Abstract
Salmonella enterica is a gram-negative intracellular pathogen that can cause a variety of diseases ranging from gastroenteritis to typhoid fever. The Typhimurium serotype causes gastroenteritis in humans; however, infection of mice results in an enteric fever that resembles human typhoid fever and has been used as a model for typhoid fever. The present study examined the role of the chemokine CCL2 in the control of Salmonella infection. Upon infection with salmonellae, mucosal expression of CCL2 is rapidly up-regulated, followed by systemic expression in the spleen. CCL2−/− mice became moribund earlier and had a higher rate of mortality compared to wild-type C57BL/6 mice. Moreover, CCL2−/− mice had significantly higher levels of bacteria in the liver compared to wild-type controls. Mucosal and serum interleukin-6 and tumor necrosis factor alpha levels were elevated in CCL2−/− mice compared to wild-type mice. In vitro analysis demonstrated that CCL2−/− macrophages infected with salmonellae resulted in dysregulated cytokine production compared to macrophages derived from wild-type mice. These data are the first to directly demonstrate CCL2 as a critical factor for immune responses and survival following S. enterica infection.
Salmonella enterica comprises a group of intracellular gram-negative bacteria that are pathogens of humans and livestock (49). In humans, ingestion of different Salmonella serovars leads to different diseases. For example, Salmonella serotype Typhimurium causes enterocolitis and gastroenteritis characterized by diarrhea and severe changes in the intestine and mesenteric lymph nodes (38, 39). A second serovar, Typhi, is the known causative agent of typhoid fever, a systemic infection characterized by both enteric fever and diarrhea. However in mice, infection with serovar Typhimurium gives rise to enteric fever with symptoms of those closely related to human S. enterica serovar Typhi (61), making it a widely accepted experimental model for human typhoid fever.
Lipopolysaccharide (LPS) is a major cell wall component of gram-negative bacteria, and it is a primary mediator of the resulting symptoms during Salmonella infection. The widespread activation of cells responsive to bacteria and cell wall components, such as LPS, results in the release of inflammatory cytokines, chemokines, prostaglandins, and reactive oxygen species. These compounds lead to hemodynamic and inflammatory changes that can lead to an acute illness known as sepsis, characterized by shock, coagulopathy, and organ dysfunction (61). LPS is recognized by an LPS binding protein that binds to CD14, allowing it to be recognized by Toll-like receptor 4 (31, 64). After binding TLR4, there are a series of intracellular signaling events that result in the activation of mitogen-activated protein kinase pathways and the ultimate activation of NF-κB (31, 54, 65, 66). LPS mediates many events in early stages of inflammation by stimulating the innate immune system to secrete important factors such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), gamma interferon (IFN-γ), and CXC and CC chemokines (50, 55, 56, 68). These factors are important in the activation of the host defense network in terms of the innate and adaptive immune response and the mobilization of effector cell types such as macrophages and neutrophils necessary for the clearance of bacterial infection (15). Cytokine and chemokine expression by intestinal epithelial cells is stimulated by Salmonella infection (16, 21, 40, 48, 68). This results in the chemoattraction of neutrophils as one of the first-line host defense mechanisms (6, 33). Eventually, salmonellae infect macrophages (42) and if the bacteria are not killed they can be disseminated to peripheral organ systems (59). The innate immune cytokine responses such as IL-12, IL-6, and TNF-α can function to shape the adaptive immune responses to Salmonella proteins and effect long term immunity (34, 60).
CCL2 is a member of the CC family of chemokines that is a chemoattractant for T cells, monocytes, mast cells, and basophils (43) and is produced by both immune and nonimmune cells in response to stimuli such as endotoxin (3). In addition to its effects of inflammation, CCL2 has been implicated in the regulation of antigen-specific immune responses by inhibiting IL-12 production by granuloma macrophages (7) and promoting Th2 differentiation (28). Furthermore, CCL2 has been identified as a critical molecule for the induction of oral tolerance by affecting cytokine regulation in the mucosal compartment (10). CCL2 has also been identified as a potent mediator of sepsis. Following administration of endotoxin to both baboons (26) and humans (57), plasma levels of CCL2 increase substantially. Furthermore, when mice were treated with anti-CCL2 antibodies prior to endotoxin exposure there was an increase in mortality from 10% to 65% (69). Conversely, administration of CCL2 during endotoxin challenge protected the mice from death (69). Finally, Nakano and colleagues (44) demonstrated that administration of CCL2 increased the survival of mice lethally infected with salmonellae by increasing macrophage activation. These results strongly suggest a potential role for CCL2 in the immune response to salmonellae necessary for its control and clearance by the host.
In the present study, we investigated the role of CCL2 in the response to S. enterica infection using CCL2-deficient mice. The results are the first to directly demonstrate the requirement for CCL2 in the innate immune response to intestinal mucosal salmonellae resulting in control and clearance of peripheral infection.
MATERIALS AND METHODS
Animals.
Female C57BL/6 (H2b) (B6) mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). CCL2−/− (H2b) (22) mice were previously described and have been backcrossed eight times onto the B6 background (24). All mice were maintained at the Center for Comparative Medicine at Northwestern University. Mice were 6 to 7 weeks old at the initiation of the experiment and were maintained on standard laboratory chow and water ad libitum. Animal care and use were performed in accordance with Northwestern University and Public Health Service policies.
Bacterial culture and infections.
S. enterica serovar Typhimurium strain SL1334 (provided by Kasturi Haldar, Northwestern University) was grown to stationary phase in Luria-Bertani (LB) medium at 37°C with constant shaking overnight. The next morning, bacteria were diluted 1:50 and grown in log phase for 4 to 5 h. The bacteria were quantified by reading the optical density at 600 nm. SL1334 was then washed three times in sterile phosphate-buffered saline (PBS) and resuspended at different concentrations ranging from 105 bacteria/ml to 109 bacteria/ml. Mice were fed 100 μl by intragastric gavage and monitored twice a day and were euthanized if they became moribund.
CFU.
Tissue was extracted, weighed, and placed in 1 ml of sterile PBS. The tissue was homogenized, and 50 μl of homogenate was plated onto LB plates in multiple dilutions containing streptomycin. Twenty-four hours after plating, the colonies were counted and the number of CFU per milligram of tissue was calculated.
