Abstract
Strains of Shiga toxin (Stx)-producing Escherichia coli, also called enterohemorrhagic E. coli (EHEC), are important food-borne pathogens for humans. Most EHEC strains intimately adhere to the intestinal mucosa in a characteristic attaching and effacing (A/E) pattern, which is mediated by the bacterial adhesin intimin. Subsequent release of Stx1 and/or Stx2 leads to the frequent development of hemorrhagic colitis and, less commonly, to hemolytic-uremic syndrome. The aim of the present study was to develop an attenuated A/E E. coli strain for use as a vaccine against EHEC infection encoding a truncated intimin lacking adhesive capacity, but which would still express somatic antigens, other products of the locus of enterocyte effacement pathogenicity island, and an immunogenic remnant of the intimin molecule. A single-nucleotide deletion was generated in the eae gene in the prototype rabbit A/E E. coli strain RDEC-1 (O15:H−), which resulted in truncation of intimin by 81 C-terminal residues (860 to 939 amino acids) containing a disulfide loop. Inoculation of rabbits with large doses of the truncated intimin mutant (RDEC-1Δeae860-939) was well tolerated, as observed by the absence of clinical signs of disease or evidence of intestinal A/E lesions. The efficacy of RDEC-1Δeae860-939 as a vaccine was evaluated by orogastric inoculation of rabbits with RDEC-1Δeae860-939 followed by challenge with the virulent strain RDEC-H19A, an Stx1-producing derivative of wild-type RDEC-1 capable of inducing hemorrhagic colitis in rabbits. Following RDEC-H19A challenge, nonimmunized control rabbits exhibited characteristic weight loss with watery to bloody diarrhea and demonstrated intimate bacterial attachment, effacement of microvilli, submucosal edema, mucosal heterophile infiltrates, and Shiga toxin-induced vascular lesions. In contrast, the RDEC-1Δeae860-939-immunized rabbits showed no clinical signs of disease, maintained normal weight gain, had reduced fecal shedding of challenge organisms, and showed an absence of gross or microscopic lesions in the intestinal mucosa. Serum antibodies specific to intimin were detected among rabbits immunized with RDEC-1Δeae860-939, indicating that truncation of the intimin functional domain not only attenuated bacterial virulence, but also retained at least some of the immunogenicity of native intimin. Although it is not possible to gauge the exact contribution of residual intimin immunity to protection, this attenuation strategy for A/E E. coli strains shows promise for the development of effective vaccines to prevent EHEC infection in humans and animals.
Strains of Shiga toxin (Stx)-producing Escherichia coli, or enterohemorrhagic E. coli (EHEC), are important food-borne pathogens for humans. The clinical manifestations of EHEC infections range from watery diarrhea, bloody diarrhea, or hemorrhagic colitis to life-threatening hemolytic-uremic syndrome (HUS) (29, 40). The majority of EHEC strains induce attaching and effacing (A/E) lesions characterized by intimate bacterial attachment to the epithelial cells with destruction of microvilli. Genes encoding the A/E phenotype are clustered on a pathogenicity island termed the locus of enterocyte effacement (LEE), the complete nucleotide sequences of which have been obtained for human EHEC O157:H7 strain EDL933 (43), and other related A/E organisms, including human enteropathogenic E. coli (EPEC) O127:H6 strain E2348/69 (17), rabbit EPEC (REPEC) O15:H− strain RDEC-1 (50), and Citrobacter rodentium, associated with colonic hyperplasia in mice (11). LEEs of EPEC, EHEC, RDEC-1, and C. rodentium share a 34-kb conserved region containing 40 (RDEC-1) or 41 (EPEC, EHEC, and C. rodentium) open reading frames organized into five major polycistronic operons: LEE1, LEE2, LEE3, LEE5(tir), and LEE4 (17). The LEE1, LEE2, and LEE3 operons encode components of the type III secretion system (esc and sep genes) and the ler (LEE-encoded regulator) gene. The LEE5 operon encodes the adhesin intimin, Tir (translocated intimin receptor), and CesT (the chaperone for Tir) (27, 31). The LEE4 operon encodes the secreted proteins EspA, -D, and -B involved in delivering Tir and other proteins to host cells, and EspF, which is translocated into host epithelial cells and alters their tight junction permeability (37).
Intimin is the outer membrane protein (OMP) which is crucial for the A/E phenotype because bacterial intimate adherence to host epithelial cells is mediated by binding of intimin to Tir, which is delivered by A/E organisms to eukaryotic cells (19-21, 24, 25, 31). All intimin alleles (1, 42, 45, 48) currently known demonstrate high homology in their N-terminal regions, but great diversity in their C-terminal regions, which are essential for binding to Tir. Intimins of A/E E. coli (AEEC), including human EPEC O127:H6 (intimin-α), EHEC O157:H7 (intimin-γ), or REPEC O15:H− (intimin-β), show greater than 94% amino acid (aa) identity over the N-terminal two-thirds of the molecule while showing only 55% homology over the remaining one-third at the C terminus (50), which includes the region responsible for Tir binding. The crystal structure of the EPEC intimin C-terminal fragment (residues 658 to 939) has been solved and reveals three adjacent domains with separate functions (18, 34): the immunoglobulin (Ig)-like D1 (residues 658 to 751) and D2 (residues 752 to 841) domains and the C-type lectin-like D3 (residues 842 to 929) domain. Binding of intimin to Tir is mediated primarily by interaction of the lectin-like D3 domain of intimin with the intimin-binding domain of Tir (34). Within the Tir-binding region of intimin, two conserved cysteine residues (aa 860 and 937 of EPEC intimin) form a disulfide loop essential for intimin function (18, 25, 34). This disulfide loop is conserved in Yersinia invasin and all the intimin variants (17, 42, 45, 48, 50). The immunodominant regions of the molecule have been demonstrated to be in the domains D1 and D2, as shown by reaction with intimin-specific antiserum (1).
Attenuation of virulence by deletion of intimin has been demonstrated for human EPEC (O127:H6) (14), human EHEC (O157:H7) (15), REPEC (O103:H2) (35), and C. rodentium (12). Further evidence of the crucial role of intimin in bacterial virulence is provided by studies showing that anti-intimin immune responses directed against the C-terminal Tir-binding fragment can favorably modulate the outcome of infection by A/E organisms (10, 23, 28). Such studies demonstrating protection include active immunization of mice with either an intimin C-terminal binding domain (Int280α) from a human EPEC strain or the EHEC O157:H7 intimin C-terminal domain expressed in transgenic tobacco plant cells (28). Both strategies protected against infection with A/E organisms expressing the homologous intimin fragment. Passive immunization of neonatal piglets (suckling colostrums from dams immunized with an intact intimin molecule) was also shown to protect against EHEC O157:H7 colonization and intestinal damage (10). All of these studies used vaccines containing the entire C terminus of intimin, including the D3 binding domain, suggesting that the binding domain might be essential for the observed protective immunity.
