Abstract
Toxoplasmosis is a ubiquitous parasitic infection causing a wide spectrum of diseases. It is usually asymptomatic but can lead to severe ocular and neurological disorders. Among the small-animal models available to study factors that determine susceptibility to toxoplasmosis, the rat appears to be rather similar to humans, particularly in terms of resistance to acute infection. Here, we demonstrate that the Lewis (LEW) rat strain displays an unexpected refractoriness to Toxoplasma infection. Complete resistance was assessed by both negative anti-Toxoplasma serology and lack of detection of the parasite during the course of infection. In this model, sex, age, major histocompatibility complex, and inoculum size had no effect on resistance. Interestingly, progeny from F1 hybrid crosses between Fischer (F344) or Brown Norway susceptible rats and LEW resistant rats were also fully resistant, showing a dominant effect of the gene or set of genes. Furthermore, resistance of the LEW rat was shown to be dependent on hematopoietic cells and partially abrogated by neutralization of endogenous gamma interferon. To our knowledge, this is the first observation of a rodent strain that is refractory to Toxoplasma infection. This model is therefore an attractive and powerful tool to dissect host genetic factors involved in susceptibility to toxoplasmosis.
Toxoplasma gondii is an obligate, intracellular parasite which can infect all mammals, including humans. In natural oral infection, the parasite initially crosses the intestinal barrier and disseminates, during the acute disease, as replicating cytolytic tachyzoites. The development of a vigorous immune response leads to a chronic infection characterized by the persistence of encysted parasites within the host's muscular and nervous tissues.
In the human population, toxoplasmosis is usually asymptomatic, and substantial morbidity and mortality are most often found in immunocompromised patients (e.g., in those with AIDS, with organ transplants, or who received anticancer therapies) and in congenitally infected infants (10). Despite the fact that the host immunologic status is known to be critical in the outcome of Toxoplasma infection (7, 12), the severity of the disease caused by Toxoplasma infection varies widely depending on the host species (8, 30, 33) and remains unpredictable among individuals.
Up to now, genetic studies on susceptibility to toxoplasmosis have been confined to the mouse model (2, 3, 23). A limitation of this model is the high susceptibility of certain strains of mice to toxoplasmosis, with a high rate of mortality during acute infection. Interestingly, in respect to clinical course and in utero transmission, toxoplasmoses in rats and humans are similar, and the infection in rats can serve as a model for human toxoplasmosis (6, 26, 33-35). Hence, like humans, rats do not succumb to acute toxoplasmosis even with a high inoculum of Toxoplasma strains that are highly virulent in mice. In a comparative study using various strains of rats, we have previously shown that the rat genetic background influences the number of brain cysts (18). In that study, the Lewis (LEW) strain was of particular interest, since no brain cysts seemed to develop during chronic infection. We therefore decided to further explore this apparent resistance.
Here, we demonstrate that the LEW rat is of particular interest, since it exhibits a complete resistance to Toxoplasma infection and dissemination. Immunological and genetic data indicate that LEW rat resistance is a dominant trait that is intrinsic to bone marrow-derived cells. This is, to our knowledge, the first report of an experimental model in which there is a complete refractoriness to Toxoplasma infection.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free LEW (RT1l), Fischer (F344) (RT1l), Brown Norway (BN) (RT1n), and F1 progeny (LEW × F344 and LEW × BN) rats were purchased from IFFA CREDO (L'Arbresle, France) and maintained in our specific-pathogen-free animal house facility. The major histocompatibility complex (MHC) congenic BN-1L rats were obtained by the cross-intercross-backcross method and have been backcrossed 20 times (H. J. Hedrich [Medizinische Hochschule, Hannover, Germany], unpublished observation). They were originally purchased from the Zentralinstitut für Versuchstierzucht (Hannover, Germany) and were used as a breeding nucleus in Maastricht (The Netherlands) from 1994 until 1999 and in Toulouse (France) since 1999. Rats were male or female and 8 to 16 weeks of age at the start of the experiment. Breeding and experimental procedures were in accordance with national and European guidelines.
Radiation bone marrow chimeras.
F1 (LEW × BN) recipient male rats (29 rats) were given 8.5 Gy total body irradiation by using an IBL (Paris, France) 437C cesium-135 irradiation machine 1 day before bone marrow transplantation. Recipient rats were given 2 × 108 viable nucleated bone marrow cells intravenously (i.v.) into the penis vein. The donor rats were LEW rats (LEW→F1, 10 rats), BN rats (BN→F1, 10 rats), or F1 rats (F1→F1, 9 rats). The extent of hematopoietic cell replacement by donor phenotype cells upon reconstitution was analyzed 8 weeks after transfer of bone marrow cells. Peripheral blood cells were collected and analyzed for the origin of immune cells by using RT1-A haplotype-specific monoclonal antibodies (MAbs). The results showed that the chimerism ranged from 70 to 95%. At 12 weeks postengraftment, the animals were infected with Toxoplasma.
Parasites and infection.
T. gondii tachyzoites from the RH strain were used for immunofluorescence and for preparation of parasite extracts. T. gondii Prugniaud-β-galactosidase (Pru-β-gal) tachyzoites, cysts, or oocysts were used for rat infections. Construction of the recombinant Prugniaud strain which expresses constitutively the Escherichia coli lacZ gene has been recently described (5). Tachyzoites were propagated in human foreskin fibroblasts (HFF) maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and 25% gentamicin. Parasites were harvested after complete lysis of the monolayer, purified through 3.0-μm filters, and washed in phosphate-buffered saline (PBS). Cysts were produced by oral infection of 2-month-old Swiss mice with 10 cysts of the Pru-β-gal strain. The mice chronically infected with Pru-β-gal were sacrificed 3 months after infection, and numbers of cysts were determined after homogenization of the brains in PBS. Rats were infected perorally with either 20 or 200 Pru-β-gal cysts or 1 million Pru-β-gal sporulated oocysts produced as previously described (5). Intraperitoneal (i.p.), i.v., or subcutaneous (s.c.) infection was performed with an inoculum of 1 × 106 Pru-β-gal tachyzoites.