Chemokine ELISA.
Assessment of CCL2 was quantified from tissue samples using a previously described enzyme-linked immunosorbent assay (ELISA) (35, 36). Briefly, mucosal samples were harvested and homogenized in 1 ml PBS and clarified by centrifugation (400 × g) for 10 min. Flat-bottom microtiter plates were coated with capture antibody diluted in sodium phosphate coating buffer and incubated at 4°C overnight. Nonspecific binding sites were blocked by incubation with 10% fetal calf serum in PBS for 1 h at 37°C, samples were subsequently added in triplicate, and the mixture was incubated for 2 h at 37°C. Horseradish peroxidase-conjugated anti-CCL2 antibody was added, and the mixture was incubated for an additional 1 h at 37°C. Plates were developed using o-phenylenediamine substrate, and absorbance was read at 490 nm. Standard curves for CCL2 were generated using a series of dilutions of purified recombinant CCL2 (R&D Systems). The detection limit of these assays is 31.25 pg/ml, and the ELISA is specific and does not cross-react with any other cytokine or chemokine. Chemokine levels in the mucosa and spleen were quantified by comparison to the standard curve and expressed as picograms per milliliter of tissue.
Cytokine bead array.
Fifty-microliter experimental samples and standards were placed in a 96-well plate, 25 μl of Beadlyte anti-mouse cytokine beads (IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IFN-γ, and TNF-α) was mixed with each sample, and the mixture was incubated for 2 h in the dark. Twenty-five microliters of Beadlyte biotin anti-mouse cytokine reporters (IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IFN-γ, and TNF-α) was added to the samples, and the mixture was incubated for 1.5 h in the dark while mixing. Streptavidin-phycoerythrin was then added to each well, and the mixture was incubated for 30 min in the dark. To stop the reaction in each well, 10 μl of stop solution was added and the plate was incubated for an additional 5 min. Samples were read on a Luminex100 instrument.
Salmonella LPS.
Mice were injected intraperitoneally with 50 μg Salmonella LPS purchased from Sigma (St. Louis, MO) and evaluated twice daily. Mice that were moribund were euthanized. Mice were anesthetized and bled retroorbitally to obtain serum to test for cytokines.
In vitro stimulation of macrophages.
Peritoneal macrophages were harvested from normal CCL2−/− and wild-type mice by rinsing the peritoneal cavity with Hanks balanced salt solution. Cells (5 × 106 /ml) were plated in a 24-well plate and rested for 1 h at 37°C. SL1344 bacteria were grown overnight as described above. The bacteria were diluted to 4 × 106/ml (for a multiplicity of infection of 10), and 1 ml was added to the macrophages. The plate was centrifuged at 400 × g for 5 min to create more contact between the bacteria and the monolayer. Another set of macrophages received 1 μg/ml of Salmonella LPS (Sigma). The plates were placed at 37°C, and infection was allowed to proceed for 30 min. The bacteria were removed by washing the plates three times with serum-free medium, and fresh Dulbecco modified Eagle medium (with 10% fetal bovine serum) containing 100 μg/ml gentamicin was added to the wells. The gentamicin concentration was reduced to 10 μg/ml after 1.5 h. Medium was collected at various time points following infection for analysis of cytokines and chemokine secretion.
Statistical analysis.
Statistical significance of cytokine levels was analyzed using Student's t test for comparisons of two means. Differences between percentages of mice affected were determined by using the χ2 Fischer exact test. Values of P ≤ 0.05 were considered significant.
RESULTS
CCL2 is expressed mucosally and systemically after infection with S. enterica serotype Typhimurium.
C57BL/6 mice were infected orally with 108 virulent S. enterica serotype Typhimurium strain SL1334 bacteria. Gut-associated lymphoid tissues (GALT; Peyer's patch and mesenteric lymph nodes), spleen tissue, and serum were harvested at various days after infection and analyzed for production of CCL2 by ELISA. The results in Fig. 1 demonstrate that infection with salmonellae resulted in a dramatic up regulation of CCL2 in the GALT 1 day after infection, followed by a rapidly decline. In the spleen and serum, Salmonella infection induced little CCL2 1 day after infection; however, the levels of CCL2 increased dramatically as late as 11 days postinfection (Fig. 1). These data suggest that Salmonella infection induces a temporal pattern of spatial CCL2 expression as infection progresses.
FIG. 1.
CCL2 expression in mucosal and systemic tissues after Salmonella infection. C57BL/6 mice were infected intragastrically with 108 salmonellae. At different time points after infection, CCL2 levels in the mucosa (black bars), spleen (diagonal stripes), and serum (hatched bars) were measured by ELISA.
CCL2−/− mice show reduced survival following infection with salmonellae.
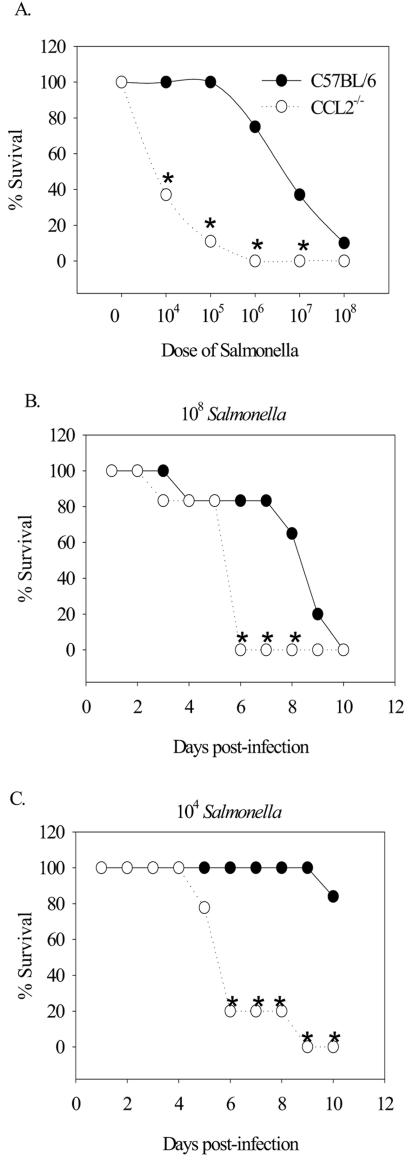
The increase in CCL2 expression in the mucosal tissue soon after infection suggested that CCL2 may be an important innate immune factor in modulating Salmonella infection. To directly test the contribution of CCL2 to the pathogenesis of Salmonella infection, CCL2−/− and C57BL/6 control mice were inoculated with different doses of virulent salmonellae ranging from 104 to 108 bacteria. The results shown in Fig. 2A demonstrate that CCL2−/− mice became significantly more moribund and had to be euthanized compared to C57BL/6 controls at doses of 104, 105, 106, and 107 bacteria. When CCL2−/− mice were infected with 108 organisms, they showed significantly lower survival compared to control mice at days 6 to 8 following infection (Fig. 2B). Similarly, when CCL2−/− mice were infected with 104 organisms, they showed significantly decreased survival compared to controls at days 6 to 10 postinfection (Fig. 2C). These data strongly suggest that CCL2 is critical for survival following Salmonella infection.
