Abstract
Ornithobacterium rhinotracheale is a bacterial pathogen known for causing respiratory disease in poultry. In this study, we demonstrate for the first time that cross-protective immunity against different O. rhinotracheale serotypes can be induced by live vaccination. Sera from these live-vaccinated and cross-protected birds were used to identify new vaccine targets by screening an O. rhinotracheale expression library. Out of 20,000 screened plaques, a total of 30 cross-reactive clones were selected for further analysis. Western blot analysis and DNA sequencing identified eight different open reading frames. The genes encoding the eight cross-reactive antigens were amplified, cloned in an expression vector, and expressed in Escherichia coli. Purified recombinant proteins with a molecular mass ranging from 35.9 kDa to 62.9 kDa were mixed and tested as a subunit vaccine for (cross-)protection against challenge with homologous and heterologous O. rhinotracheale serotypes in chickens. Subunit vaccination resulted in the production of antibodies reactive to the recombinant proteins on Western blot, and this eight-valent vaccine conferred both homologous and heterologous protection against O. rhinotracheale challenge in chickens.
A major challenge in vaccine development against bacterial infections is the existence of different serotypes within a pathogen species, since the use of subunits or inactivated bacterin vaccines mostly provides low or only partial cross-protection and not always for all serotypes. Therefore, the use of a directed approach in order to identify cross-protective antigens or epitopes will greatly contribute to improvement of current vaccines. Here, we describe an efficient approach that allows the identification of potential cross-protective antigens of a poultry pathogen, Ornithobacterium rhinotracheale.
Ornithobacterium rhinotracheale is a gram-negative bacterial pathogen most known for causing respiratory tract infections, such as airsacculitis and pneumonia, in birds all over the world. The pathogen may also cause systemic diseases e.g., hepatitis, joint lesions, and cerebrovascular pathology (4, 9, 32, 35). The outcome of O. rhinotracheale infection varies from a mild disease to death and can be influenced by host factors and environmental conditions. Furthermore, other infectious agents of the respiratory tract such as Escherichia coli (24), Bordetella avium (7), and Newcastle diseases (ND) virus (31) have a triggering effect on the manifestation of O. rhinotracheale infection. Treatment of O. rhinotracheale infection is becoming more and more difficult, as most isolates have acquired resistance against the regularly used antibiotics (8, 17, 29). Therefore, administration of a suitable vaccine inducing protective immunity against O. rhinotracheale infection is a good solution.
Current O. rhinotracheale vaccines, based on inactivated bacterin formulations, have proven to be efficacious against O. rhinotracheale infection with homologous serotypes (34). Because 18 different O. rhinotracheale serotypes have currently been identified (4, 33), an immunization strategy is needed that protects birds, especially turkeys, from infections against multiple serotypes. With respect to poultry pathogens and cross-protection, vaccination with live vaccines is generally of higher quality than vaccination with killed whole-cell vaccines (2, 10, 26). Most likely, cross-protective immunity is elicited by specific antigens absent in killed in vitro-grown (bacterin) preparations but produced specifically in vivo after live vaccination or infection. In addition, a living bacterium will reach certain niches in the body of the host that can be important for the induction of the proper protective immune responses (11, 20, 28). Therefore, live avirulent O. rhinotracheale vaccine strains can be an option for a new immunization strategy, but for now, it is still difficult to genetically engineer this bacterium, and knowledge about the molecular pathogenesis of O. rhinotracheale infections is still limited. An alternative strategy is the identification of cross-protective antigens in combination with a suitable vaccine application.
By means of immune depletion and reconstitution experiments, we previously demonstrated that the antibody-mediated immune response can provide cross-protective immunity against O. rhinotracheale infection in chickens (27). In this study, we made use of cross-protective antisera for the detection of relevant antigens by screening an O. rhinotracheale genomic expression library. Specific antisera used in immunoscreening were obtained from (partially) cross-protected birds that had experienced cross-infection by live vaccination with one serotype and subsequent challenge with a different serotype. Our hypothesis was that, as a cause of a heterologous booster, birds would produce higher titers of cross-reactive and also cross-protective antibodies. Using this approach, different cross-reactive antigens were identified, and the immunogenicity of these antigens was assessed by subunit vaccination of broiler chickens followed by homologous and heterologous O. rhinotracheale challenge.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
O. rhinotracheale serotype A strain B3263/91, O. rhinotracheale serotype B strain GGD 1261, O. rhinotracheale serotype G strain O-95029 nr.16279, and O. rhinotracheale serotype M strain TOP 98036 4500 were grown on 5% sheep blood agar at 37°C in a 5% CO2 atmosphere for 48 h. For liquid culture, single colonies were inoculated in Todd Hewitt (TH) medium (Difco, Detroit, MI) and grown for 24 h at 37°C on a 100-rpm shaker. E. coli strain XL1-Blue was obtained from Clontech Laboratories (Palo Alto, CA) and grown in Luria Bertani (LB) broth supplemented with 10 mM MgSO4 and 0.2% maltose. E. coli strains TOP10 (Invitrogen, Carlsbad, CA) and BL21(DE3) codon RIL pLysS (Novagen, Madison, WI) were used for cloning and protein expression, respectively. Both strains were grown in Terrific broth for protein expression supplemented with 10 mM MgSO4. All E. coli liquid cultures were grown for 16 to 20 h at 37°C on a 200-rpm shaker.
Chickens.
Commercial Ross broiler chickens were used in live vaccination experiments, and specific-pathogen-free (SPF) broiler chickens (Intervet, Boxmeer, The Netherlands) were used for subunit vaccine trials. All animals were placed at the day of hatch in negative-pressure isolators of approximately 1.5 m3 with a maximum of 12 birds per isolator in a 7-week study. The animals received sterilized food (Hendrix, Boxmeer, The Netherlands) and water ad libitum. In the subunit vaccine experiments, where there was no risk of cross-contamination, the birds of different test groups were housed in a mixed population to diminish isolator effects. All animal studies were approved by the committee for animal experiments in The Netherlands (DEC) according to international regulations.
Live vaccination study.
At 2 weeks of age, Ross broiler chickens were live vaccinated with O. rhinotracheale serotype B strain GGD 1261, O. rhinotracheale serotype G strain O-95029 nr.16279, or O. rhinotracheale serotype M strain TOP 98036 4500. Birds, housed in isolators, were vaccinated by aerosol spraying of 100 ml of a fresh bacterial culture containing approximately 108 CFU per ml TH medium (Difco, Detroit, MI). During aerosol spraying, the bacterial culture was administered as a fine spray to the birds using a commercial paint sprayer. The developed mist in the isolators was maintained for at least 10 min with the air circulation closed. A challenge control group of unvaccinated birds was included in the test. At 30 days of age, 5 days before aerosol challenge, birds were triggered with ND strain LaSota by aerosol spray of approximately 106 egg infectious doses (EID) per bird. At 5 weeks of age, 3 weeks after live vaccination, blood samples were collected for serological analysis, and after that, chickens were challenged with O. rhinotracheale serotype A strain B3263/91. Challenge was done either by aerosol spraying of 100 ml of a fresh bacterial culture containing approximately 109 CFU per ml, as described above, or by administration of 0.5 ml of the same bacterial culture intravenously (i.v.). An unvaccinated and unchallenged ND control group was included in the test. One week after challenge, at 6 weeks of age, birds were bled, blood samples were collected for serology, and postmortem analysis was performed as described below. Each treatment group contained 11 animals.
Postmortem examination and parameters of infection.
