitoneal injection of metformin at a dose of 50 mg/kg for the remaining 4 days. Blood glucose levels were measured daily using a OneTouch Glucometer via tail vein sampling. In the Rap1V12 mouse study, Rap1V12 mice were generated by bilateral injection of AAV-DJ-Rap1V12-GFP into the VMH of C57BL/6J male mice at 3 months of age. AAV-DJ-GFP was injected as a control. After AAV injection, mice were subjected to HFD for 3 months. Both groups of mice received a daily oral gavage of 150 mg/kg metformin for 4 days. Blood glucose levels were measured daily using a OneTouch Glucometer with tail vein sampling.
GTT after metformin
For GTTs after metformin treatment, Rap1ΔCNS female mice and their controls that had been maintained on an HFD for 3 months were fasted for 16 hours. They were then given a single intraperitoneal dose of metformin (50, 100, or 250 mg/kg). After 30 min, the mice were challenged with an intraperitoneal administration of glucose (1.5 g/kg of body weight). Blood glucose was measured via tail vein sampling using a OneTouch Glucometer. For the Rap1V12 mouse study, C57BL/6J male mice were maintained on an HFD for 5 months to induce DIO. They were then randomized to receive bilateral injections of either AAV-DJ-Rap1V12-GFP or AAV-DJ-GFP (n = 9 to 11). Three months later, both groups of mice received either metformin (150 mg/kg, ip) or vehicle 30 min before GTT (1.5 mg/kg of glucose, ip). Blood glucose levels were measured using a OneTouch Glucometer by tail vein sampling. The area under the glucose curve (AUC) was calculated and plotted using GraphPad Prism 10.3.0.
ICV metformin treatment
HFD-fed male mice were housed singly for at least 1 week. On experimental days, the mice were infused with 1 μl of each solution: vehicle or metformin (1 to 30 μg per mouse). Blood glucose, food consumption, and body weight were measured at the indicated time points during the metformin treatment. To examine the effect of food on the glucose-lowering effect of metformin, food was removed from the cages after the ICV injection of metformin. For the ICV metformin study in DIO/STZ mice, C57BL/6J male mice were fed HFD for 8 months to induce DIO and underwent LV cannulation. Two weeks later, they received three consecutive daily intraperitoneal injections of 40 mg of STZ (Sigma-Aldrich) (DIO/STZ) to induce moderate hyperglycemia (~150 to 200 mg/dl). One week later, mice were treated with ICV metformin at a dose of 3 μg per brain. Blood glucose levels, food consumption, and body weight were recorded at the indicated time points.
ICV metformin treatment for c-Fos studies
After inducing DIO in C57BL/6J male mice by feeding them an HFD for 3 months, the mice underwent LV cannulation. They then received three consecutive daily ICV injections of either a low dose of 1 μg metformin or vehicle. Two hours after the final injection, food was removed, and the mice were subjected to transcardial perfusion under deep anesthesia, followed by immunohistochemistry for c-Fos.
Histology
Brains were perfused with PBS, followed by 4% formaldehyde in PBS. Brains were extracted, postfixed in 10% formalin, infiltrated with 20% sucrose, and sectioned at 25-μm thickness. The sections were rinsed six times for 5 min each in PBS and then treated with 0.3% hydrogen peroxide in 0.25% Triton X-100 in PBS (PBT) for 30 min. The sections were then incubated with c-Fos antibody (1:1000; Synaptic Systems, 226003) or GFP antibody (1:1000; Aves Labs, NC9510598) in 3% normal donkey serum in PBT with 0.02% sodium azide for 48 to 72 hours. They were then treated with biotinylated secondary antibody against rabbit immunoglobulin G (1:1000; Vector Laboratories, BA-1000) or the avidin-biotin-peroxidase complex kit (1:1000; Vector Laboratories, VECTASTAIN Elite ABC kit). Immunoreactivities were visualized with fluorescence-conjugated streptavidin (1:500; Life Technologies, Thermo Fisher Scientific). Last, images were analyzed using a Leica 5500 OptiGrid fluorescence microscope.
