Abstract
Neutrophils are important in controlling early infections with the intracellular bacterium Rhodococcus equi. Antineutrophil monoclonal antibody (RB6-8C5)-induced neutrophil deficiency during the first week after experimental infection of mice with R. equi resulted in more severe disease and significantly increased tissue concentrations of R. equi.
Rhodococcus equi is an opportunistic facultative-intracellular bacterium that causes severe disease, principally pneumonia, in immunocompromised people (e.g., AIDS patients and organ transplant recipients) and in very young horses (foals) (13). It survives and replicates in macrophages and shares many phenotypic, phylogenetic, and pathogenic characteristics with Mycobacterium species (13).
Specific host factors that determine the outcome of exposure to R. equi have not been identified; however, evidence indicates that innate immune responses are important in the control of early infections (4, 7, 9, 10) and that cell-mediated immunity, via T-helper 1 (Th1) immune responses, is important in resistance to infection (2).
Neutrophils provide protection against a variety of intracellular organisms by both phagocytic and nonphagocytic mechanisms (1, 3, 5, 7, 10-12, 14). They phagocytize and kill many pathogens, including R. equi (7, 10), and produce an array of cytokines and chemokines that modulate immune responses to infection by directing the recruitment and activation of other effector cells. Such actions influence the development of T-cell responses that favor a Th1-type response. Neutrophils, therefore, are considered to be important in linking innate and acquired immune functions.
Neutrophil responses and disease control contributions, however, are not the same for all intracellular bacterial infections, suggesting that microorganism and perhaps host factors may be important determinants. For instance, early infections with fast-replicating intracellular bacteria (e.g., Salmonella enterica serovar Typhimurium) are controlled by neutrophils (3, 14), whereas infections with slow-replicating Mycobacterium tuberculosis organisms are not adequately controlled because of the neutrophils' inconsistent or lack of ability to kill the bacteria (14). Neutrophils do, however, appear to have a nonphagocytic immunomodulatory protective role against early systemic M. tuberculosis infections (12). The protective role of neutrophils in the pathogenesis of infection with M. tuberculosis, however, is unclear: following M. tuberculosis infection of mice genetically susceptible to pulmonary tuberculosis, neutrophil degranulation contributes to lung tissue destruction over time, rather than to microbe destruction (6). To our knowledge, there are no published reports on the in vivo effects of neutrophils on R. equi-induced disease.
The purpose of this study was to determine the importance of neutrophils in the control of early R. equi infections in mice. Herein we present evidence that mice deficient in neutrophils are more susceptible to experimental infection with R. equi and develop substantially greater bacterial burdens than do neutrophil-replete mice.
Female BALB/c mice (Harlan Laboratories, Indianapolis, IN), 7 to 9 weeks of age, were segregated based on their treatment with an antineutrophil monoclonal antibody (MAb; RB6-8C5) (principal mice; n = 10) or normal mouse serum(control mice; n = 8). This study was approved by the Texas A&M University Animal Care and Use Committee. Principal mice received 200 μg of RB6-8C5 (obtained from T.A.M.) intraperitoneally (i.p.), in a 0.5-ml volume, and control mice received 0.5 ml of normal mouse serum (Sigma Chemical Co., St. Louis, MO) i.p (11). Treatments were administered 18 h before R. equi infection and at 1 and 4 days postinfection (p.i.) in those that survived.
Virulent R. equi (ATCC 33701) bacteria were grown in R. equi minimal medium (8) for 48 h at 37°C with rotation (10 rpm), centrifuged at 1,600 × g for 10 min, resuspended in phosphate-buffered saline (PBS), and stored at −80°C until used. Bacterial suspensions were thawed at 37°C prior to infection, and R. equi concentrations were assessed by duplicate plate counts on Trypticase soy agar with 10% sheep red blood cells (Beckton-Dickinson Microbiology Systems, Cockeysville, MD) and reported in CFU/ml. All mice were injected i.p. with 0.2 ml of bacterial suspension, containing approximately 3 × 105 R. equi bacteria (15).
At 7 days p.i., mice were anesthetized with methoxyflurane (Pitman-Moore, Mundelein, IL) and euthanized by cardiac snip, and blood was collected for hemograms. Livers, spleens, and lungs were aseptically harvested, weighed, and homogenized in sterile PBS using tissue grinders (Wheaton, Millville, N.J.). Aliquots (100 μl) of 10-fold serial PBS-homogenate dilutions were cultured, in duplicate, on plates containing Trypticase soy agar and 10% sheep red blood cells to quantify the number of CFU of R. equi per gram of tissue. Lung, liver, and spleen tissues from representative mice in each group, principal (n = 4) and control (n = 2), were collected at 7 days p.i. and examined histologically.