The birds were bled, and organ lesions were macroscopically scored for typical respiratory and systemic pathology caused by O. rhinotracheale using the following scoring system: for thoracic air sacs, a score of 0 was given for no abnormalities, 1 was given if one air sac was seriously affected by fibrinous airsacculitis or both air sacs containing limited pinhead-sized foci of fibrinous exudates, and 2 was given if both air sacs were seriously affected by fibrinous airsacculitis; for abdominal air sacs, a score of 0 was given for no abnormalities, 1 was given for pinhead-sized foci of fibrinous exudates or slight diffuse fibrinous airsacculitis, and 2 was given for severe fibrinous airsacculitis (the airsacculitis score is given as the sum of both scores); for lungs, a score of 0 was given for no abnormalities, 1 was given for unilateral pneumonia, and 2 was given for bilateral pneumonia; for liver, a score of 0 was given for no abnormalities, 1 was given for pinhead-sized foci, and 2 was given for severe hepatitis; and for the joints, 0 was given for no abnormalities, and 1 point was given for each joint that showed purulent exudates. The average group scores are given as a percentage of the maximum possible respiratory or systemic score. Statistical analysis was performed using the Kruskal-Wallis nonparametric one-way analysis of variance test.
Serological investigation and antiserum preparation.
Serum samples were collected at the beginning of each experiment by bleeding 10 control birds. During the animal experiments, serum samples were collected before challenge and postmortem investigation. Serum samples were tested in an enzyme-linked immunosorbent assay (ELISA) against boiled extract antigens of the O. rhinotracheale serotypes as described previously by van Empel et al. (33). Serum antibody levels were represented as log2 titers including standard deviations.
For use in immunoscreening or Western blot analysis, sera from all birds within a treatment group (9 to 11 animals) were pooled. Preadsorption with E. coli XL1-Blue cell lysate was done as described previously by Sambrook et al. (25) before use in screening the expression library in order to reduce the aspecific background signal.
Construction of a genomic expression library and immunoscreening.
O. rhinotracheale serotype G genomic DNA was isolated from liquid-cultured cells according to the method described previously by Sambrook et al. (25), partially digested with Tsp509I restriction enzyme (New England Biolabs, Beverly, MA) to obtain 1- to 4-kb fragments, and cloned into the EcoRI-digested and dephosphorylated λTriplEx vector arms (Clontech, Palo Alto, CA). Packaging was performed using the Stratagene (La Jolla, CA) in vitro packaging extracts, and phage particles containing O. rhinotracheale DNA were transfected into E. coli-XL1-Blue, resulting in a genomic expression library containing 97% recombinants and a complexity of 6.9. The immunoscreening procedure was performed as described by the manufacturer (Clontech Manual; Clontech, Palo Alto, CA) under native conditions. In short, phage-infected E. coli XL1-Blue cells were plated in LB top agar onto LB agar plates and incubated at 42°C for 4 h, during which small clear plaques became visible. These plaques were covered with nitrocellulose filters (Schleicher and Schuell, Dassel, Germany) which were saturated with 10 mM of isopropyl-β-d-thiogalactopyranoside (IPTG) and incubated for a further 4 h at 37°C. Filters were removed from the plates, blocked in 0.04 M phosphate-buffered saline (PBS)-0.5% polysorbate-20-1% skim milk, and treated with a 1:250 dilution of primary antiserum (in 0.04 M PBS-0.05% polysorbate-20-1% skim milk). Rabbit anti-chicken immunoglobulin G (IgG) peroxidase conjugate (Nordic, Tilburg, The Netherlands) in a 1:1,000 dilution (in 0.04 M PBS--0.05% polysorbate-20-1% skim milk) was used as secondary antibody. Finally, filters were washed, and the substrate solution Vector SG (Vector, Burlingame, CA) was added. Positive (reactive) plaques located on the agar plates were picked and rescreened twice to obtain single purified clones.
PCR and sequencing.
Oligonucleotide primers used for both PCR amplification and (partial) sequencing of the DNA inserts of the selected plaques were specifically designed for the λTriplEx vector arms and synthesized by Life Technologies (Invitrogen, Carlsbad, CA). The 5′ primer used was 5′-GCG CCA TTG TGT TGG TAC-3′, and the 3′ primer used was 5′-TTT TTC TCG GGA AGC GCG-3′. PCR was performed in an automated thermal cycler (GeneAmp 9700; Perkin-Elmer, CA). The final PCR volume was 50 μl containing 50 μM of deoxynucleoside triphosphates (Promega, Madison, WI), 10 pmol of both primers, 20 U/ml of Supertaq plus polymerase, and Supertaq buffer (both HT Biotechnology Ltd., Cambridge, United Kingdom) in water. Phage DNA was added to the reaction mix by picking a freshly plated plaque. The following conditions were used: denaturation at 94°C for 3 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 2 min, and elongation at 68°C for 2 min 30 s, followed by a final extension at 68°C for 10 min.
To determine the nucleotide sequence of the DNA inserts, a sequence reaction was done (94°C for 10 s, 50°C for 5 s, and 60°C for 2 min for 25 cycles in an automated thermal cycler as described above) using Big Dye Terminator Ready reaction mix, 50 ng PCR product, and 2.4 pmol primer in a 20-μl reaction volume. Sequencing was done with an ABI 310 automated sequencer (Perkin-Elmer, CA). Data were collected using ABI 310 Collection software version 1.0.4 and analyzed with Sequence Analysis version 3.1 (Perkin-Elmer, CA). By using the partial sequences from the ends, new internal primers were designed to obtain the complete nucleotide sequence of the inserts. Contigs and alignments were made using Sequencer version 4.1.4 (Gene Codes Corporation).
The 5′-end sequence of the open reading frames (ORFs) cloned in fusion with the λTriplEx vector was determined by sequencing as described above, with genomic O. rhinotracheale serotype G DNA (2 μg) as a template and internal 30-mer primers using the following reaction conditions: 95°C for 5 min; 95°C for 30 s and 65°C − 0.5°C/cycle for 4 min 20 s for 10 cycles; 95°C for 30 s, 60°C − 0.5°C/cycle for 20 s, and 60°C for 4 min 20 s for 10 cycles; and 95°C for 30 s, 55°C for 30 s, and 60°C for 4 min 20 s for 80 cycles.
Bioinformatic analysis.
Hydrophilicity was analyzed by the method described previously by Kyte and Doolittle (16) using Sci Ed Central 2002 software. Signal sequence prediction was performed with SignalP (http://www.cbs.dtu.dk/services/SignalP) (21). Analysis for sequence homologies, protein families, and conserved domains was done using Prosite (http://au.expasy.org/prosite/) (13), Pfam (http://www.sanger.ac.uk/cgi-bin/Pfam), NCBI BLAST (http://ncbi.nlm.nih.gov/) (1, 19), and TIGR CRM (http://www.tigr.org/tdb/) (23).
Polyacrylamide gel electrophoresis (PAGE) and Western blot analysis.
NOVEX NuPAGE was used for protein electrophoresis under denaturating conditions in 4 to 12% polyacrylamide gels according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Protein bands were made visible by using Coomassie brilliant blue. For immunoanalysis, polypeptides were electroblotted onto an Immobilon polyvinylidene difluoride 0.45-μm membrane (Millipore, Bedford, MA) by semidry Western blotting according to a method described previously by Towbin et al. (30). Membranes were blocked in 0.04 M PBS-0-0.5% polysorbate-20-1% skim milk, washed with 0.04 M PBS-0-0.5% polysorbate-20, treated with a 1:250 dilution of primary antiserum (in 0.04 M PBS-0-0.05% polysorbate-20-1% skim milk), and washed with 0.04 M PBS-0-0.5% polysorbate-20. Rabbit anti-chicken IgG peroxidase conjugate (Nordic, Tilburg, The Netherlands) in a 1:1,000 dilution (in 0.04 M PBS-0-0.05% polysorbate-20-1% skim milk) was used as secondary antibody. Finally, filters were washed, and the substrate solution Vector SG (Vector, Burlingame, CA) was added.
Cloning and protein expression.
