Abstract
An expanding area of interest is the utilization of microbe-based components to augment mucosal and systemic immune responses to target antigens. Thus, the aim of the present study was to assess if the flagellin component FljB from Salmonella enterica serovar Typhimurium could act as a mucosal adjuvant and then to determine the cellular mechanism(s) by which FljB mediates its adjuvant properties. To determine if FljB could act as a mucosal adjuvant, mice were immunized by the intranasal (i.n.) route with antigen alone or in conjunction with FljB. Additionally, we assessed how FljB affected the levels of the costimulatory molecules B7-1 and B7-2 on dendritic cells by flow cytometry and determined the functional role these costimulatory molecules played in the adjuvant properties of FljB in vivo. Mice immunized by the i.n. route with antigen and FljB exhibited significantly elevated levels of mucosal and systemic antibody and CD4+-T-cell responses compared to mice given antigen only. Stimulation of dendritic cells in vitro with FljB resulted in a pronounced increase in the surface expression of B7-1 and B7-2. The percentage of dendritic cells expressing B7-2 but not B7-1 increased significantly when stimulated with FljB over a concentration range of 10 to 10,000 ng/ml. Immunization of wild-type and B7-1, B7-2, and B7-1/2 knockout mice by the i.n. route revealed that the ability of FljB to increase B7-2 expression is largely responsible for its adjuvant effect in vivo. These findings demonstrate that FljB can act as an effective mucosal adjuvant and that its ability to enhance the level of B7-2 expression is predominantly responsible for its adjuvant properties.
The innate immune system in mammals plays a critical role in the initial host defense against microbial pathogens. The ability of the innate immune system to recognize and respond to bacterial and viral components has been attributed to a family of type I transmembrane proteins called Toll-like receptors (TLRs) (2, 26, 32, 39). Studies have shown that TLRs can be expressed by a variety of host cells, including antigen-presenting cells (APC), such as monocytes/macrophages and dendritic cells (2, 34). Recognition of microbial products by TLRs can lead to the activation of a variety of signal transduction pathways and the subsequent induction of cytokines and costimulatory molecules (24, 26). In this regard, several known microbe-based adjuvants have been shown to augment both humoral and cell-mediated immune responses to coadministered antigens (Ags) in which their adjuvant properties have been shown to be dependent upon the utilization of TLRs (6, 17, 30). Thus, the ability of microbial products to interact with specific TLRs on APC is believed to play a critical role in their immunostimulatory properties and highlights the potential use of TLR agonists as adjuvants (26, 27).
The ability of TLRs to influence the production of pro- and anti-inflammatory cytokines, e.g., interleukin-10 (IL-10) and IL-12, and modulate the expression of the major histocompatibility complex I/II and B7 costimulatory molecules on APC demonstrates the critical role these receptors play in linking the innate and adaptive immune systems (3, 18, 26). B7 costimulatory molecules and regulatory cytokines have been shown to play key roles for the activation of naïve CD4+ T cells. In this regard, the binding of B7-1 or B7-2 to its ligand CD28 expressed on T cells provides a second signal necessary for the optimal activation, proliferation, and effector function of naïve CD4+ T cells (1, 22). Differing results have been reported on the relative contribution of B7 costimulatory molecules in mediating the induction of immune responses. While some studies have demonstrated that B7-1 and B7-2 costimulatory molecules can mediate unique immunoregulatory effects, such as their preferential involvement in the development of CD4+ T helper 1 (Th1)- and Th2-type immune responses, respectively (19, 22), others have reported that B7-1 and B7-2 costimulatory molecules mediate similar effects on the generation of Th1- and Th2-type immune responses and thus play redundant roles (4, 20, 28).
Recent studies using mice naturally deficient in TLR5 expression have identified that the flagellin component FliC and/or FljB of Salmonella enterica serovar Typhimurium is capable of activating the innate immune system via specific interaction with TLR5 to elicit immunoprotection to Salmonella infection (7, 10, 11, 14, 35, 36, 38). Additional studies have also demonstrated that systemic administration of FliC with Ag augmented systemic host immune responses (8, 30). However, the underlying mechanism responsible for the adjuvant properties of FliC or other variants of flagellin is currently unresolved. In this regard, differing results have been reported on the ability of FliC to enhance the levels of the costimulatory molecules B7-1 and B7-2 on APC. McSorley et al. (30) have reported that systemic administration of FliC augmented B7-1 but not B7-2 expression on splenic dendritic cells (DC), whereas others have reported that systemic administration of FliC resulted in the upregulation of both B7-1 and B7-2 on splenic or bone marrow-derived DC (8). Additionally, past studies assessing the functional role of the B7 costimulatory molecules in mediating the adjuvant properties of FliC utilized the fusion protein CTLA4-immunoglobulin (Ig) in vivo to demonstrate the importance of B7 costimulatory molecules in its adjuvant properties (8, 30). However, the use of CTLA4-Ig inhibits the binding of both B7-1 and B7-2 to its cognate ligand CD28 on T cells, thus making potential differences in the relative contribution of these costimulatory molecules in its adjuvanticity unclear (12). Moreover, findings by Grohmann et al. (12) have shown that CTLA4-Ig can inhibit T-cell responses not only by blocking B7-CD28 interactions but also by inducing the production of indoleamine 2,3-dioxygenase from APC.