FIG. 2.
Survival following Salmonella infection. (A) C57BL/6 and CCL2−/− mice were infected intragastrically with increasing doses of salmonellae, and the percent survival was determined 5 days after infection. If the mice became moribund, they were euthanized and considered to have succumbed to infection. Shown are survival percentages for infecting doses of 108 (B) and 104 (C) bacteria as a function of time postinfection. An asterisk denotes P < 0.05 when comparing the percent survival of CCL2−/− mice to that of C57BL/6 mice for each day.
CCL2−/− mice show an enhanced systemic bacterial burden.
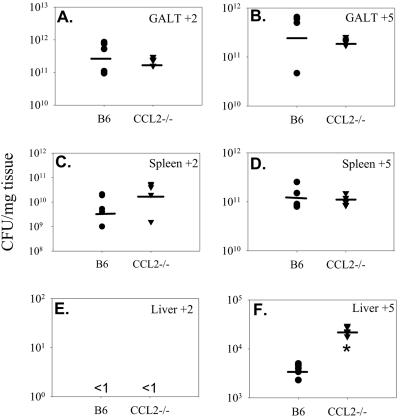
The rapid morbidity and mortality of the CCL2−/− mice following infection with 104 or 108 salmonellae could be due to a number of different possibilities. The first was that the salmonellae were disseminating and replicating at a higher rate in the CCL2−/− mice. To test this possibility, bacterial burdens were evaluated by enumerating the number of CFU in the GALT (defined as Peyer's patches), spleens, and livers of CCL2−/− and wild-type C57BL/6 mice following infection with 108 Salmonella organisms. We chose to evaluate bacterial burdens at two time points following infection: days 2 and 5 following bacterial inoculation. At these time points, the GALT, spleen, and liver were harvested and homogenized and extracts plated onto LB agar plates. The results in Fig. 3 show that CCL2−/− mice had similar amounts of salmonellae in the GALT and spleen at days 2 and 5 after infection compared to C57BL/6-infected mice (panels A to D). However, CCL2−/− mice had a significantly greater bacterial burden in the liver at day 5 postinfection compared to wild-type controls (Fig. 3F). These data suggest that CCL2−/− mice cannot control the systemic dissemination of salmonellae and that this may be a contributing factor in the decreased survival rate.
FIG. 3.
Systemic bacterial burdens following gastric Salmonella infection. C57BL/6 and CCL2−/− mice were fed 108 virulent salmonellae. At 2 and 5 days postinfection, four mice were euthanized and the GALT (A and B), spleens (C and D), and livers (E and F) were homogenized and plated on LB agar plates to determine numbers of CFU. The horizontal bar shows the average number of CFU per group. The data are representative of three separate experiments. An asterisk denotes P < 0.05 comparing the bacterial counts of CCL2−/− mice to those of wild-type mice.
Increased levels of inflammatory cytokines in CCL2−/− mice after infection with salmonellae.
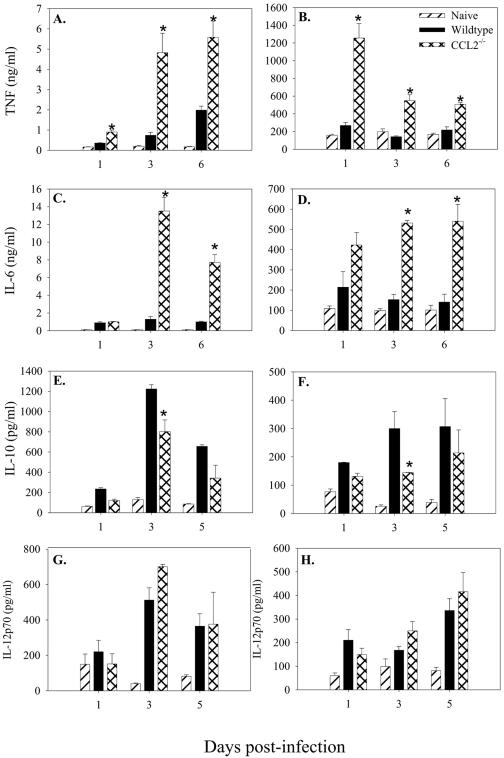
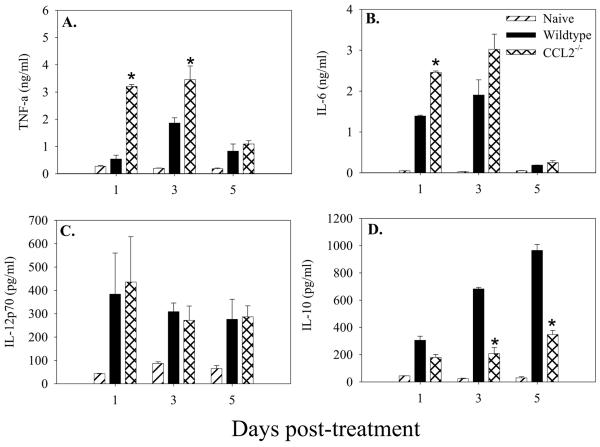
In order to gain insight into the mechanism of CCL2 regulation of immunity to salmonellae, we considered the possibility that CCL2 regulated inflammatory cytokine expression following infection. Serum and mucosal tissue from mice infected with 104 salmonellae were examined for expression of cytokines that have been shown to be important during infection (TNF-α, IL-6, IL-10, and IL-12). The results in Fig. 4 show that levels of TNF-α were significantly higher in both the serum and mucosal tissue of the CCL2−/− animals at each time point following infection compared to wild-type mice. Serum and mucosal IL-6 was also significantly elevated following Salmonella infection in the CCL2−/− mice (Fig. 4C and D, respectively). Interestingly, 3 days following infection with salmonellae, CCL2−/− mice had significantly lower levels of serum and mucosal IL-10 (Fig. 4E and F) and no observable differences in IL-12p70 (Fig. 4G and H) or IFN-γ (data not shown) compared to C57BL/6 mice. These data suggest that following Salmonella infection, CCL2−/− mice express higher levels of TNF-α and IL-6 and that the high mortality rate seen in the CCL2−/− mice may be due to this dysregulated cytokine response.