PCR-amplified gene products were cloned with a TOPO-TA cloning system (Invitrogen, Carlsbad, CA) and digested with the appropriate restriction enzymes (obtained from New England Biolabs, Beverly, MA) for directional cloning in the expression vector of interest. Ligation products were initially transformed into E. coli TOP10 competent cells (Invitrogen, Carlsbad, CA) and were subsequently transformed to E. coli BL21(DE3) codon RIL pLysS host cells (Novagen, Madison, WI) for expression. Genes were cloned into pET plasmid vector pET22b, and therefore, the recombinant proteins were expressed with an E. coli PelB leader peptide fused at the amino-terminal portion (O. rhinotracheale leader peptides were replaced) and six histidine residues at the carboxy-terminal portion of the protein. E. coli strain BL21(DE3) codon RIL pLysS (Novagen, Madison, WI) was used for high-level expression as described in the pET system manual. Recombinant antigens were isolated from supernatant or purified by (i) metal affinity chromatography using talon resin (Clontech Inc., Palo Alto, CA) in the presence of 8 M urea as described by the manufacturer or (ii) repeated freeze-thawing, sonification, and centrifugation steps in 5 mM Tris-2 mM EDTA, pH 7.5.
To collect non-O. rhinotracheale proteins as control vaccine antigens, E. coli BL21(DE3) codon RIL pLysS expression cells harboring an empty pET22b vector were followed by an equal procedure of metal affinity chromatography or repeated freeze-thawing, sonification, and centrifugation cycling as described above for the recombinant antigens.
Subunit vaccination studies.
The eight purified cross-reactive antigens were blended into one subunit vaccine in a water-in-oil emulsion (mineral oil adjuvant) and tested for antigenicity and (cross-)protective capacity in SPF broilers. In the first subunit vaccination study, at 2 weeks of age, birds were injected subcutaneously with 0.5 ml vaccine containing approximately 25 μg of each present antigen per dose. At 5 weeks of age, birds were primed with ND strain LaSota by aerosol spraying of approximately 106 EID per bird. At 6 weeks of age, blood samples were collected from all birds to analyze the antigenicity of the different antigens by Western blot. After blood sampling, at 6 weeks of age, chickens were challenged with either O. rhinotracheale serotype G strain O-95029 nr.16279 (homologous challenge) or O. rhinotracheale serotype A strain B3263/91 (heterologous challenge). The challenge was done by aerosol spraying of 100 ml of a fresh bacterial culture containing approximately 109 CFU per ml TH medium (Difco, Detroit, MI) as described above. One week after challenge, at 7 weeks of age, birds were sacrificed and respiratory organ lesions of air sacs and lungs were scored. Each treatment group contained 11 birds.
The above-described experiment in which cross-protection was analyzed was repeated in a second subunit vaccination study where a negative control group was included. In this experiment, birds were vaccinated at 2 weeks of age with either 0.5 ml of the cross-protective eight-component vaccine or 0.5 ml of a subunit vaccine containing approximately 25 μg per dose of proteins purified from the E. coli expression strain in a water-in-oil emulsion (mineral oil adjuvant [water-oil ratio, 40:60]). At 5 weeks of age, birds were primed with ND strain LaSota by aerosol spraying of approximately 106 EID per bird. At 6 weeks of age, chickens were challenged with O. rhinotracheale serotype A strain B3263/91 (heterologous challenge) as described above. One week after challenge, at 7 weeks of age, birds were sacrificed and respiratory organ lesions were scored. Each treatment group contained 11 birds.
Nucleotide sequence accession numbers.
Newly determined sequence data were deposited in the EMBL Nucleotide Sequence Database (http://www.ebi.ac.uk), and accession numbers were assigned as follows: for Or01, AJ748732; Or02, AJ748733; Or03, AJ748734; Or04, AJ748735; Or11, AJ748736; Or77, AJ748737; Or98A, AJ748738; Or98B, AJ748739.
RESULTS
Generation of cross-reactive antibodies.
In order to obtain O. rhinotracheale-specific antiserum containing potentially cross-protective antibodies directed against the complete repertoire of in vivo-expressed antigens, the following animal experiment was performed. Two-week-old broiler chickens were live vaccinated by aerosol spraying with an O. rhinotracheale serotype B, G, or M strain. Although natural infection was mimicked, no abnormalities or disease symptoms were observed, and 3 weeks later, at 5 weeks of age, only minor serological responses could be detected. (Table 1). The average serotype-specific serum IgG antibody titers of vaccinated birds (which ranged from 5.7 [±0.6] and 5.8 [±0.8] to 6.1 [±1.0] 3 weeks after serotype M, B, and G vaccination, respectively) remained close to the average levels of unvaccinated birds (5.2 [±0.3]). To boost the production of cross-reactive antibodies and to determine cross-protection by live vaccination (see below), at 5 weeks of age, after blood sampling, birds were reinfected with a heterologous O. rhinotracheale serotype A strain via the natural route, i.e., aerosol spraying (after an additional ND priming), or via an artificial route, i.e., intravenous injection. One week later, at 6 weeks of age, the average, serotype-specific IgG titers of serotype B-vaccinated birds increased from 5.8 (±0.8) to 9.8 (±1.3) and 12.9 (±1.2) after aerosol and intravenous challenge, respectively (Table 1). Serotype G- and serotype M-vaccinated birds that were aerosol challenged with serotype A showed no elevation but a minor reduction of serotype-specific IgG antibody levels, from 6.1 (±1.0) to 5.9 (±0.9) and from 5.7 (±0.6) to 5.4 (±0.5) (Table 1). However, intravenous challenge with serotype A did result in increased titers, from 6.1 (±1.0) to 8.0 (±1.5) in serotype G-vaccinated birds and from 5.7 (±0.6) to 7.5 (±1.2) in serotype M-vaccinated birds (Table 1). Furthermore, the unvaccinated serotype A challenge control birds also showed elevated antibody titers (Table 1).
TABLE 1.
Serological responses after live vaccination and (cross-)reactivity between different serotypes before and after challenge
| Treatment | Avg O. rhinotracheale serotype-specific ELISA titers (log2) (±SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 wks pva | Aerosol challengeb
|
Intravenous challengeb
|
|||||||
| A | B | G | M | A | B | G | M | ||
| Vaccination with serotype B | 5.8c (±0.8) | 11.2 (±1.4) | 9.8 (±1.3) | 6.5 (±1.4) | 6.2 (±0.6) | 14.6 (±1.1) | 12.9 (±1.2) | 8.1 (±1.6) | 8.5 (±0.7) |
| Vaccination with serotype G | 6.1 (±1.0) | 8.5 (±1.2) | 6.8 (±1.0) | 5.9 (±0.9) | 5.4 (±0.5) | 13.5 (±1.4) | 10.8 (±1.1) | 8.0 (±1.5) | 8.0 (±1.7) |
| Vaccination with serotype M | 5.7 (±0.6) | 8.4 (±2.1) | 6.2 (±1.7) | 5.2 (±0.3) | 5.4 (±0.5) | 11.9 (±2.5) | 9.7 (±2.8) | 7.2 (±1.3) | 7.5 (±1.2) |
| Challenge control | 5.2d (±0.3) | 9.9 (±2.3) | 8.6 (±2.0) | 5.8 (±0.5) | 5.9 (±0.5) | 13.4 (±0.9) | 11.2 (±1.3) | 7.0 (±0,9) | 7.9 (±1.0) |
Serum samples were obtained at 5 weeks of age, 3 weeks after live vaccination (pv) with O. rhinotracheale serotype B, G, or M.
Serum samples were obtained at 6 weeks of age, 1 week after challenge with O. rhinotracheale serotype A.
Serotype-specific ELISA titers are presented in boldface type.
Serum samples of unvaccinated control birds were analyzed for reactivity against serotype B, G, and M antigens, and values are represented as an average of these titers.
The serological cross-reactivity was analyzed in four different ELISAs for O. rhinotracheale serotypes A, B, G, and M. Antigenic cross-reactivity between the different treatment groups could be observed 1 week after both aerosol and intravenous challenge (Table 1), indicating the presence of common antigens and the presence of cross-reactive serum IgG antibodies. The highest antibody titers and strongest serological cross-reactivity were found against serotypes A and B.