Since past studies have assessed only the systemic adjuvanticity of FliC, it is currently unknown if a flagellin component of serovar Typhimurium can act as a mucosal adjuvant or if it can exert a preferential influence on the generation of Th1- and Th2-type immune responses to a mucosally coapplied Ag. Moreover, it is presently unknown if differences exist in the ability of FljB to enhance B7-1 and B7-2 expression on APC and if there is a functional difference between the ability of a flagellin component to influence B7-1 and B7-2 expression on APC and its ability to augment host immune responses to a coadministered antigen. Therefore, the purpose of the present study was to assess the ability of the flagellin component FljB to act as a mucosal adjuvant by assessing both humoral and cell-mediated immune responses to a coadministered Ag given by a mucosal route to determine if FljB enhances B7-1 and B7-2 costimulatory molecule expression on APC and to determine if the ability of FljB to enhance B7-1 or B7-2 on APC plays a preferential role in its adjuvant properties.
MATERIALS AND METHODS
Purification of SBR and FljB.
The recombinant saliva-binding region (SBR) of the adhesin AgI/II of Streptococcus mutans was purified from the cell lysate of Escherichia coli BL21(DE3)(pSBR) using a nickel-charged affinity chromatography column (Novagen, Madison, Wis.) as previously described (13). The affinity of the SBR for nickel arises from a six-residue histidine sequence (at its C-terminal end), which was derived from the expression vector. The Salmonella flagellin component FljB was kindly provided by Andrew Gerwitz (Emory University, Atlanta, Ga.) and was purified as previously described (11). Analysis of FljB for endotoxin content was performed by means of a quantitative chromogenic Limulus amebocyte lysate assay kit (BioWhittaker, Walkersville, MD) using E. coli K235 lipopolysaccharide as standard. The endotoxin content of FljB was <50 pg of lipopolysaccharide per mg of protein.
Mice.
BALB/c wild-type (wt), B7-1 knockout (ko), B7-2 ko, B7-1/2 ko, C57BL/6, and TLR4 ko mice were bred and maintained within an environmentally controlled, pathogen-free animal facility at the University of Alabama at Birmingham and generated as previously described (4, 40). The original B7 ko breeding pairs were obtained from Arlene Sharpe (Brigham and Women's Hospital, Boston, Mass.). Female mice that were 8 to 12 weeks old were used in these studies. All studies were performed according to the National Institutes of Health guidelines, and protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Immunizations.
BALB/c mice (eight mice/group) were immunized via the intranasal (i.n.) route by using a pipette fitted with a sterile plastic tip to slowly apply 10-μl aliquots to each naris. Groups of mice were immunized on days 0 and 18 with phosphate-buffered saline (PBS) (control), 20 μg SBR, or 20 μg SBR coadministered with 5 μg FljB. In a separate series of experiments, groups of BALB/c wt, B7-1 ko, B7-2 ko, and B7-1/2 ko mice were immunized with 20 μg SBR coadministered with 5 μg FljB. An additional group of wt mice was immunized with 20 μg SBR alone to serve as controls.
Collection of mucosal secretions and plasma.
Saliva, vaginal wash, and plasma samples were collected, as previously described (13), from individual mice 1 day prior to the first immunization (day 0), on day 10 after the first immunization, 2 weeks (day 32) after the second immunization, and on day 75. Briefly, saliva was collected after stimulation of salivary flow by injecting each mouse via the intraperitoneal route with 5 μg of carbachol (Sigma Chemical Co., St. Louis, Mo.) in 0.1 ml of sterile saline. Vaginal washes were collected by inserting and aspirating 100 μl of sterile PBS into the vagina of each mouse three times. Plasma samples were obtained following centrifugation of blood collected from the retro-orbital plexus using a calibrated heparinized capillary tube. Mucosal secretions were clarified by centrifugation (13,000 rpm, 6 min), and samples of secretions and plasma were stored at −70°C until they were assayed for anti-SBR antibody activity by enzyme-linked immunosorbent assay (ELISA).
Quantification of antibody responses.
