Abstract
It is currently accepted that malaria-parasitized red blood cells (pRBC) are eliminated, like senescent erythrocytes, phagocytically by macrophages in the red pulp of the spleen. Here, however, we show that self-healing Plasmodium chabaudi malaria activates spleen closure in C57BL/6 mice. Confocal laser scanning microscopy revealed that spleen closing was manifested by elimination of entry into the red pulp of 3-μm polystyrol particles, pRBC, and nonparasitized red blood cells but not of bovine serum albumin. This spleen closure did not reflect a reduction in the number of phagocytic cells, as shown by flow cytometry, whereas marginal zone macrophages (MZM) were lost and red pulp macrophages entered the white pulp. Splenic trapping of pBRC was strongly reduced in the absence of MZM and marginal metallophilic macrophages (MMM), as it is in noninfected mice with a disrupted lymphotoxin β receptor (LTβR−/−), and it was still significantly reduced when the number of MZM and MMM was diminished, as in tumor necrosis factor alpha-deficient (TNF-α−/−) mice. Moreover, mice deficient in TNF-α, tumor necrosis factor receptor I (TNFRI−/−), and LTβR exhibited progressive impairment in malaria-induced spleen closing. Treatment of C57BL/6 mice with TNF-α induced loss of MZM and spleen closing by about 20%. Our data indicate that TNF/TNFRI signaling is involved in regulating malaria-induced spleen closure, which is maximal during crisis, when parasitemia declines more than 100-fold. Consequently, the vast majority of pRBC cannot be destroyed by the spleen during crisis, suggesting that the known sophisticated sequestration system of Plasmodium parasites did not evolve to avoid splenic clearance.
Natural immunity to malaria is directed against the blood stages of Plasmodium parasites, and the spleen is a key effector for parasite killing through production of free radicals and phagocytosis by activated macrophages. Currently, the spleen is thought to destroy parasites basically by making use of the same phagocytic mechanisms which evolved to clear senescent and other aberrant erythrocytes (5, 18). It is a widely accepted, although never formally proven, view that parasitized red blood cells (pRBC) escape splenic trapping and phagocytic clearance by sequestration at endothelia of postcapillary venuoles (3, 9, 13, 17, 25) which is mediated by a large family of antigens on the surface of the pRBC undergoing antigenic variation.
In the spleen, there are two regions with intense phagocytic activity, the marginal zone (MZ), which is responsible for elimination of inert particles, bacteria, and viruses (19), and the red pulp, which is engaged in removal of senescent and aberrant red blood cells (RBC) (19, 24). Blood enters the spleen through the splenic artery, which branches and gives rise to arterioles and capillaries. The latter terminate either in the marginal sinus, in the MZ, or in the red pulp (14). While the larger portion of blood takes a fast route directly from the marginal sinus to the draining veins, about 90% of the blood enters the extravascular beds of the “open” circulation of the MZ and red pulp. These beds contain about 91% of the total splenic blood and are characterized by a high hematocrit, a very low flow rate, and close contact between resident macrophages and blood-borne material. Passage through these extravascular beds results in efficient percolation and filtration of blood.
During avirulent Plasmodium yoelii 17XNL malaria in BALB/c mice, the ability of the spleen to trap carbon particles was found to be transiently reduced (32). In the so-called precrisis phase, when parasitemia is continuously rising, fusion of activated stromal cells has been reported to give rise to barrier cells which restrict entrance to the extravascular beds of the red pulp. When the precrisis phase culminates in peak parasitemia, reopening of the filtration beds is associated with an increased trapping activity that is presumably due to malaria-induced splenomegaly (32), and this has been correlated with rapidly decreasing parasitemia in the following crisis phase. In another avirulent malaria model, Plasmodium chabaudi adami infection of BALB/c mice, such transient changes in trapping were further substantiated using fluorescent polystyrol particles (2). By contrast, Yadava et al. (36) did not find, with exactly the same parasite/host combination, any evidence for reduced splenic trapping of either Salmonella enterica serovar Typhimurium in the MZ or pRBC in the red pulp, and they concluded that the pRBC avoid a fully functional MZ.
In contrast to previous studies, we show here that malaria induces dysfunction of the MZ and activates a spleen-inherent gating mechanism that locks out pRBC, particularly in the crisis phase of infection characterized by massive destruction of pRBC. As a model we chose a more virulent strain of P. chabaudi, a parasite species that exhibits sequestration and antigenic variation similar to the sequestration and antigenic variation of Plasmodium falciparum (12).
MATERIALS AND METHODS
Antibodies.
Fluorescein isothiocyanate-labeled rat primary antibodies L3T4 (anti-CD4), Ly-2 (anti-CD8), Ra3-6B2 (anti-CD45R/B220), and RB6-8C5 (anti-Gr-1) (all obtained from BD PharMingen, Heidelberg, Germany), as well as F4/80 (ImmunoKontact, Wiesbaden, Germany), were used. In addition, unconjugated monoclonal antibodies MOMA1 and ER-TR7 (both obtained from BMA Biomedicals, Augst, Switzerland), 3D6.112 (anti-CD169; Serotec, Düsseldorf, Germany), and biotinylated ER-TR9 (BMA Biomedicals) were used.
Animals.