ICV injection of metformin in DIO mice
C57BL/6J male mice were fed HFD for 3 months to induce obesity. After cannulation, the mice received an ICV injection of 3 μg of metformin. Four hours after injection, the mice were euthanized, and their brains were removed and immediately snap frozen in liquid nitrogen and stored at −80°C for the Rap1 pull-down assay. Brain tissue was homogenized in Pierce IP lysis buffer containing 25 mM tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 5% glycerol, supplemented with a 1× protease and phosphatase inhibitor cocktail. Tissue lysis was performed by gentle sonication using a probe sonicator. Lysates were centrifuged at 16,000g for 15 min at 4°C. The resulting supernatant was collected for use in the subsequent Rap1 pull-down assay.
Rap1 pull-down assay
Briefly, the protein concentration was determined using BCA protein assay reagent (Pierce, 23225) with bovine serum albumin as a standard. Equal amounts of protein were subjected to affinity precipitation of GTP-Rap1 using the active Rap1 pull-down and detection kit (Thermo Fisher Scientific, 16120). The amount of Rap1 was assessed by performing Western blotting with the supplied antibody (1:1000). To assess the levels of total Rap1 or β-actin, cell lysates were applied directly to Western blotting without a pull-down assay.
Electrophysiology
Male SF1-Cre/tdTomato or Rap1afl/fl/Rap1bfl/fl/SF1-Cre/tdTomato mice (10 to 12 weeks old) were used for electrophysiology recordings as described previously (66). Briefly, mice were deeply anesthetized with isoflurane and transcardially perfused with a modified ice-cold sucrose-based cutting solution (pH 7.3) containing 10 mM NaCl, 25 mM NaHCO3, 195 mM sucrose, 5 mM glucose, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM Na-pyruvate, 0.5 mM CaCl2, and 7 mM MgCl2, bubbled continuously with 95% O2 and 5% CO2 (66). The mice were then decapitated, and the entire brain was removed and immediately submerged in the cutting solution. Slices (250 μm) were cut with a HM 650V vibratome (Thermo Fisher Scientific). Three to four brain slices containing the VMH were obtained for each animal. The slices were recovered at 34°C for 1 hour and then maintained at room temperature in artificial cerebrospinal fluid (aCSF; pH 7.3) containing 126 mM NaCl, 2.5 mM KCl, 2.4 mM CaCl2, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 5.0 mM glucose, and 21.4 mM NaHCO3 saturated with 95% O2 and 5% CO2 before recording.
Slices were transferred to a recording chamber and allowed to equilibrate for at least 10 min before recording. The slices were superfused at 34°C in oxygenated aCSF at a flow rate of 1.8 to 2 ml/min. VMH SF1 neurons were visualized using epifluorescence and infrared–differential interference contrast imaging. Patch pipettes with resistances of 3 to 5 megohms were filled with intracellular solution (pH 7.3) containing 128 mM K-gluconate, 10 mM KCl, 10 mM Hepes, 0.1 mM EGTA, 2 mM MgCl2, 0.05 mM Na-GTP, and 0.05 mM Mg–adenosine 5′-triphosphate. Recordings were made using a MultiClamp 700B amplifier (Axon Instruments), sampled using Digidata 1440A, and analyzed offline with pClamp 10.3 software (Axon Instruments). Series resistance was monitored during the recording, and the values were generally <10 megohms and were not compensated. The liquid junction potential was +12.5 mV and was corrected after the experiment. Data were excluded if the series resistance increased markedly during the experiment or if there was no overshoot for the action potential. Currents were amplified, filtered at 1 kHz, and digitized at 10 kHz. Currents were amplified, filtered at 1 kHz, and digitized at 20 kHz. The current clamp was used to test neuronal firing and resting membrane potential before and after vehicle or metformin (at 1, 10, or 100 μM) perfusion treatment. Values for resting membrane potential and firing frequency were averaged within a 2-min bin before and after metformin treatment.
Quantitation of metformin
Sample collection
Male Rap1ΔCNS (Rap1afl/fl/Rap1bfl/fl/CaMKCre) and control (Rap1afl/fl/Rap1bfl/fl) mice maintained on an HFD (60% kcal fat for 57 weeks; Research Diets, D12492) were used for the study. Both groups of mice received a single injection of metformin (150 mg/kg, ip) or vehicle. The blood, liver, fat, and hypothalamus samples were collected at 4 hours posttreatment. The serum was prepared by centrifuging the blood samples at 15,000g for 15 min. To prevent the potential contamination of metformin from circulating blood, mice were transcardially perfused with a saline solution following blood collection.