The levels of isotype-specific antibodies in plasma, saliva, and vaginal wash samples and of total salivary and vaginal wash IgA were determined by an ELISA, as previously described (16). Briefly, polystyrene Maxisorp 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 2 μg/ml SBR diluted in borate-buffered saline. Total IgA levels in saliva and vaginal wash samples were determined by coating plates with goat anti-mouse IgA antibodies (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Plates were then washed and blocked for 4 h at room temperature with 0.01 M phosphate buffer (pH 7.2) containing 0.5 M NaCl and 0.15% Tween 20. Serial twofold dilutions of plasma, saliva, or vaginal wash were added in duplicate, and plates were incubated at 4°C overnight. Plates were washed with 0.01 M phosphate buffer containing 0.5 M NaCl and 0.15% Tween 20 (pH 7.2). Plates were developed following incubation with the appropriate horseradish peroxidase-conjugated goat anti-mouse isotype-specific immunoglobulin (IgA, IgG, IgG1, IgG2a, and IgG2b) reagent (Southern Biotechnology Associates, Inc.) and then the addition of o-phenylenediamine and hydrogen peroxide. The optical density was measured at a wavelength of 490 nm. The levels of antibodies and of total immunoglobulin were calculated by interpolation on calibration curves generated at the same time using a mouse immunoglobulin reference serum (ICN Biomedicals, Aurora, Ohio) and constructed by a computer program based on a four-parameter logistic algorithm (Softmax/Molecular Devices Corp., Menlo Park, Calif.).
Generation of bone marrow-derived dendritic cells.
Femurs from wt BALB/c, wt C57BL/6, and TLR4 ko mice (8 to 12 weeks old) were collected by dissecting the rear limbs of each mouse, and the epiphyses were removed from each end of the femurs using scissors to expose the bone marrow (25). Using a 5-ml syringe with a 22-gauge needle, 15 ml of ice-cold Hanks' balanced salt solution (HBSS) was used to aspirate the bone marrow out of the femur into a polystyrene petri dish. The dendritic cells were generated from the bone marrow by culturing the bone marrow cells in the presence of 20 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (Atlanta Biologicals, Atlanta, Ga.) for 10 days in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 20 mM HEPES, 1 mM sodium pyruvate, 50 μg/ml penicillin, and 50 μg/ml streptomycin (RMPI 1640 complete medium) in a humidified 5% CO2 incubator at 37°C (25). The resulting nonadherent dendritic cells were harvested after 10 days, and the purity of the dendritic cell population was determined by fluorescence-activated cell sorter (FACS) analysis of CD11c+ cells using a FACScaliber (Immunocytometry Systems, San Jose, Calif.) (27). This procedure routinely results in >70% of the cells staining positive for CD11c+.
Isolation of CD4+ T cells.
CD4+ T cells were purified as previously described (27). Briefly, mice were sacrificed and spleens were surgically removed and placed in ice-cold HBSS. Using sterile technique, spleens were dispersed into a polystyrene petri dish using a 40-μm cell strainer and a rubber stopper from a sterile 5-ml syringe. The single-cell suspensions were transferred to sterile 15-ml tubes. The tubes were centrifuged at 1,200 rpm for 5 min at 4°C. The supernatant was discarded, and the pellet was resuspended in 25 ml ammonium chloride lysis buffer for 5 min at room temperature to lyse erythrocytes. The cell preparations were washed twice with 25 ml HBSS by centrifugation at 1,200 rpm for 5 min at 4°C. The supernatant was discarded, and the pellet was resuspended in 25 ml HBSS. CD4+ T cells were purified from the spleen cell suspension by negative selection using a Mouse CD4+ Subset column, according to the manufacturer's protocol (R&D Systems, Minneapolis, Minn.). This procedure routinely results in >95% of the cells staining positive for CD4+.
Cytokine and proliferation analysis.
Cultures of dendritic cells (2 × 105 cells) were incubated with various concentrations of FljB (10 ng/ml to 10,000 ng/ml) in RPMI 1640 complete medium in a humidified CO2 incubator at 37°C for 24 to 48 h. Following incubation, the cells were stained with CD11c allophycocyanin, counterstained with phycoerythrin-conjugated anti-mouse B7-1 and fluorescein isothiocyanate-conjugated anti-mouse B7-2 (eBioscience, San Diego, Calif.), and analyzed for B7-1 and B7-2 expression by FACS.
Purified splenic CD4+ T cells (2.5 × 106 cells/ml) from immunized wt mice were cocultured with irradiated (2,500 rad) splenic feeder cells (4 × 106 cells/ml) in RPMI 1640 complete medium alone or with SBR (2 μg/ml) in a humidified CO2 incubator at 37°C for 4 days. Following incubation, culture supernatants were collected, and the levels of gamma interferon (IFN-γ), IL-4, and IL-5 were determined using Ready-SET-Go ELISA kits (eBioscience). CD4+-T-cell proliferation was determined by adding 0.5 μCi/well of [3H]thymidine for the last 18 to 24 h of incubation. The amount of [3H]thymidine incorporated into cells was determined by using a liquid scintillation counter (27).
Statistical analysis.
The significance of differences between groups was evaluated by analysis of variance and the Tukey multiple comparison test using the Instat program (GraphPad, San Diego, Calif.). Differences between groups were considered significant at the level of P < 0.05.
RESULTS
Adjuvanticity of FljB.