We previously developed a rabbit model for EHEC infection by introducing an Stx1-converting phage into a rabbit A/E pathogen, RDEC-1, to obtain strain RDEC-H19A (46). Infection of rabbits with this strain reproduces both the A/E pathology and the vascular damage and intestinal inflammation attributable to Stx and is a suitable model for evaluating various EHEC interventions (46). In this report, we constructed an eae mutant of RDEC-1 and examined both the level of attenuation in virulence and the effectiveness of this eae mutant as a vaccine candidate. We demonstrate that RDEC-1Δeae860-939 is fully attenuated and well tolerated in rabbits. We further show that immunization of rabbits with RDEC-1Δeae860-939 protected animals from occurrence of diarrhea, weight loss, and tissue damage induced by virulent RDEC-H19A. Thus, the attenuation of an AEEC strain by the truncation of intimin, as we describe, shows promise as a means to provide an effective vaccine for the prevention of EHEC infection.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains used in this study are listed in Table 1. The prototype RDEC-1 (O15:H−) was originally isolated from rabbits with diarrhea (7). Strain RDEC-H19A is an RDEC-1 derivative containing the Stx1-producing phage H19A from human O26 EHEC (46). The laboratory E. coli strain DH5α was used for plasmid transformation, except in the case of suicide plasmids (pCVD442 and its derivatives), which were maintained in E. coli SY327 or SM10 (13). Bacterial strains were stored at −70°C in Luria-Bertani (LB) broth containing 20% glycerol and grown on LB agar or broth unless otherwise described. Appropriate antibiotics were added to the media when needed at the following concentrations: ampicillin (Amp), 50 μg/ml; nalidixic acid (Nal), 20 μg/ml; and tetracycline (Tet), 25 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | Laboratory E. coli strain | |
| RDEC-1 | O15:H−, Nalr | 7 |
| RDEC-H19A | RDEC-1 transduced with phage H19A, Nalr Tetr | 46 |
| SM10 | SM10 λpir, recipient for suicide vector pCVD442 | 13 |
| TSA01 | SM10 containing pM381 | This study |
| RDEC-1Δeae860-939 | RDEC-1Δeae860-939, Nalr | This study |
| Plasmids | ||
| pALT417-3 | pALTER-1::RDEC-1eae, Ampr | |
| p368 | Derived from pALT417-3 with 1-bp deletion in eae, Ampr | This study |
| pCVD442 | Suicide vector, Ampr | 13 |
| pM381 | pCVD442::Δeae, Kanr Ampr | This study |
Construction of an eae truncation mutation in RDEC-1.
A 4.36-kb HindIII DNA fragment containing the RDEC-1 eae gene (excluding the first 100 bp) and downstream sequences was cloned into pALTER-1, and site-directed mutagenesis of the eae gene was achieved using the single-stranded phagemid protocol and the mutant oligonucleotide 5′-GATGCCGAAAACAACTGTAAGACAAATAGCGCAA-3′ following the manufacturer's guidance (Promega Inc., Madison, WI) to obtain plasmid p368. This oligonucleotide contains a single-base-pair deletion at the position of nucleotide (nt) 2565 in the eae coding sequence (Fig. 1B), thus resulting in a frameshift which generates a stop codon 23 bp downstream of the deletion (Fig. 1B). A DNA fragment containing the mutated eae gene was excised from pALTER plasmid by XbaI digestion and cloned into XbaI-digested suicide plasmid pCVD442, resulting in plasmid pM381. Plasmid pM381 was transformed into E. coli strain SY327, and the resultant plasmid was transformed subsequently into strain SM10 to obtain strain TSA01 (Table 1). A chromosomal eae mutation in RDEC-1 was generated by allelic exchange as described by Donnenberg and Kaper (13). The Amps Nalr sucrose-resistant bacteria that underwent allelic exchange were screened for FAS (fluorescence actin staining) activity on HEp-2 cells as previously described (30). Strains that failed to induce actin aggregation on HEp-2 cells were further examined by sequencing the intimin C-terminal portion, using PCR-amplified DNA with a pair of primers (Agin1, 5′-CCAGTATTACTGAGATTAAG, nt 27351 to 27371; Agin2, 5′-TCCGGGATTTGAGATGTAAT, nt 28223 to 28204) derived from RDEC-1 LEE (GenBank accession no. AF200363) (50). Expression of the truncated intimin by RDEC-1Δeae860-939 was examined by separation of bacterial outer membrane preparations by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (49).
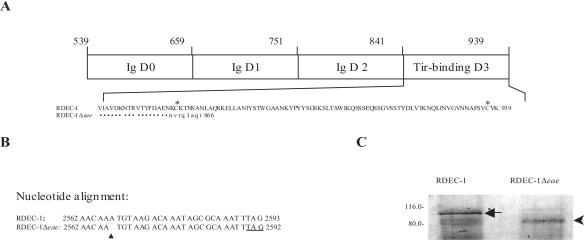
FIG. 1.
(A) Schematic representation of RDEC-1 intimin C-terminal fragment (539 to 939 aa) showing Ig-like (Ig) and Tir-binding domains. Numbers above the diagram represent the amino acid position of intimin deduced from RDEC-1 LEE (50). The residues of the intimin Tir-binding domain (842 to 939 aa) and of the truncated intimin mutant are shown below the diagram. Asterisks indicate two conserved cysteine residues (aa 860 and 937) involved in the formation of a disulfide loop essential for intimin function. Identical amino acids in the mutant are shown by dots. (B) DNA alignment of partial sequence of the eae gene and the mutant showing a single-base deletion (arrow) which introduced a stop codon (underlined) 24 bp immediately after deletion. (C) Comparison of OMP profiles showing native intimin (denoted by a white arrowhead) produced by RDEC-1 in the left panel or truncated intimin in the right panel (arrowhead) expressed by RDEC-1Δeae860-939. Molecular mass markers are indicated in kDa.
Examination of in vivo virulence of RDEC-1Δeae860-939.
The in vivo virulence of RDEC-1Δeae860-939 was determined in 2-month-old New Zealand White rabbits. Six animals were inoculated with the wild-type (WT) RDEC-1, and six were inoculated with the truncated intimin mutant. Bacterial strains were streaked onto MacConkey agar supplemented with Nal, and an individual colony was then inoculated into 100 ml Penassay broth (antibiotic medium 3; Difco) and cultured at 37°C overnight without shaking. Bacteria were washed once and suspended in sterile phosphate-buffered saline (PBS) and adjusted to a concentration represented by an optical density at 600 nm (OD600) of 0.70 (approximately 1 × 108 CFU/ml). Bacterial viable counts were determined by standard plating methods. Rabbits were fasted overnight, and unsedated animals were inoculated intragastrically via a pediatric feeding tube with 10 ml 10% bicarbonate solution to neutralize gastric contents followed by the inoculum. Inocula consisted of a total volume of 3 ml of PBS containing RDEC-1 or its derivative RDEC-1Δeae860-939. The feeding tube was then flushed with 5 ml sterile PBS to ensure that the total inocula were delivered into the stomach. Rabbits were weighed daily and observed for clinical signs of illness and stool characteristics. Stools were graded on a scale of 1 (normal, hard pellets), 2 (soft), 3 (mixed soft and liquid), or 4 (completely liquid). Fecal shedding of the strains was determined semiquantitatively by culture of rectal swabs. In brief, rectal swabs were rolled onto MacConkey plates (supplemented with Tet and Nal or Nal alone for RDEC-H19A or RDEC-1Δeae860-939, respectively), grown overnight at 37°C, and graded as 0 (no CFU), 1+ (1 to 50 CFU), 2+ (50 to 200 CFU), 3+ (>200 CFU), or 4+ (confluent growth). Rabbits were sacrificed 14 days postinoculation for histopathologic evaluation of bacterial mucosal adherence.