C57BL/6 mice, mice with a disrupted lymphotoxin β receptor (LTβR−/−), mice deficient in tumor necrosis factor receptor I (TNFRI−/−), and tumor necrosis factor alpha-deficient (TNF-α−/−) mice with a C57BL/6 background were bred under specific-pathogen-free conditions in our central animal facilities. Experiments were performed with 12- to 14-week-old female mice which were housed in plastic cages and received a standard diet (Wohrlin, Bad Salzuflen, Germany) and water ad libitum. The research complied with all relevant guidelines and institutional policies.
Infections and TNF-α treatment.
We used a nonclonal line of P. chabaudi (33) which exhibits a restriction fragment length polymorphism pattern that is very similar but not identical to that of P. chabaudi chabaudi AS (G. Snounou and F. Wunderlich, unpublished data). Blood stages of P. chabaudi were weekly passaged in NMRI mice (34). Experimental animals were challenged with 1 × 106 P. chabaudi-parasitized erythrocytes. Parasitemia was evaluated in Giemsa-stained blood smears, and total erythrocytes were counted in a Neubauer chamber. For treatment with TNF-α, mice were injected intraperitoneally with 104 or 105 U recombinant mouse TNF-α (Roche, Mannheim, Germany) in 200 μl phosphate-buffered saline (PBS) on three consecutive days.
Isolation and labeling of pRBC.
Isolation of pRBC from the blood of infected NMRI mice with 30 to 40% parasitemia was performed as described previously (33). For labeling with PKH-26 (Sigma, Taufkirchen, Germany), 1 × 1010 RBC or pRBC were resuspended in 1 ml diluent C and mixed with 1 ml diluent C containing 4 μM PKH-26. Cells were incubated for 5 min at room temperature in the dark before 2 ml of a bovine serum albumin (BSA) solution (1% in PBS) was added to stop the reaction. After three washing steps with IMDM cell culture medium (Invitrogen, Karlsruhe, Germany) containing 5% fetal calf serum (PAA Laboratories, Cölbe, Germany), cells were washed once with PBS and finally resuspended in PBS.
Determination of filtration capacity.
Mice were anesthetized with diethyl ether before 200 μl PBS containing 1.3 × 108 green fluorescent beads (Duke Scientific, Palo Alto, CA) with a diameter of 3 μm were injected intravenously (i.v.). After 5 min, the mice were killed by cervical dislocation. The spleen of each mouse was removed, weighed, and squeezed with a glass rod in 8 ml 2.3 M KOH, 0.5% Tween 80 in ethanol. After addition of 5 × 105 red beads (diameter, 2.9 μm; Duke Scientific) as a control for losses during extraction, the tissues were dissolved by shaking at 50°C for 48 h interrupted several times by vigorous vortexing. Samples were centrifuged at 2,000 × g at 20°C for 20 min, and each pellet with beads was washed with 8 ml 1% Triton X-100 and then with 8 ml PBS and finally resuspended in 1 ml distilled water. Fluorescence intensity was measured with a luminescence spectrometer (LS 55; Perkin-Elmer, Langen, Germany) at excitation and emission wavelengths of 450 and 480 nm, respectively, for green beads and 520 and 590 nm, respectively, for red beads. The filtration capacity for infected erythrocytes was measured by i.v. injection of 1 × 109 PKH-26-labeled P. chabaudi-parasitized erythrocytes. Splenic uptake of BSA was quantified by i.v. injection of 500 μg red fluorescent BSA-tetramethyl rhodamine isocyanate (TRITC) conjugate (Molecular Probes, Karlsruhe, Germany) in 200 μl PBS as described by Nolte et al. (23). After 30 min, the mice were killed, and the spleens were processed for immune fluorescence microscopy. Sections without any counterstaining were used for quantification of trapping. Pictures of red fluorescence were taken with a Leica DMLB microscope (Leica Microsysteme Vertrieb, Bensheim, Germany). Signal intensity was evaluated using the ImageJ software.
Isolation of spleen cells.
Spleens were gently dissociated through a stainless steel sieve into RPMI medium (Invitrogen, Karlsruhe, Germany) supplemented with 5% fetal calf serum. Erythrocytes were removed by NH4Cl lysis as described previously (4). Total leukocytes were counted in a Neubauer chamber.
Flow cytometry.
Splenic leukocytes were labeled with antibodies and analyzed by flow cytometry (FACScan; BD Biosciences, Heidelberg, Germany) as detailed previously (4). Specifically, cells were preincubated with anti-mouse CD16/CD32 (FcIII/II receptor) Fc block (BD PharMingen, Heidelberg, Germany) for 3 min and then labeled with anti-mouse F4/80 (ImmunoKontact, Wiesbaden, Germany) or anti-mouse Gr-1 (BD PharMingen) fluorescein isothiocyanate-labeled monoclonal antibodies. Fluorescence-activated cell sorting analysis was done with a sample size of 10,000 cells gated on the basis of forward and side scatter. Data were stored and processed using the Cell Quest Pro software (BD Biosciences).
Fluorescence microscopy.
Spleen pieces were embedded in OCT compound (Sakura, Zouterwede, The Netherlands) and snap frozen on dry ice before serial sections were mounted on superfrost plus slides (Menzel-Glaser, Braunschweig, Germany). Slides were air dried at room temperature for at least 1 h, fixed in acetone for 20 min, and washed with PBS. For localization of fluorescent beads, sections were counterstained with hematoxylin and mounted in Mowiol (Polyscience, Niles, IL) containing 2.5% DABCO (Merck, Darmstadt, Germany), and photographs were taken with a Leica DMLB fluorescence microscope. After injection of PKH-26-labeled P. chabaudi-parasitized erythrocytes, sections were fixed for 20 min in 1% paraformaldehyde in PBS and mounted without counterstaining. Evaluation of fluorescence intensity was performed using the ImageJ software.