Sample preparation
Twenty microliters of serum was added to 60 μl of ice-cold methanol containing internal standard (IS; 0.1 μM agomelatine), vortexed, and centrifuged at 15,000 relative centrifugal force for 10 min at 4°C. Fifty microliters of each supernatant was transferred into a sample vial for the Thermo Fisher Scientific TSQ Quantis mass spectrometer (MS) coupled with a Thermo Fisher Scientific Vanquish ultrahigh-performance liquid chromatography (UHPLC-TSQ MS) analysis. For tissue samples, 10 mg of tissue was mixed with a 4× volume of 50% methanol-water, added with beads, and thoroughly homogenized with a bullet blender. A 20-μl aliquot of tissue homogenate was then added to 100 μl (for liver) or 60 μl (for hypothalamus and fat) of ice-cold methanol containing IS, vortexed, and centrifuged at 15,000 rcf for 15 min at 4°C. Sixty microliters of supernatant was transferred into a sample vial for analysis. To quantify metformin in the liquid or tissue samples, methanol stock solutions with different concentrations of metformin were spiked into blank mouse plasma or tissue homogenate, respectively, and the calibration samples were prepared in the same way as actual samples. The linear ranges of the calibration curves for all liquid or tissue samples were 10 to 10,000 nM with good linearity [all coefficients of determination (R2) > 0.98; weight 1/x2].
Liquid chromatography–MS/MS analysis
All samples were analyzed with the UHPLC-TSQ MS. The analyte and the IS were separated on an XDB-C18 Agilent column (4.6 mm by 50 mm; 3.5 μm) and eluted by a water-acetonitrile mobile phase system (both containing 0.1% formic acid) with isocratic 70% acetonitrile in a 6-min run. The flow rate was set at 0.3 ml/min. The column temperature was 40°C, and the injection volume was 3 μl. Metformin and agomelatine were monitored under the selected reaction monitoring mode coupled with a positive electrospray ionization source. The quantification ion transition was 130.11 → 60.05 for metformin and 244.10 → 185.05 for agomelatine. The ion spray voltage was 3500 V. High-purity nitrogen was used as the sheath gas (50 arbitrary units), auxiliary gas (10 arbitrary units), and sweep gas (1.0 arbitrary units), and high-purity argon was used as the collision gas. The temperatures of ion transfer tube and vaporizer were 325° and 350°C, respectively.
Statistics
Data were presented as the mean ± SEM. Statistical analyses were performed using GraphPad Prism 10.3.0 with unpaired Student’s t test or one- or two-way analysis of variance (ANOVA), followed by appropriate post hoc tests. A significance level of P < 0.05 was used to determine statistical significance (67).
Acknowledgments
We acknowledge P. Huang for technical assistance. We especially thank P. Chen for providing AAV-Rap1V12 and A. Morozov for providing Rap1a/Rap1b-floxed mice.
Funding: This work was funded by the National Institutes of Health grant nos. R01DK136627 (C.W.), R01DK121970 (F.L.), R01DK093587 (Y.X.), R01DK101379 (Y.X.), P30-DK079638 (M.F.), R01DK104901 (M.F.), and R01DK126655 (M.F.); USDA/ARS 6250-51000-055 (M.F.); the American Heart Association 14BGIA20460080 (M.F.) and 15POST22500012 (K.K.); the American Diabetes Association 1-17-PDF-138 (Y.H.); the Uehara Memorial Foundation (K.K.); the Takeda Science Foundation (K.K.); and the Japan Foundation for Applied Enzymology (K.K.). This project was also supported, in part, by the NMR and Drug Metabolism Core at Baylor College of Medicine (F.L.).