Examination of protection against RDEC-H19A challenge following immunization.
To determine if RDEC-1Δeae860-939 immunization would protect animals from experimental challenge with a virulent A/E strain producing Shiga toxin, 12 rabbits were orogastrically inoculated with RDEC-1Δeae860-939 as described above and boosted with the same dose 14 days later. Six of these immunized rabbits were sacrificed at day 28 for determination of biliary antibody and histology postvaccination but prior to challenge. The remaining six rabbits, and six rabbits orogastrically inoculated with PBS to serve as controls, were challenged with 5 × 107 CFU of RDEC-H19A, an Stx1-producing RDEC-1 derivative which is highly virulent in weanling rabbits (46).
Animals were observed daily for clinical signs of disease. Rabbit weights were recorded, and stool consistency was scored daily. Fecal shedding of inoculated bacteria was determined for each rabbit by semiquantitative cultures of rectal swabs as described above (46). Rabbits were euthanized according to standard protocols. At necropsy, the intestinal tract was inspected for serosal hemorrhage and bowel edema and the degree of liquidity of cecal contents was observed and recorded. Transmural sections from the ileum, cecum (the major site of mucosal adherence and pathological changes), and colon were excised and fixed in 10% buffered formalin for sectioning. Tissues were stained with hematoxylin and eosin or with Giemsa stain. Microscopic examination of histopathology was based on 10 sequential, well-oriented ×400 fields from each sample. The following parameters, reflecting bacterial adherence and toxin-induced vascular damage characteristic of RDEC-H19A infection (46), were evaluated. Bacterial enteroadherence was graded as the percentage of surface area in the field that is covered by closely adherent bacteria (46). Edema depth was quantitated with an ocular micrometer by measuring the distance from the muscularis mucosa to the muscularis propria and graded from 0 to 4 as previously described (46). Heterophiles were counted in the mucosa, in fields immediately adjacent to the muscularis mucosa extending luminally to the limits of the 250-μm diameter field (×40 objective). Counts from 10 fields were tabulated, averaged, and expressed as the number of heterophiles per high-power field (HPF). Vascular changes, defined as endothelial swelling, adherent heterophiles, endothelial denudation, and thrombus formation, were graded separately in 10 sequential vessels in the mucosal, submucosal, and serosal compartments and recorded on a scale from 0 to 4 as previously described (46). These parameters were separately analyzed and also examined as a composite score for vascular changes based on summation of the scores for the four individual parameters for each of the three tissue compartments (mucosa, submucosa, and serosa).
Detection of antibodies specific to RDEC-1 intimin.
Serum was collected for antibody detection prior to immunization (day 0), prior to the first booster immunization (day 14), prior to challenge with RDEC-H19A (day 28), and at the time of sacrifice (day 35). Bile was aspirated from the rabbit gall bladder for determination of IgA antibody titers to intimin from a group of six immunized animals which were euthanized at day 28 and from immunized and nonimmunized groups of animals following challenge with RDEC-H19A (day 35). For these assays, a maltose-binding protein (MBP)-intimin fusion protein was constructed by cloning a PCR-amplified 843-bp fragment containing the C-terminal 280-aa portion of RDEC-1 intimin into pMAL-p2 (New England Biolabs) according to the manufacturer's instructions. The MBP-intimin fusion protein was isolated from E. coli periplasm by affinity chromatography (2). Serum IgG or bile IgA specific to RDEC-1 intimin was determined by enzyme-linked immunosorbent assay as previously described (36). Briefly, microtiter wells were coated with MBP-intimin fusion protein at a concentration of 4 μg/ml in bicarbonate buffer at pH 9.6. Serial dilutions of rabbit sera or bile were added, and bound antibodies were detected with horseradish peroxidase-conjugated sheep anti-rabbit IgG or goat anti-rabbit IgA and developed with ABT peroxidase substrate (KPL, Gaithersburg, MD). Serum and biliary antibody titers were determined by endpoint dilutions subtracting a background OD value determined as 2 standard deviations from the negative control. Twofold dilutions were used for serum, and threefold dilutions were used for biliary samples.
Statistical analysis.
Values for differences in rabbit weight gain, heterophiles/HPF, bacterial adherence, and antibody titers between experimental groups were compared by Student's t test. Because of the obvious non-Gaussian nature of score data, a nonparametric test (Wilcoxon rank sum) was used to compare scores for fecal bacterial shedding, stool consistency, edema, and microvascular lesions in the tissue compartments (adherent heterophiles, endothelial swelling, endothelial denudation, and thrombus formation) between experimental groups.
RESULTS
Frameshift mutation resulted in intimin truncation.
A single-nucleotide deletion was made at nucleotide 2565 in the eae coding sequence of RDEC-1. As a result of the frameshift, a stop codon was introduced 24 bp immediately after the deletion (Fig. 1B). Thus, the intimin C-terminal portion was truncated by 81 residues (860 to 939 aa) containing a disulfide loop and replaced by a series of eight new residues (NVRGIAGI, Fig. 1A). As a result, the predicted truncated intimin is composed of 866 residues and is 73 aa shorter than the native intimin molecule.
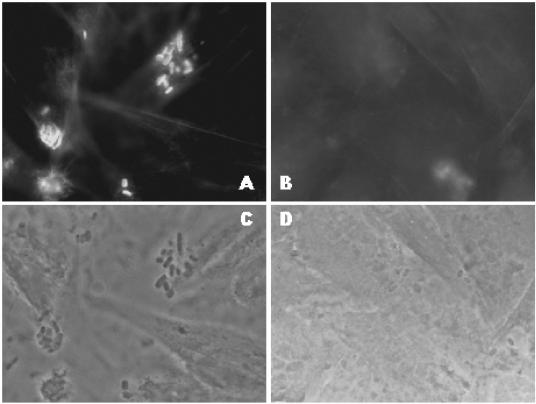
Conjugation of RDEC-1 with conjugative strain TSA01 (SM10 containing suicide plasmid pCVD442) yielded numerous colonies grown on LB agar supplemented with Amp and Nal, one of which was then cultured overnight in LB broth without antibiotics and subsequently plated on LB agar containing 5% sucrose. Individual colonies grown on sucrose agar were examined for antibiotic resistance profiles. The Amp-resistant bacteria were further characterized by the FAS test on HEp-2 cells (30). Among 60 colonies tested, we recovered four isolates that were negative by FAS assay (data not shown). Nucleotide sequencing of PCR amplicons using primers Agin1/Agin2 for the FAS-negative isolates showed a nucleotide deletion at the position of nucleotide 2565 in the eae gene. One of these isolates was designated as RDEC-1Δeae860-939 and used in the subsequent study. RDEC-1Δeae860-939 was further examined by FAS. The intimin C-terminal 76-aa disulfide loop has been shown to be essential for Tir binding (18, 25, 34). Thus, elimination of this disulfide loop was expected to abolish the Tir-binding capacity of RDEC-1Δeae860-939. Compared to the WT strain RDEC-1, which showed FAS-positive reactions (Fig. 2A and B), RDEC-1Δeae860-939 was less adherent and FAS negative (Fig. 2C and D).