Parasite quantitation by β-galactosidase detection.
For quantitation of Pru-β-gal cysts, each rat brain was removed and homogenized in 5 ml PBS (50 mM sodium phosphate buffer pH 7.4, 150 mM NaCl) with a Potter homogenizer (Thoma, Philadelphia, PA). This suspension was fixed by adding 5 ml of a 10% paraformaldehyde solution at room temperature. After two washes in PBS, the pellet was resuspended in 5 ml of PBS and frozen at −80°C in liquid nitrogen. For staining, the frozen brain suspension was warmed to 37°C and incubated with β-gal detection reagent [5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0.30 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) in PBS]. Following 2 h of incubation at 37°C, the suspension was distributed into two six-well culture plates, diluted in PBS, and scanned by light microscopy (magnification, ×20) to count the blue-stained cysts. Determination of parasite burden in the organs was performed by a tissue culture method adapted from that of Piketty et al. (25): (i) blood (200 μl) of infected rats was collected from the retro-orbital sinus into Vacutainer Systems tubes (HEMOGARD SST; Becton Dickinson), diluted into 4 ml of PBSA (0.01 M PBS, pH 7.4, supplemented with 100 U/ml penicillin, 50 μl/ml streptomycin, 0.04 μl/ml gentamicin, and 2.5 μg/ml amphotericin B), and (ii) the organs were removed, washed in PBSA, weighed, and homogenized, using a Potter homogenizer, into various volumes of PBSA (4 ml for the spleen and 2 ml for mesenteric lymphatic nodes). Serial fourfold dilution of homogenates were prepared in Dulbecco's modified Eagle's medium supplemented with antibiotics. Forty microliters of each dilution was inoculated in duplicates onto confluent HFF cells in 24-well tissue culture plates. After 72 h of incubation at 37°C, cultures were fixed for 10 min with 0.1% formaldehyde-0.016% glutaraldehyde. The β-galactosidase activity was revealed by incubating each well, overnight at 37°C, with the β-galactosidase reagent solution. The presence of parasitic foci in each well was recorded; the final dilution was the last one for which the tissue culture contained at least one parasitic focus. The number of parasites per gram of organ or per milliliter of blood (parasite burden) was calculated as follows: parasite burden = reciprocal titer in tissue culture/volume (milliliters) or weight (grams). For each organ, the parasite burden was expressed as a mean log value ± standard deviation (SD).
Detection of parasites in the gut.
Rats were killed at day 3 postinfection with 1 million Pru-β-gal oocysts. Their ileums were removed, opened longitudinally, washed in PBS, and fixed for 30 min at room temperature in 4% paraformaldehyde-0.05% glutaraldehyde prepared in PBS. The tissue was then rinsed in PBS, rapidly frozen in liquid nitrogen, and then thawed. Parasites were detected by overnight incubation in β-galactosidase chromogenic substrate as described above for cyst suspensions. Pieces of tissue showing the tiny blue dots characteristic of infection (5) were embedded in paraffin. Five-micrometer sections were prepared, deparaffined, stained with 0.1% nuclear red prepared in 5% Al2(SO4)3, and mounted with Eukit.
Gel electrophoresis and Western blotting.
Tachyzoites extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose sheets (Schleicher and Schuell). Blots were saturated for 1 h with 5% nonfat powdered milk in PBS and were incubated first with rat sera (dilution, 1:100) and further with anti-rat immunoglobulin G (IgG)-alkaline phosphatase conjugate (Jackson). They were developed with a chromogenic substrate (BCIP [5-bromo-4-chloro-3-indolylphosphate]-nitroblue tetrazolium).
Immunofluorescence.
Purified tachyzoites of the RH strain were dried onto immunofluorescence slides and fixed for 15 min in 4% paraformaldehyde. After three washes in PBS, slides were saturated twice with 5% FCS-5% goat serum in PBS for 20 min. They were further incubated for 30 min at 37°C with serial dilutions of rat sera. After three washes in 1% FCS-PBS, slides were incubated with anti-rabbit IgG-fluorescein isothiocyanate conjugate (Sigma). Observations were performed with a Leitz Ortholux microscope equipped for epifluorescence.
ELISA.
The anti-Toxoplasma IgG response was measured by specific enzyme-linked immunosorbent assay (ELISA). Total Toxoplasma antigens were prepared as previously described (13). Nunc Immuno modules were coated at 5 mg/ml with Toxoplasma antigens and left for 3 h at 37°C. After washing, serial dilutions of sera were added in PBS-0.1% Tween 20 and incubation was done overnight at 4°C. Plates were then washed with PBS-0.1% Tween 20, and peroxidase-conjugated anti-rat IgG1, IgG2a, IgG2b, and IgG2c antibodies (MARG-1, MARG-2a, MARG-2b, and MARG-2c, respectively; LO-IMEX, Brussels, Belgium) diluted at 1/8,000 (except for MARG-2c, which was at 1/3,000) were added. Plates were then incubated for 90 min at 37°C. Finally, after five washes, 100 μl of substrate solution (1 mg/ml of o-phenylenediamine dihydrochloride [Sigma]) and 0.03% H2O2 in 0.1 M citrate buffer, pH 5.0) was added to the wells. The color reaction was stopped by the addition of 100 μl of 2 N HCl. Optical density at 492 nm was measured by using a Multiskan MCC/340 spectrophotometer (Labsystems, Helsinki, Finland). Anti-Toxoplasma positive and negative sera were used as controls. Results are expressed as serum titers. The titer corresponds to the dilution which gave an optical density reading twofold higher than the mean background of normal rat serum. Titers correspond to the means from five infected animals ± SD.
Treatment of rats with MAb against IFN-γ.