Continuous data were non-Gaussian in distribution and were compared using the Wilcoxon rank-sum test. Comparisons of categorical data were made using the Fisher exact test. P values of ≤0.05 were considered significant.
Three principal mice appeared ill (i.e., ruffled fur, lethargy, and hunched stature) 24 h p.i., did not receive a second dose of MAb, and died at 36 to 48 h p.i. Although the proportion of principal mice that died (30%; 3 of 10) was greater than that of control mice (0%), the difference was not significant.
At 7 days p.i., the remaining principal mice exhibited early signs of disease (i.e., slightly ruffled fur and moderately reduced activity), whereas all control mice were normal in appearance and activity. Concurrently, principal mice had significantly (P = 0.0015) lower concentrations of peripheral blood neutrophils (median, 44 neutrophils/μl; range, 0 to 174 neutrophils/μl) than control mice (median, 480 neutrophils/μl; range, 156 to 1,144 neutrophils/μl). The finding of a significant neutropenia 3 days after administration of RB6-8C5 MAb is consistent with previous observations (11, 12, 14).
There were no apparent gross lesions in the organs of any surviving mice, but the spleens of principal mice were larger and significantly (P = 0.0015) heavier (median weight, 251 mg; range, 215 to 315 mg) than those of control mice (median weight, 82 mg; range, 55 to 94 mg). The livers of surviving principal mice were significantly (P = 0.0489) heavier (median weight, 1,026 mg; range, 909 to1,134 mg) than those of control mice (median weight, 894 mg; range 853 to 1,118 mg).
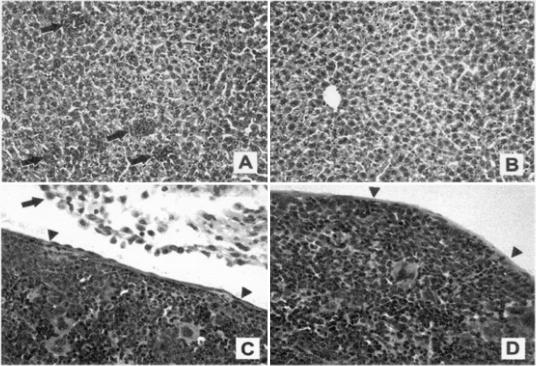
In comparison to the findings for control mice, histologic examination of tissues from principal mice that survived 7 days p.i. revealed (i) severe peritonitis, characterized by mononuclear inflammation; (ii) intense splenic extramedullary myelopoiesis with occasional granulomas containing bacteria; and (iii) increased hepatic myeloid extramedullary myelopoiesis (Fig. 1). The lungs of both groups exhibited similar degrees of mild acute interstitial pneumonia. The splenic and hepatic myeloid hyperplasia correlated with the increased organ weights and most likely represented the host's responses to RB6-8C5-induced neutrophil destruction, peritoneal neutrophil sequestration, and ongoing systemic disease. The peritonitis in principal mice may also explain their increased severity of clinical signs.
FIG. 1.
Tissue responses representative of those seen in principal (neutrophil-deficient) and control (neutrophil-replete) mice at 7 days p.i. with R. equi. (A and B) Note islands of myeloid hyperplasia (arrows) scattered in liver sinusoids from a principal mouse (A), compared to the absence of myeloid hyperplasia in liver tissue from a control mouse (B). Hematoxylin and eosin stain; magnification, ×10. (C) In the principal mouse, mononuclear peritonitis (arrow) and increased areas of splenic myeloid hyperplasia, characterized by a proliferating neutrophilic population having bean-shaped to circularized linear nuclei and expanding the parenchyma below the capsule (arrowheads), are evident. (D) In the control mouse, there is no evidence of peritonitis, and the spleen parenchyma below the capsule (arrowheads) is normal. Hematoxylin and eosin stain; magnification, ×20.
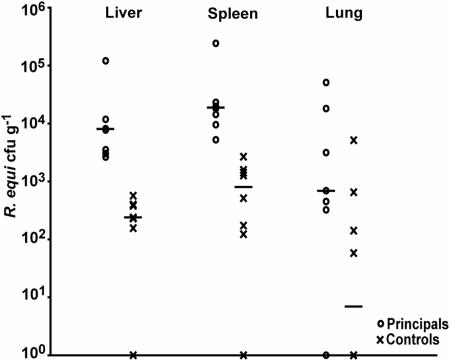
There were significantly (P = 0.0014) greater concentrations of R. equi in the livers of principal mice (median, 7,720 CFU/g; range 2,630 to 121,000 CFU/g) than in control mice (median, 234 CFU/g; range, 0 to 568 CFU/g) and significantly (P = 0.0015) greater concentrations of R. equi in the spleens of principal mice (median, 18,100 CFU/g; range, 5,240 to 242,000 CFU/g) than in control mice (median, 891 CFU/g; range 0 to 2,670 CFU/g). Although not significant (P = 0.067), there were more R. equi organisms in the lungs of surviving principal mice (median, 692 CFU/g; range, 0 to 51,000 CFU/g) than in control mice (median, 29 CFU/g; range 0 to 5,150 CFU/g) (Fig. 2).