Live vaccination and cross-protection.
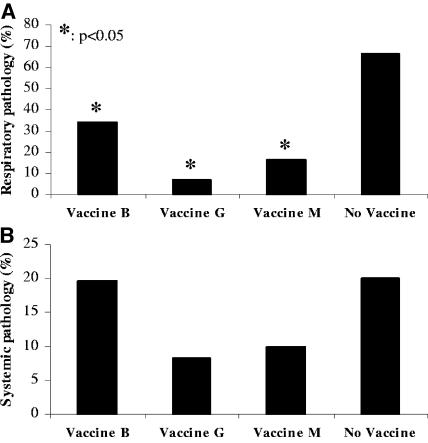
Cross-protection due to live vaccination was determined in the experiment described above. At 30 days of age, 5 days before aerosol challenge (at 5 weeks of age), birds were primed with ND strain LaSota in order to make the birds more susceptible to infection after the second challenge (since they already received a primary challenge by means of live vaccination at 2 weeks of age). One week after challenge, at 6 weeks of age, necropsy was performed, and organs were scored with respect to macroscopic pathology. Respiratory pathology (scored in air sacs and lungs) observed after aerosol infection, corrected for background pathology due to ND priming, is summarized in Fig. 1A. Birds that were live vaccinated with O. rhinotracheale serotypes B, G, or M followed by an aerosol challenge with O. rhinotracheale serotype A showed significantly lower (P < 0.05) (34.4%, 7.1%, and 16.7%, respectively) organ lesion scores than unvaccinated but aerosol-challenged birds (66.7%). Systemic pathology (scored in joints and liver) observed after intravenous infection is summarized in Fig. 1B. Birds live vaccinated with O. rhinotracheale serotype B before i.v. serotype A challenge showed no reduction in organ lesion score (19.7%) compared to that of the unvaccinated challenge control group (20%). Systemic symptoms of birds live vaccinated with O. rhinotracheale serotypes G (8.3%) and M (10%) showed a twofold but statistically nonsignificant (P > 0.05) reduction in comparison to the challenge control group.
FIG. 1.
Live vaccination and cross-protection. (A) Respiratory pathology after aerosol challenge with O. rhinotracheale serotype A. Lesions in air sacs and lungs were macroscopically scored and represented as the percentage of the maximum possible respiratory score. Values of groups live vaccinated with O. rhinotracheale serotype B, G, or M were significantly different compared to the value of the unvaccinated challenge control group (P < 0.05). (B) Systemic pathology after i.v. challenge with O. rhinotracheale serotype A. Lesions in joints and liver were macroscopically scored and represented as the percentage of the maximum possible systemic score.
Selection of cross-reactive clones by screening of a genomic expression library.
Since live vaccination with O. rhinotracheale serotype G induced the highest level of cross-protection against serotype A challenge, this serotype was selected for the construction of a genomic expression library. Approximately 2 × 104 plaques (representing the total genome) of this recombinant DNA library were screened under native conditions using the cross-protective sera obtained from O. rhinotracheale serotype G live-vaccinated and O. rhinotracheale serotype A i.v. challenged chickens. Using serum from i.v. challenged birds was preferred over using serum from aerosol-challenged birds since this serum contained the highest levels of O. rhinotracheale-specific IgG antibodies (Table 1). The primary screening of the complete library resulted in a selection of the 200 most intensely reactive plaques, which were rescreened twice, resulting in 175 single, pure, positive plaques.
Antiserum obtained from O. rhinotracheale serotype B or serotype M live-vaccinated and O. rhinotracheale serotype A i.v. challenged birds (Table 1) was used in the rescreening of the 175 plaques for cross-reactivity. Thirty plaques reacted positively in immunoscreening for all three serotypes, G, B, and M. No reactivity against the 30 plaques could be observed using negative serum from unvaccinated and unchallenged control birds (data not shown).
Identification of open reading frames encoding antigens.
The 30 cross-reactive clones were analyzed for the presence of cloned insert DNA by PCR; inserts ranged in size between 1.2 and 3.6 kb (data not shown). Sequence analysis of these inserts revealed that the total selection represented seven different sequence groups or loci with a variable number of matching clones. These groups were named Or01 (six clones), Or02 (five clones), Or03 (12 clones), Or04 (four clones), Or11 (one clone), Or77 (one clone), and Or98 (one clone). Analysis of the DNA sequences of the cloned inserts revealed multiple ORFs per sequence group.
To determine which ORFs were translated into the proteins recognized by antibodies during screening, clones were induced and phage plaques were collected for Western blot analysis under denaturating conditions. With a 1:1:1 mixture of the three sera previously used to select the cross-reactive plaques, protein products of Or01, Or02, Or03, Or04, and Or77 could be detected (Table 2). The molecular masses of the expressed proteins encoded by members of these different groups varied between 37 and 65 kDa (data not shown). Reactive bands of a certain clone varied in size within a 5-kDa range, indicating that proteins were expressed as a fusion protein with the λTriplEx vector. The sizes of the expressed fusion proteins detected on Western blot were comparable to the deduced proteins sizes of predicted ORFs present in the five different DNA sequences. As expected, these ORFs were in frame with coding sequences of the expression vector. The bands of cross-reactive proteins Or11 and Or98 could not be visualized using Western blot analysis. The DNA sequence of fragment Or11 also contained an ORF cloned in fusion with the vector. Only fragment Or98 contained two complete ORFs of comparable sizes (Or98A and Or98B) that were not cloned in frame with the expression vector. Therefore, both ORFs were considered to encode a possible cross-reactive antigen.
TABLE 2.
Summary of serological analysis of cross-reactive antigens
| Plaque | Immunoscreening (serum liveb) | Western blota (serum liveb) | Recombinant protein | Western blota
|
|
|---|---|---|---|---|---|
| Serum liveb | Serum subunitc | ||||
| Or01 | + | + | Or01 | + | + |
| Or02 | + | + | Or02 | + | + |
| Or03 | + | + | Or03 | + | + |
| Or04 | + | + | Or04 | + | + |
| Or11 | + | − | Or11 | − | − |
| Or77 | + | + | Or77 | + | + |
| Or98 | + | − | Or98A | − | − |
| Or98B | − | + | |||
Western blot analysis was performed under denaturating conditions.
Serum live, 1:1:1 mixture of sera obtained from birds live vaccinated with O. rhinotracheale serotype B, G, or M and i.v. challenged with serotype A.
Serum subunit, serum obtained from birds vaccinated with the subunit vaccine containing all eight recombinant proteins.
Since six out of eight proteins were expressed as a fusion protein, the 5′ end of these ORFs was missing. Therefore, sequence reactions were performed on genomic DNA of O. rhinotracheale serotype G using internal primers. Sequencing was done until the most upstream ATG start codon, corresponding to the ORF of interest, was reached. The sizes of the eight complete ORFs and encoded proteins were as follows: Or01, 1,614 bp and 59.8 kDa; Or02, 1,572 bp and 58.2 kDa; Or03, 1,242 bp and 46.0 kDa; Or04, 1,023 bp and 37.9 kDa; Or11, 1,230 bp and 45.6 kDa; Or77, 1,140 bp and 42.2 kDa; Or98A, 918 bp and 34.0 kDa; Or98B, 888 bp and 32.9 kDa (Table 3).
TABLE 3.