FIG. 4.
Cytokine expression after infection with salmonellae. C57BL/6 and CCL2−/− mice were infected with 104 virulent salmonellae. At days 1, 3, and 6 following infection, three mice were euthanized per group and TNF-α (A and B), IL-6 (C and D), IL-10 (E and F), and IL-12p70 (G and H) were measured in the serum (A, C, E, and G) and GALT (B, D, F, and H). The data are expressed as the mean cytokine production of three mice and are representative of three separate experiments. An asterisk denotes P < 0.05 comparing the mean CCL2−/− cytokine production to that of the wild type for that day.
CCL2−/− mice have decreased survival after Salmonella LPS administration.
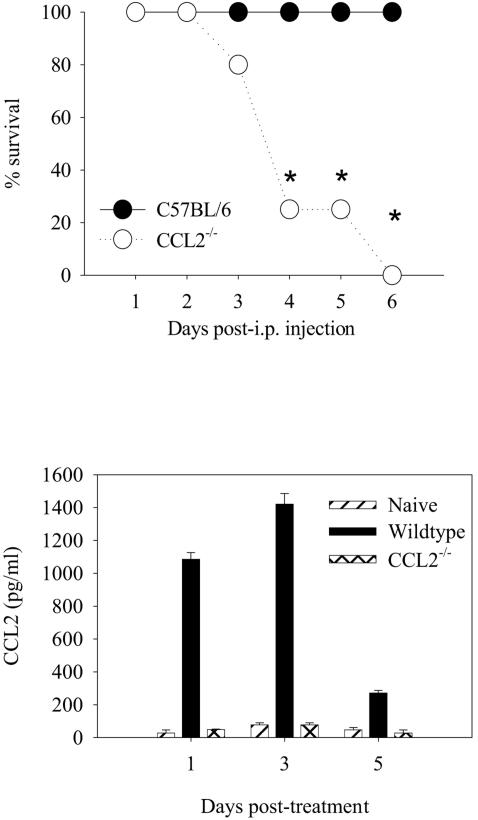
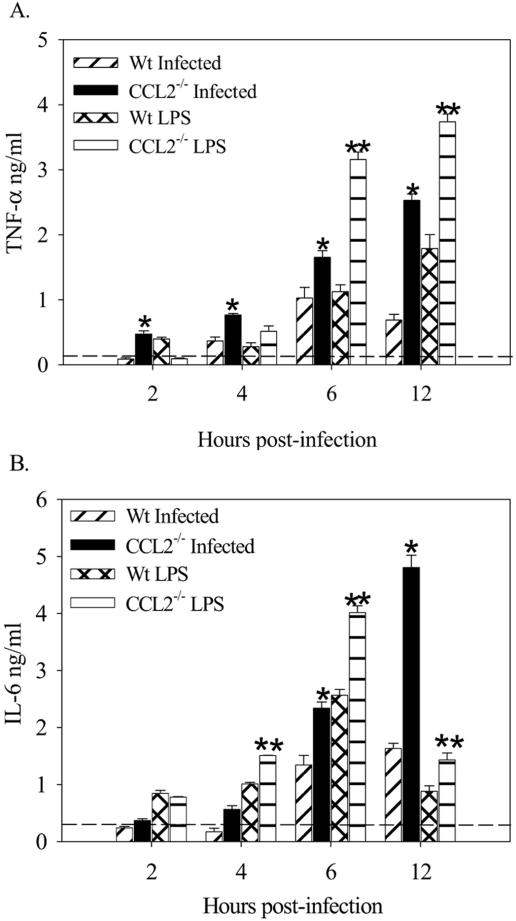
CCL2−/− mice succumb to infection with salmonellae at a dose at which wild-type mice effectively clear the bacteria (Fig. 2C). In an effort to understand the increased morbidity and mortality and inflammatory cytokine production of the CCL2−/− mice, we administered Salmonella-derived LPS to CCL2−/− and C57BL/6 mice. The rationale behind this experiment was the possibility that the increased bacterial load seen in the livers of CCL2−/− mice 5 days after infection could be contributing an increased LPS load that is known to stimulate TNF-α expression. The results in Fig. 5 show that control C57BL/6 mice administered 50 μg of purified Salmonella LPS had little morbidity. In contrast, CCL2−/− mice that were given the same dose of purified Salmonella LPS became moribund and were euthanized on the third day after the LPS challenge (Fig. 5). Figure 5B shows that serum levels of CCL2 are also elevated following treatment with purified Salmonella LPS in the wild-type mice between days 1 and 3 postchallenge. Serum was collected at three time points post LPS administration to evaluate levels of TNF-α, IL-6, IL-12p70, and IL-10 in CCL2−/− and C57BL/6 control mice. The results in Fig. 6 show that CCL2−/− mice that received 50 μg purified Salmonella LPS expressed significantly higher levels of serum TNF-α (Fig. 6Α) and IL-6 (Fig. 6B) at all time points compared to C57BL/6 mice. Interestingly, the CCL2−/− mice had significantly less serum IL-10 compared to wild-type mice (Fig. 6C). IL-12p70 levels were not significantly different between the groups (Fig. 6D). The results in Fig. 7 show that macrophages derived from CCL2−/− mice infected with salmonellae or stimulated with Salmonella LPS produced significantly larger amounts of TNF-α and IL-6. The data in Fig. 5 and 6 obtained using purified Salmonella LPS recapitulate the findings obtained with Salmonella-infected CCL2−/− mice. Collectively, these data suggest that CCL2−/− mice exhibit increased morbidity and mortality due to increased inflammatory cytokine expression induced by an elevated systemic LPS burden.