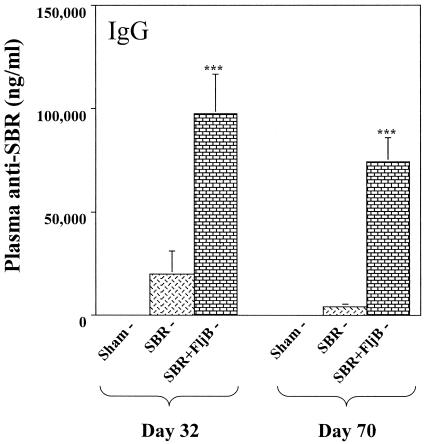
To determine whether FljB can act as a mucosal adjuvant, wt mice were immunized by the i.n. route with SBR or SBR coadministered with FljB. On days 32 and 70, the levels of plasma IgG anti-SBR antibody activity in mice immunized with SBR and FljB were significantly greater (P < 0.001 and P < 0.001, respectively) than that seen in plasma from mice immunized with SBR alone (Fig. 1). Essentially no plasma IgG anti-SBR antibody activity was seen in control mice. A similar response pattern was seen in terms of the plasma IgA response, where the levels of plasma IgA anti-SBR antibody activity in mice immunized with SBR and FljB were significantly greater (P < 0.001) than that seen in plasma from mice immunized with SBR alone (data not shown).
FIG. 1.
Adjuvant effect of FljB on the plasma IgG response to SBR. Mice were immunized by the i.n. route on days 0 and 18 with 20 μg SBR or 20 μg SBR coadministered with 5 μg FljB. Wild-type mice receiving PBS via the i.n. route served as controls. The plasma anti-SBR IgG response was analyzed 2 weeks following the second i.n. immunization (day 32) and on day 70. Results represent the arithmetic mean ± the standard error of the mean (SEM) of eight mice per group. *** indicates significance differences between the groups of mice at P < 0.001.
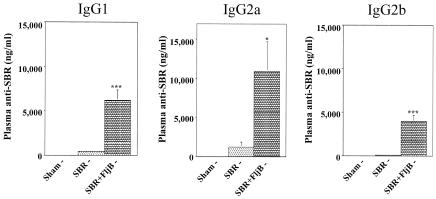
In order to determine the nature of the IgG response, we next assessed the subclass of the plasma IgG response. On day 32, the levels of IgG1, IgG2a, and IgG2b were significantly higher (P < 0.001, P < 0.05, and P < 0.001, respectively) in mice immunized with SBR and FljB than control mice (Fig. 2). These results indicate that FljB potentiates a mixed Th1- and Th2-type systemic response to SBR following i.n. immunization with SBR plus FljB.
FIG. 2.
Adjuvant effect of FljB on the plasma IgG subclass responses to SBR. Mice were immunized by the i.n. route on days 0 and 18 with 20 μg SBR or 20 μg SBR coadministered with 5 μg FljB. Wild-type mice receiving PBS via the i.n. route served as controls. SBR-specific plasma IgG, IgG1, IgG2a, and IgG2b responses were analyzed 2 weeks following the second i.n. immunization (day 32). Results represent the arithmetic mean ± SEM of eight mice per group. * and *** indicate significance differences between the groups of mice at P < 0.05 and P < 0.001, respectively.
We next assessed the mucosal IgA anti-SBR response in the saliva and vaginal wash samples from the various groups of mice. On days 32 and 70, the levels of salivary IgA anti-SBR antibody activity in mice immunized with SBR and FljB were significantly greater (P < 0.01 and P < 0.01, respectively) than that seen in saliva from mice immunized with SBR alone (Fig. 3). Also on days 32 and 70, the levels of vaginal wash IgA anti-SBR antibody activity in mice immunized with SBR and FljB were significantly greater (P < 0.01 and P < 0.05, respectively) than that seen in vaginal washes from mice immunized with SBR alone (Fig. 3). Taken together, these results demonstrate that FljB can act as a mucosal adjuvant in potentiating plasma and mucosal responses to SBR following i.n. immunization.
FIG. 3.
Adjuvant effects of FljB on mucosal IgA responses to SBR. SBR-specific salivary and vaginal IgA responses were analyzed 2 weeks (day 32) after and at day 70 following a secondary i.n. immunization of mice with 20 μg SBR or 20 μg SBR coadministered with 5 μg FljB. Wild-type mice receiving PBS via the i.n. route were used as controls. Results represent the arithmetic mean ± SEM of eight mice per group. * and ** indicate significant differences between SBR- and SBR-plus-FljB-immunized mice at P < 0.05 and P < 0.01, respectively.
FljB differentially upregulates B7-1 and B7-2 on DC.
To investigate the potential mechanism of FljB adjuvanticity, we initially determined if there was a differential expression of B7-1 and B7-2 on DC following stimulation with FljB and if the expression levels were dependent upon the dose of FljB. DC were treated with various amounts of FljB (0 to 10,000 ng/ml) for 24 h and then analyzed for B7 expression by FACS (Fig. 4A). The mean fluorescence intensity of both B7-1 and B7-2 expression increased significantly (P < 0.05) on DC stimulated with FljB in the presence or absence of 1 μg/ml of polymyxin B compared to nonstimulated controls (Fig. 4A). Moreover, wt or TLR4 ko DC stimulated with FljB exhibited similar profiles in B7-1 and B7-2 upregulation (Fig. 4C).