Author contributions: M.F. conceived the study. H.-Y.L., W.L., Y.H., Y.F., K.K., C.W., Y.Y., F.L., Y.X., and M.F. designed the experiments. H.-Y.L., W.L., Y.H., Y.F., K.K., P.H., and F.L. performed the experiments. C.W. and Y.Y. contributed reagents and intellectually assisted with studies with AAVs and microscopes. H.-Y.L., W.L., Y.H., Y.F., K.K., F.L., Y.X., and M.F. analyzed the data and interpreted the results. Most of the manuscript was written by M.F. with some help from W.L., Y.H., F.L., and Y.X. All authors approved the final version of the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
Table S1
REFERENCES AND NOTES
- 1.Hesse E., Taubmann G., Die wirkung des biguanids und seiner derivate auf den zuckerstoffwechsel. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 142, 290–308 (1929). [The effect of biguanide and its derivatives on carbohydrate metabolism]. [Google Scholar]
- 2.Slotta K. H., Tschesche R., Über biguanide, II.: Die blutzucker-senkende wirkung der biguanide. Ber. Dtsch. Chem. Ges. 62, 1398–1405 (1929). [On biguanides, II: The blood-sugar-lowering effect of biguanides]. [Google Scholar]
- 3.Diabetes Prevention Program Research Group , Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35, 731–737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fullerton M. D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z. P., O’Neill H. M., Ford R. J., Palanivel R., O’Brien M., Hardie D. G., Macaulay S. L., Schertzer J. D., Dyck J. R., van Denderen B. J., Kemp B. E., Steinberg G. R., Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumvoll M., Nurjhan N., Perriello G., Dailey G., Gerich J. E., Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 333, 550–554 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Coll A. P., Chen M., Taskar P., Rimmington D., Patel S., Tadross J. A., Cimino I., Yang M., Welsh P., Virtue S., Goldspink D. A., Miedzybrodzka E. L., Konopka A. R., Esponda R. R., Huang J. T. J., Tung Y. C. L., Rodriguez-Cuenca S., Tomaz R. A., Harding H. P., Melvin A., Yeo G. S. H., Preiss D., Vidal-Puig A., Vallier L., Nair K. S., Wareham N. J., Ron D., Gribble F. M., Reimann F., Sattar N., Savage D. B., Allan B. B., O’Rahilly S., GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day E. A., Ford R. J., Smith B. K., Mohammadi-Shemirani P., Morrow M. R., Gutgesell R. M., Lu R., Raphenya A. R., Kabiri M., McArthur A. G., McInnes N., Hess S., Paré G., Gerstein H. C., Steinberg G. R., Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 1, 1202–1208 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Foretz M., Guigas B., Viollet B., Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 15, 569–589 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Rena G., Pearson E. R., Sakamoto K., Molecular mechanism of action of metformin: Old or new insights? Diabetologia 56, 1898–1906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaMoia T. E., Shulman G. I., Cellular and molecular mechanisms of metformin action. Endocr. Rev. 42, 77–96 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L., Wondisford F. E., Metformin action: Concentrations matter. Cell Metab. 21, 159–162 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Marshall S. M., 60 years of metformin use: A glance at the past and a look to the future. Diabetologia 60, 1561–1565 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Aroda V. R., Knowler W. C., Crandall J. P., Perreault L., Edelstein S. L., Jeffries S. L., Molitch M. E., Pi-Sunyer X., Darwin C., Heckman-Stoddard B. M., Temprosa M., Kahn S. E., Nathan D. M., G., Diabetes Prevention Program Research , Metformin for diabetes prevention: Insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 60, 1601–1611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flory J., Lipska K., Metformin in 2019. JAMA 321, 1926–1927 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer F., Ipaktchi M., Clauser H., Specific inhibition of gluconeogenesis by biguanides. Nature 213, 203–204 (1967). [DOI] [PubMed] [Google Scholar]
- 16.Hundal R. S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S. E., Schumann W. C., Petersen K. F., Landau B. R., Shulman G. I., Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cusi K., Consoli A., DeFronzo R. A., Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 81, 4059–4067 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Natali A., Ferrannini E., Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 49, 434–441 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E., Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C., The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B., Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Mir M. Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X., Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Owen M. R., Doran E., Halestrap A. P., Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000). [PMC free article] [PubMed] [Google Scholar]