For localization of cell populations in the spleen, sections were air dried, fixed for 20 min in 1% paraformaldehyde in PBS, and blocked for 20 min with Fc block (1:200 in PBS) before incubation for 1 h with primary antibodies diluted 1:20 (anti-CD4, anti-CD8), 1:50 (F4/80), or 1:100 (ER-TR7, MOMA-1, anti-CD169, B220, GR-1, biotinylated ER-TR9) in PBS. Signal amplification was performed using a biotinylated secondary mouse anti-rat antibody (Dianova, Hamburg, Germany) and Alexa fluor 488-coupled streptavidin (Molecular Probes). The biotinylated ER-TR9 antibody was directly detected with Alexa fluor 488-coupled streptavidin. After counterstaining with hematoxylin, sections mounted in Mowiol-DABCO were analyzed with a Leica confocal laser scanning microscope (Leica Lasertechnik, Heidelberg, Germany). Linear enhancement of the brightness, contrast, and intensity of red fluorescence pictures was performed using Corel PhotoPaint 10. All pictures were treated in exactly the same way.
RESULTS
Malaria-induced spleen closing.
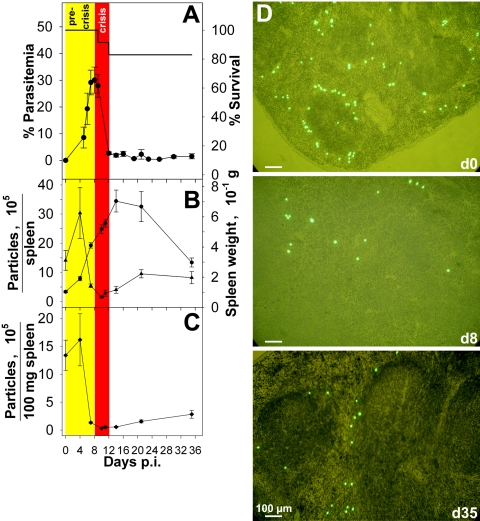
Blood stage infections with P. chabaudi took a self-healing course in female C57BL/6 mice (Fig. 1A). The precrisis phase culminated in peak parasitemia of about 30% on day 8 postinfection (p.i.), and the subsequent crisis phase was characterized by massive destruction of pRBC which resulted in less than 1% pRBC on days 12 and 13 p.i., before passage into a chronic phase of persistent low-grade parasitemia controlled by protective immune mechanisms. Figure 1B shows that P. chabaudi infections induced dramatic splenomegaly and that the maximal size, with an approximately sevenfold increase in spleen weight, was reached shortly after the crisis phase on day 14 p.i. In the precrisis phase, there was a dramatic drop in the total and specific capacities of the spleen to trap injected 3-μm fluorescent polystyrol beads (Fig. 1B and C). This state of the spleen, referred to as “closed” below, has been reported to occur transiently in nonlethal infections with P. yoelii 17XNL (32) and P. chabaudi adami (2). However, the data in Fig. 1 show that the closed state was maintained throughout the entire crisis phase and even after the crisis phase. On day 21 p.i., slight reopening occurred, but even on day 35 p.i. the spleen exhibited less than 25% of its initial specific trapping capacity. Fluorescence microscopy of spleen sections confirmed that there was reduced trapping of beads on days 8 and 35 p.i. (Fig. 1D). While only a few beads appeared to be randomly scattered on day 8 p.i., trapping of beads occurred preferentially in the MZ on days 0 and 35 p.i. (Fig. 1D). This is consistent with the view that the MZ is responsible for removal of inert particles, various bacteria, and viruses by MZ macrophages (MZM) (1, 19) and demonstrated that there was a loss of functional MZM during the crisis phase.
FIG. 1.
Malaria-induced spleen closing. (A) Parasitemia and survival after challenge of female C57BL/6 mice (n = 12) with 1 × 106 P. chabaudi pRBC. (B) Splenic trapping of green fluorescent polystyrol particles. Spleen weight (circles) and total splenic uptake of particles (triangles) were determined. (C) Specific uptake of particles per 100 mg spleen. (D) Localization of green fluorescent particles in splenic cryosections stained with hematoxylin and eosin. All values are means ± standard errors of the means.
Splenic exclusion of pRBC during the crisis phase.