Identification and characterization of open reading frames
| Gene product | Size of ORF (bp) | Size of protein (aa) | Mol mass of protein (kDa) | Signal sequence | Similar protein in database (organism [% identity]) |
|---|---|---|---|---|---|
| Or01 | 1,614 | 537 | 59.8 | No | Dihydrolipoamide acetyltransferase (Bradyrhizobium japonicum [35]) |
| Or02 | 1,572 | 523 | 58.2 | Yesa | Putative outer membrane protein (Leptospira interrogans [31]) |
| Or03 | 1,242 | 413 | 46.0 | Yes | Hypothetical protein (Bacteroides thetaiotaomicron [23]) |
| Or04 | 1,023 | 340 | 37.9 | No | Hypothetical protein (Clostridium perfringens [25]) |
| Or11 | 1,230 | 409 | 45.6 | Yes | OmpA-like outer membrane protein (Desulfovibrio vulgaris [27]) |
| Or77 | 1,140 | 379 | 42.2 | Yesa | Hypothetical membrane-associated lipoprotein (Ureaplasma urealyticum [27]) |
| Or98A | 918 | 305 | 34.0 | No | Hypothetical protein (Actinobacillus pleuropneumoniae [30]) |
| Or98B | 888 | 295 | 32.9 | No | RecT protein (Clostridium tetani [44]) |
Signal sequence is characteristic for a gram-negative lipoprotein.
Characterization of identified open reading frames.
The amino acid sequences of the identified ORFs were compared with published microbial genome sequences and analyzed for protein families and conserved domains (1, 13, 16, 19, 21, 23). The results of this analysis are summarized in Table 3.
Sequence analysis indicated that Or01 codes for a dihydrolipoamide acetyltransferase (E2) component of a 2-oxo acid dehydrogenase with highest similarity to gram-negative bacteria Bradyrhizobium japonicum (35% identity and 51% similarity) and Brucella suis and Brucella melitensis (both 35% identity and 52% similarity). The protein contains two conserved lipoyl-binding sites and strongly hydrophobic regions. The N terminus of the protein showed a highly hydrophilic region.
Or02 shows similarity to a putative outer membrane protein of the bacterium Leptospira interrogans (31% identity and 43% similarity). The protein has a hydrophobic N-terminal end with the characteristics of a gram-negative signal peptide. A lipoprotein attachment site could also be identified.
Or03 shows similarity to a hypothetical protein with unknown function of gram-negative Bacteroides thetaiotaomicron (23% identity and 39% similarity). The hydrophobic N terminus of the protein has the characteristics of a gram-negative signal peptide.
Or04 codes for a protein with unknown function with closest homology to a hypothetical protein of the bacterium Clostridium perfringens (25% identity and 38% similarity). No signal sequence or transmembrane regions could be detected.
A domain search of Or11 detected an OmpA-like transmembrane domain, and among the BLAST hits, several outer membrane proteins were found. The most significant similarity was found with the OmpP1/FadL/TodX family from the bacterium Desulfovibrio vulgaris (27% identity and 41% similarity). Therefore, the sequence of Or11 probably represents an outer membrane protein for long-chain fatty acid transport. The hydrophobic N terminus of the protein has the characteristics of a gram-negative signal peptide.
No significant homologies were found for Or77. A domain search identified a cleavage site for a lipoprotein signal peptidase II and a lipoprotein-associated domain of approximately 100 amino acids in length (positions 164 to 266), which has 27% identity with a conserved hypothetical membrane lipoprotein of Ureaplasma urealyticum.
Or98A showed the closest similarity to a hypothetical protein of the bacterium Actinobacillus pleuropneumoniae (30% identity and 44% similarity). No significant domains could be detected.
A domain search of Or98B revealed a region characteristic for the RecT family with the most significant similarity with the RecT protein of the bacterium Clostridium tetani (44% identity and 66% similarity).
Cloning, expression, and purification of recombinant proteins.
In order to amplify the ORFs for expression of the different antigens, PCR primers containing specific restriction sites for directional cloning (Table 4) were designed. PCR products were cloned in pET22b, and recombinant proteins were expressed in E. coli with an E. coli PelB leader secretion signal peptide fused at the amino-terminal portion (putative O. rhinotracheale leader peptides were omitted in Or02, Or03, Or11, and Or77 [Table 4]) and six histidine residues at the carboxy-terminal portion of the protein (except for Or02).
TABLE 4.
Oligonucleotide primer sets for amplification of selected O. rhinotracheale genesa
| Gene product | 5′ oligonucleotide | 3′ oligonucleotide | PCR product size (bp) | 5′ start,d Mol mass of expression product (kDa)b |
|---|---|---|---|---|
| Or01 | 5′-GCTGGCCATGGCTGAAATTATAAAAATGCC-3′ | 5′-CCGCTCGAGCACAAGCATAGACATTGG-3′ | 1,627 | M1, 62.9 |
| MscI | XhoI | |||
| Or02 | 5′-CAGTCCATGGCATGTAGCGATTTTGAT-3′ | 5′-CCGCTCGAGGTGGTCTTTATAAAAATG-3′ | 1,578 | A19, 58.2c |
| NcoI | XhoI | |||
| Or03 | 5′-CAGTCCATGGCGATGATAATCAGTTCTTATG-3′ | 5′-CCGCTCGAGAATAAATTCATCATTAAGC-3′ | 1,092 | D31, 43.1 |
| NcoI | XhoI | |||
| Or04 | 5′-CGATGGCCATGAAAGATATATTTGAAT-3′ | 5′-CCGCTCGAGTTCTTCACTTGGTATTTTGA-3′ | 1,034 | M1, 41.0 |
| MscI | XhoI | |||
| Or11 | 5′-CGATGGCCATGGGGGCACAAGGTGTAGC-3′ | 5′-GCGGCCGCTACGATAAACCTAGACCAAA-3′ | 1,129 | M35, 44.5 |
| MscI | NotI | |||
| Or77 | 5′-CATGCCATGGTCTGTAGCAGTGATGATTAC-3′ | 5′-CCGCTCGAGGTTAATTGAAACTCTTAAGC-3′ | 1,107 | C17, 43.7 |
| NcoI | XhoI | |||
| Or98A | 5′-CAGTCCATGGTAAAAGACTTTTCAG-3′ | 5′-CCGCTCGAGTGCTATTAATTCTAATCG-3′ | 927 | V3, 37.0 |
| NcoI | XhoI | |||
| Or98B | 5′-CAGTCCATGGAATTAGCGAAAAACGAC-3′ | 5′-CCGCTCGAGTTTTAATTCATTTTTTCTG-3′ | 897 | E3, 35.9 |
| NcoI | XhoI |
Restriction sites are underlined, ATG start codons are in boldface type, and the gene of interest is in italics.
Including the E. coli PelB leader peptide and His tail.
Cloned without the C-terminal His tail.
First amino acid of the O. rhinotracheale protein sequence.
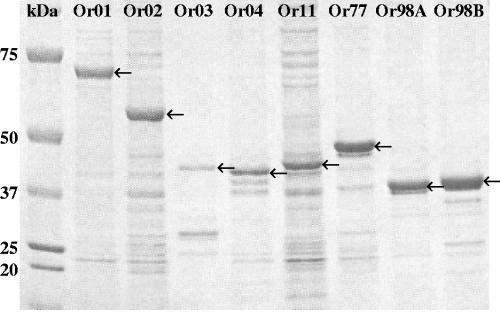
Even though a PelB leader peptide was cloned at the N terminus of all eight proteins, microscopic analysis of the induced E. coli cultures showed inclusion bodies, except for E. coli cells containing the pET22b-Or77 expression construct, which secreted the protein into the supernatant. Recombinant proteins Or03, Or04, Or98A, and Or98B were purified by metal affinity chromatography, facilitated by the confirmed presence of the cloned histidine tag, solubilized in 8 M urea, and dialyzed against PBS. After dialysis of the affinity-purified proteins, protein aggregates were formed, which were collected and used for formulation of a vaccine and immunization of birds. Recombinant proteins Or01, Or02, and Or11 were partially purified by repeated freeze-thawing, sonification, centrifugation, and ultrafiltration. Purity of the recombinant proteins was assessed by PAGE and Coomassie brilliant blue staining (Fig. 2). It could be observed that recombinant proteins Or01, Or77, and Or98B migrated more slowly during electrophoresis since their molecular masses on PAGE were higher than expected, based on sequence data (62.9 kDa, 43.7 kDa, and 35.9 kDa for recombinant Or01, Or77, and Or98B, respectively [Table 4]).