- 24.LaMoia T. E., Butrico G. M., Kalpage H. A., Goedeke L., Hubbard B. T., Vatner D. F., Gaspar R. C., Zhang X. M., Cline G. W., Nakahara K., Woo S., Shimada A., Hüttemann M., Shulman G. I., Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 119, e2122287119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller R. A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M. J., Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madiraju A. K., Erion D. M., Rahimi Y., Zhang X. M., Braddock D. T., Albright R. A., Prigaro B. J., Wood J. L., Bhanot S., MacDonald M. J., Jurczak M. J., Camporez J. P., Lee H. Y., Cline G. W., Samuel V. T., Kibbey R. G., Shulman G. I., Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter R. W., Hughey C. C., Lantier L., Sundelin E. I., Peggie M., Zeqiraj E., Sicheri F., Jessen N., Wasserman D. H., Sakamoto K., Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 24, 1395–1406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey C. J., Mynett K. J., Page T., Importance of the intestine as a site of metformin-stimulated glucose utilization. Br. J. Pharmacol. 112, 671–675 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCreight L. J., Bailey C. J., Pearson E. R., Metformin and the gastrointestinal tract. Diabetologia 59, 426–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu T., Horowitz M., Rayner C. K., New insights into the anti-diabetic actions of metformin: From the liver to the gut. Expert Rev. Gastroenterol. Hepatol. 11, 157–166 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Wang D., Day E. A., Townsend L. K., Djordjevic D., Jørgensen S. B., Steinberg G. R., GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 17, 592–607 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Maida A., Lamont B. J., Cao X., Drucker D. J., Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 54, 339–349 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Duca F. A., Cote C. D., Rasmussen B. A., Zadeh-Tahmasebi M., Rutter G. A., Filippi B. M., Lam T. K., Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 21, 506–511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foretz M., Guigas B., Viollet B., Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 19, 460–476 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonge K. M., D’Alessio D. A., Schwartz M. W., Brain control of blood glucose levels: Implications for the pathogenesis of type 2 diabetes. Diabetologia 64, 5–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brüning J. C., Fenselau H., Integrative neurocircuits that control metabolism and food intake. Science 381, eabl7398 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Grayson B. E., Seeley R. J., Sandoval D. A., Wired on sugar: The role of the CNS in the regulation of glucose homeostasis. Nat. Rev. Neurosci. 14, 24–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz M. W., Seeley R. J., Tschop M. H., Woods S. C., Morton G. J., Myers M. G., D’Alessio D., Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.C. Bernard, De l’origine du sucre dans l’économie animale (Société de biologie, 1848). [On the origin of sugar in animal physiology].
- 40.Kaneko K., Lin H. Y., Fu Y., Saha P. K., De la Puente-Gomez A. B., Xu Y., Ohinata K., Chen P., Morozov A., Fukuda M., Rap1 in the VMH regulates glucose homeostasis. JCI Insight 6, e142545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneko K., Xu P., Cordonier E. L., Chen S. S., Ng A., Xu Y., Morozov A., Fukuda M., Neuronal Rap1 regulates energy balance, glucose homeostasis, and leptin actions. Cell Rep. 16, 3003–3015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham G. G., Punt J., Arora M., Day R. O., Doogue M. P., Duong J. K., Furlong T. J., Greenfield J. R., Greenup L. C., Kirkpatrick C. M., Ray J. E., Timmins P., Williams K. M., Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50, 81–98 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Bailey C. J., Wilcock C., Scarpello J. H., Metformin and the intestine. Diabetologia 51, 1552–1553 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Dowling R. J. O., Lam S., Bassi C., Mouaaz S., Aman A., Kiyota T., Al-awar R., Goodwin P. J., Stambolic V., Metformin pharmacokinetics in mouse tumors: Implications for human therapy. Cell Metab. 23, 567–568 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Gantois I., Khoutorsky A., Popic J., Aguilar-Valles A., Freemantle E., Cao R., Sharma V., Pooters T., Nagpal A., Skalecka A., Truong V. T., Wiebe S., Groves I. A., Jafarnejad S. M., Chapat C., McCullagh E. A., Gamache K., Nader K., Lacaille J.-C., Gkogkas C. G., Sonenberg N., Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat. Med. 23, 674–677 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Thinnes A., Westenberger M., Piechotta C., Lehto A., Wirth F., Lau H., Klein J., Cholinergic and metabolic effects of metformin in mouse brain. Brain Res. Bull. 170, 211–217 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Kim H. J., Zhang X. H., Park E. Y., Shin K. H., Choi S. H., Chun B. G., Kim D. H., Metformin decreases meal size and number and increases c-Fos expression in the nucleus tractus solitarius of obese mice. Physiol. Behav. 