FIG. 4.
Effects of FljB on B7-1 and B7-2 expression on murine bone marrow-derived dendritic cells. For panels A and B, wt dendritic cells were incubated with various concentrations (10 to 10,000 ng/ml) of FljB in the presence or absence of polymyxin B (1 μg/ml) for 24 h. For panels C and D, wt or TLR4 ko dendritic cells were incubated with various concentrations (10 to 10,000 ng/ml) of FljB for 24 h. Expression profiles represent the mean fluorescence intensity (MFI) of B7-1 and B7-2 expression on CD11c+ cells (A and C) and the percentage of CD11c+ cells expressing B7-1 and B7-2 (B and D). Data are the means of six separate experiments. *, **, and *** indicate significance differences between nonstimulated and FljB-stimulated DC at P < 0.05, P < 0.01, and P < 0.001, respectively.
We also assessed the effect of FljB on the percentage of DC expressing either B7-1 or B7-2. The percentage of DC expressing B7-1 increased slightly following exposure to 10 ng/ml FljB, but no significant change was seen following exposure to increasing doses of FljB (Fig. 4B). However, a significant increase (P < 0.05) was observed in the percentage of DC expressing B7-2 following exposure to 10 ng/ml FljB (Fig. 4B). The addition of polymyxin B (1 μg/ml) to cultures resulted in no discernible effect on the ability of FljB to augment B7-1 or B7-2 expression on DC (Fig. 4B). Furthermore, the percentage of cells expressing B7-2 continued to increase significantly (P < 0.05) with increasing concentrations of FljB in a dose-dependent manner (Fig. 4B). Moreover, stimulated DC from TLR4 ko mice exhibited patterns of B7-1 and B7-2 expression similar to those of wt controls (Fig. 4D). Thus, at 10 ng/ml or higher, FljB preferentially augmented B7-2 over B7-1 on DC.
Functional role of B7-1 and B7-2 in the adjuvanticity of FljB.
To define the in vivo functional role of B7-1 and B7-2 in the adjuvant activity of FljB, wt, B7-1 ko, B7-2 ko, or B7-1/2 ko mice were immunized by the i.n. route twice with SBR in the presence of FljB. A control group of wt mice were immunized with SBR only. While the level of plasma IgG anti-SBR antibody activity in B7-1 ko mice was similar to that seen in wt mice, B7-2 ko mice had significantly lower (P < 0.01) levels of plasma IgG anti-SBR than those seen in wt and B7-1 ko mice (Fig. 5). B7-1/2 ko mice exhibited little or no IgG anti-SBR antibody activity following immunization with SBR and FljB. These results demonstrate that the ability of FljB to augment B7-2 and not B7-1 levels is largely responsible for its systemic adjuvant properties.
FIG. 5.
Functional role of B7 for the adjuvant activity of FljB for plasma IgG responses to SBR following mucosal immunization. BALB/c wt, B7-1, B7-2, and B7-1/2 ko mice were immunized via the i.n. route on days 0 and 18 with 20 μg SBR coadministered with 5 μg FljB. BALB/c wt mice immunized with 20 μg SBR only on days 0 and 18 served as controls. SBR-specific plasma IgG, IgG1, IgG2a, and IgG2b responses were analyzed 2 weeks following the second immunization. Results represent the arithmetic mean ± SEM of six mice per group. ** and *** indicate significant differences between the wt and the other groups of mice at P < 0.01 and P < 0.001, respectively.
We next sought to determine how the ability of FljB to influence B7 costimulatory molecule expression influences the nature of the IgG subclass response in wt and B7 ko mice following immunization with SBR and FljB (Fig. 5). Whereas the levels of IgG2b anti-SBR antibody activity in B7-1 ko mice were significantly lower (P < 0.01) than that seen in wt mice following immunization with SBR and FljB, the levels of IgG1 and IgG2a anti-SBR in B7-1 ko mice were not significantly different. Interestingly, the induction of IgG1, IgG2a, and IgG2b anti-SBR responses in B7-2 ko mice were all significantly lower (P < 0.01, P < 0.001, and P < 0.001, respectively) than that seen in wt mice (Fig. 5). Moreover, B7-1/2 ko mice exhibited little or no IgG1, IgG2a, or IgG2b anti-SBR antibody activities, which were all significantly lower than those seen in wt, B7-1 ko, and B7-2 ko mice (P < 0.001). These results demonstrate that the ability of FljB to augment IgG1 (Th2-type) and IgG2a (Th1-type) responses to SBR was predominantly mediated by its ability to enhance B7-2 levels.
SBR-specific CD4+-T-cell responses of wt and B7 ko mice immunized with SBR and FljB as adjuvant.