One day before oral infection with 20 cysts from the Pru-β-gal strain, LEW and BN rats were injected intraperitoneally with 5 mg of anti-gamma interferon (anti-IFN-γ) MAb (DB1) (31), and they were then injected with 3 mg of DB1 at days 0, 2, and 4 postinfection. Control rats were injected intraperitoneally with isotype control MAb (OX21) (15).
Statistical analysis.
The significance of differences found between groups of rats was evaluated by the Mann-Whitney U test and, for dichotomous variables, by the Fischer exact test.
RESULTS
LEW rats develop no brain cyst following oral infection by the T. gondii Pru-β-gal strain.
Previous work had shown a remarkable difference between the numbers of cysts detected in the brains of LEW and F344 rats (18). Whereas brains of F344 rats contained a high number of cysts 2 months after an infection with either the CT1 or the NED Toxoplasma strain, no cysts could be detected in the brains of infected LEW rats. However, the method used to determine the average number of cysts per brain was not sensitive enough to determine whether there was a complete absence of intracerebral cysts in LEW rats. Therefore, more sensitive methods based on detection of parasites constitutively expressing β-galactosidase (5) (Fig. 1) were developed to quantify the number of brain cysts by scanning the entire brain.
FIG. 1.
Histological detection of T. gondii in the brain and gut of an F344 rat. A. β-Gal staining detection of brain cysts from an F344 rat orally infected by the Pru-β-gal strain. Blue cysts were easily visualized (arrows) and counted following plating a homogenized infected brain in a six-well plate. B. Histological section of the ileum of an F344 rat at day 3 following infection with 1 × 106 oocysts of the Pru-β-gal strain. After β-gal staining, a vacuole containing several blue tachyzoites was observed.
Initially, histological analyses of brain sections from orally infected LEW and F344 rat were scanned. The scanning of about 50 vibratome sections (200 μm thick) allowed a complete view of a rat brain, and this was performed on 10 infected LEW rats and 3 infected F344 rats. Using this method, we confirmed the presence of numerous blue cysts in the brains of F344 rats and the complete lack of intracerebral cysts in the brains of LEW rats (data not shown). Since histological analysis is time-consuming and labor-intensive, we also developed another method of cyst quantitation that is more appropriate for studying larger numbers of samples. As described in Materials and Methods, this method involved plating an entire infected brain following homogenization and β-gal staining onto two six-well plates (Fig. 1A). By this method, the abilities of three distinct rat strains to form brain cysts were compared. Rats of the F344, BN, and LEW strains were infected with 20 or 200 cysts of the Pru-β-gal strain. At 6 weeks postinfection, the numbers of cysts counted in the brains of these three rat strains were significantly different (Table 1). Brains from F344 rats contained a high number of Pru-β-gal cysts, fewer were counted in the BN brains (F344 versus BN, P < 0.01), and no cysts were detected in the LEW brains (BN or F344 versus LEW, P = 0.0079). Together, these results were consistent with those obtained by histological detection and demonstrated that LEW rats do not develop any detectable brain cysts after oral infection by Toxoplasma gondii.
TABLE 1.
Comparative study of cyst burdens detected in the brains of different rat strains
| Rat strain | No. of brain cystsa with the following inoculum size:
|
|
|---|---|---|
| 20 cysts | 200 cysts | |
| F344 | 170 ± 55 | NDb |
| BN | 4 ± 2.3 | 5 ± 3.6 |
| LEW | 0 | 0 |
Values are the average number of cysts per rat brain ± SD (n = 5 per group). The number of brain cysts was statistically higher in F344 rats than in BN rats (20 cysts; P < 0.01), and it was higher in BN rats than in LEW rats (20 and 200 cysts; P < 0.01). In BN rats no difference was found in the number of brain cysts between rats infected with 20 cysts and rats infected with 200 cysts. The absence of brain cysts in LEW rats (n = 5) compared to the presence of brain cysts in BN and F344 rats (n = 10) was highly significant (P = 0.0079 by Fischer's exact test).
ND, not done.
Parasites are not detected in the organs of LEW rats during acute infection.
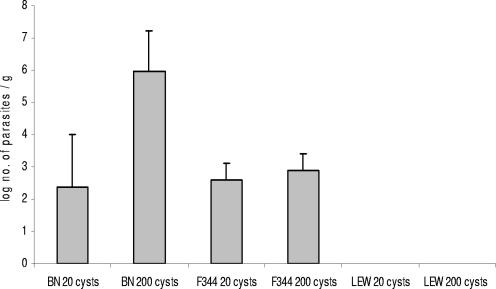
The lack of detectable brain cysts in the orally infected LEW rat suggested that parasites might not disseminate during the acute infection in this particular strain of rat. β-Gal quantitation of parasitic burdens in the organs of the three rat strains orally infected with 20 or 200 cysts of the Pru-β-gal strain showed that there were parasites in the mesenteric lymph nodes (MLN) (Fig. 2) and in the spleens and the blood (not shown) of both BN and F344 rats at day 4 following infection. By contrast, no parasites were detected in any of the LEW organs analyzed (P = 0.0079). These results, which were confirmed by PCR analysis performed on MLN (1) at day 5 postinfection of BN and LEW rats (data not shown), demonstrated that parasites do not disseminate through LEW tissues.
FIG. 2.
Tachyzoites were detected in the MLN of F344 and BN rats but not in those of LEW rats. Parasitic burdens in the MLN of rats at day 4 following oral infection with 20 or 200 Pru-β-gal cysts were determined. Five rats of each strain (F344, BN, and LEW) were infected. At day 4 postinfection, MLN were removed and homogenized. Serial fourfold dilutions of homogenates were plated onto confluent HFF cells in 24-well tissue culture plates for 72 h. After β-galactosidase staining, the presence of blue parasitic foci in each well was recorded. The final dilution was the last one for which the tissue culture contained at least one parasitic focus. The number of parasites per gram of organ (parasite burden), was calculated as the reciprocal titer in tissue culture/weight (grams). Values represent the means + SDs of the parasitic load per gram of MNL for five rats. The parasite burden observed in BN rats following oral infection with 200 cysts was significantly higher than that observed in BN rats following oral infection with 20 cysts (P < 0.05). In F344 rats no significant difference according to the number of administered cysts was observed.