FIG. 5.
Survival following Salmonella LPS challenge. (A) C57BL/6 and CCL2−/− mice were treated with 50 μg Salmonella LPS intraperitoneally (i.p.). Mice were evaluated twice daily for signs of illness and euthanized if they became moribund. These data are representative of three identical experiments. (B) Serum samples were collected from three mice per time point and pooled. CCL2 levels were evaluated in the pooled samples by cytokine bead array. An asterisk denotes P < 0.05 for CCL2−/− mouse percent survival compared to that of C57BL/6 mice for that day.
FIG. 6.
Cytokine expression after Salmonella LPS challenge. At 3 and 5 days after Salmonella LPS challenge, C57BL/6 and CCL2−/− mice were evaluated for proinflammatory cytokine secretion in the serum. TNF-α (A), IL-6 (B), IL-12 (C), and IL-10 (D) were measured by cytokine bead matrix. The data are the mean cytokine production from three individual mice per time point. These data are representative of three identical experiments. An asterisk denotes P < 0.05 when comparing CCL2−/− mouse cytokine levels to those of C57BL/6 mice on the same day.
FIG. 7.
Macrophage cytokine response to Salmonella infection and LPS challenge. Wild-type (Wt) and CCL2−/− mouse peritoneal macrophages were isolated, plated at a density of 5 × 106/well, and incubated with S. enterica serovar Typhimurium SL1334 (at a multiplicity of infection of 10) or with 1 μg/ml Salmonella LPS. Medium was collected at various time points, and the supernatants were analyzed for production of TNF-α (A) and IL-6 (B). These data are representative of three identical experiments. An asterisk denotes P < 0.05 for CCL2−/− mouse macrophages infected with salmonellae compared to wild-type macrophages infected with salmonellae. A double asterisk denotes P < 0.05 comparing CCL2−/− mouse macrophages to wild-type mouse macrophages incubated with Salmonella LPS.
DISCUSSION
The present study demonstrates that CCL2 acts as a critical effector molecule in the response against the gram-negative pathogen S. enterica serotype Typhimurium by regulating the host inflammatory cytokine response. After infection with salmonellae, mice secrete a large amount of CCL2 in the intestinal mucosal sites. As the salmonellae disseminate, CCL2 is found at high levels in systemic sites such as the spleen (Fig. 1) and serum. Mice deficient in CCL2 succumb to an endotoxic-disease-like illness regardless of the infecting dose of salmonellae (Fig. 2) and are less able to control a systemic Salmonella infection (Fig. 3). The CCL2−/− mice show significantly elevated levels of the inflammatory cytokines TNF-α and IL-6 (Fig. 4). The CCL2−/− mice also demonstrated decreased survival after administration of purified Salmonella LPS with accompanying elevated levels of TNF-α and IL-6 in the serum. Taken together, our results suggest that CCL2 is an important factor in the innate response against intestinal Salmonella infection and LPS load by regulating TNF-α and IL-6.
Three immediate possibilities exist that could explain how CCL2 regulates the immune response to and subsequent control of Salmonella infection. The first is that CCL2 could be regulating the migration of neutrophils and/or macrophages to tissue sites for the clearance of bacteria. We do not favor this possibility as we did not detect any changes in neutrophil or macrophage migration in mucosal, splenic, or liver tissue of infected CCL2−/− or control mice at any time after bacterial infection using both flow cytometric and standard histologic methods (data not shown). Despite our results, others have reported that administration of CCL2 to Salmonella-infected mice resulted in enhanced macrophage activation and accumulation (44). Although CCL2 addition can modulate leukocyte accumulation, it does not appear to be necessary for control of leukocyte accumulation in the regulation of Salmonella dissemination.
The second possibility that arises from our results is that CCL2 stimulates the expression of regulatory cytokines, such as IL-10, that inhibit inflammatory immune responses. Therefore, the lack of CCL2 resulted in the lack of IL-10. Interestingly, we found that CCL2−/− mice infected with salmonellae or treated with purified Salmonella LPS had lower levels of serum and mucosal IL-10 (Fig. 4 and 6). Our data partially support the results of another study examining the role of CCL2 during sepsis, where administration of anti-CCL2 antibody resulted in decreased serum levels of IL-10 and sepsis (69). IL-10 has been shown to be important in the pathogenesis of Salmonella infection and regulation of subsequent host immune responses. IL-10 levels are increased in susceptible strains of mice (47), suggesting that those strains producing IL-10 at high levels cannot adequately control the infection (15). Conversely, anti-IL-10 treatment of infected mice resulted in enhanced host resistance, suggesting that removing the down-regulating effect of IL-10 on host defense mechanisms actually boosted the ability to control the infection (1). The fact that our present work with salmonellae and the aforementioned study on sepsis (69) both found decreased levels of IL-10 in the absence of functional CCL2 is important because of the many immune suppressive effects of IL-10. IL-10 has been shown to block the antigen-presenting capacity of macrophages and dendritic cells (13, 14, 37). IL-10 can inhibit costimulatory molecule expression (13, 63) and block cytokine production (19). The major role for IL-10 in decreasing cytokine production is stabilization of IκBα (54) in the cytoplasm and preventing LPS-induced NFκB activation (62) by inhibiting the IκB kinases (52), which by phosphorylating the NFκB inhibitor IκBα induces the proteolysis of IκBα and the release of NFκB. IL-10 also induces suppressor of cytokine signaling 3, which can inhibit many aspects of IL-6-initiated signaling (46). Therefore, if there is a CCL2-mediated decrease in IL-10 function, one might expect a concomitant increase in inflammatory cytokines such as TNF-α. In models of endotoxemia and sepsis, IL-10 has been shown by a number of groups to inhibit TNF-α production (32, 58). The direct effect of CCL2 on the regulation of IL-10 in the present model of Salmonella infection is unknown but currently under investigation.