110-111, 213–220 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Portela L. V., Gnoatto J., Brochier A. W., Haas C. B., de Assis A. M., de Carvalho A. K., Hansel G., Zimmer E. R., Oses J. P., Muller A. P., Intracerebroventricular metformin decreases body weight but has pro-oxidant effects and decreases survival. Neurochem. Res. 40, 514–523 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Minichiello L., Korte M., Wolfer D., Kuhn R., Unsicker K., Cestari V., Rossi-Arnaud C., Lipp H. P., Bonhoeffer T., Klein R., Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24, 401–414 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Kim K. W., Sohn J. W., Kohno D., Xu Y., Williams K., Elmquist J. K., SF-1 in the ventral medial hypothalamic nucleus: A key regulator of homeostasis. Mol. Cell. Endocrinol. 336, 219–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhari K., Wang J., Xu Y., Winters A., Wang L., Dong X., Cheng E. Y., Liu R., Yang S. H., Determination of metformin bio-distribution by LC-MS/MS in mice treated with a clinically relevant paradigm. PLOS ONE 15, e0234571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lv W. S., Wen J. P., Li L., Sun R. X., Wang J., Xian Y. X., Cao C. X., Wang Y. L., Gao Y. Y., The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 1444, 11–19 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Koenig A. M., Mechanic-Hamilton D., Xie S. X., Combs M. F., Cappola A. R., Xie L., Detre J. A., Wolk D. A., Arnold S. E., Effects of the insulin sensitizer metformin in alzheimer disease: Pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis. Assoc. Disord. 31, 107–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Łabuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopień B., Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 62, 956–965 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Sharma S., Zhang Y., Patel D., Akter K. A., Bagchi S., Sifat A. E., Nozohouri E., Ahn Y., Karamyan V. T., Bickel U., Abbruscato T. J., Evaluation of systemic and brain pharmacokinetic parameters for repurposing metformin using intravenous bolus administration. J. Pharmacol. Exp. Ther. 392, 100013 (2025). [DOI] [PubMed] [Google Scholar]
- 56.Cook S. J., Rubinfeld B., Albert I., McCormick F., RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 12, 3475–3485 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin B. Y., Kim H. J., Oh M. J., Ha N. H., Jeong Y. T., Choi S. H., Lee J. S., Kim N. H., Kim D. H., Metformin acts as a dual glucose regulator in mouse brain. Front. Pharmacol. 14, 1108660 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim Y. B., Park S. H., Kang Y. J., Kim S. S., Kim C. H., Kim S. J., Jung J. S., Ryu O. H., Choi M. G., Suh H. W., Central anti-diabetic action of biguanide and thizolidinediones in d-glucose fed and streptozotocin-treated mouse models. Neurosci. Lett. 528, 73–77 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., Housman D. E., Graybiel A. M., A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Ma T., Tian X., Zhang B., Li M., Wang Y., Yang C., Wu J., Wei X., Qu Q., Yu Y., Long S., Feng J. W., Li C., Zhang C., Xie C., Wu Y., Xu Z., Chen J., Yu Y., Huang X., He Y., Yao L., Zhang L., Zhu M., Wang W., Wang Z. C., Zhang M., Bao Y., Jia W., Lin S. Y., Ye Z., Piao H. L., Deng X., Zhang C. S., Lin S. C., Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603, 159–165 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pizon V., Méchali F., Baldacci G., RAP1A GTP/GDP cycles determine the intracellular location of the late endocytic compartments and contribute to myogenic differentiation. Exp. Cell Res. 246, 56–68 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Mutvei A. P., Nagiec M. J., Hamann J. C., Kim S. G., Vincent C. T., Blenis J., Rap1-GTPases control mTORC1 activity by coordinating lysosome organization with amino acid availability. Nat. Commun. 11, 1416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan B. X., Vautier F., Ito W., Bolshakov V. Y., Morozov A., Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J. Neurosci. 28, 2089–2098 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.G. Paxinos, K. B. J. Franklin, The Mouse Brain in Stereotaxic Coordinates (Elsevier Science, 2007).
- 65.Virtue S., Vidal-Puig A., GTTs and ITTs in mice: Simple tests, complex answers. Nat. Metab. 3, 883–886 (2021). [DOI] [PubMed] [Google Scholar]
- 66.He Y., Xu P., Wang C., Xia Y., Yu M., Yang Y., Yu K., Cai X., Qu N., Saito K., Wang J., Hyseni I., Robertson M., Piyarathna B., Gao M., Khan S. A., Liu F., Chen R., Coarfa C., Zhao Z., Tong Q., Sun Z., Xu Y., Estrogen receptor-α expressing neurons in the ventrolateral VMH regulate glucose balance. Nat. Commun. 11, 2165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boutaleb S., Pouget J. P., Hindorf C., Pelegrin A., Barbet J., Kotzki P. O., Bardies M., Impact of mouse model on preclinical dosimetry in targeted radionuclide therapy. Proc. IEEE 97, 2076–2085 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7
Table S1