Since no parasites were detected in the blood or MLN of LEW rats, we analyzed the earliest stages of infection, i.e., events occurring during the intestinal stage of the Toxoplasma cycle in the rat intestine. This study of early stages has not been described so far for rats, but by analogy to mice, one should expect the multiplication of parasites in cells of the lamina propria within the first days of infection (5). Therefore, F344 and LEW rats were infected with a high inoculum of parasites, which consisted of 1 million oocysts from the Pru-β-gal strain. Their ileums were removed at day 2 or 3 postinfection and incubated overnight in β-galactosidase chromogenic substrate to detect parasites. In F344 susceptible rats, a few tiny blue dots were seen in the distal part of the ileum, and histology confirmed the presence of vacuoles containing dividing parasites (Fig. 1B), whereas no parasites could be detected in the guts of LEW rats.
Together these data provide evidence that in orally infected LEW rats, there is no parasite burden or dissemination and that the lack of parasite propagation in LEW rats occurs very early following oral infection.
LEW rats do not mount any detectable IgG response following oral infection with Toxoplasma.
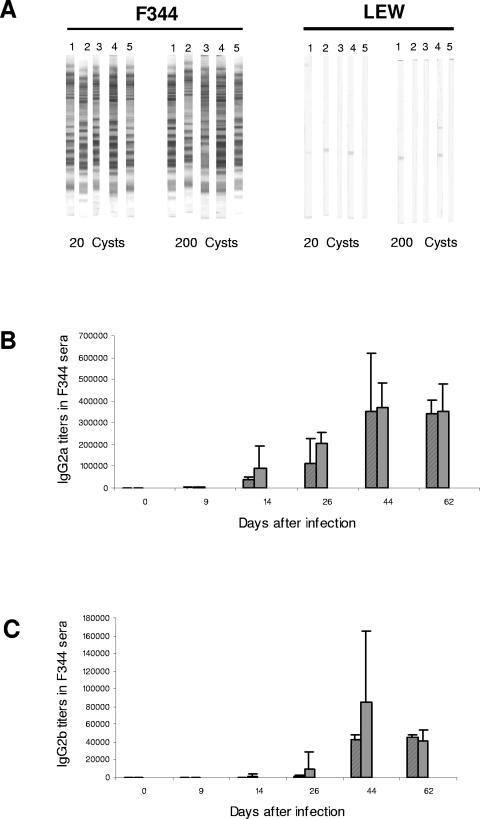
To analyze the involvement of the immune response in the resistance of LEW rats to Toxoplasma infection, we compared the IgG responses of susceptible (F344 and BN) and resistant (LEW) rat strains after peroral infection with Toxoplasma. Sera obtained from the three rat strains 6 weeks after oral infection with 20 or 200 cysts of the Pru-β-gal strain were tested in Western blotting against T. gondii tachyzoites (Fig. 3A). Whereas strong immunoblot staining was found in the case of the F344 rat sera, no significant staining was observed for the LEW rat sera (F344 or BN versus LEW, P = 0.0079). Strong immunoblot staining was also found using sera from infected BN rats (data not shown).
FIG. 3.
LEW rats do not mount any detectable anti-T. gondii IgG response following oral infection with Toxoplasma. A. Western blot analysis of the anti-Toxoplasma antibody response in LEW and F344 rat sera. Strips corresponding to LEW rat sera remained negative, whereas strong staining with multiple bands was observed for each individual serum of five infected F344 rats (P < 0.0079). The staining was independent of the inoculum (20 and 200 cysts of the Pru-β-gal strain). B and C. Kinetics of IgG2a (B) and IgG2b (C) isotype production in sera of infected F344 rats. IgG2a and Ig2b titers were quantified by ELISA. Titers were defined as the dilution which gave an optical density reading at least twofold higher than the mean background in uninfected rat serum. Results are represented as mean values obtained from individual F344 rats infected either with 20 cysts (striped bars) or 200 cysts (gray bars) of the Pru-β-gal strain. No significant difference in antibody titers between rats infected with 20 cysts and rats infected with 200 cysts was observed at any time.
The kinetics of production of the four IgG subclasses (IgG1, IgG2a, IgG2b, and IgG2c) specific for Toxoplasma antigens in the sera obtained from infected F344 and LEW rats were also compared. Blood was collected at days 0, 9, 14, 26, 44, and 62 postinfection. All LEW rat sera remained negative in the IgG ELISA (data not shown). In contrast, F344 rats mounted a strong humoral response against Toxoplasma that was dominated by IgG2a (Fig. 3B) and IgG2b (Fig. 3C) antibody responses. These data demonstrate that the resistance of LEW rats to oral infection with Toxoplasma is associated with the absence of a detectable IgG humoral response against the parasite.
Resistance of LEW rats to Toxoplasma infection does not depend on the inoculation route and is a dominant trait that is not linked to the MHC haplotype.
After having demonstrated that the LEW rat strain is resistant to oral Toxoplasma infection, we further examined if the inoculation route may influence the susceptibility phenotype. Infection experiments with both LEW and F344 rats were performed using either the i.p., the i.v., or the s.c. inoculation route. The results presented in Table 2 showed that no brain cyst burden was detected in infected LEW rats by using either the i.p., i.v., or s.c. route. In these i.p.-, i.v.-, or s.c.-infected LEW rats, as in the case of peroral infection, we observed very weak titers of antitoxoplasma antibodies (titers of <1/100 for i.v. and s.c. and <1/500 for i.p.), which were not comparable to the high titers observed in the case of an active parasite dissemination as in the susceptible F344 rats (titer of ≥1/6,400) (Table 2). Moreover, in these infected F344 rats, the three inoculation routes (i.p., i.v., and s.c.) led to highly reduced numbers of brain cysts compared to those counted in the brains of orally infected F344 rats (Table 2). This result indicated that in rats, unlike in mice, these routes of inoculation are much less infectious than the oral route. Together, these data showed that the resistance patterns observed in the LEW rat are similar whatever the route used. They also revealed that, in rats, the peroral infection route is the most infectious one and therefore the most accurate in terms of resistance/susceptibility readout.