The third possibility to explain our present results is that CCL2 stimulation directly represses TNF-α and IL-6 expression. We are currently exploring this possibility in depth in this model. Hildebrandt and colleagues (23) have demonstrated that in CCR2−/− mice unable to respond to CCL2 there was a reduction in TNF-α levels. Therefore, we favor the interpretation in the present study that CCL2 is directly regulating TNF-α expression and the effects on IL-10 are secondary. This conclusion is also based on the observation that at days 1, 3, and 6 post Salmonella infection, TNF-α is dramatically enhanced in the CCL2−/− mice whereas IL-10 expression is not affected until day 3 postinfection (Fig. 4). This idea is also supported by a previous study that demonstrated that the endogenous TNF-α expression in response to LPS challenge functioned to inhibit early IL-10 production but enhance later IL-10 production in the liver (2). However, this does not rule out the possibility that the down regulation of IL-10 contributes to enhanced inflammatory cytokine expression at later time points. During septic shock with gram-negative microorganisms, mortality is determined by two independent factors: high concentrations of circulating proinflammatory cytokines and multiplication of the microorganisms in the organs of the host. This is best demonstrated by the study that showed that TNF−/− LT−/− mice were more resistant to LPS challenge and more susceptible to Salmonella infection (11). It is well accepted that Salmonella infection results in activation of the TLR4 signaling cascade culminating with TNF-α expression (51) via the NF-κB pathway (17). Furthermore, macrophages and neutrophils (30), as well as dendritic cells (67), have been shown to be sources of this proinflammatory cytokine. Neutralization of IL-1 or TNF-α has been shown to result in increased infection (12). These examples demonstrate the central nature of TNF expression during Salmonella infection and host defense.
That CCL2 can directly regulate inflammatory cytokine expression was previous demonstrated in oral tolerance systems. Mice fed specific soluble antigen and treated with anti-CCL2 antisera demonstrated an inability to generate oral tolerance and showed enhanced mucosal IL-12 expression (27). Furthermore, mice lacking either CCL2 or its receptor CCR2 also did not develop oral tolerance due to enhanced IL-12 expression (10). Additionally, Braun et al. (5) demonstrated that direct stimulation of monocytes and dendritic cells with CCL2 resulted in decreased IL-12 and, to a lesser extent, TNF-α. The regulation of inflammatory cytokine expression by CCL2 can be modulated by other chemokines such as CCL5 (9), further illustrating the complex network of cytokines involved in host immune responses outside the realm of chemoattraction.
In addition to IL-10 and TNF-α as mediators in the host response to Salmonella infection, the IL-12 family members (53), as well as IL-18 (41, 45), have been shown to be involved in the host defense against salmonellae. Anti-IL-12 treatment of infected mice resulted in enhanced infection and increased disease (4, 8, 29). However, the importance of IL-12 appears to be linked to the p40 subunit as p40−/−, but not p35−/−, mice showed high mortality due to disseminated bacterial infection (34). Despite these examples, we did not find that CCL2 plays a significant role in the regulation of IL-12 expression during the host defense against Salmonella infection (Fig. 4). It is possible that the major effect of IL-12 in the host defense against salmonellae is further downstream in the generation of the adaptive immune response. In our experiments, the time period of 1 to 6 days postinfection is too early for the generation of a fully polarized Th1 response, thereby making CCL2 regulation of IL-12 during this period unlikely to be involved in the immediate control of infection.
Finally, additional chemokines have been implicated in the host defense against salmonellae. These include CCL20 (macrophage inflammatory protein 3α), where it has been demonstrated that infection of dendritic cells (20) and intestinal epithelial cells (25) with Salmonella induced the expression of CCL20. Treatment of Salmonella-infected mice with anti-CCL20 demonstrated an increased bacterial load in the Peyer's patches but not in the spleen (18), demonstrating a regional role for this chemokine. Whether CCL2 can directly regulate CCL20 expression is unknown.
The present report details the critical importance of CCL2 during Salmonella infection through modulating cytokines of the innate immune system. Taken together, these data suggest an important regulatory function that CCL2 plays during innate immune responses to gram-negative bacteria and could be useful in potential therapies and drug design.
Acknowledgments
This work was supported in part by NIH grant R01 AI035934 (W.J.K.).
Editor: F. C. Fang
REFERENCES
- 1.Arai, T., K. Hiromatsu, H. Nishimura, Y. Kimura, N. Kobayashi, H. Ishida, Y. Nimura, and Y. Yoshikai. 1995. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defense mechanism against murine Salmonella infection. Immunology 85:381-388. [PMC free article] [PubMed] [Google Scholar]
- 2.Barsig, J., S. Kusters, K. Vogt, H. D. Volk, G. Tiegs, and A. Wendel. 1995. Lipopolysaccharide-induced interleukin-10 in mice: role of endogenous tumor necrosis factor alpha. Eur. J. Immunol. 25:2888-2893. [DOI] [PubMed] [Google Scholar]
- 3.Bossink, A. W., L. Paemen, P. M. Jansen, C. E. Hack, L. G. Thijs, and J. Van Damme. 1995. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood 86:3841-3847. [PubMed] [Google Scholar]
- 4.Bost, K. L., and J. D. Clements. 1995. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect. Immun. 63:1076-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, M. C., E. Lahey, and B. L. Kelsall. 2000. Selective suppression of IL-12 production by chemoattractants. J. Immunol. 164:3009-3017. [DOI] [PubMed] [Google Scholar]