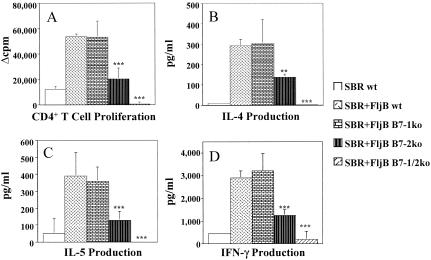
Due to the importance of the B7 costimulatory molecules in influencing both qualitative and quantitative aspects of CD4+-T-cell responses (4, 19-22, 29, 37), we next examined the effect of the B7 costimulatory molecules on the ability of FljB to augment SBR-specific CD4+-T-cell proliferation and cytokine production (Fig. 6). The proliferation of SBR-stimulated CD4+ T cells from mice deficient in B7-1 was similar to that seen with CD4+ T cells from wt mice immunized with SBR and FljB (Fig. 6A). In contrast, the proliferation of SBR-specific CD4+ T cells from B7-2 ko mice immunized with SBR and FljB was significantly lower (P < 0.001) than that seen in wt or B7-1 ko mice immunized with SBR and FljB (Fig. 6A). Assessment of SBR-specific CD4+-T-cell responses from B7-1/2-deficient mice demonstrated essentially no proliferation after stimulation with SBR, which was significantly lower (P < 0.001) than wt, B7-1 ko, and B7-2 ko mice (Fig. 6A).
FIG. 6.
Functional role of B7 on SBR-specific CD4+-T-cell proliferation and cytokine production. CD4+ T cells from the spleens of immunized mice were cultured in the presence of naïve feeder cells and SBR for 24 h. Proliferation of SBR-specific CD4+ T cells was determined by a liquid scintillation counter following the addition of 0.5 μCi/well of [3H]thymidine for the last 18 to 24 h of culture. The results are expressed as the Δcpm (experimental cpm − nonstimulated cpm) (A). Cytokine production (IL-4, IL-5, and IFN-γ) in culture supernatants of SBR-stimulated cultures was assessed at 24 h (B, C, and D, respectively). Results represent the arithmetic mean ± SEM of six mice per group. ** and *** indicate significant differences between the wt and the other groups of mice at P < 0.01 and P < 0.001, respectively.
The levels of Th1- and Th2-type cytokines in supernatants of SBR-stimulated CD4+ T cells isolated from B7-1 ko mice revealed that the levels of IL-4 (Fig. 6B), IL-5 (Fig. 6C), and IFN-γ (Fig. 6D) were similar to those of wt mice. In contrast, significantly lower levels of the Th2-type cytokines IL-4 and IL-5 (P < 0.01 and P < 0.001, respectively) were produced by CD4+ T cells isolated from B7-2 ko mice than from the wt or B7-1 ko groups (Fig. 6B and C). Furthermore, the level of the Th1-type cytokine IFN-γ produced by CD4+ T cells from B7-2 ko mice was also significantly lower (P < 0.001) than that seen with CD4+ T cells from wt or B7-1 ko mice (Fig. 6D). The levels of IL-4, IL-5, and IFN-γ produced by CD4+-T-cell cultures from B7-1/2 ko mice in response to SBR were negligible and were significantly lower (P < 0.001) than that seen in the wt, B7-1, and B7-2 ko mice (Fig. 5). Taken together, these results demonstrate that the ability of FljB to augment both Th1- and Th2-type CD4+-T-cell responses is largely dependent upon B7-2 expression.
DISCUSSION
Previous studies characterizing the immunostimulatory properties of bacterial flagellin have demonstrated that it has limited immunogenicity while concurrently exhibiting potent adjuvant properties (8, 30). In this regard, it has been shown that the flagellin component FliC of serovar Typhimurium can augment both CD4+-T-cell and antibody responses to a coadministered Ag upon parenteral administration (8, 30). While these studies demonstrate the efficacy of FliC acting as a systemic adjuvant, it was unknown whether FliC or other flagellin components of serovar Typhimurium, including FljB, could act as mucosal adjuvants and the cellular mechanism(s) responsible for these adjuvant properties. Our present study demonstrates that the flagellin component FljB of serovar Typhimurium enhanced both Th1- and Th2-associated antibody and CD4+-T-cell responses to the coadministered Ag SBR when given by the i.n. route. Furthermore, to define the cellular mechanisms responsible for the ability of FljB to act as a mucosal adjuvant, we assessed how FljB was modulating the expression of the costimulatory molecules B7-1 and B7-2 on dendritic cells. Previous studies examined the role of B7 on splenic dendritic cells to influence the adjuvanticity of FliC; however, the use of CTLA4-Ig raises questions in the reported results on the functionality of B7 isoforms due to the ability of CTLA4-Ig to block the binding of both B7-1 and B7-2 to CD28 (8, 30), as well as the reported effects of CTLA4-Ig on the production of indoleamine 2,3-dioxygenase from APC that can potently inhibit T-cell responses (31, 33). In the present study, stimulation of dendritic cells with FljB at concentrations greater than 10 ng/ml resulted in enhanced expression of B7-1 and especially B7-2. By using B7 ko mice, we have shown that the ability of FljB to augment both humoral and cell-mediated immune responses to a mucosally applied Ag is predominantly dependent on its ability to enhance B7-2 expression, whereas its ability to influence B7-1 expression does not appear to significantly affect its adjuvanticity. Thus, our present study extends those of others (8) by demonstrating that the flagellin monomer variant FljB possesses potent mucosal adjuvant properties that are highly dependent upon its ability to enhance B7-2 expression.