TABLE 2.
Effect of route of inoculation on rat susceptibility to Toxoplasma infection
| Inoculation route | F344 rats
|
LEW rats
|
||
|---|---|---|---|---|
| Brain cysts (n)a | Serology (n)b | Brain cysts (n) | Serology (n) | |
| p.o.c | 170 ± 55 (5) | ≥1/6,400 (5) | 0 (5) | ≤1/100 (5) |
| i.p. | 4 ± 3 (4) | ≥1/6,400 (4) | 0 (5) | ≤1/500 (5) |
| i.v. | 12 ± 6.5 (5) | ≥1/6,400 (5) | 0 (5) | ≤1/100 (5) |
| s.c. | 7 ± 3 (5) | ≥1/6,400 (5) | 0 (5) | ≤1/100 (5) |
Quantitative analysis of brain cysts. Values shown are the average number of cysts per rat brain ± SD. The number of rats per group is in parentheses.
Semiquantitative analysis of anti-T. gondii antibody titers by immunofluorescence. The number of rats per group is in parentheses.
p.o., peroral.
We also examined the genetic transmission of the LEW rat resistance trait by analyzing the susceptibility of LEW × F344 and LEW × BN F1 rats to infection with 20 cysts of the Pru-β-gal strain. We showed that both the LEW × F344 and LEW × BN hybrid rats behaved as resistant LEW rats did, with no detectable intracerebral cysts and negative anti-Toxoplasma serology (data not shown). These results demonstrated that the resistance of the LEW rat to Toxoplasma infection is a dominant trait
Finally, we examined the role of MHC genes in the resistance of the LEW rats to Toxoplasma infection by analyzing the susceptibility of BN rats congenic for the MHC of the LEW strain (BN-1L). All infected BN-1L rats were found to be susceptible (by the presence of intracerebral cysts and seropositivity for Toxoplasma), indicating that the LEW MHC genes are not involved in the resistance of LEW rats to Toxoplasma infection.
Resistance of LEW rats to Toxoplasma infection is intrinsic to hematopoietic cells.
To investigate if resistance of LEW rats to Toxoplasma infection is intrinsic to hematopoietic cells or is dependent on cells from other tissues, such as cells from the digestive compartment, we generated bone marrow chimeras between LEW × BN F1 recipients and LEW, BN, and F1 donors. First, we analyzed the state of T-cell chimerism by using anti-T-cell-receptor MAb and anti-MHC class I haplotype-specific MAbs. We showed that the chimerism was similar in F1 recipients of BN and LEW bone marrow cells (72% ± 5% and 75% ± 8%, respectively). These chimeras were infected with 20 cysts of the Pru-β-gal strain 12 weeks after bone marrow reconstitution. The 10 LEW × BN F1 rats reconstituted with bone marrow from the resistant strain (LEW) were found to have the resistant phenotype (Table 3). In particular, they remained seronegative, while the 10 LEW × BN F1 rats reconstituted with bone marrow from susceptible rats (BN) developed high titers of anti-Toxoplasma antibodies (P = 0.0006). The absence of brain cysts in the 20 LEW × BN F1 rats reconstituted with bone marrow from resistant rats (LEW rats or LEW × BN F1 hybrids), compared to the presence of brain cysts in some LEW × BN F1 rats reconstituted with bone marrow from susceptible rats (BN), was also statistically highly significant (P = 0.0077). These results indicate that the resistance to Toxoplasma infection is genetically controlled by bone marrow-derived cells.
TABLE 3.
Role of hematopoietic cells in the difference in susceptibility to Toxoplasma infection between BN and LEW rats
| Rats (donor→recipient) | Anti-Toxoplasma serologya
|
No. of rats with brain cysts/totalb | |
|---|---|---|---|
| By WB | By IF | ||
| BN→F1 | Positive (10/10) | ≥1/40,000 (10/10) | 4/10 |
| LEW→F1 | Negative (10/10) | <1/100 (10/10) | 0/10 |
| F1→F1 | Negative (9/9) | <1/100 (9/9) | 0/10 |
| BN→BN | Positive (3/3) | ≥1/40,000 (3/3) | 2/3 |
Anti-T. gondii antibodies were detected by Western blotting (WB) or analyzed semiquantitatively by immunofluorescence (IF). The number of rats per group is indicated in parentheses. Highly significant differences were found between BN→F1 or BN→BN rats on one hand and LEW→F1 or F1→F1 rats on the other hand, with P values ranging from 0.0045 (BN→BN versus F1→F1) to 0.0006 (BN→F1 versus LEW→F1).
The differences were significant, with P values of 0.038 (BN→BN versus F1→F1) and 0.043 (BN→F1 versus LEW→F1). For brain cysts, when BN→F1 rats were compared to LEW→F1 plus F1→F1 rats, the difference was highly significant (P = 0.0077).
Endogenous production of IFN-γ is partially involved in the resistance of LEW rats to Toxoplasma infection.