FIG. 2.
FAS tests (corresponding actin fluorescence [A and B] and phase-contrast micrographs [C and D]) showing adherence pattern to HeLa cells of WT RDEC-1 (A and C) and RDEC-1Δeae860-939 (B and D) following 5 h of incubation.
Because the truncated intimin has 73 fewer residues than the native intimin, a molecular mass shift is expected. The predicted sizes for the native and truncated intimins are 101.7 and 97.0 kDa, respectively. Since posttranslational modification of intimin eliminates the signal peptide of 39 aa (49), the mature intimins of RDEC-1 and RDEC-1Δeae860-939 are 97.1 kDa and 89.2 kDa, respectively. Consistent with our previous observations (49), Coomassie brilliant blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated OMP extracts from the WT strain RDEC-1 revealed an intimin protein band with estimated molecular mass of 97 kDa (Fig. 1C). This 97-kDa protein band was absent in strain RDEC-1Δeae860-939 and replaced with a protein band with an estimated molecular mass of 90 kDa (Fig. 1C), showing a molecular mass shift from native intimin expressed by the WT RDEC-1 to the truncated intimin expressed by RDEC-1Δeae860-939.
Effect of the intimin mutation on in vivo pathogenicity.
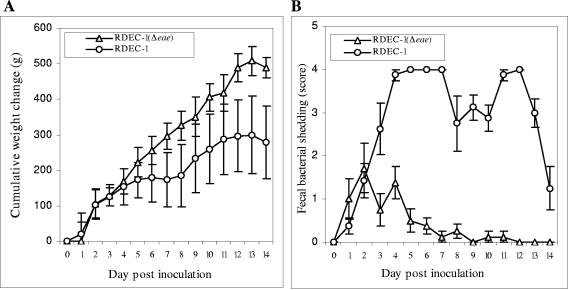
We first compared the level of in vivo virulence of the WT RDEC-1 and its isogeneic intimin mutant, RDEC-1Δeae860-939. Rabbitsinoculated with RDEC-1Δeae860-939 remained clinically normal, and they gained an average weight of 488 g by day 14 postinoculation (Fig. 3A). Fecal shedding of RDEC-1Δeae860-939 occurred within 24 h, peaked at day 2, and persisted in some animals at very low levels for nearly 10 days (Fig. 3B). Light microscopic examination of tissues from ileum, cecum, and colon revealed no mucosally adherent bacteria and normal morphology (data not shown).
FIG. 3.
Clinical observations in rabbits inoculated with WT RDEC-1 (open circles) or its derivative eae mutant (open triangles). Comparisons of cumulative weight change (A) and semiquantitative determination of fecal bacterial shedding of the administered strain (B) are shown. Averages were derived from six rabbits in each group. Bars designate standard errors.
All rabbits receiving WT RDEC-1 developed mild diarrhea ranging from soft stools to watery discharge. These rabbits initially gained weight at the same rate as those inoculated with RDEC-1Δeae860-939. Beginning on day 3 postinoculation, RDEC-1-infected rabbits continued to gain weight, but at a significantly lower rate than RDEC-1Δeae860-939-inoculated rabbits. By day 14 postinoculation, the RDEC-1-inoculated group had an average weight gain of only 220 g, which is significantly (P < 0.006) less than the weight gain of 480 g in the RDEC-1Δeae860-939 group (Fig. 3A). Animals receiving WT RDEC-1 had high scores for shedding of the inoculated organisms during the whole observation period (Fig. 3B).
Consistent with previous studies, we observed typical A/E lesions with epithelial cupping of adherent bacteria and effacement of microvilli among rabbits inoculated with the WT RDEC-1 (data not shown), but not in the rabbits inoculated with the mutant strain. This initial comparison clearly indicates the attenuated virulence of RDEC-1Δeae860-939.
Immunization with RDEC-1Δeae860-939.
Further experiments were carried out to determine the level of protection provided by RDEC-1Δeae860-939 immunization against challenge with RDEC-H19A. Following vaccination, the pattern of fecal bacterial shedding following prime vaccination was similar to the pattern observed above in the pathogenicity study (Fig. 3B). However, fecal shedding lasted for only 4 days following boost immunization, and rectal swabs from all the rabbits remained free of inoculated organisms thereafter. All immunized and nonimmunized rabbits gained the expected amount of body weight and showed no signs of clinical illness, as we previously observed in the pathogenicity study.
Protection of rabbits against challenge with RDEC-H19A following RDEC-1Δeae860-939 immunization.
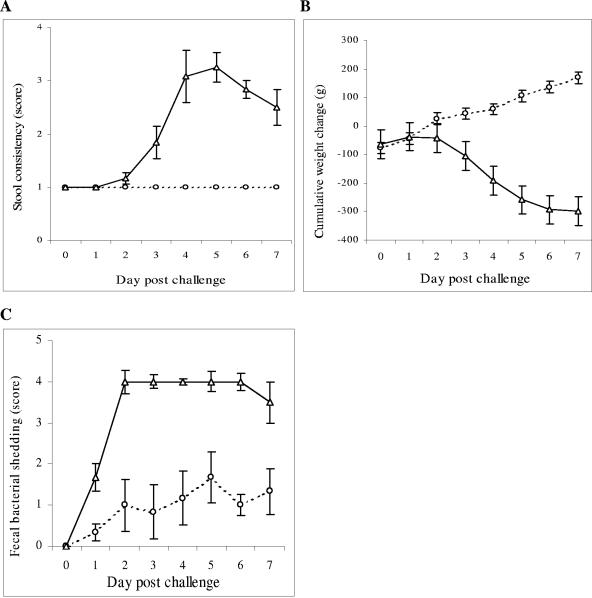
Following RDEC-H19A challenge, all RDEC-1Δeae860-939-immunized rabbits consistently discharged stool of normal consistency (Fig. 4A). In contrast, all nonimmunized rabbits manifested abnormal stools ranging from soft stools to frank watery diarrhea (Fig. 4A). The Wilcoxon rank sum test confirmed (P < 0.01) that the mean measure of abnormal stool consistency was higher for the nonimmunized group. Rabbits in the immunized group demonstrated an average cumulative weight gain of 170 g by day 7 postchallenge (Fig. 4B). In contrast, the nonimmunized animals lost an average of 350 g body weight (50 g/day) during the same period (Fig. 4B). The differences between the groups were significant at the level of P < 0.01. We observed fecal shedding of inoculated RDEC-H19A organisms in rabbits in both RDEC-1Δeae860-939-immunized and PBS control groups; however, this shedding was decreased in the immunized group. While confluent growth of Nalr Tetr RDEC-H19A was observed in fecal pellets of nonimmunized rabbits, only an inoculation of approximately 50 CFU per swab was seen for the RDEC-1Δeae860-939-immunized rabbits. A two-sample Wilcoxon rank sum test indicated significantly lower scores for fecal bacterial shedding of the challenge strain in RDEC-1Δeae860-939-immunized rabbits compared to those in the nonimmunized group (P < 0.01) (Fig. 4C).
