Obesity must be ranked among the most intractable of our health problems. In 1999, the Centers for Disease Control and Prevention reported (1) that obesity, defined as a body mass index ≧30,† had risen in the adult United States population from 12.0% in 1991 to 17.9% in 1998. At this time we have little evidence for abatement of the “epidemic,” although warnings from health officials and physicians are numerous and most individuals consider obesity both unsightly and hazardous. Treatments with many different diets, behavior modification, nutrition counseling, over-the-counter drugs, and dietary supplements are the basis for a lucrative industry that has hardly made a dent in the problem. This issue of PNAS includes a report (2) on an agent affecting fatty acid synthesis that may or may not enter the battle against obesity but is sure to cast some light on the nature of the problem. How can we understand the intractability of this national nuisance?
The unwanted weight of the obese is that of triglyceride stored in adipose tissue. Storage of this calorically dense substance is held to account by the simple thermodynamic expression, Δ E = Q − W. One cannot increase energy storage (E) without an inequality between what you eat (Q) and what you burn (W).
But there is some surprising arithmetic in the account ledger of calories. Consider the all-too-common case of Mr. X who joined the ranks of the obese by gradually accumulating 75,000 kcal (1 kcal = 4.18 kJ) in his adipose depot over the first 5 years of happy marriage. The extra 75,000 kcal is just a ripple in the vast stream of 4 million or 5 million calories that flux in and out of Mr. X's body over 5 years. In fact, his excess intake averages only 40 kcal per day or about 1.5% of what normal or nonobese intake might be. Our choices of foods and the amount we eat at any meal are likely to be determined by the time of day, social context, level of culinary excellence, and just caprice. This being the case, it is all of the more amazing that just a bite or two less at each meal, to create the small deficit in Q needed for the slow abatement of obesity, is such a difficult behavior to sustain over time. In some way, seemingly random variations in food intake are shaped over long periods of time so as to maintain the level of caloric storage, whether obese or normal. This speaks for the existence of a regulatory system that senses the level of caloric storage and makes long-term adjustments of food intake and/or caloric expenditure.
Studies of adipose tissue histology many years ago showed that the number of adipocytes, like the level of fat storage, remains quite constant, but adipocytes readily enlarge or shrink with overfeeding and underfeeding (3). This response of cell size to nutritional alterations led me to wonder whether something related to cell size might generate a signal to hypothalamic centers involved in the control of food intake, thereby constituting a system for maintaining a fixed weight of adipose tissue at either normal or excess levels (4).
An agent affecting fatty acid synthesis may or may not enter the battle against obesity.
The advent of molecular genetics brought powerful new tools for addressing this issue. Various genetically based obesities in mice and rats had been under study in many different ways without uncovering the mechanism for obesity; however, positional cloning found the molecular defect in the obese ob/ob mouse (5). The inability of the adipocyte of ob/ob mice to secrete leptin, a novel peptide, is the central abnormality in this genetic form of obesity; defects in the detection of leptin by central nervous system receptors are the basis for a number of other genetic obesities in rats and mice. Along with leptin, the adipocyte elaborates and secretes adipsin, adiponectin, resistin, and such a remarkable number of other substances (6) that reviews have appeared on the “adipocyte as an endocrine organ” (7). Which adipocyte product, other than leptin, senses energy storage is a lively area for research, but it is likely that more than one signal from adipose tissue can up- or down-regulate a group of food intake-controlling neuropeptides found in the arcuate nucleus of the ventro-medical hypothalamus.