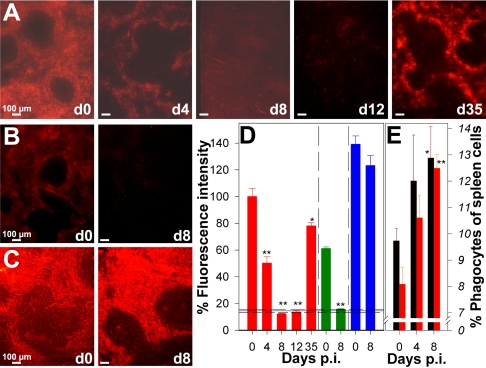
In contrast to inert particles, it has been shown that pRBC avoid a fully functional MZ and the spleen forms no blood-spleen barrier against P. chabaudi adami-infected pRBC (36). However, our data demonstrate that malaria-induced spleen closing also occurs for pRBC. After injection of red fluorescent PKH-26-labeled pRBC, noninfected mice displayed bright fluorescence in the red pulp (Fig. 2A). The uptake of pRBC was approximately twofold greater than the uptake of noninfected erythrocytes (Fig. 2A, B, and D). Presumably, this was due to decreased deformability of pRBC and altered surface properties compared to those of RBC, which facilitated trapping in the red pulp meshwork and interaction with macrophages (6, 28). On day 4 p.i., pRBC trapping by the red pulp was already decreased, and it even ceased during the crisis phase between days 8 and 12 p.i. On day 35 p.i., the spleen had largely recovered and was again able to trap pRBC (Fig. 2A and D). Remarkably, spleen closing also occurred with noninfected RBC (Fig. 2B and D), indicating the independence of the closing from pathogen-associated molecular patterns. By contrast, entry of soluble material was not significantly impaired during the crisis phase (Fig. 2C and D). Injected BSA-TRITC was found predominantly in the red pulp before infection and on day 8 p.i., demonstrating that the barrier between the blood and white pulp remained intact and confined soluble material to narrow conduits in the white pulp (23).
FIG. 2.
(A to C) Trapping capacity of the spleen during P. chabaudi infection. Splenic uptake of pRBC (A), RBC (B), and BSA-TRITC (C) was analyzed by fluorescence microscopy on the days indicated (d0 to d35) after injection of 1 × 109 PKH-26-labeled pRBC or noninfected RBC or 500 μg BSA-TRITC. (D) Semiquantitative evaluation of fluorescence intensity for 10 cryosections per mouse with three to five mice per time after injection of PKH-26-labeled pRBC (red bars), noninfected RBC (green bars), and BSA-TRITC (blue bars). Each bar indicates the mean, and each error bar indicates 0.5 standard error of the mean. The horizontal lines indicate means ± standard errors of the means for background fluorescence for cryosections of mice that did not receive an injection. (E) Numbers of F4/80+ macrophages (black bars) and Gr-1+ granulocytes (red bars) expressed as percentages of the total spleen cells. Each bar indicates the mean, and each error bar indicates 0.5 standard error of the mean. One asterisk, P < 0.05 compared with day 0 p.i.; two asterisks, P < 0.01 compared with day 0 p.i.
Redistribution of phagocytic cells during spleen closing.
The reduced trapping activity of the spleen at peak parasitemia was not due to a decrease in the number of phagocytic cells. By contrast, there was even a significant increase in the proportion of F4/80+ macrophages and Gr-1+granulocytes (Fig. 2E). However, phagocytic cells became dramatically redistributed in the spleen during malaria.
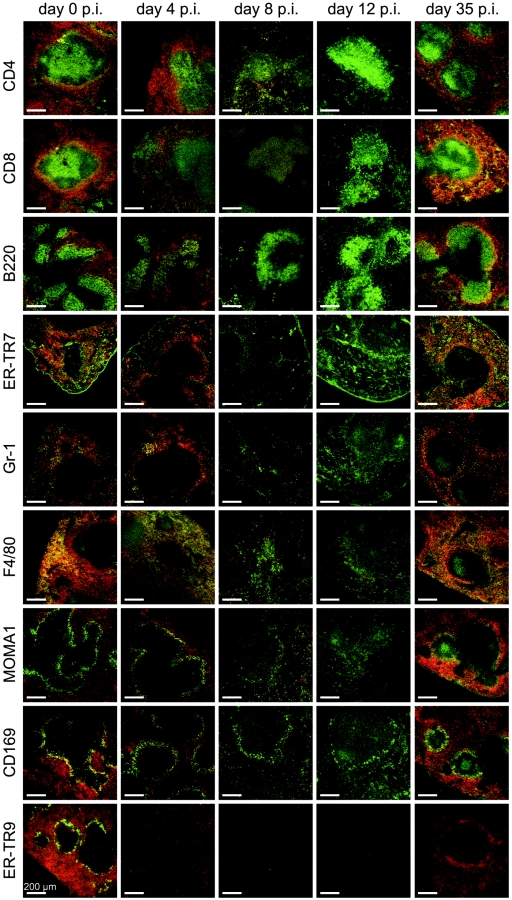
On day 0 p.i., injected red fluorescent pRBC colocalized predominantly with F4/80+ macrophages and only rarely with Gr-1+ granulocytes and the ER-TR7+ reticular meshwork in the red pulp of the spleen (Fig. 3). Moreover, pRBC colocalized with CD169+/MOMA1+ marginal metallophilic macrophages (MMM) between the marginal sinus and white pulp but only very rarely with the ER-TR9+ MZM localized between the marginal sinus and red pulp.
FIG.3.
Localization of cell types in the spleen. Mice were injected with 1 × 109 PKH-26-labeled (red) pRBC. Cryosections were incubated with the primary antibodies indicated and were developed using biotinylated secondary antibodies and streptavidin-Alexa 488 (green). Separate green and red images were taken by confocal laser scanning microscopy and merged.
With the beginning of spleen closing on day 4 p.i., F4/80+ macrophages entered the white pulp, and the labeling patterns for T cells, MMM, and MZM became slightly diffuse (Fig. 3). Only a few MMM and some F4/80+ macrophages colocalized with PKH-26-labeled pRBC since uptake of the latter was already significantly reduced. ER-TR9+ MZM completely disappeared from the spleen, as reported previously for mice infected with P. chabaudi (29, 36) or Leishmania donovani (8).