FIG. 2.
PAGE analysis of eight expressed recombinant vaccine proteins.
Serological reaction of recombinant proteins was determined by Western blotting using the 1:1:1 mixture of sera that was initially used to screen the library and recognized the initial 30 cross-reactive λTriplEx clones. Recombinant proteins Or01, Or02, Or03, Or04, and Or77 reacted positive. No reactivity could be detected with recombinant proteins Or11, Or98A, and Or98B (Table 2).
Antigenic analysis of the eight proteins.
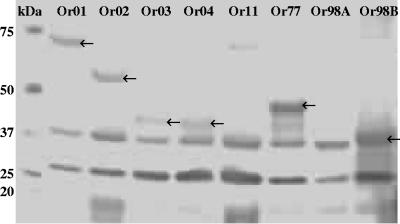
To test the antibody-inducing capacity of the eight proteins, a subunit vaccine was formulated in a water-in-oil emulsion that contained all eight proteins in approximately equal concentrations of 25 μg per antigen per dose. Two-week-old SPF broiler chickens were subcutaneously injected with the vaccine, and subsequent induction of serum antibody levels directed against the vaccine was tested by Western blot analysis using blood samples collected 4 weeks after vaccination. The proteins in the water phase of the subunit vaccine were run on PAGE, blotted, and incubated with pooled serum from vaccinated birds or unvaccinated control birds. Serum obtained from animals that were vaccinated showed protein-specific IgG reactivity to all antigens except Or11 and Or98A (Fig. 3 and Table 2). The 27-kDa and 37-kDa protein bands as observed in each lane were also observed using serum from unvaccinated control birds (data not shown). However, using negative control sera, no reactivity could be observed against any band of the same size as the recombinant vaccine proteins (data not shown). These results indicate that subunit vaccination induced the humoral immune system to produce protein-specific antibodies.
FIG. 3.
Western blot demonstrating the reactivity of sera from birds vaccinated with the subunit vaccine to the eight recombinant vaccine proteins. Note that reactivity against proteins Or11 and Or98A could not be detected.
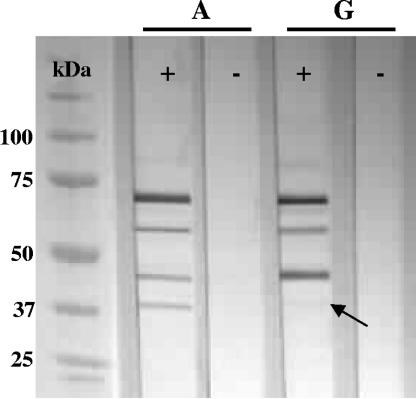
Reactivity of IgG antibodies induced by vaccination against in vitro-expressed O. rhinotracheale proteins was determined by Western blotting of serotype A and serotype G challenge cultures containing both cells and supernatant. Specific reactivity against four different O. rhinotracheale proteins with molecular masses of approximately 40, 45, 55, and 65 kDa could be detected for both serotypes (Fig. 4), although the 40-kDa protein of O. rhinotracheale serotype G showed very weak reaction. No reactivity could be observed using sera from unvaccinated control animals.
FIG. 4.
Western blot demonstrating the reactivity of sera from vaccinated (+) and unvaccinated (−) birds against four O. rhinotracheale serotype A and G in vitro-expressed antigens with molecular masses ranging from 40 to 65 kDa. Note that the reactivity of the 40-kDa protein of O. rhinotracheale serotype G is very weak (indicated with an arrow).
Subunit vaccination and cross-protection.
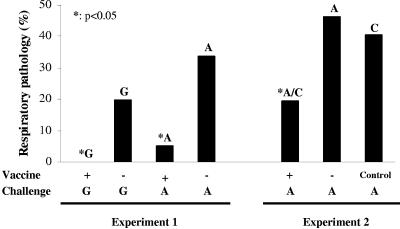
Two different subunit vaccination experiments were performed. First, the protective capacity of the induced protein-specific antibodies (see above) was tested. The birds that were vaccinated at 2 weeks of age were primed with ND virus at 5 weeks of age and challenged with O. rhinotracheale at 6 weeks of age. Challenge was performed using serotype G, to study homologous protection, or serotype A, to study heterologous (cross-)protection. Birds were infected via the natural route, i.e., aerosol spray application, causing mainly respiratory lesions in air sacs and lungs. Necropsy was performed at 7 weeks of age, 1 week after challenge. Organs were scored with respect to macroscopic pathology and corrected for background pathology due to ND priming. The results are summarized in Fig. 5 (experiment 1).
FIG. 5.
Protective effect of subunit vaccination to O. rhinotracheale serotype G (homologous) and serotype A (heterologous) challenge. Lesions in air sacs and lungs were macroscopically scored 1 week after challenge. Values are represented as the percentages of the maximum possible respiratory score. Note that the values of the vaccinated groups were significantly different compared to those of their respective challenge control groups (P < 0.05).
Birds that received the subunit vaccine containing the eight antigens showed complete protection (0.0% pathology [P = 0.002]) against homologous serotype G challenge compared to the unvaccinated challenge control group (19.7% pathology). The average organ lesion score of the birds that did receive subunit vaccine before challenge with heterologous serotype A was also significantly lower (P = 0.0004) (5.0%) than that of the unvaccinated birds (33.7%).
To verify the cross-protection induced by the recombinant antigens, the O. rhinotracheale serotype A challenge experiment as described above was repeated with the inclusion of an extra control group that was vaccinated at 2 weeks of age with a subunit vaccine containing proteins of the E. coli expression strain but no O. rhinotracheale antigens. Again, birds were primed with ND at 5 weeks of age and infected at 6 weeks of age via aerosol spray application. Necropsy was performed at 7 weeks of age, 1 week after challenge. Air sacs and lungs were scored with respect to macroscopic pathology, and the results were corrected for background pathology due to ND priming. The results are summarized in Fig. 5 (experiment 2).
Birds that received the eight-component vaccine again showed a significant (P < 0.05) reduction in respiratory pathology (19.4%) in comparison to the unvaccinated challenge control group (46.1%). The pathology score of the vaccinated group was also significantly different from the pathology score of the group vaccinated with the negative-control vaccine containing the E. coli proteins (40.6%). The pathology scores of the unvaccinated group and the E. coli control group were comparable.
DISCUSSION
In this paper, we describe an effective approach for the identification of potential vaccine targets using the poultry pathogen O. rhinotracheale. By screening an O. rhinotracheale expression library using polyclonal antiserum obtained from cross-protected birds, seven different cross-reactive clones were selected. The corresponding eight open reading frames were identified and subsequently expressed in E. coli, and eight protein antigens were purified for formulation into one multicomponent subunit vaccine. Vaccination of chickens induced the humoral immune system to produce antibodies reactive against most of the recombinant vaccine proteins. Moreover, vaccinated birds showed a high level of protection against infection with different O. rhinotracheale serotype strains.
In this study, we demonstrate for the fist time that vaccination of broilers with live O. rhinotracheale bacteria induced cross-protective immunity against heterologous challenge. Previously, cross-protection by live vaccination was observed against infection with heterologous serotypes or serovars of other poultry pathogens such as Pasteurella multocida (10), Riemerella anatipestifer (26), and Haemophilus paragallinarum (2). In general, inactivated vaccines do not provide high-level protection against a different immunotype not contained within the vaccine. This suggests that antigens involved in cross-protection are expressed in vivo and are either not expressed or expressed at low levels in vitro or that live bacteria reach a certain niche during infection essential for the induction of a proper immune response (11, 20, 28).