The involvement of B7-1 and B7-2 in mediating Th1 and Th2 responses has been controversial (4, 19, 20, 22). Studies characterizing the functional role of the B7 costimulatory molecules in influencing Th1- and Th2-type responses have reported that B7-1 and B7-2 differentially activate Th1 and Th2 responses, respectively (19, 22), while other studies have demonstrated that B7-1 and B7-2 can mediate similar, if not overlapping, roles in the priming of Th1- and Th2-type responses (4, 20, 23). However, analysis of cytokines from SBR-specific CD4+ T cells from B7-2 ko mice immunized with FljB as adjuvant showed significantly lower levels of both Th1- and Th2-associated humoral and cell-mediated immune responses than wt and B7-1 ko mice. Thus, our results suggest that FljB primarily enhances B7-2 expression that mediates its ability to augment both Th1- and Th2-type responses.
Studies assessing cellular mechanisms responsible for the adjuvant properties of several mucosal adjuvants, including cholera toxin, the heat-labile toxin of E. coli, and CpG DNA (5, 9, 24, 27, 41, 42), have shown that the ability of these adjuvants to selectively influence B7-1 or B7-2 costimulatory molecule expression on APC is largely responsible for their immunostimulatory effects. The present study demonstrates that the ability of FljB to augment both mucosal and systemic humoral immune responses to the mucosally coadministered Ag SBR was largely dependent on the expression of B7 costimulatory molecules. Moreover, although the current findings showing a lack of adjuvant activity in B7-1/2 ko mice suggest that there is some level of compensation between the ability of B7-1 and B7-2 to influence the adjuvant properties of FljB, analysis of the adjuvant properties of FljB in B7-1- or B7-2-deficient mice demonstrated that mice lacking B7-2 exhibited a significant reduction in both humoral and cell-mediated immune responses compared to wt or B7-1-deficient mice. These in vivo results are consistent with our in vitro data showing that FljB preferentially enhanced the percentage of dendritic cells expressing B7-2 and are in agreement with past studies demonstrating a strong correlation between the ability of an adjuvant to selectively upregulate B7-1 or B7-2 and the preferential role the upregulated costimulatory molecule plays in potentiating the subsequent immune response (15, 26, 27).
In summary, we have demonstrated that the FljB flagellin component of serovar Typhimurium can act as a potent mucosal adjuvant and that its ability to enhance B7-2 expression is largely responsible for its immunostimulatory properties on both humoral and cell-mediated immune responses. These findings provide a cellular mechanism by which FljB mediates its adjuvanticity.
Acknowledgments
We thank Russell Johnson for his excellent technical assistance.
This work was supported by USPHS grant DE09081 from the National Institute of Dental and Craniofacial Research.
Editor: J. D. Clements
REFERENCES
- 1.Appleman, L. J., and V. A. Boussiotis. 2003. T cell anergy and costimulation. Immunol. Rev. 192:161-180. [DOI] [PubMed] [Google Scholar]
- 2.Basset, C., J. Holton, R. O'Mahony, and I. Roitt. 2003. Innate immunity and pathogen-host interaction. Vaccine 21:S2/12-S2/23. [DOI] [PubMed] [Google Scholar]
- 3.Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74:479-485. [DOI] [PubMed] [Google Scholar]
- 4.Borriello, F., M. P. Sethna, S. D. Boyd, A. N. Schweitzer, E. A. Tivol, D. Jacoby, T. B. Strom, E. M. Simpson, G. J. Freeman, and A. H. Sharpe. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6:303-313. [DOI] [PubMed] [Google Scholar]
- 5.Cong, Y., C. T. Weaver, and C. O. Elson. 1997. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J. Immunol. 159:5301-5308. [PubMed] [Google Scholar]
- 6.Cox, J. C., and A. R. Coulter. 1997. Adjuvants—a classification and review of their modes of action. Vaccine 15:248-256. [DOI] [PubMed] [Google Scholar]
- 7.Cuadros, C., F. J. Lopez-Hernandez, A. L. Dominguez, M. McClelland, and J. Lustgarten. 2004. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect. Immun. 72:2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didierlaurent, A., I. Ferrero, L. A. Otten, B. Dubois, M. Reinhardt, H. Carlsen, R. Blomhoff, S. Akira, J. Kraehenbuhl, and J. Sirard. 2004. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J. Immunol. 172:6922-6930. [DOI] [PubMed] [Google Scholar]
- 9.Elson, C. O. 1987. Cholera toxin as a mucosal adjuvant—the effect of H-2 genes. Fed. Proc. 46:1778. [Google Scholar]