In the closed state on days 8 and 12 p.i., B cells, T cells, and the reticular meshwork remained largely confined to the white pulp and red pulp, respectively, while there was a dramatic redistribution of Gr-1+ granulocytes and F4/80+ macrophages, which were scattered almost uniformly throughout the spleen (Fig. 3). Labeling of MMM for MOMA1 was only weak, indicating that expression of the MOMA1 antigen is downregulated in MMM during infection, as recently also suggested for infections with L. donovani (8). However, a thin, perforated ring of MMM persisted around the white pulp throughout infection, as observed with anti-CD169 (Fig. 3).
On day 35 p.i., the spleen architecture and trapping activity for pRBC had largely recovered (Fig. 2 and 3). A few F4/80+ macrophages were still detectable in the white pulp, but the majority were found in the red pulp, with considerable colocalization with PKH-26-labeled pRBC. However, this colocalization was not as pronounced as that on day 0 p.i., since the red fluorescence of pRBC was brighter in the MZ than in the red pulp (Fig. 2). An inner MZ ring composed of CD169+/MOMA1+ MMM was clearly visible, again showing remarkable colocalization with pRBC. ER-TR9+ MZM were still absent from the spleen, which is consistent with the low trapping activity for polystyrol beads at this time (Fig. 1). Furthermore, there was considerable colocalization of pRBC with B220+ B cells and the ER-TR7+ reticular meshwork in the MZ.
Role of TNF/TNFR family members in spleen closing.
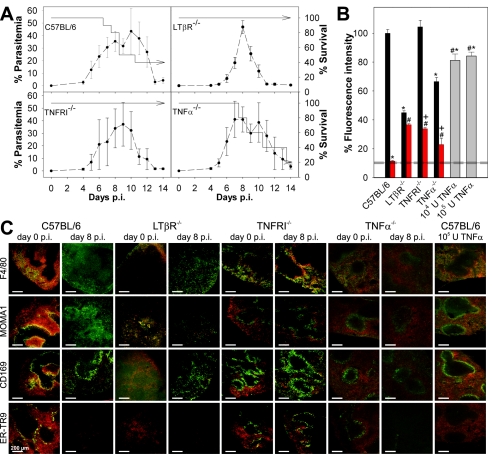
The data described above suggest that the MZ plays a critical role in the control of spleen closing. This view was further supported by analyzing mice with different defects in their MZ. For instance, LTβR−/− mice completely lack MZM and MMM (10, 11). All LTβR−/− mice survived an acute P. chabaudi infection, while a large proportion of C57BL/6 mice, used as controls in this experiment, succumbed to infection (Fig. 4A). The latter observation resulted from an abrupt increase in virulence of the parasite strain used, which occasionally occurs after more than 40 passages. Although this effect complicates comparison of the survival data shown in Fig. 1 and 4, it extends the difference in susceptibility between LTβR−/− mice and C57BL/6 mice that we reported recently (35), thus allowing better correlation of changes in the MZ and splenic trapping activity with resistance to blood stage malaria. A constitutive property of the LTβR−/− mice was their significantly diminished ability to trap pRBC on day 0 p.i. (P < 0.01) (Fig. 4B and C), and no significant spleen closing occurred after infection on day 8 p.i.
FIG. 4.
Impaired spleen closing in mice with a defect in the marginal zone. (A) Parasitemia (dashed line) and survival of C57BL/6, LTβR−/−, TNFRI−/−, and TNF-α−/− mice in the acute phase of a P. chabaudi infection. (B) Mice were injected on days 0 and 8 p.i. with 1 × 109 PKH-26-labeled (red) pRBC. Alternatively, C57BL/6 mice were treated on three consecutive days with 104 U or 105 U recombinant mouse TNF-α and injected on day 4 with 1 × 109 PKH-26-labeled pRBC. Semiquantitative evaluation of PKH-26 fluorescence intensity in cryosections was performed by fluorescence microscopy on day 0 (black bars) and on day 8 p.i. (red bars) and after TNF-α treatment (gray bars). The horizontal lines indicate the means ± standard error of the means for background fluorescence for cryosections of mice that did not receive an injection. Asterisk, P < 0.01 compared with C57BL/6 and TNFR−/− mice on day 0 p.i.; number sign, P < 0.01 compared with C57BL/6 mice on day 8 p.i.; plus sign, P < 0.01 for a comparison of both groups. (C) Cryosections from the spleens of mice were incubated with the primary and secondary antibodies indicated, developed using biotinylated secondary antibodies and streptavidin-Alexa 488 (green), and analyzed as described in the legend to Fig. 3.