IFN-γ production by NK and T cells plays an important role in the host defense against Toxoplasma infection, mainly by triggering macrophage anti-Toxoplasma activity (7, 11, 28). It is known that, compared to BN rats, LEW rats are strong producers of IFN-γ. In fact, activated T or NK cells from LEW rats produce more IFN-γ than those from BN rats (4, 24), and BN rats do not develop Th1-mediated autoimmune diseases (9). To investigate if the resistance of LEW rats could be related to the ability of immune cells of this strain to produce large amounts of IFN-γ, the effect of treatment with an IFN-γ-neutralizing monoclonal antibody was investigated. As shown in Table 4, whereas untreated LEW rats were seronegative, all anti-IFN-γ treated LEW rats developed seropositivity for Toxoplasma (P = 0.0078), indicating an effect of endogenous IFN-γ on the resistance phenotype. However, their antibody titers were significantly lower than those of both treated and untreated BN rats (P < 0.01), and no cysts could be detected in the brains of treated LEW rats, whereas all treated BN rats developed brain cysts. Both the lack of brain cysts and the weakness of the serology in anti-IFN-γ-treated LEW rats strongly suggested that tachyzoites were unable to disseminate throughout the treated LEW rats. We therefore analyzed the effect of a similar anti-IFN-γ treatment on parasite burden in MLN of rats at day 5 postinfection. As expected, the parasite burden was significantly higher (P < 0.01) in organs of treated BN rats (455 ± 36 tachyzoites per gram) than in those of untreated BN rats (51 ± 47 tachyzoites per gram). By contrast, no tachyzoites could be detected in the MLN of either treated or control LEW rats, suggesting that parasites had not disseminated through LEW rats despite IFN-γ neutralization. These results were confirmed by PCR analyses performed on MLN at day 5 postinfection after neutralization of IFN-γ (data not shown). Together, these results indicate that IFN-γ neutralization had a partial effect on LEW rat resistance in allowing seropositivity but not development of a parasite burden and dissemination.
TABLE 4.
Effect of treatment with MAb against IFN-γ on resistance of LEW rats
| Rat strain | Brain cystsa
|
Anti-T. gondii serologyb
|
||
|---|---|---|---|---|
| Anti-IFN-γ | Isotype control | Anti-IFN-γ | Isotype control | |
| BNc | 191 ± 63 | 14 ± 9 | 1/40,000-1/80,000 | 1/20,000-1/40,000 |
| LEWc | 0 | 0 | 1/1,600-1/12,800 | <1/100 |
Quantitative analysis of brain cysts. Values shown are the average number of cysts per rat brain ± SD (n = 5 in each group).
Quantitative analysis of anti-T. gondii antibodies by immunofluorescence. Values are the range of titers (n = 5 in each group).
In BN rats, increases in the number of brain cysts and in antibody titers were found in anti-IFN-γ-treated rats compared to control rats (P < 0.01 and P < 0.05, respectively). In LEW rats, the appearance of anti-T. gondii antibodies in anti-IFN-γ-treated rats compared to the absence of an antibody response in control rats was highly significant (P = 0.0078). In anti-IFN-γ-treated rats, titers of anti-T. gondii antibodies were significantly lower in LEW rats than in BN rats (P < 0.01).
DISCUSSION
In this study, we demonstrated that the LEW rat strain exhibits an unexpected innate refractoriness to Toxoplasma infection. In this particular rat strain, there is no trace of parasite dissemination (negative anti-Toxoplasma serology and no detectable parasites during both the acute and the chronic phases of infection). By contrast, both the F344 and BN rat strains are susceptible and develop positive anti-Toxoplasma serology and a chronic infection characterized by the presence of encysted parasites in their brains. Interestingly, the LEW rat resistance was found to be a dominant trait, since it is transmitted to the entire progeny of first-generation hybrid rats (LEW × BN and LEW × F344). To our knowledge, this provides an unprecedented experimental model to explore mechanisms of innate resistance to Toxoplasma infection.
In the mouse model, it is well known that the inoculation route, the inoculum size, and the age and the sex of the host may influence Toxoplasma infection (8, 16, 20, 32). We showed that these factors have no effect on LEW rat resistance. No difference was noticed between male and female or young and old animals, and the size of the inoculum (20 or 200 cysts) did not modify the resistance of the rats, even when very high doses, such as 1,000 cysts, were used for infection (data not shown). Moreover, the resistance patterns were similar when other routes of infection were used (i.e., parenteral, subcutaneous, or intravenous). Also, in the mouse model susceptibility to toxoplasmosis appears to be influenced by genes within the MHC region (2, 3, 17, 22, 23). It was demonstrated that at least five genes influenced survival and that they were linkages to the H-2 and H-13 complex and to the Wnt1 locus (17). Hence, mouse strains with the H-2b haplotype, are highly susceptible and succumb to oral infection, whereas those with the H-2d haplotype survive the acute infection (21, 29). Such an MHC-dependent mechanism is not likely to be involved in the resistance of LEW rats to Toxoplasma infection, since BN rats congenic for the MHC of the LEW strain (BN-1L) remained susceptible (as shown by positive anti-Toxoplasma serology and presence of brain cysts).
Several hypotheses have been envisaged to explain the refractoriness of the LEW rat to Toxoplasma infection. The parasite could be unable to invade LEW cells because of a lack of a cellular receptor(s). However, invasion assays indicated that medullar macrophages and fibroblasts from both susceptible and resistant rat strains may be infected by Toxoplasma (data not shown). Moreover the “receptor” hypothesis would not fit with genetic evidence that the LEW refractoriness is a dominant trait. Another possibility could be that a physiologic peculiarity of LEW rats, such as intestinal mucus of a particular composition, might also explain failure of Toxoplasma per os infection. However, our data showing that chimeric BN→F1 rats become susceptible to the infection whereas LEW→F1 rats become resistant strongly support involvement of hematopoietic cells and, therefore, that an immunologically based mechanism is involved in resistance of LEW rats. This mechanism must act very early during acute infection, since LEW rats develop no detectable anti-Toxoplasma humoral response. Cells from the innate compartment are therefore likely to be involved in the resistance mechanism, and we have recently accumulated evidence that Toxoplasma replication is reduced within LEW compared to BN macrophages in vitro (P. Cavailles et al., unpublished results).