The hypothalamus has long been known as a major locus for food intake regulation (8). Thus, ventromedial hypothalamic ablation produces extreme obesity in experimental animals and tumors or infection in the same site can lead to obesity in humans. The neuropeptides involved are of two classes: promoters of food intake (orexigens), such as neuropeptide Y, agouti-related protein, and melanin concentrating hormone, and anorexigens, such as proopiomelanocortin and the cocaine (amphetamine)-related transcript. This system of central controllers, as modulated by energy storage signals, very likely interacts with other control systems, for example, the endocrine system of the gastro-intestinal tract that secretes hormones such as cholecystokinin and ghrelin, regulating the inauguration and termination of meals. There is a long and growing list of the components in these interacting systems controlling food intake and energy metabolism. When a new one is found or its actions are evaluated, the public is soon informed. Thus, the front page of The New York Times on May 23 reported on a fascinating study of changes in plasma ghrelin that occur when obesity is treated by gastric bypass (9). This intense public interest reflects the hope that the malfunction of one of these elements will be uncovered as the root cause of obesity and a new and fully effective treatment will soon follow. Yet, it is by no means clear that malfunction of any single component of the regulatory system will be the long-sought answer. Single gene lesions as have been found responsible for genetic obesities in rodents appear to be only rare causes for human obesity. More likely, complex genetic interactions with environmental factors occurring at different stages in development will emerge as causes of the human ailment. The only agents now approved for long-term treatment are Sibutramine, an amphetamine-like food intake suppressant, and Xenical, which blocks gastro-intestinal absorption of fat. Helpful as these may be for some of the obese, neither agent is eradicating obesity. C75, an inhibitor of fatty acid synthesis described in this issue of PNAS (2), brings hope that another family of agents will be useful in treatment.
Fatty acids must be consumed or synthesized from carbohydrate to be available for storage as triglyceride in adipose tissue. Thirty years ago, cerulenin, (2,3-epoxy-4-oxo-6-dodecadienoylamide) (Fig. 1), an antifungal antibiotic found in cultures of Cephalosporium caerulens, was found to be a strong inhibitor of fatty acid synthesis, blocking the condensation of acetyl and malonyl CoA to acetoacetyl CoA (10). The group at Johns Hopkins reporting on C75 in this issue of PNAS has examined the effects of inhibition of fatty acid synthesis in experimental animals (11–13). They reasoned that the epoxide of cerulenin might render it too toxic for use as a drug and synthesized C75, a closely related compound (Fig. 1). Over the past 2 years they have shown the remarkable capacity of cerulenin and C75 to induce rapid weight loss in BALB/c mice and greater weight loss in genetically obese ob/ob mice. Tolerance to the drug occurred after several days, more rapidly in lean mice than in genetically obese mice or animals with dietary-induced (high fat) obesity. At least part of the decline in caloric storage can be ascribed to effects of C75 on the hypothalamus. During fasting the orexigenic system of neuropeptide Y and the agouti-related protein is activated, but with C75 administration this system is inhibited. In lean animals the fasting induced down-regulation of anorexigenic substances, proopiomelanocortin, and the cocaine (amphetamine)-related transcript of fasting was prevented by C75, but with obese mice only orexigens were affected. The expression of melanin-concentrating hormone and its receptor was increased by C75 in both obese and lean mice. These complex interactions summate to reduce food intake. The trigger for the hypothalamic changes is unknown, but there are good reasons to believe that malonyl CoA plays a particularly important role.
Figure 1.
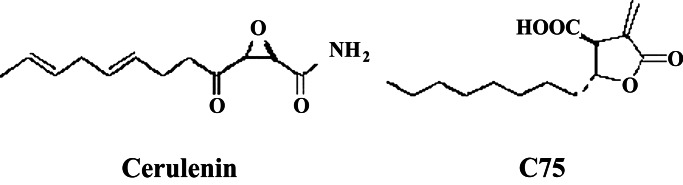
Structural formulae of a naturally occurring inhibitor of fatty acid synthesis, cerulenin, and a newly synthesized, related compound. [Reproduced with permission from ref. 11 (Copyright 2000, AAAS).]
It has been hypothesized that malonyl CoA is a prime agent in the sensing of fuel supply in brain and peripheral organs (14), and one of the consequences of fatty acid synthesis inhibition by C75 is an elevation in malonyl CoA. Cytosolic citrate is a more likely regulator of brain malonyl CoA than variations in fatty acid synthesis, because fatty acids are not significant substrates for energy in nerve tissue. Malonyl CoA also affects fatty acid oxidation by inhibition of carnitine palmityltransferase-1 (CPT-1), which controls fatty acid entry into the mitochondrion for oxidation. Thus, rises in malonyl CoA with C75 should lower fat oxidation, but the Johns Hopkins group showed that mice receiving C75 have an increase in fat oxidation. The report in this issue of PNAS (2) clarifies this problem with evidence for an unanticipated action of C75, the stimulation of CPT-1. Thus, C75 affects both intake (Q) and expenditure (W) to induce unusually rapid decline in fat stores (E). This finding is surprising because the lowering of fat stores by dietary means is usually compensated for by a decline in caloric expenditure (15). Thus, further elucidation of these effects of C75 to upset the balances in energy regulation may lead to a better appreciation of how the system of caloric storage could be adjusted to maintain lower weight. The inhibition of fatty acid synthesis by C75 might also help to lessen some adverse effects of dietary carbohydrates, the source of most of our dietary calories.