This finding was further corroborated with TNFRI−/− mice, which were also highly resistant to P. chabaudi (Fig. 4A). In contrast to LTβR−/− mice, TNFRI−/− mice have been reported to have an MZ with MZM and MMM, although the number was reduced (26). Moreover, the two cell populations are not separated into two layers but are intermingled. However, our confocal laser scanning microscopy analysis did not reveal the expected reduction in labeling for ER-TR9+ MZM or CD169+ MMM, although the labeling pattern was more diffuse than that in C57BL/6 mice and expression of MMM on MOMA1 was very weak (Fig. 4C). One reason for this difference might be an effect of the different genetic backgrounds of the mice used. TNFRI−/− mice exhibited normal pRBC trapping activity on day p.i. (Fig. 4B and C), but their spleens were only partially closed upon infection on day 8 p.i., with about one-third of the uptake occurring on day 0 p.i.. This indicates that signaling through TNFRI is involved in the regulation of malaria-inducible spleen closing. Conspicuously, TNFRI−/− mice did not exhibit any malaria-induced redistribution of F4/80+ macrophages and exhibited no alterations in labeling patterns of CD169/MOMA1+ MMM and ER-TR9+ MZM at peak parasitemia (Fig. 4C). In contrast to C57BL/6 mice, colocalization of PKH-26-labeled pRBC with F4/80+ red pulp macrophages and MMM was observed even on day 8 p.i., which further supports the hypothesis that the MZ play a role in the control of pRBC entry into the red pulp.
TNFRI is known to mediate not only the effects of TNF-α but also the effects of lymphotoxin alpha (LTα) (16). Therefore, we also investigated TNF-α−/− mice to determine if production of TNF-α is sufficient or necessary to provoke the changes in histology and function associated with malaria-induced spleen closing. In contrast to TNFRI−/− mice, the proportion of TNF-α−/− mice which succumbed to P. chabaudi infection during the crisis phase was similar to the proportion of wild-type C57BL/6 mice which succumbed (Fig. 4A). Also in contrast to the TNFRI−/− mice, the TNF-α−/− mice showed only weak labeling for MMM and MZM that was correlated with significantly reduced trapping of pRBC on day 0 p.i. (Fig. 4C). On day 8 p.i., however, the redistribution of F4/80+ macrophages, the loss of ER-TR9+ MZM, and the downregulation of MOMA1 antigen on MMM were similar to the redistribution of F4/80+ macrophages, the loss of ER-TR9+ MZM, and the downregulation of MOMA1 antigen on MMM in wild-type control mice, indicating that LTα signaling is sufficient to cause these malaria-induced effects. Moreover, the uptake of pRBC was reduced to about one-third that on day 0 p.i. which resembles the situation in TNFRI−/− mice. The uptake of PKH-26-labeled pRBC at peak parasitemia was lower in TNF-α−/− mice than in TNFRI−/− mice but still significantly higher than the uptake in wild-type mice (Fig. 4B).
Since disruption of the TNF-α gene impaired spleen trapping, we analyzed the possibility that TNF-α has a direct role in spleen closing by injecting 104 U or 105 U TNF-α per day for 3 days into noninfected C57BL/6 mice. This treatment resulted in a small but significant reduction in pRBC uptake (about 20%) (Fig. 4B) and in a loss of ER-TR9+ MZM (Fig. 4C), indicating that both the absence and strong activation of TNFRI signaling lead to changes in the MZ.
DISCUSSION
This study demonstrated that there is a malaria-inducible spleen-inherent closing mechanism that controls the uptake of both inert beads and parasitized and nonparasitized RBC. The spleen became largely closed shortly before peak parasitemia and remained in the closed state throughout and after the crisis phase of infection, which is characterized by massive destruction of pRBC. Only about 1 week after crisis did a slow reopening of the spleen begin to occur. Our data therefore differ from previous findings. For instance, Yadava et al. (36) did not detect any reduced uptake of pRBC by the red pulp or bacteria by the MZ during P. chabaudi adami infection. Moreover, only transiently reduced trapping of inert particles has been reported for infections with P. yoelii 17XNL during the late precrisis phase (32) and with P. chabaudi adami at peak parasitemia (2), followed by reopening of the spleen with even higher trapping capacity during the midcrisis to late crisis phase. One reason for this difference may be the higher virulence of the parasite strain which we used than of P. chabaudi adami and P. yoelii 17XNL.
A functional MZ appears to be important for trapping of pRBC in the red pulp. In noninfected mice, a decrease in the number of MZM and MMM in TNF-α−/− mice and the complete absence of MZM and MMM in LTβR−/− mice correlate with weakly reduced and strongly reduced trapping, respectively. In addition, the MZ appears to be involved in malaria-induced spleen closing since no closing occurred in LTβR−/− mice during malaria, although the red pulp is apparently normal in these mice. Moreover, our data demonstrate that spleen closure is partially mediated by signaling through TNFRI. Thus, disrupted signaling through TNFRI impaired splenic closing, and, coincidently, it prevented loss of MZM and migration of F4/80+ macrophages into the white pulp. In accordance with this view, treatment of C57BL/6 mice with recombinant TNF-α diminished splenic trapping and eliminated MZM, whereas no migration of F4/80+ macrophages and no changes in localization of MMM were observed. Although MZM were implicated in regulation of lymphocyte entry into the white pulp (22), they presumably also contribute to entry of pRBC into the red pulp. In the absence of MZM (e.g., after treatment of noninfected C57BL/6 mice with recombinant TNF-α or on day 35 p.i. in C57BL/6 mice), splenic trapping of pRBC was reduced by at least ∼20%. In addition, MMM are also candidates for regulation of entry into the red pulp, since significant colocalization between MMM and pRBC was always observed in an open spleen. In this context, it is also noteworthy that, besides TNF-α/LTα, additional proinflammatory cytokines, such as interleukin-1, have been found to be involved in malaria-induced spleen closure (2).