Immunologic events occurring at the early phase of Toxoplasma infection have been well characterized in murine models (for reviews, see references 7 and 12). Activated neutrophils, macrophages, and dendritic cells are recruited to the area of infection and produce large amounts interleukin-12, which initiates NK cell IFN-γ production (7, 11, 28). The IFN-γ produced protects the host from acute infection by triggering microbicidal and microbistatic functions of macrophages and, together with IL-12 and dendritic cells, drives the development of an adaptive immune response (11). In our study we showed that in vivo neutralization of IFN-γ of LEW rats prior and during acute infection led to the development of a weak anti-Toxoplasma antibody response, but without a detectable parasite burden at day 5 postinfection or brain cysts. Although it cannot be excluded that the anti-IFN-γ treatment did not completely neutralize this cytokine, our results suggest that IFN-γ plays some role in the resistance of LEW rats. IFN-γ neutralization, during acute infection, might have delayed the mechanism of resistance, allowing a limited proliferation of tachyzoites within LEW cells.
The LEW and BN rat strains have already been shown to exhibit differences in their susceptibilities to some infectious agents, such as Salmonella enteritidis (14), murine cornavirus JHM (27), and Cryptococcus neoformans (19). However, in all these cases the disease susceptibility was found to be associated to the LEW background. It is also well established that BN and LEW strains differ markedly with respect to polarization of their immune responses as well as in their susceptibility to develop distinct immune-mediated diseases, including autoimmunity and allergic manifestations (9). These differences between LEW and BN rats are much more marked than in mouse strains, making the rat a model of choice for genetic and immunological studies of autoimmune, allergic, and infectious diseases.
The nature of the precise mechanism(s) involved in the LEW resistance to Toxoplasma infection remains to be determined. The availability of two strains of rat, one being fully resistant to toxoplasmosis and the other being susceptible, is unique. This simple model will allow genetic analysis to decipher the mechanism of this dominant trait. Linkage analysis of a cohort of LEW × BN F2 hybrid rats is in progress to look for quantitative trait loci controlling the immune response to Toxoplasma infection (P. Cavaillez et al., unpublished data). Further clarification of the gene(s) involved will require studies of congenic rat lines to identify the locus (or loci) of major importance and to look for candidate genes according to the positional cloning strategy. Using this approach, we aim to characterize the gene(s) responsible for the refractoriness of the LEW rats to toxoplasmosis and to investigate its mechanism(s) of action. Understanding the genetic and pathophysiological bases of this phenomenon could open the way to new avenues in the prevention and/or treatment of toxoplasmosis in human and veterinary medicine. It could also point to a new important disease pathway involved in parasitic diseases in which infection proceeds through the intestinal route and similar cellular mechanisms.
Acknowledgments
This work was supported in part by the Institut Pasteur of Lille, the French Ministry of Research and Technology (A0 PRFMMIP and ACI “Programme de Microbiologie”), the Institut National de la Santé et de la Recherche Médicale (INSERM “ATC Environnement Santé”), The Paul Sabatier University Toulouse III, SIDACTION, and the Toxoplasma Research Institute.
We thank Cordelia Bisanz for critical reading of the manuscript, Isabelle Bernard for excellent technical assistance, and Maryline Calise, Patrick Aregui, and Audrey Boyer (Zootechnic Unit, IFR 30, Toulouse, France) for taking care of the animal house.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Bretagne, S., J. M. Costa, M. Vidaud, J. Tran, V. Nhieu, and J. Fleury-Feith. 1993. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J. Infect. Dis. 168:1585-1588. [DOI] [PubMed] [Google Scholar]
- 2.Brown, C. R., and R. McLeod. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 152:4537-4541. [PubMed] [Google Scholar]
- 3.Brown, C. R., C. Hunter, R. Estes, E. Beckmann, J. Forman, C. David, J. S. Remington, and R. McLeod. 1995. Definitive identification of a gene that confers resistance against toxoplasmosis. Immunology 85:419-429. [PMC free article] [PubMed] [Google Scholar]
- 4.Cautain, B., J. Damoiseaux, I. Bernard, E. Xystrakis, E. Fournie, P. van Breda Vriesman, P. Druet, and A. Saoudi. 2002. The CD8 T cell compartment plays a dominant role in the deficiency of Brown-Norway rats to mount a proper type 1 immune response. J. Immunol. 168:162-170. [DOI] [PubMed] [Google Scholar]
- 5.Dao, A., M. Soete, V. Sergent, D. Deslee, B. Fortier, and J. F. Dubremetz. 2002. Potential of β-galactosidase-expressing Toxoplasma gondii for in situ localization and observation of rare stages of the parasite life cycle. Parasitol. Res. 88:69-72. [DOI] [PubMed] [Google Scholar]
- 6.Darcy, F., and L. Zenner. 1993. Experimental models of toxoplasmosis. Res. Immunol. 144:16-23. [DOI] [PubMed] [Google Scholar]
- 7.Denkers, E. Y., and R. T. Gazinelli. 1998. Regulation and function of T-cell mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 1:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey, J. P. 1987. Toxoplasmosis. Vet. Clin. North. Am. Small Anim. Pract. 17:1389-1404. [DOI] [PubMed] [Google Scholar]
- 9.Fournie, G. J., B. Cautain, E. Xystrakis, J. Damoiseaux, M. Mas, D. Lagrange, I. Bernard, J. F. Subra, L. Pelletier, P. Druet, and A. Saoudi. 2001. Cellular and genetic factors involved in the difference between Brown Norway and Lewis rats to develop respectively type-2 and type-1 immune-mediated diseases. Immunol. Rev. 184:145-160. [DOI] [PubMed] [Google Scholar]