FIG. 4.
Clinical observations in RDEC-1Δeae860-939-immunized (open circles) and nonimmunized (open triangles) rabbits following challenge with RDEC-H19A (day 0). Comparisons of stool consistency (A), cumulative weight change (B), and semiquantitative determination of fecal bacterial shedding of the challenge strain RDEC-H19A (C) are shown. Bars designate standard errors.
Major differences in gross appearance of the intestinal tissues were observed between immunized and nonimmunized rabbits challenged with virulent RDEC-H19A. All immunized and challenged rabbits exhibited normal appearance of the intestinal mucosa (ileum, cecum, and colon). However, nonimmunized, challenged rabbits showed various degrees of gross pathological changes, including pale cecum and proximal colon, watery intestinal contents, and edematous and thickened cecal walls.
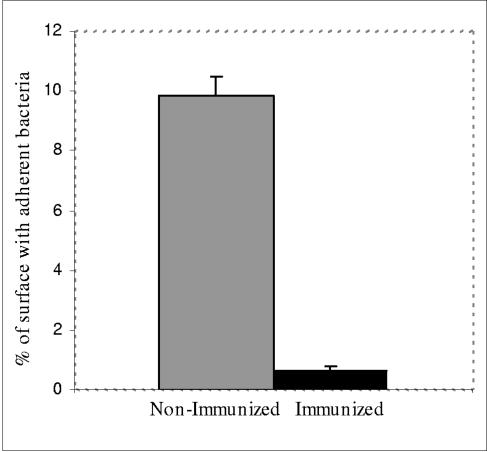
Microscopically, mucosally closely adherent RDEC-H19A organisms were seen covering 10% of the surface area of the cecal mucosa (Fig. 5) of nonimmunized animals. The severity of A/E lesions induced by RDEC-H19A varied from extensive attachment and effacement to small scattered focal lesions with a small cluster of adherent bacteria and irregularity of intestinal mucosa. In areas of adherence, the epithelial cells became irregular with reduced cytoplasm (Fig. 6A). Clusters of cells could be observed desquamating from the mucosal surface (Fig. 6A). The A/E lesions, when observed, were most severe in the cecum and in the proximal colon (data not shown). In contrast, less than 1% of the cecal surface had observable associated bacteria among the RDEC-1Δeae860-939-immunized rabbits challenged with RDEC-H19A (Fig. 5).
FIG. 5.
Comparison of percentages of mucosal surface (cecum) with adherent bacteria between RDEC-1Δeae860-939-immunized and nonimmunized groups following challenge with RDEC-H19A. Bars designate standard errors.
FIG. 6.
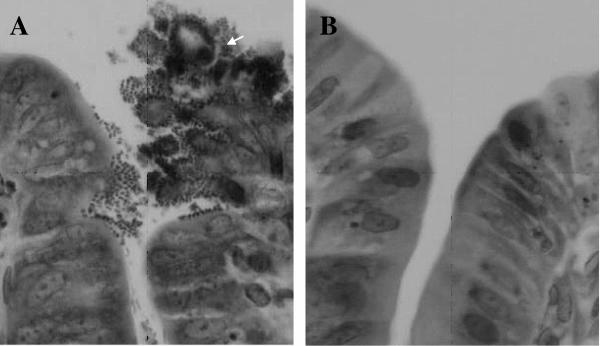
(A) Histological section from a nonimmunized rabbit challenged with RDEC-H19A showing intimate bacterial adherence and effacement of microvilli. Bacteria are adhering to the cecal enterocytes, and the mucosal surface has become irregular with a cluster of desquamating cells (white arrow). In comparison, the mucosa from an immunized and challenged animal remained normal (B). Hematoxylin and eosin staining; magnification, ×400.
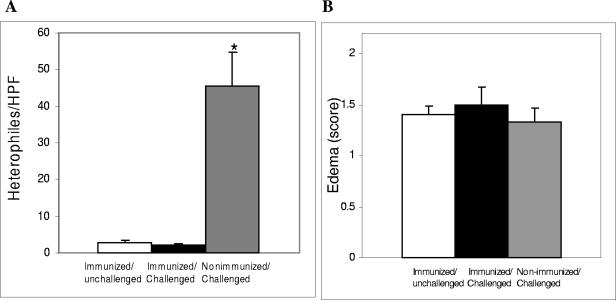
RDEC-H19A challenge induced inflammatory infiltrates of polymorphonuclear heterophiles in the nonimmunized rabbits, but not the immunized ones (Fig. 7A). The degree of inflammatory infiltrate in immunized and challenged rabbits was indistinguishable from that seen in animals which were immunized but not challenged. In all groups of animals, similar degrees of submucosal edema were observed (Fig. 7B).
FIG. 7.
Comparisons of enumeration of heterophiles per HPF (A) and scoring of edema (B) between immunized, unchallenged (day 28) animals (open columns), immunized, challenged (day 35) animals (solid columns), and nonimmunized, challenged (day 35) animals (semisolid columns). Bars designate standard errors. *, statistically significant difference versus both other groups.