Spleen closing may have both protective and adverse effects on the malaria-infected host. In the precrisis phase, several protective effects of spleen closing can be envisaged (2, 32). First, the release of toxic and proinflammatory products upon destruction of pRBC by activated macrophages in the spleen might be limited, and, therefore, the inflammatory response could be mitigated. Second, exclusion of pRBC allows adaptive immune responses to develop largely unperturbed in the spleen. For instance, adhesion of pRBC to dendritic cells impairs their activation in vitro, and this effect has been invoked to explain T-cell anergy at peak parasitemia (30, 31), involving perturbed expansion of malaria-specific T- and B-cell populations. By contrast, however, fully functional dendritic cells were found in vivo in the spleens of malaria-infected mice (20, 21, 27), which is in accordance with our findings that the spleen became largely closed throughout the crisis phase and therefore locked out pRBC. Third, the spleen has been shown to be the major erythropoietic organ in malaria-infected mice (15), and exclusion of parasites from the spleen could efficiently protect splenic reticulocytes from infection. Fourth, it is known that the spleen also destroys noninfected RBC during malaria and thus contributes to malaria-induced anemia (5, 7), which would therefore be diminished by spleen closure.
At peak parasitemia, however, spleen closure may be a disadvantage for the host, as indicated by our results. Indeed, TNFRI−/− and LTβR−/− mice displayed only incomplete splenic closing, which correlated with increased resistance to blood stage malaria, compared with substantial spleen closing and higher susceptibility in TNF-α−/− and wild-type C57BL/6 mice. It is therefore tempting to speculate that incomplete closing, like that in P. chabaudi-infected LTβR−/− and TNFRI−/− mice, is optimal for survival of the host. This view is also supported by the finding that interleukin-1 treatment of P. chabaudi adami-infected BALB/c mice causes an additional drop in particle trapping activity compared to untreated infected mice, which inversely correlates with an approximately twofold increase in peak parasitemia (2).
Although we are only beginning to understand the mechanisms that regulate the so-called closing of the spleen, our data indicate at least that P. chabaudi infections induce spleen closure which is manifested by a locking out of pRBC, particularly during the crisis phase. Therefore, the spleen cannot destroy pRBC during the crisis phase when parasitemia decreases by at least 100-fold within 4 days. This indicates that the sophisticated sequestration system of Plasmodium parasites did not evolve to avoid clearance by the spleen.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aichele, P., J. Zinke, L. Grode, R. A. Schwendener, S. H. Kaufmann, and P. Seiler. 2003. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 171:1148-1155. [DOI] [PubMed] [Google Scholar]
- 2.Alves, H. J., W. Weidanz, and L. Weiss. 1996. The spleen in murine Plasmodium chabaudi adami malaria: stromal cells, T lymphocytes, and hematopoiesis. Am. J. Trop. Med. Hyg. 55:370-378. [DOI] [PubMed] [Google Scholar]
- 3.Baruch, D. I., S. J. Rogerson, and B. M. Cooke. 2002. Asexual blood stages of malaria antigens: cytoadherence. Chem. Immunol. 80:144-162. [DOI] [PubMed] [Google Scholar]
- 4.Benten, W. P. M., U. Bettenhaeuser, F. Wunderlich, E. Van Vliet, and H. Mossmann. 1991. Testosterone-induced abrogation of self-healing of Plasmodium chabaudi malaria in B10 mice: mediation by spleen cells. Infect. Immun. 59:4486-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor, J., C. C. Pak, and A. J. Schroit. 1994. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J. Biol. Chem. 269:2399-2404. [PubMed] [Google Scholar]
- 6.Cooke, B. M., N. Mohandas, and R. L. Coppel. 2004. Malaria and the red blood cell membrane. Semin. Hematol. 41:173-188. [DOI] [PubMed] [Google Scholar]
- 7.Ekvall, H. 2003. Malaria and anemia. Curr. Opin. Hematol. 10:108-114. [DOI] [PubMed] [Google Scholar]
- 8.Engwerda, C. R., M. Ato, S. E. Cotterell, T. L. Mynott, A. Tschannerl, P. M. Gorak-Stolinska, and P. M. Kaye. 2002. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am. J. Pathol. 161:429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick, K., and Q. Chen. 2004. var genes, PfEMP1 and the human host. Mol. Biochem. Parasitol. 134:3-9. [DOI] [PubMed] [Google Scholar]
- 10.Fu, Y. X., and D. D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399-433. [DOI] [PubMed] [Google Scholar]
- 11.Fütterer, A., K. Mink, A. Luz, M. H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9:59-70. [DOI] [PubMed] [Google Scholar]
- 12.Gilks, C. F., D. Walliker, and C. I. Newbold. 1990. Relationships between sequestration, antigenic variation and chronic parasitism in Plasmodium chabaudi chabaudi—a rodent malaria model. Parasite Immunol. 12:45-64. [DOI] [PubMed] [Google Scholar]