The commonest of all recent dietary recommendations for North Americans, whether obese or not, is to lower fat intake and make up needed calories with carbohydrate. There is a widespread conviction that lowering fat intake will prevent obesity, heart disease, and even some cancers. When this advice is followed, saturated fats are endogenously synthesized from carbohydrate (16). An unrivalled example of fat synthesis in vivo from carbohydrate is found by examining s.c. adipose tissue laid down in the fetus during the last trimester of pregnancy. The transport of fatty acids across the placental barrier is limited and maternal carbohydate becomes the necessary substrate for fetal lipogenesis (17), thus the s.c. fat of the term newborn infant contains an abundance of saturated acids (18). When high-carbohydrate diets are consumed in adult life, saturated fat is synthesized as in the prenatal state. Increasing saturation of lipids and a frequently associated rise in serum triglyceride levels gives one pause in recommending very low-fat diets for all. It would be most interesting to determine whether C75 could lessen the adverse effects of a high-carbohydrate diet.
Whether C75 will ever be a useful drug is uncertain, but further study of its mechanism of action will surely enrich our understanding of both carbohydrate-lipid interactions and the complex regulatory system that maintains a fixed level of caloric storage in adipose tissue. Such new knowledge must bring us closer to a victory over obesity.
Footnotes
See companion article on page 9498.
Body mass index (BMI) = weight in kg/height in (m)2. For those unfamiliar with this measure, BMI ≧ 30 is a substantial and easily discerned degree of obesity.
References
- 1.Nokdad A H, Serdula M K, Dietz W H, Bowman B A, Marks J S, Koplan J P. J Am Med Assoc. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 2.Thupari J N, Landree L E, Ronnett G V, Kuhajda F P. Proc Natl Acad Sci USA. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch J. In: Advances in Internal Medicine. Stollerman G H, editor. Vol. 17. Chicago: Yearbook Medical Publishers; 1971. pp. 289–300. [Google Scholar]
- 4.Hirsch J. Adv Psychosom Med. 1972;7:229–242. [PubMed] [Google Scholar]
- 5.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 6.Shuldiner A R, Yang R, Gong D W. N Engl J Med. 2001;345:1345–1346. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 7.Ahima R S, Flier J S. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum M, Leibel R L, Hirsch J. N Engl J Med. 1997;337:396–407. doi: 10.1056/NEJM199708073370606. [DOI] [PubMed] [Google Scholar]
- 9.Cummings D E, Weigle D S, Frayo R S, Breen P A, Ma M K, Patchen Dellinger E, Purnell J Q. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 10.Vance D, Goldberg I, Mitsuhashi O, Bloch K. Biochem Biophys Res Commun. 1972;48:649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- 11.Loftus T M, Jaworsky D E, Frehywot G L, Townsend C A, Ronnett G V, Lane M D, Kuhajda F P. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa T, Kumar M V, Lane D M. Proc Natl Acad Sci USA. 2002;99:66–71. doi: 10.1073/pnas.012606199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar M V, Shimokawa T, Nagy T R, Lane M D. Proc Natl Acad Sci USA. 2002;99:1921–1925. doi: 10.1073/pnas.042683699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruderman N B, Saha A K, Vavvas D, Witters L A. Am J Physiol. 1999;276:E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 15.Leibel R L, Rosenbaum M, Hirsch J. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 16.Hudgins L C, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch J. In: Handbook of Physiology. Renold A E, Cahill G F Jr, editors. Washington, DC: Am. Physiol. Soc.; 1965. pp. 181–189. [Google Scholar]
- 18.Bagdade J D, Hirsch J. Proc Soc Exp Biol Med. 1966;122:616–619. doi: 10.3181/00379727-122-31206. [DOI] [PubMed] [Google Scholar]