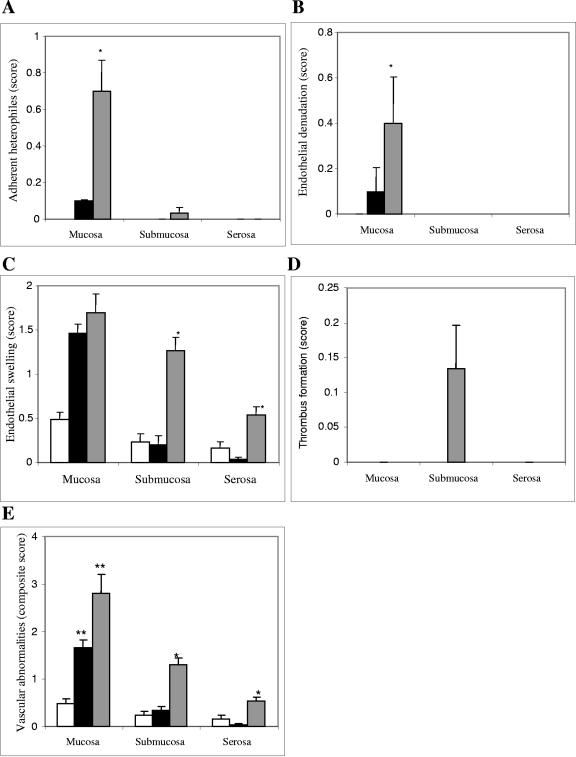
Microvascular changes, as measured by adherent heterophiles (Fig. 8A), endothelial denudation (Fig. 8B), and endothelial swelling (Fig. 8C), expressed either as individual parameters or as a composite vascular score (Fig. 8E), were more prominent among nonimmunized rabbits challenged with RDEC-H19A than in those which were immunized. Consistent with the results for the absolute heterophile counts (Fig. 7A), heterophiles adherent to the microvascular endothelium (Fig. 8A) were greater in the mucosa of the nonimmunized and challenged animals (P < 0.01, Wilcoxon rank sum test) than in the other two immunized groups. Such adherent cells were not observed in the other two compartments. Similarly, frank endothelial denudation (Fig. 8B) was seen more prominently in the mucosal compartment of the nonimmunized, challenged animals than in the immunized groups (P < 0.01). These changes were not observed in the submucosa and serosa in any groups. Endothelial swelling (Fig. 8C) greater than that seen in immunized but unchallenged animals was present in all compartments of the nonimmunized and challenged animals. However the differences in this parameter between the challenged animals, which were immunized or nonimmunized, were statistically different only in the submucosal and serosal compartments (P < 0.01). Frank thrombus formation (Fig. 8D) was rarely seen and did not serve to statistically distinguish the groups. Thrombus formation was seen only in submucosa among immunized rabbits following challenge with RDEC-H19A.
FIG. 8.
Microvascular changes in mucosal, submucosal, and serosal compartments, as measured by scores for adherent heterophiles (A), endothelial denudation (B), endothelial swelling (C), and thrombus formation (D) expressed as individual parameters and as a composite vascular score (E) among immunized, unchallenged animals (open columns), immunized, challenged animals (solid columns), and nonimmunized, challenged animals (semisolid columns). Bars designate standard errors. *, statistically significant difference between the nonimmunized, challenged group and the immunized, challenged group; **, statistically significant difference versus the immunized, unchallenged group.
When the composite vascular score was examined (Fig. 8E), immunized animals appeared completely protected against vascular changes induced by the challenge strain in the submucosa and serosa. In the mucosa, immunized and challenged animals also demonstrated reduced total vascular changes as compared to those observed in the nonimmunized and challenged group, but the differences did not reach statistical significance.
Immune responses.
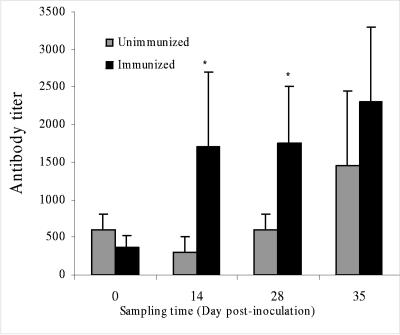
The intimin-specific serum antibody responses among nonimmunized animals maintained a low titer during the sampling periods of 0, 14, and 28 days but rose 1 week following challenge with RDEC-H19A (day 35). In contrast, the intimin-specific titers increased fourfold following initial immunization with RDEC-1Δeae860-939 and remained elevated but did not show significant additional increases following boost immunization or following challenge with RDE-H19A (Fig. 9). Immune responses to somatic antigens of the vaccine strain or to other secreted proteins encoded on the LEE were not measured in this study.
FIG. 9.
Rabbit serum IgG titers specific to MBP-Int280 prior to immunization (day 0), following prime (day 14) and boost (day 28) immunizations with RDEC-1Δeae860-939, and after RDEC-H19A challenge (day 35). Bars designate standard errors. *, statistically significant difference between the nonimmunized, challenged group and the immunized, challenged group.
Because of our experimental design, biliary antibody responses specific for intimin were unable to be obtained in any group prior to immunization; however, a group of six animals which were immunized and boosted were sacrificed at day 28 to determine antibody titers following immunization, but prior to challenge. These animals demonstrated a biliary antibody titer of 1:60 (data not shown). Higher titers were found in animals sacrificed at experimental day 35, 1 week following challenge with RDEC-H19A, both in groups which had been immunized (1:140) and in those which had not (1:170), but the differences among these groups were not statistically significant.
DISCUSSION
Interaction of intimin with its receptor, Tir, plays a key role in intimate adherence of AEEC to the host intestinal mucosa (27, 29, 31). Early studies demonstrated that the isogeneic intimin mutants are deficient in virulence, as examined in human volunteers (14), in piglets (15), and in rabbits (35). Further supporting this are the reports that intimin immune responses, directed against the C-terminal Tir-binding fragment of intimin or the entire molecule, can modulate the outcome of A/E organism infection (10, 23). These results strongly suggest that intimin may serve as a candidate virulence factor for attenuation of A/E organisms by deletion. Since intimin is also likely a potentially important protein for inclusion as a protective antigen in a vaccine, we sought to retain as much of the intimin molecule as possible in our vaccine strain by deleting only the Tir-binding portion of the molecule located in the D3 region of native intimin.
We constructed in this study an eae mutant with a single-nucleotide deletion, which truncates the native intimin by its C-terminal 81 residues that include a disulfide loop essential for intimin binding function. In our initial studies, we demonstrated that RDEC-1Δeae860-939 does not induce A/E lesions, either in vitro or in vivo, indicating attenuation of virulence of the WT RDEC-1 by our targeted deletion. Our results are in full agreement with previous reports that defined mutations in eae attenuated virulence of human EPEC, EHEC, and REPEC strains (13-15, 35). Donnenberg and Kaper created an internal 1,848-bp deletion in the EPEC eae gene (13) and demonstrated that while all of the human volunteers receiving WT EPEC developed diarrhea, the isogeneic eae mutant caused diarrhea among only 4 of 11 individuals (14). In a separate study, the isogeneic eae mutant of EHEC strain 86-24 (O157:H7), generated by replacing the internal 1.1-kb eae DNA with a 2.9-kb NDA fragment containing a Tet marker, was unable to colonize in experimentally inoculated piglets (15). In another REPEC strain belonging to the serogroup O103:H2, an insertion of aphT encoding Kan resistance in the eae gene (between 993 nt and 994 nt) disrupted the expression of intimin and abolished bacterial virulence when tested by experimental inoculation of its natural rabbit host (35).
One of the unique features of the truncated eae mutant constructed in the present study is the elimination of the intimin functional D3 domain with preservation of the remaining intimin molecule unaltered. This novel strategy retains an immunogenic portion of intimin, which might represent an important component of an attenuated live bacterial vaccine. The elevated serum IgG levels specific to intact intimin seen among rabbits immunized with the RDEC-1Δeae860-939 are an indication that the truncated intimin is immunogenic and retains sufficient native structure to generate immune responses to intimin.