We screened an expression library of O. rhinotracheale serotype G with chicken antiserum exhibiting cross-reactive IgG antibodies induced by live vaccination with low to moderately virulent strains of O. rhinotracheale serotypes B, G, and M, followed by infection with a virulent serotype A strain. Although the vaccination serotypes were randomly selected, the challenge serotype was chosen, since serotype A is the most prevalent serotype in both chickens and turkeys. The particular strains of these serotypes were selected since these are biochemically and serologically characterized (33). This strategy allowed us to select for clones encoding proteins that are expressed during an infection and are able to induce an immune response. The observation that antibodies can induce cross-protection (27) was the most important evidence for following this strategy. In this screening, we decided to use antisera from intravenously challenged animals since these animals showed higher IgG antibody titers than aerosol-challenged animals, although the latter group showed less reduction of pathology. We reasoned that until the day of challenge, both groups were treated identically and should have had identical antibody populations. Intravenous challenge appeared to be more severe than aerosol challenge and therefore caused lower protection levels, but it could give a better booster of cross-reactive antibodies. On the contrary, there is a reasonable chance that interesting proteins are missed in our immunoscreening approach. For example, some potentially cross-protective antigens might be poorly antigenic during O. rhinotracheale infection, resulting in low serum antibody titers, and therefore, those genes will not be identified. Furthermore, when the O. rhinotracheale expression library was screened, antibodies of the IgA and IgM class were not detected because as a secondary antibody anti-chicken IgG was used.
A detailed analysis of the 30 selected clones revealed eight different ORFs encoding cross-reactive proteins. As most ORFs were expressed as translational fusions with a peptide encoded by the expression vector, the 5′ sequence of the gene was lacking. This 5′ end of the ORFs was obtained by means of sequence analysis using genomic DNA of O. rhinotracheale serotype G as a template. Since nothing is known about translation initiation sites of O. rhinotracheale, the N-terminal prediction of the encoded protein from the DNA sequence was in some cases ambiguous, as the start codon had to be selected from a set of possible start sites close to each other. For example, consensus sequences like AGGA of ribosome binding sites were rarely found. For our experiments, we selected the 5′-most upstream ATG that was still in frame with the rest of the ORF. It should be noted that the recombinant proteins can differ from the wild-type O. rhinotracheale proteins by the introduction of extra amino acids.
Bioinformatic analysis was performed in order to characterize the proteins encoded by the identified ORFs. The amino acid sequences of Or02, Or03, Or11, and Or77 were predicted to contain a gram-negative signal sequence (21) that is involved in the transport of the protein across the cell inner membrane. Comparison of amino acid sequences with available databases showed that the functions of five identified ORFs, Or02, Or03, Or04, Or77, and Or98A, are not defined, and they don't exhibit significant similarity to any sequence in the databases. Analysis for protein families and conserved domains did reveal that Or02 and Or77 contain the characteristics of a lipoprotein. Only the ORFs encoding Or01, Or11, and Or98B showed strong similarities with published sequences of characterized proteins: protein Or01 showed high similarity with dihydrolipoamide acetyltransferase, the E2 component of the pyruvate dehydrogenase complex, which is involved in energy production and conversion (6); Or11 has the characteristics of an outer membrane fatty acid transport protein containing an OmpA-like transmembrane domain (15); and Or98B has 66% similarity with the RecT protein family that is involved in DNA recombination pathways (22).
Although antibodies raised during infection were used for detection, the selected antigens do not necessarily have to be surface-exposed or secreted proteins. They can also be intracellular or periplasmic proteins that are usually inaccessible to antibodies, except during infection, when they are released by cell lysis. Whether the identified antigens were localized on the bacterial surface has to be determined. Surface components of bacteria can play an important role in virulence, and therefore, they are also of interest for studying the molecular pathogenesis of O. rhinotracheale infection. Analysis of virulence of gene-specific O. rhinotracheale mutant strains can possibly indicate the role of the eight proteins in pathogenesis, but genetic manipulation of this bacterium is still difficult.
Cloning of the eight ORFs in an expression vector and expression of the cross-reactive proteins in E. coli resulted in the production of recombinant proteins. Only protein Or77 was secreted into the supernatant of the growth medium. Despite the presence of a PelB leader peptide cloned at the N terminus of all recombinant proteins, expression of seven out of eight proteins in E. coli resulted in the formation of inclusion bodies. Active proteins are usually recovered from inclusion bodies by solubilization of the aggregated protein in urea and subsequent refolding by dialysis (5). Unfortunately, renaturation of the protein can result in inactive, misfolded proteins and new aggregates. The proteins Or03, Or04, Or98A, and Or98B were purified by metal affinity chromatography in the presence of urea followed by dialysis which resulted in the formation of precipitates. However, if the protective epitope(s) is in a correct conformation, aggregate formation or inclusion bodies do not necessarily affect the protective capacity of a protein. Since Or77 was secreted in the growth medium, no additional purification steps were needed, and thereby the possibility of isolating a misfolded protein was reduced.
The purified recombinant antigens were clearly visible by PAGE. However, not all recombinant proteins reacted on Western blot, even when positive serum was used from the previous expression library screening. This could be explained by the presence of antibodies that are cross-reactive against conformational epitopes that are destroyed under denaturating conditions of PAGE and Western blotting. Initial expression library screening was done using the same antiserum but under native conditions.
The antisera used for the library screening were obtained from cross-protected birds. However, it is important that the antibodies that recognized the cross-reactive clones in expression library screening were not necessarily the antibodies that conferred cross-protective immunity. The capacity of the selected proteins to induce protective immunity was studied in an animal experiment. Chickens were injected with a subunit vaccine containing all eight recombinant proteins. Again, not all vaccine proteins could be detected on Western blot using antiserum obtained from vaccinated birds. Surprisingly, one protein, Or98B, that was previously undetectable (after E. coli protein expression and purification) using the immunoscreening serum from cross-protected birds reacted positively with protein-specific serum obtained after subunit vaccination. This can be explained by the presence of other or different epitopes on the recombinant protein in comparison to the same antigen expressed in vivo during infection, which affirms conformational issues as described above.
Successful vaccination using recombinant subunit vaccines depends on the induction of antibodies that recognize the wild-type proteins as expressed by the infectious pathogen. Reactivity of antibodies induced after subunit vaccination with the recombinant proteins against in vitro-expressed O. rhinotracheale antigens was assessed by Western blot analysis. Specific reactivity against four different O. rhinotracheale bands could be observed, but whether these antigens are the cross-reactive proteins selected by our immunoscreening approach is unclear. Future studies using antigen-specific sera should give a better indication of whether the expressed O. rhinotracheale antigens detected on Western blot correspond to the eight identified antigens.
The (cross-)protective capacity of the selected proteins was determined in two animal experiments in which vaccinated chickens were challenged with O. rhinotracheale serotype G (homologous challenge) or O. rhinotracheale serotype A (heterologous challenge). Vaccination followed by challenge resulted in reduced and significantly different respiratory pathology scores compared to unvaccinated challenged birds or birds that received the E. coli control proteins. These results indicated that the selection of eight cross-reactive proteins contains a new vaccine candidate(s) with cross-protective capacity. However, as most recombinant proteins induced a specific antibody response, it is unclear which antigen(s) is responsible for the observed cross-protection. Although a variation in the pathology scores of the unvaccinated serotype A challenge control groups could be observed between the different subunit vaccination experiments, this variation did not influence the results, as control groups were included in each experiment.
So far, several approaches have been reported that allowed in vitro identification of antigens out of proteomic samples or expression libraries by using specific antiserum raised upon encountering a given pathogenic microorganism, for example, the detection of enteropathogenic Escherichia coli antigens using secretory immunoglobulin A antibodies isolated from human breast milk (18); the application of in vitro protein selection methods, such as ribosome display, to identify and map immunologically relevant proteins of Staphylococcus aureus (36); and the characterization of Vibrio vulnificus antigens preferentially expressed during infection by using in vivo-induced antigen technology (14). The application of an immunoscreening approach has also been described previously (3, 12, 37, 38). However, when prophylactic studies were performed, animal models were used instead of the natural host-pathogen interaction. The major advantage of this study is the use of O. rhinotracheale and its natural host, the chicken. Consequently, this approach is applicable for infectious diseases in animals and is less applicable for humans.