- 6.Cheminay, C., D. Chakravortty, and M. Hensel. 2004. Role of neutrophils in murine salmonellosis. Infect. Immun. 72:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chensue, S. W., K. S. Warmington, J. H. Ruth, P. S. Sanghi, P. Lincoln, and S. L. Kunkel. 1996. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation—relationship to local inflammation, Th cell expression, and IL-12 production. J. Immunol. 157:4602-4608. [PubMed] [Google Scholar]
- 8.Chong, C., K. L. Bost, and J. D. Clements. 1996. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonella spp. Infect. Immun. 64:1154-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePaolo, R. W., R. Lathan, and W. J. Karpus. 2004. CCR5 regulates high dose oral tolerance by modulating CC chemokine ligand 2 levels in the GALT. J. Immunol. 173:314-320. [DOI] [PubMed] [Google Scholar]
- 10.DePaolo, R. W., B. J. Rollins, W. Kuziel, and W. J. Karpus. 2003. CC chemokine ligand 2 and its receptor regulate mucosal production of IL-12 and TGF-β in high dose oral tolerance. J. Immunol. 171:3560-3567. [DOI] [PubMed] [Google Scholar]
- 11.Dharmana, E., M. Keuter, M. G. Netea, I. C. Verschueren, and B. J. Kullberg. 2002. Divergent effects of tumor necrosis factor-alpha and lymphotoxin-alpha on lethal endotoxemia and infection with live Salmonella typhimurium in mice. Eur. Cytokine Netw. 13:104-109. [PubMed] [Google Scholar]
- 12.Dinarello, C. A. 2003. Anti-cytokine therapeutics and infections. Vaccine 21:S24-S34. [DOI] [PubMed] [Google Scholar]
- 13.Ding, L., P. S. Linsley, L.-Y. Huang, R. N. Germain, and E. M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 14.Ding, L., and E. M. Shevach. 1992. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol. 148:3133-3139. [PubMed] [Google Scholar]
- 15.Eckmann, L., and M. F. Kagnoff. 2001. Cytokines in host defense against Salmonella. Microbes Infect. 3:1191-1200. [DOI] [PubMed] [Google Scholar]
- 16.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elewaut, D., J. A. DiDonato, J. M. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 163:1457-1466. [PubMed] [Google Scholar]
- 18.Fahy, O. L., S. L. Townley, N. J. Coates, I. Clark-Lewis, and S. R. McColl. 2004. Control of Salmonella dissemination by macrophage inflammatory protein (MIP)-3α/CCL20. Lab. Investig. 84:1511. [DOI] [PubMed] [Google Scholar]
- 19.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 20.Fu, G., O. L. C. Wijburg, P. U. Cameron, J. D. Price, and R. A. Strugnell. 2005. Salmonella enterica serovar Typhimurium infection of dendritic cells leads to functionally increased expression of the macrophage-derived chemokine. Infect. Immun. 73:1714-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 22.Gu, L., S. Tseng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins. 2000. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407-411. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt, G. C., U. A. Duffner, K. M. Olkiewicz, L. A. Corrion, N. E. Willmarth, D. L. Williams, S. G. Clouthier, C. M. Hogaboam, P. R. Reddy, B. B. Moore, W. A. Kuziel, C. Liu, G. Yanik, and K. R. Cooke. 2004. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 103:2417-2426. [DOI] [PubMed] [Google Scholar]
- 24.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 25.Izadpanah, A., M. B. Dwinell, L. Eckmann, N. M. Varki, and M. F. Kagnoff. 2001. Regulated MIP-3α/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G710-G719. [DOI] [PubMed] [Google Scholar]
- 26.Jansen, P. M., T. C. van der Pouw Kraan, I. W. de Jong, G. van Mierlo, J. Wijdenes, L. Aarden, A. A. Chang, F. B. Taylor, Jr., and C. E. Hack. 1996. The release of interleukin-12 in Escherichia coli-induced lethal and sublethal primate sepsis. Ann. N. Y. Acad. Sci. 795:351-353. [DOI] [PubMed] [Google Scholar]
- 27.Karpus, W. J., K. J. Kennedy, S. L. Kunkel, and N. W. Lukacs. 1998. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J. Exp. Med. 187:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpus, W. J., N. W. Lukacs, K. J. Kennedy, W. S. Smith, S. D. Hurst, and T. A. Barrett. 1997. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 158:4129-4136. [PubMed] [Google Scholar]
- 29.Kincy-Cain, T., J. D. Clements, and K. L. Bost. 1996. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect. Immun. 64:1437-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 169:4450-4459. [DOI] [PubMed] [Google Scholar]
- 31.Kirschning, C. J., H. Wesche, A. T. Merrill, and M. Rothe. 1998. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauw, F. N., D. Pajkrt, C. E. Hack, M. Kurimoto, S. J. van Deventer, and P. T. van Der. 2000. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 165:2783-2789. [DOI] [PubMed] [Google Scholar]
- 33.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann, J., S. Bellmann, C. Werner, R. Schroder, N. Schutze, and G. Alber. 2001. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J. Immunol. 167:5304-5315. [DOI] [PubMed] [Google Scholar]
- 35.Lukacs, N. W., S. W. Chensue, R. E. Smith, R. M. Strieter, K. Warmington, C. Wilke, and S. L. Kunkel. 1994. Production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α by inflammatory granuloma fibroblasts. Am. J. Pathol. 144:711-718. [PMC free article] [PubMed] [Google Scholar]
- 36.Lukacs, N. W., S. L. Kunkel, R. M. Strieter, K. Warmington, and S. W. Chensue. 1993. The role of macrophage inflammatory protein 1α in Shistosoma mansoni egg-induced granulomatous inflammation. J. Exp. Med. 177:1551-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macatonia, S. E., T. M. Doherty, S. C. Knight, and A. O'Garra. 1993. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-γ production. J. Immunol. 150:3755-3765. [PubMed] [Google Scholar]
- 38.Mandal, B. K., and J. Brennand. 1988. Bacteraemia in salmonellosis: a 15 year retrospective study from a regional infectious diseases unit. Br. Med. J. 297:1242-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandal, B. K., and V. Mani. 1976. Colonic involvement in salmonellosis. Lancet i:887-888. [DOI] [PubMed] [Google Scholar]