- 13.Gratepanche, S., B. Gamain, J. D. Smith, B. A. Robinson, A. Saul, and L. H. Miller. 2003. Induction of crossreactive antibodies against the Plasmodium falciparum variant protein. Proc. Natl. Acad. Sci. USA 100:13007-13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groom, A. C., E. E. Schmidt, and I. C. MacDonald. 1991. Microcirculatory pathways and blood flow in spleen: new insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc. 5:159-173. [PubMed] [Google Scholar]
- 15.Halder, R. C., T. Abe, M. K. Mannoor, S. R. Morshed, A. Ariyasinghe, H. Watanabe, H. Kawamura, H. Sekikawa, H. Hamada, Y. Nishiyama, H. Ishikawa, K. Toba, and T. Abo. 2003. Onset of hepatic erythropoiesis after malarial infection in mice. Parasitol. Int. 52:259-268. [DOI] [PubMed] [Google Scholar]
- 16.Hehlgans, T., and K. Pfeffer. 2005. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology 115:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, M., and N. J. White. 1999. Molecular mechanisms of cytoadherence in malaria. Am. J. Physiol. 276:C1231-C1242. [DOI] [PubMed] [Google Scholar]
- 18.Janicik, J. M., R. Schauer, K. H. Andres, and M. von During. 1978. Sequestration of neuraminidase-treated erythrocytes. Studies on its topographic, morphologic and immunologic aspects. Cell Tissue Res. 186:209-226. [DOI] [PubMed] [Google Scholar]
- 19.Kraal, G. 1992. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132:31-74. [DOI] [PubMed] [Google Scholar]
- 20.Langhorne, J., F. R. Albano, M. Hensmann, L. Sanni, E. Cadman, C. Voisine, and A. M. Sponaas. 2004. Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection. Immunol. Rev. 201:35-47. [DOI] [PubMed] [Google Scholar]
- 21.Leisewitz, A. L., K. A. Rockett, B. Gumede, M. Jones, B. Urban, and D. P. Kwiatkowski. 2004. Response of the splenic dendritic cell population to malaria infection. Infect. Immun. 72:4233-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons, A. B., and C. R. Parish. 1995. Are murine marginal-zone macrophages the splenic white pulp analog of high endothelial venules? Eur. J. Immunol. 25:3165-3172. [DOI] [PubMed] [Google Scholar]
- 23.Nolte, M. A., J. A. Belien, I. Schadee-Eestermans, W. Jansen, W. W. Unger, N. van Rooijen, G. Kraal, and R. E. Mebius. 2003. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 198:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldenborg, P. A., A. Zheleznyak, Y. F. Fang, C. F. Lagenaur, H. D. Gresham, and F. P. Lindberg. 2000. Role of CD47 as a marker of self on red blood cells. Science 288:2051-2054. [DOI] [PubMed] [Google Scholar]
- 25.Pasloske, B. L., and R. J. Howard. 1994. Malaria, the red cell, and the endothelium. Annu. Rev. Med. 45:283-295. [DOI] [PubMed] [Google Scholar]
- 26.Pasparakis, M., S. Kousteni, J. Peschon, and G. Kollias. 2000. Tumor necrosis factor and the p55TNF receptor are required for optimal development of the marginal sinus and for migration of follicular dendritic cell precursors into splenic follicles. Cell. Immunol. 201:33-41. [DOI] [PubMed] [Google Scholar]
- 27.Perry, J. A., A. Rush, R. J. Wilson, C. S. Olver, and A. C. Avery. 2004. Dendritic cells from malaria-infected mice are fully functional APC. J. Immunol. 172:475-482. [DOI] [PubMed] [Google Scholar]
- 28.Sherman, I. W., S. Eda, and E. Winograd. 2004. Erythrocyte aging and malaria. Cell. Mol. Biol. 50:159-169. [PubMed] [Google Scholar]
- 29.Stevenson, M. M., and G. Kraal. 1989. Histological changes in the spleen and liver of C57BL/6 and A/J. mice during Plasmodium chabaudi AS infection. Exp. Mol. Pathol. 51:80-95. [DOI] [PubMed] [Google Scholar]
- 30.Urban, B. C., D. J. P. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 31.Urban, B. C., and D. J. Roberts. 2002. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr. Opin. Immunol. 14:458-465. [DOI] [PubMed] [Google Scholar]
- 32.Weiss, L., U. Geduldig, and W. Weidanz. 1986. Mechanisms of splenic control of murine malaria: reticular cell activation and the development of a blood-spleen barrier. Am. J. Anat. 176:251-285. [DOI] [PubMed] [Google Scholar]
- 33.Wunderlich, F., H. Stübig, and E. Konigk. 1982. Development of Plasmodium chabaudi in mouse red blood cells: structural properties of the host and parasite membranes. J. Protozool. 29:60-66. [DOI] [PubMed] [Google Scholar]
- 34.Wunderlich, F., G. Schillinger, and M. Helwig. 1985. Fractionation of Plasmodium chabaudi-infected erythrocytes into parasites and ghosts. Z. Parasitenkd. 71:545-551. [DOI] [PubMed] [Google Scholar]
- 35.Wunderlich, F., M. A. Dkhil, L. I. Mehnert, J. V. Braun, M. El-Khadragy, E. Borsch, D. Hermsen, W. P. M. Benten, K. Pfeffer, H. Mossmann, and J. Krucken. 2005. Testosterone responsiveness of spleen and liver in female lymphotoxin β receptor-deficient mice resistant to blood-stage malaria. Microbes Infect. 7:399-409. [DOI] [PubMed] [Google Scholar]
- 36.Yadava, A., S. Kumar, J. A. Dvorak, G. Milon, and L. H. Miller. 1996. Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc. Natl. Acad. Sci. USA 93:4595-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]