Based on the results presented in this paper, it can be concluded that immunoscreening of an O. rhinotracheale expression library with cross-protective antisera, and subsequent analysis of the (cross-)protective capacity of selected proteins within the natural host, was a successful method for the identification of potential vaccine antigens. Further vaccination and antigen characterization studies will have to determine which of the eight identified antigens have the highest cross-protective capacity and have the best potential for further use in vaccine development.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackall, P. J., and M. Matsumoto. 2003. Infectious Coryza, p. 691-703. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Poultry diseases, 11th ed. Iowa State Press, Ames, Iowa.
- 3.Brodeur, B. R., M. Boyer, I. Charlebois, J. Hamel, F. Couture, C. R. Rioux, and D. Martin. 2000. Identification of group B streptococcal Sip protein which elicits cross-protective immunity. Infect. Immun. 68:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin, R. P., P. C. M. van Empel, and H. M. Hafez. 2003. Ornithobacterium rhinotracheale infection, p. 683-690. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Poultry diseases, 11th ed. Iowa State Press, Ames, Iowa.
- 5.Clark, E. D. B. 2001. Protein refolding for industrial processes. Curr. Opin. Biotechnol. 12:202-207. [DOI] [PubMed] [Google Scholar]
- 6.De Kok, A., A. F. Hengeveld, A. Martin, and A. H. Westphal. 1998. The pyruvate dehydrogenase multi-enzyme complex from Gram-negative bacteria. Biochim. Biophys. Acta 1385:353-366. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa, M., R. Droual, R. P. Chin, and H. L. Shivaprasad. 1997. Interaction of Ornithobacterium rhinotracheale and Bordetella avium in turkey poults, p. 52-53. In Proceedings of the 46th Western Poultry Disease Conference.
- 8.Devriese, L., J. Hommez, P. Vandamme, K. Kersters, and F. Haesebrouck. 1995. In vitro antibiotic sensitivity of Ornithobacterium rhinotracheale strains from poultry and wild birds. Vet. Rec. 137:435-436. [DOI] [PubMed] [Google Scholar]
- 9.Glisson, J. R. 1998. Bacterial respiratory diseases of poultry. Poultry Sci. 77:1139-1142. [DOI] [PubMed] [Google Scholar]
- 10.Glisson, J. R., C. L. Hofacre, and J. P. Christensen. 2003. Fowl cholera, p. 658-676. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Poultry diseases, 11th ed. Iowa State Press, Ames, Iowa.
- 11.Heithoff, D. M., C. P. Conner, and M. J. Mahan. 1997. Dissecting the biology of a pathogen during infection. Trends Microbiol. 5:509-513. [DOI] [PubMed] [Google Scholar]
- 12.Hocking, D., E. Webb, F. Radcliff, L. Rothel, S. Taylor, G. Pinczower, C. Kapouleas, H. Braley, A. Lee, and C. Doidge. 1999. Isolation of recombinant protective Helicobacter pylori antigens. Infect. Immun. 67:4713-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulo N., C. J. A. Sigrist, V. Le Saux, P. S. Langendijk-Genevaux, L. Bordoli, A. Gattiker, E. De Castro, P. Bucher, and A. Bairoch. 2004. Recent improvements to the PROSITE database. Nucleic Acids Res. 32:D134-D137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, Y. R., S. E. Lee, C. M. Kim, S. Y. Kim, E. K. Shin, D. H. Shin, S. S. Chung, H. E. Choy, A. Progulske-Fox, J. D. Hillman, M. Handfield, and J. H. Rhee. 2003. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 71:5461-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koebnik, R., K. P. Locher, and P. van Gelder. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239-253. [DOI] [PubMed] [Google Scholar]
- 16.Kyte, J., and Doolittle, R. F. 1982. A simple method for displaying the hydrophatic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 17.Malik, Y. S., K. Olsen, K. Kumar, and S. M. Goyal. 2003. In vitro antibiotic resistance profiles of Ornithobacterium rhinotracheale strains from Minnesota turkeys during 1996-2002. Avian Dis. 47:588-593. [DOI] [PubMed] [Google Scholar]
- 18.Manjarrez-Hernandez, H. A., S. Gavilanes-Parra, E. Chavez-Berrocal, A. Navarro-Ocana, and A. Cravioto. 2000. Antigen detection in enteropathogenic Escherichia coli using secretory immunoglobulin A antibodies isolated from human breast milk. Infect. Immun. 68:5030-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker,V. Simonyan, J. S. Song, R. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Noirot, P., R. C. Gupta, C. M. Radding, and R. D. Kolodner. 2003. Hallmarks of homology recognition by RecA-like recombinases are exhibited by the unrelated Escherichia coli RecT protein. EMBO J. 22:324-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson, J. D., L. A. Umayam, T. M. Dickinson, E. K. Hickey, and O. White. 2001. The Comprehensive Microbial Resource. Nucleic Acids Res. 29:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai, E., Y. Tokuyama, F. Nonaka, S. Ohishi, Y. Ishikawa, M. Tanaka, and A. Taneno. 2000. Ornithobacterium rhinotracheale infection in Japan: preliminary investigations. Vet. Rec. 146:502-503. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sandhu, T. S. 2003. Riemerella anatipestifer infection, p. 676-682. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Poultry diseases, 11th ed. Iowa State Press, Ames, Iowa.
- 27.Schuijffel, D. F., P. C. M. van Empel, A. M. M. A. Pennings, J. P. M. van Putten, and P. J. M. Nuijten. 2005. Passive immunization of immune-suppressed animals: chicken antibodies protect against Ornithobacterium rhinotracheale infection. Vaccine 23:3404-3411. [DOI] [PubMed] [Google Scholar]
- 28.Smith, H. 1998. What happens to bacterial pathogens in vivo? Trends Microbiol. 6:239-243. [DOI] [PubMed] [Google Scholar]
- 29.Soriano, V. E., N. A. Vera, C. R. Salado, R. P. Fernandez, and P. J. Blackall. 2003. In vitro susceptibility of Ornithobacterium rhinotracheale to several antimicrobial drugs. Avian Dis. 47:476-480. [DOI] [PubMed] [Google Scholar]
- 30.Towbin, H., T. Staehlin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travers, A. F. 1996. Concomitant Ornithobacterium rhinotracheale and Newcastle disease infection in broilers in South Africa. Avian Dis. 40:488-490. [PubMed] [Google Scholar]
- 32.Van Empel, P. C. M. 1996. Ornithobacterium rhinotracheale. Ph.D. thesis. Utrecht University, Utrecht, The Netherlands.
- 33.van Empel, P., H. van den Bosch, P. Loeffen, and P. Storm. 1997. Identification and serotyping of Ornithobacterium rhinotracheale. J. Clin. Microbiol. 35:418-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Empel, P. C. M., and H. van den Bosch. 1998. Vaccination of chickens against Ornithobacterium rhinotracheale infection. Avian Dis. 42:572-578. [PubMed] [Google Scholar]
- 35.Van Empel, P. C. M., and H. M. Hafez. 1999. Ornithobacterium rhinotracheale: a review. Avian Pathol. 28:217-227. [DOI] [PubMed] [Google Scholar]
- 36.Weichhart, T., M. Horky, J. Söllner, S. Gangl, T. Henics, E. Nagy, A. Meinke, A. von Gabain, C. M. Fraser, S. R. Gill, M. Hafner, and U. von Ahsen. 2003. Functional selection of vaccine candidate peptides from Staphylococcus aureus whole-genome expression libraries in vitro. Infect. Immun. 71:4633-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu, Y., L. Jiang, B. E. Murray, and G. M. Weinstock. 1997. Enterococcus faecalis antigens in human infections. Infect. Immun. 65:4207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, G., K. Kiss, R. Seshadri, L. R. Hendrix, and J. E. Samuel. 2004. Identification and cloning of immunodominant antigens of Coxiella burnetii. Infect. Immun. 72:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]