- 40.McCormick, B. A., P. M. Hofman, J. Kim, D. K. Carnes, S. I. Miller, and J. L. Madara. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol. 131:1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuno, Y., H. Takada, A. Nomura, C. H. Jin, H. Hattori, K. Ihara, T. Aoki, K. Eguchi, and T. Hara. 2003. Th1 and Th1-inducing cytokines in Salmonella infection. Clin. Exp. Immunol. 131:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monack, D. M., D. M. Bouley, and S. Falkow. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFN-γ neutralization. J. Exp. Med. 199:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy, P. M., M. Baggiolini, I. F. Charo, C. A. Hebert, R. Horuk, K. Matsushima, L. H. Miller, J. J. Oppenheim, and C. A. Power. 2000. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52:145-176. [PubMed] [Google Scholar]
- 44.Nakano, Y., T. Kasahara, N. Mukaida, Ko, Y. C., M. Nakano, and K. Matsushima. 1994. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect. Immun. 62:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Netea, M. G., G. Fantuzzi, B. J. Kullberg, R. J. Stuyt, E. J. Pulido, R. C. McIntyre, Jr., L. A. Joosten, J. W. Van der Meer, and C. A. Dinarello. 2000. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644-2649. [DOI] [PubMed] [Google Scholar]
- 46.Niemand, C., A. Nimmesgern, S. Haan, P. Fischer, F. Schaper, R. Rossaint, P. C. Heinrich, and G. Muller-Newen. 2003. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J. Immunol. 170:3263-3272. [DOI] [PubMed] [Google Scholar]
- 47.Pie, S., P. Matsiota-Bernard, P. Truffa-Bachi, and C. Nauciel. 1996. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect. Immun. 64:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed, K. A., M. E. Hobert, C. E. Kolenda, K. A. Sands, M. Rathman, M. O'Connor, S. Lyons, A. T. Gewirtz, P. J. Sansonetti, and J. L. Madara. 2002. The Salmonella typhimurium flagellar basal body protein FliE is required for flagellin production and to induce a proinflammatory response in epithelial cells. J. Biol. Chem. 277:13346-13353. [DOI] [PubMed] [Google Scholar]
- 49.Robbins, J. B., R. Schneerson, I. L. Acharya, C. U. Lowe, S. S. Szu, E. Daniels, Y. H. Yang, and B. Trollfors. 1988. Protective roles of mucosal and serum immunity against typhoid fever. Monogr. Allergy 24:315-320. [PubMed] [Google Scholar]
- 50.Rodenburg, R. J., R. F. Brinkhuis, R. Peek, J. R. Westphal, F. H. Van Den Hoogen, van W. J. Venrooij, and L. B. van de Putte. 1998. Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interleukin-1β, tumor necrosis factor alpha, and lipopolysaccharide. J. Leukoc. Biol. 63:606-611. [DOI] [PubMed] [Google Scholar]
- 51.Royle, M. C., S. Totemeyer, L. C. Alldridge, D. J. Maskell, and C. E. Bryant. 2003. Stimulation of Toll-like receptor 4 by lipopolysaccharide during cellular invasion by live Salmonella typhimurium is a critical but not exclusive event leading to macrophage responses. J. Immunol. 170:5445-5454. [DOI] [PubMed] [Google Scholar]
- 52.Schottelius, A. J., M. W. Mayo, R. B. Sartor, and A. S. Baldwin, Jr. 1999. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J. Biol. Chem. 274:31868-31874. [DOI] [PubMed] [Google Scholar]
- 53.Schuetze, N., S. Schoeneberger, U. Mueller, M. A. Freudenberg, G. Alber, and R. K. Straubinger. 2005. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int. Immunol. 17:649-659. [DOI] [PubMed] [Google Scholar]
- 54.Shames, B. D., C. H. Selzman, D. R. Meldrum, E. J. Pulido, H. A. Barton, X. Meng, A. H. Harken, and R. C. McIntyre, Jr. 1998. Interleukin-10 stabilizes inhibitory κB-α in human monocytes. Shock 10:389-394. [PubMed] [Google Scholar]
- 55.Sozzani, S., P. Allavena, D'Amico, G., W. Luini, G. Bianchi, M. Kataura, T. Imai, O. Yoshie, R. Bonecchi, and A. Mantovani. 1998. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161:1083-1086. [PubMed] [Google Scholar]
- 56.Sprenger, H., A. Krause, A. Kaufmann, S. Priem, D. Fabian, G. R. Burmester, D. Gemsa, and M. G. Rittig. 1997. Borrelia burgdorferi induces chemokines in human monocytes. Infect. Immun. 65:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sylvester, I., A. F. Suffredini, A. J. Boujoukos, G. D. Martich, R. L. Danner, T. Yoshimura, and E. J. Leonard. 1993. Neutrophil attractant protein-1 and monocyte chemoattractant protein-1 in human serum. Effects of intravenous lipopolysaccharide on free attractants, specific IgG autoantibodies and immune complexes. J. Immunol. 151:3292-3298. [PubMed] [Google Scholar]
- 58.van Der, P. T., P. M. Jansen, W. J. Montegut, C. C. Braxton, S. E. Calvano, S. A. Stackpole, S. R. Smith, S. W. Swanson, C. E. Hack, S. F. Lowry, and L. L. Moldawer. 1997. Effects of IL-10 on systemic inflammatory responses during sublethal primate endotoxemia. J. Immunol. 158:1971-1975. [PubMed] [Google Scholar]
- 59.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 60.Vazquez-Torres, A., B. A. Vallance, M. A. Bergman, B. B. Finlay, B. T. Cookson, J. Jones-Carson, and F. C. Fang. 2004. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J. Immunol. 172:6202-6208. [DOI] [PubMed] [Google Scholar]
- 61.Vidal, S., P. Gros, and E. Skamene. 1995. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J. Leukoc. Biol. 58:382-390. [DOI] [PubMed] [Google Scholar]
- 62.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor κB (NFκB) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 63.Willems, F., A. Marchant, J. P. Delville, C. Gerard, A. Delvaux, T. Velu, M. de Boer, and M. Goldman. 1994. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur. J. Immunol. 24:1007-1009. [DOI] [PubMed] [Google Scholar]
- 64.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 65.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 66.Yang, R. B., M. R. Mark, A. L. Gurney, and P. J. Godowski. 1999. Signaling events induced by lipopolysaccharide-activated Toll-like receptor 2. J. Immunol. 163:639-643. [PubMed] [Google Scholar]
- 67.Yrlid, U., and M. J. Wick. 2002. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J. Immunol. 169:108-116. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, S., L. G. Adams, J. Nunes, S. Khare, R. M. Tsolis, and A. J. Baumler. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 71:4795-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zisman, D. A., S. L. Kunkel, R. M. Strieter, W. C. Tsai, K. Bucknell, J. Wilkowski, and T. J. Standiford. 1997. MCP-1 protects mice in lethal endotoxemia. J. Clin. Investig. 99:2832-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]