Our experimental design did not permit us to determine the contribution of the observed antibody to intimin in providing the observed protection. Other studies from our laboratory have shown that a mutant severely defective in intimin expression (a ler deletion mutant) also provided protection against challenge with strain RDEC-H19A, although it did not induce antibodies to intimin (C. Zhu and E. C. Boedeker, unpublished data). This protection has been shown in our laboratory to be serotype specific, indicating that a non-intimin-expressing mutant can generate protective responses directed at REPEC antigens other than intimin. In addition, in a study from another laboratory, an intimin mutant of a REPEC strain protected against challenge with the virulent parent strain (35).
In future studies, we intend to explore the ability of our truncated intimin mutant to protect against Shiga toxin-producing REPEC strains of different serotypes, such as the O153 Stx1-producing strain recently reported by Garcia et al. (22). This kind of study would help to determine the importance of the anti-intimin antibody induced by our vaccine in providing protection, since it would eliminate the effect of serotype-specific immunity. However, such a study is beyond the scope of the present report. All other reports demonstrating protection against infection induced by intimin have utilized vaccines containing the D3 binding domain of the molecule (10, 23). We speculate that the intimin-specific immunity induced by RDEC-1Δeae860-939, combined with immune responses to serotype-specific antigens, secreted virulence proteins, and other as-yet-unidentified factors, may contribute to the protection of rabbits from pathological effects caused by virulent A/E strain RDEC-H19A.
With respect to mucosal IgA responses, as represented by biliary IgA, we immunized a separate group of animals and sacrificed them prior to challenge to look for the presence of intimin-specific IgA. In these animals, we detected a low but measurable titer of IgA directed toward intimin. Since measurement of a biliary IgA requires sacrifice of the animal, we were unable to formally compare the observed antibody titers to preimmune values in the same animals. However, the titer was increased above background. Higher anti-intimin IgA titers were found in bile after RDEC-H19A challenge in both immunized and nonimmunized animals. Since the postchallenge titers in both groups were similar, these results did not permit us to determine whether our immunization strategy primed a subsequent response to intimin expressed by the pathogen. Further studies will be required to determine conclusively whether our vaccination regimen induced a robust mucosal IgA response to intimin, such as we observed for serum IgG.
Although intimin mutation abolishes the capacity of RDEC-1 to attach closely to intestinal epithelial cells, RDEC-1Δeae860-939 organisms are still able to persist transiently in the rabbit intestinal tract, albeit at low levels, for 10 days after initial inoculation. The observed low-level shedding, apparent after the first but not subsequent inoculation, suggests that other adhesive factors, in addition to the intimin, are involved in RDEC-1 interaction with the immune system. RDEC-1 cells express plasmid-encoded AF/Rl fimbriae which mediate a species-specific mucosal attachment by interacting with a sialo-glycoprotein complex on the microvillus (5, 8, 44) and also mediate interaction with M cells overlying the Peyer's patch. In addition, in nearly all A/E E. coli strains, a large-molecular-weight protein, Efa1 (EHEC factor for adherence)/LifA (lymphocyte inhibitory factor), has been shown to be an adhesive factor (4, 32, 38). RDEC-1 cells express EspA and Efa1/LifA homologues (50). In a separate study, we have demonstrated that LifA/Efa1 plays a crucial role in in vivo colonization of RDEC-1 because deletion mutation in the lifA/efa1 gene resulted in significantly reduced bacterial colonization by 100-fold (Y. Mao, C. Zhu, and E. C. Boedeker, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. B438, 2003). Studies also implicate the filamentous EspA-containing surface appendages in attachment of A/E organisms (16). Collectively these adhesive factors may contribute to the localization in the intestinal tract of the RDEC-1 intimin mutant and its interaction with immune inductive sites, independent of intimin-Tir binding, which may be favorable to the development of systemic and local immunity against subsequent infection.
Most importantly, our studies demonstrate that immunization with the RDEC-1 intimin mutant strain was able to prevent colonization and shedding of RDEC-H19A, a highly virulent A/E strain which produces Shiga toxin and causes hemorrhagic colitis disease in rabbits. Immunization with the attenuated strain protected rabbits from RDEC-H19A-induced disease, as demonstrated by reduced colonization and maintenance of normal clinical signs. Moreover, the immunized rabbits were also protected from Shiga toxin-mediated disease. In the immunized animals, there were reduced pathological changes attributable to the interaction of Shiga toxin with the intestinal microvasculature, including transmigration of heterophiles and endothelial denudation and swelling. Since the vaccine strain contains no toxin components, it is likely that the prevention of toxin-induced damage is secondary to the prevention of A/E lesions by the vaccine, as we observed.
The hallmark of EHEC pathogenicity is the production of Stx (29, 41). Another approach to prevent EHEC infection is the neutralization of toxin activity, as represented by a number of candidate toxin-based vaccines (6, 26, 33), DNA vaccine (9), or toxin binding agents (3, 39, 47). Although approaches eliciting immunity neutralizing the toxin activity would protect against severe complications caused by Stx(s) produced during EHEC infection, they would not prevent the spread of infection. However, an attenuated live EHEC vaccine, such as the eae mutant constructed in this study, may provide more effective protection against EHEC infection by preventing bacterial colonization by a majority of clinically relevant EHEC strains based on common intimin epitopes.
In summary the truncated attenuated eae mutant has features that make it a safe and effective attenuated mucosal vaccine prototype for A/E strains, including those producing Shiga toxin. It is based on a mutation generated in a noninvasive enteric pathogen which has been well studied at the molecular level. It is not immediately cleared from the intestinal tract after initial inoculation,, but induces neither clinical evidence of disease nor pathognomonic A/E lesions. It induces measurable intimin antibody, and it provides protection against infection. Such a mutation, introduced into an A/E strain of human or bovine origin, might provide a basis for vaccine against EHEC infection.
Acknowledgments
The authors wish to thank Tom Bowen for technical assistance.
This work was supported by NIDDK/NIH grant R01-DK51507 (E.C.B.).