- 10.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grohmann, U., C. Orabona, F. Fallarino, C. Vacca, F. Calcinaro, A. Falorni, P. Candeloro, M. L. Belladonna, R. Bianchi, M. C. Fioretti, and P. Puccetti. 2002. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 3:1097-1101. [DOI] [PubMed] [Google Scholar]
- 13.Harokopakis, E., G. Hajishengallis, T. E. Greenway, M. W. Russell, and S. M. Michalek. 1997. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect. Immun. 65:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 15.Hoebe, K., E. M. Janssen, S. O. Kim, L. Alexopoulou, R. A. Flavell, J. Han, and B. Beutler. 2003. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat. Immunol. 4:1223-1229. [DOI] [PubMed] [Google Scholar]
- 16.Huang, Y., G. Hajishengallis, and S. M. Michalek. 2001. Induction of protective immunity against Streptococcus mutans colonization after mucosal immunization with attenuated Salmonella enterica serovar Typhimurium expressing an S. mutans adhesin under the control of in vivo-inducible nirB promoter. Infect. Immun. 69:2154-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter, R. L. 2002. Overview of vaccine adjuvants: present and future. Vaccine 20:S7-S12. [DOI] [PubMed] [Google Scholar]
- 18.Janeway, C. A. 2001. How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad. Sci. USA 98:7461-7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 20.Lanier, L. L., S. O'Fallon, C. Somoza, J. H. Phillips, P. S. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 21.Lenschow, D. J., S. C. Ho, H. Sattar, L. Rhee, G. Gray, N. Nabavi, K. C. Herold, and J. A. Bluestone. 1995. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 181:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 23.Lespagnard, L., P. Mettens, T. De Smedt, H. Bazin, J. Urbain, O. Leo, and M. Moser. 1998. The immune response induced in vivo by dendritic cells is dependent on B7-1 or B7-2, but the inhibition of both signals does not lead to tolerance. Int. Immunol. 10:295-304. [DOI] [PubMed] [Google Scholar]
- 24.Lien, E., and D. T. Golenbock. 2003. Adjuvants and their signaling pathways: beyond TLRs. Nat. Immunol. 4:1162-1164. [DOI] [PubMed] [Google Scholar]
- 25.Lutz, M. B., N. Kukutsch, A. L. J. Ogilvie, S. Robner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 26.Marciani, D. J. 2003. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 8:934-943. [DOI] [PubMed] [Google Scholar]
- 27.Martin, M., A. H. Sharpe, J. D. Clements, and S. M. Michalek. 2002. Role of B7 costimulatory molecules in the adjuvant activity of the heat-labile enterotoxin of Escherichia coli. J. Immunol. 169:1744-1752. [DOI] [PubMed] [Google Scholar]
- 28.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8 cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdam, A. J., A. N. Schweitzer, and A. H. Sharpe. 1998. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 165:231-247. [DOI] [PubMed] [Google Scholar]
- 30.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 169:3914-3919. [DOI] [PubMed] [Google Scholar]
- 31.Mellor, A. L., P. Chandler, B. Banan, A. M. Hansen, B. Marshall, J. Pihkala, H. Waldmann, S. Cobbold, E. Adams, and D. H. Munn. 2004. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 16:1391-1401. [DOI] [PubMed] [Google Scholar]
- 32.Michalek, S. M., J. Katz, N. K. Childers, M. Martin, and D. F. Balkovetz. 2002. Microbial/host interactions. Immunol. Res. 26:223-234. [DOI] [PubMed] [Google Scholar]
- 33.Munn, D. H., M. D. Sharma, and A. L. Mellor. 2004. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 172:4100-4110. [DOI] [PubMed] [Google Scholar]
- 34.Muzio, M., D. Bosisio, N. Polentarutti, G. D'amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 35.Reichhart, J. M. 2003. TLR5 takes aim at bacterial propeller. Nat. Immunol. 4:1159-1160. [DOI] [PubMed] [Google Scholar]
- 36.Sbrogio-Almeida, M. E., and L. C. S. Ferreira. 2001. Flagellin expressed by live Salmonella vaccine strains induces distinct antibody responses following delivery via systemic or mucosal immunization routes. FEMS Immunol. Med. Microbiol. 30:203-208. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe, A. H., and A. N. Schweitzer. 1998. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 161:2762-2771. [PubMed] [Google Scholar]
- 38.Smith, K. D., E. Anderson-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Rassoulian Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4:1247-1253. [DOI] [PubMed] [Google Scholar]
- 39.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 41.Wagner, H. 2004. The immunobiology of the TLR9 subfamily. Trends Immunol. 25:381-386. [DOI] [PubMed] [Google Scholar]
- 42.Yuki, Y., and H. Kiyono. 2003. New generation of mucosal adjuvants for the induction of protective immunity. Rev. Med. Virol. 13:293-310. [DOI] [PubMed] [Google Scholar]