- 10.Frenkel, J. K. 1988. Pathophysiology of toxoplasmosis. Parasitol. Today 4:273-278. [DOI] [PubMed] [Google Scholar]
- 11.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzinelli, R. T., D. Amichay, T. Sharton-Kersten, E. Grunwald, J. M. Farber, and A. Sher. 1996. Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. Curr. Top. Microbiol. Immunol. 219:127-139. [DOI] [PubMed] [Google Scholar]
- 13.Godard, I., F., Darcy, D., Deslee, J. P., Dessaint, and A. Capron. 1990. Isotypic profiles of antibody responses to Toxoplasma gondii infection in rats and mice: kinetic study and characterization of target antigens of immunoglobulin A antibodies. Infect. Immun. 58:2246-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havelaar, A., J. Garssen, K. Takumi, M. Koedam, W. Ritmeester, L. de la Fonteyne, T. Bousema, and J. Vos. 2004. Intraspecies variability in the dose-response relationship for Salmonella Enteritidis associated with genetic differences in cellular immune response. J. Food Prot. 67:2008-2015. [DOI] [PubMed] [Google Scholar]
- 15.Hsiung, L. M., A. N. Barclay, M. R. Brandon, E. Sim, and R. R. Porter. 1982. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem. J. 203:293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, L. L., G. W. Gibson, and P. C. Sayles. 1995. Preimmune resistance to Toxoplasma gondii in aged and young adult mice. J. Parasitol. 6:894-899. [PubMed] [Google Scholar]
- 17.Johnson, J., Y. Suzuki, D. Mack, E. Mui, R. Estes, D. David, E. Skamene, J. Forman, and R. McLeod. 2002. Genetic analysis of influences on survival following Toxoplasma gondii infection. Int. J. Parasitol. 32:179-185. [DOI] [PubMed] [Google Scholar]
- 18.Kempf, M. C., M. F. Cesbron-Delauw, D. Deslee, U. Gross, T. Herrmann, and P. Sutton. 1999. Different manifestations of Toxoplasma gondii infection in F344 and LEW rats. Med. Microbiol. Immunol. 187:137-142. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, M., M. Ito, K. Sano, and M. Koyama. 2001. Granulomatous and cytokine responses to pulmonary Cryptococcus neoformans in two strains of rats. Mycopathologia 151:121-130. [DOI] [PubMed] [Google Scholar]
- 20.Liesenfeld, O., T. A. Nguyen, C. Pharke, and Y. Suzuki. 2001. Importance of gender and sex hormones in regulation of susceptibility of the small intestine to peroral infection with Toxoplasma gondii tissue cysts. J. Parasitol. 87:1491-1493. [DOI] [PubMed] [Google Scholar]
- 21.Mack, D. G., J. J. Johnson, F. Roberts, C. W. Roberts, R. G. Estes, C. David, F. C. Grumet, and R. McLeod. 1999. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int. J. Parasitol. 29:1351-1358. [DOI] [PubMed] [Google Scholar]
- 22.McLeod, R., E. Skamene, C. R. Brown, P. Eisenhauer, and D. Mack. 1989. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of AXB/BXA recombinant inbred and B10 congenic mice. J. Immunol. 143:3031-3034. [PubMed] [Google Scholar]
- 23.McLeod, R., C. Brown, and D. Mack. 1993. Immunogenetics influence outcome of T. gondii infection. Res. Immunol. Ann. Inst. Pasteur 144:61-66. [DOI] [PubMed] [Google Scholar]
- 24.Mikus, L. D., L. A. Rosenthal, R. L. Sorkness, and R. F. Lemanske. 2001. Reduced interferon-gamma secretion by natural killer cells from rats susceptible to postviral chronic airway dysfunction. Am. J. Respir. Cell Mol. Biol. 2:74-82. [DOI] [PubMed] [Google Scholar]
- 25.Piketty, C., F. Derouin, B. Rouveix, and J. J. Pocidalo. 1990. In vivo assessment of antimicrobial agents against Toxoplasma gondii by quantification of parasites in blood, lungs, and brain of infected mice. Antimicrob. Agents Chemother. 34:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoro, F., C. Auriault, P. Leite, F. Darcy, and A. Capron. 1987. Infection of the athymic rat by Toxoplasma gondii. C. R. Acad. Sci. III 304:297-300. [PubMed] [Google Scholar]
- 27.Schwender, S., H. Imrich, and R. Dorries. 1991. The pathogenic role of virus-specific antibody-secreting cells in the central nervous system of rats with different susceptibility to coronavirus-induced demyelinating encephalitis. Immunology 74:533-538. [PMC free article] [PubMed] [Google Scholar]
- 28.Sher, A., I. P. Oswald, S. Hieny, and R. T. Gazzinelli. 1993. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J. Immunol. 150:3982-3989. [PubMed] [Google Scholar]
- 29.Suzuki, Y., K. Joh, M. A. Orellana, F. K. Conley, and J. S. Remington. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732-739. [PMC free article] [PubMed] [Google Scholar]
- 30.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Meide, P. H., A. H. Borman, H. G. Beljaars, M. A. Dubbeld, C. A. D. Botman, and H. Schellekens. 1989. Isolation and characterization of monoclonal antibodies directed to rat interferon-gamma. Lymphokine Res. 8:439-449. [PubMed] [Google Scholar]
- 32.Walker, W., C. W. Roberts, D. Ferguson, H. Jebbari, and J. Alexander. 1997. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect. Immun. 65:1119-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zenner, L., F. Darcy, A. Capron, and M. F. Cesbron-Delauw. 1998. Toxoplasma gondii: kinetics of the dissemination in the host tissues during the acute phase of infection of mice and rats. Exp. Parasitol. 90:86-94. [DOI] [PubMed] [Google Scholar]
- 34.Zenner, L., A. Foulet, Y. Caudrelier, F. Darcy, B. Gosselin, A. Capron, and M. F. Cesbron-Delauw. 1999. Infection with Toxoplasma gondii RH and Prugniaud strains in mice, rats and nude rats: kinetics of infection in blood and tissues related to pathology in acute and chronic infection. Pathol. Res. Pract. 195:475-485. [DOI] [PubMed] [Google Scholar]
- 35.Zenner, L., J. Estaquier, F. Darcy, P. Maes, A. Capron, and M. F. Cesbron-Delauw. 1999. Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted-secreted antigens as vaccine components. Parasite Immunol. 21:261-272. [DOI] [PubMed] [Google Scholar]