Editor: V. J. DiRita
REFERENCES
- 1.Adu-Bobie, J., L. R. Trabulsi, M. M. S. Carneiro-Sampaio, G. Dougan, and G. Frankel. 1998. Identification of immunodominant regions within the C-terminal cell binding domain of intimin α and intimin β from enteropathogenic Escherichia coli. Infect. Immun. 66:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agin, T. S., and M. K. Wolf. 1997. Identification of a family of intimins common to Escherichia coli causing attaching-effacing lesions in rabbits, humans, and swine. Infect. Immun. 65:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, G. D., P. C. Rowe, P. Goodyer, E. Orrbine, T. P. Klassen, G. Wells, A. MacKenzie, H. Lior, C. Blanchard, and F. Auclair. 1995. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171:1042-1045. [DOI] [PubMed] [Google Scholar]
- 4.Badea, L., S. Doughty, L. Nicholls, J. Sloan, R. M. Robins-Browne, and E. L. Hartland. 2003. Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb. Pathog. 34:205-215. [DOI] [PubMed] [Google Scholar]
- 5.Berendson, R., C. P. Cheney, P. A. Schad, and E. C. Boedeker. 1983. Species-specific binding of purified pili (AF/R1) from the Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology 85:837-845. [PubMed] [Google Scholar]
- 6.Bielaszewska, M., I. Clarke, M. A. Karmali, and M. Petric. 1997. Localization of intravenously administered verocytotoxins (Shiga-like toxins) 1 and 2 in rabbits immunized with homologous and heterologous toxoids and toxin subunits. Infect. Immun. 65:2509-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantey, J. R., and R. K. Blake. 1977. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J. Infect. Dis. 135:454-462. [DOI] [PubMed] [Google Scholar]
- 8.Cantey, J. R., R. K. Blake, J. R. Williford, and S. L. Moseley. 1999. Characterization of the Escherichia coli AF/R1 pilus operon: novel genes necessary for transcriptional regulation and for pilus-mediated adherence. Infect. Immun. 67:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capozzo, A. V. E., V. Pistone Creydt, G. Dran, G. Fernández, S. Gómez, L. V. Bentancor, C. Rubel, C. Ibarra, M. Isturiz, and M. S. Palermo. 2003. Development of DNA vaccines against hemolytic-uremic syndrome in a murine model. Infect. Immun. 71:3971-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean-Nystrom, E. A., L. J. Gansheroff, M. Mills, H. W. Moon, and A. D. O'Brien. 2002. Vaccination of pregnant dams with intiminO157 protects suckling piglets from Escherichia coli O157:H7 infection. Infect. Immun. 70:2414-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg, M. S., C. O. Tacket, S. P. James, G. Losonsky, J. P. Nataro, S. S. Wasserman, J. B. Kaper, and M. M. Levine. 1993. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J. Clin. Investig. 92:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Kramer, C. Deibel, C. A. Guzman, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 17.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 18.Frankel, G., D. C. A. Candy, E. Fabiani, J. Adu-Bobie, S. Gil, M. Novakova, A. D. Phillips, and G. Dougan,. 1995. Molecular characterization of a carboxy-terminal eukaryotic-cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect. Immun. 63:4323-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. A. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 20.Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. A. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frankel, G., A. D. Philips, M. Novakova, M. Batchelor, S. Hicks, and G. Dougan. 1998. Generation of Escherichia coli intimin derivatives with differing biological activities using site-directed mutagenesis of the intimin C-terminus domain. Mol. Microbiol. 29:559-570. [DOI] [PubMed] [Google Scholar]
- 22.Garcia, A., R. P. Marini, Y. Feng, A. Vitsky, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2002. A naturally occurring rabbit model of enterohemorrhagic Escherichia coli-induced disease. J. Infect. Dis. 186:1682-1686. [DOI] [PubMed] [Google Scholar]
- 23.Ghaem-Maghami, M., C. P. Simmons, S. Daniell, M. Pizza, D. Lewis, G. Frankel, and G. Dougan. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 69:5597-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartland, E. L., M. Batchelor, R. M. Delahay, C. Hale, S. Matthews, G. Dougan, S. Knutton, I. Connerton, and G. Frankel. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol. Microbiol. 32:151-158. [DOI] [PubMed] [Google Scholar]
- 25.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa, S., K. Kawahara, Y. Kagami, Y. Isshiki, A. Kaneko, H. Matsui, N. Okada, and H. Danbara. 2003. Protection against Shiga toxin 1 challenge by immunization of mice with purified mutant Shiga toxin 1. Infect. Immun. 71:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judge, N. A., H. S. Mason, and A. D. O'Brien. 2004. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect. Immun. 72:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 30.Karaolis, D. K., T. K. McDaniel, J. B. Kaper, and E. C. Boedeker. 1997. Cloning of the RDEC-1 locus of enterocyte effacement (LEE) and functional analysis of the phenotype on HEp-2 cells. Adv. Exp. Med. Biol. 412:241-245. [DOI] [PubMed] [Google Scholar]
- 31.Kenny, B., R. Devinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 32.Klapproth, J.-M., I. C. A. Scaletsky, B. P. McNamara, L. C. Lai, C. Malstrom, S. P. James, and M. S. Donnenberg. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig, K., M. A. Karmali, C. R. Smith, and M. Petric. 2002. Cross-protection against challenge by intravenous Escherichia coli verocytotoxin 1 (VT1) in rabbits immunized with VT2 toxoid. Can. J. Microbiol. 48:99-103. [DOI] [PubMed] [Google Scholar]
- 34.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 35.Marchès, O., J. P. Nougayrède, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee, M. L., and A. D. O'Brien. 1996. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect. Immun. 64:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa, K., K. Matsuoka, E. Kita, N. Okabe, M. Mizuguchi, K. Hino, S. Miyazawa, C. Yamasaki, J. Aoki, S. Takashima, Y. Yamakawa, M. Nishijima, D. Terunuma, H. Kuzuhara, and Y. Natori. 2002. A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157:H7. Proc. Natl. Acad. Sci. USA 99:7669-7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noel, J. M., and E. C. Boedeker. 1997. Enterohemorrhagic Escherichia coli: a family of emerging pathogens. Dig. Dis. 15:67-91. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 42.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perna, N. T., G. F. Mayhew, G. Pósfai, S. Elliott, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafiee, P., H. Leffler, J. C. Byrd, F. J. Cassels, E. C. Boedeker, and Y. S. Kim. 1991. A sialoglycoprotein complex linked to the microvillus cytoskeleton acts as a receptor for pilus (AF/R1) mediated adhesion of enteropathogenic Escherichia coli (RDEC-1) in rabbit small intestine. J. Cell Biol. 115:1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramachandran, V., K. Brett, M. A. Hornitzky, M. Dowton, K. A. Bettelheim, M. J. Walker, and S. P. Djordjevic. 2003. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. J. Clin. Microbiol. 41:5022-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjogren, R., R. Neill, D. Rachmilewitz, D. Fritz, J. Newland, D. Sharpnack, C. Colleton, J. Fondacaro, P. Gemski, and E. C. Boedeker. 1994. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology 106:306-317. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe, M., K. Matsuoka, E. Kita, K. Igai, N. Higashi, A. Miyagawa, T. Watanabe, R. Yanoshita, Y. Samejima, D. Terunuma, Y. Natori, and K. Nishikawa. 2004. Oral therapeutic agents with highly clustered globotriose for treatment of Shiga toxigenic Escherichia coli infections. J. Infect. Dis. 189:360-368. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, W. L., B. Kohler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 40:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, C., J. Harel, F. Dumas, and J. M. Fairbrother. 1995. Identification of EaeA protein in the outer membrane of attaching and effacing Escherichia coli O45 from pigs. FEMS Microbiol. Lett. 129:237-242. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]