FIG. 2.
Neutrophils protect against infection with R. equi. Bacterial burdens in the liver, spleen, and lung 7 days after R. equi infection of principal mice rendered neutropenic with RB-8C5 MAb (○) and neutrophil-replete control mice (×). Individual CFU values and the median (horizontal line) values for all surviving principal (n = 7) and control (n = 8) mice are shown. Wilcoxin rank-sum analyses indicated a significant difference (P < 0.05) in median concentrations of R. equi in livers and spleens, between principal and control mice, but not a significant difference in these concentrations in their lungs.
A laboratory animal model of R. equi pneumonia is lacking, and the virulence status of R. equi isolates has been established by infecting mice either intravenously or i.p. and quantifying the concentrations of bacteria in their spleens, livers, and lungs (15). The finding of relatively lower concentrations of organisms in the lungs than in the spleens or livers is consistent with the results of previous experimental systemic infections of mice with R. equi (15). The finding of similar patterns of pneumonia in principal and control mice correlated well with the observed similarities in tissue concentrations of R. equi, thereby providing further evidence that the lung is not the primary target organ in mice infected systemically with R. equi. On the basis of increased tissue burdens of R. equi in neutrophil-deficient mice in this study and the in vitro R. equi-killing capacity of neutrophils in previous studies (7, 10), it is quite likely that a neutrophil deficiency or inefficiency would contribute to increased lung bacterial burdens and lesions in animals predisposed to R. equi pneumonia (i.e., young foals).
During primary infection with most intracellular bacteria, the host must rely on innate defenses to prevent organisms from growing to overwhelming numbers before specific adaptive immunity can be generated and expressed. This is the first report on the importance of neutrophils in disease caused by R. equi, and the results indicate that they substantially reduce tissue bacterial burdens in mice. Additional studies are needed to determine the mechanistic basis of this protective response and the means by which it might be enhanced.
Acknowledgments
This work was supported by the Link Equine Research Endowment, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University.
We thank Michael Grimm, Jessica Harrington, Andrea Taylor, and Jeane Mounce for technical support.
Editor: J. B. Bliska
REFERENCES
- 1.Bennouna, S., S. K. Bliss, T. J. Curiel, and E. Y. Denkers. 2003. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J. Immunol. 171:6052-6058. [DOI] [PubMed] [Google Scholar]
- 2.Chaffin, M. K., N. D. Cohen, R. J. Martens, R. F. Edwards, M. Nevill, and R. Smith III. 2004. Hematologic and immunophenotypic factors associated with development of Rhodococcus equi pneumonia of foals at equine breeding farms with endemic infection. Vet. Immunol. Immunopathol. 100:33-48. [DOI] [PubMed] [Google Scholar]
- 3.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darrah, P., M. C. G. Monaco, S. Jain, M. K. Hondalus, D. T. Golenbock, and D. M. Mosser. 2004. Innate immune responses to Rhodococcus equi. J. Immunol. 173:1914-1924. [DOI] [PubMed] [Google Scholar]
- 5.Denkers, E. Y., L. Del Rio, and B. Soumaya. 2003. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem. Immunol. Allergy 83:95-114. [DOI] [PubMed] [Google Scholar]
- 6.Eruslanov, E., I. Lyadova, T. Kondratieva, K. Majorov, I. Scheglov, M. Orlova, and A. Apt. 2005. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect. Immun. 73:1744-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hietala, S. K., and A. A. Ardans. 1987. Neutrophil phagocytic and serum opsonic response of the foal to Corynebacterium equi. Vet. Immunol. Immunopathol. 14:279-294. [DOI] [PubMed] [Google Scholar]
- 8.Jordan, M. C., J. R. Harrington, N. D. Cohen, R. M. Tsolis, L. J. Dangott, E. D. Weinberg, and R. J. Martens. 2003. Effects of iron modulation on growth and viability of Rhodococcus equi and expression of virulence-associated protein A. Am. J. Vet. Res. 64:1337-1346. [DOI] [PubMed] [Google Scholar]
- 9.Kohler, A. K., D. M. Stone, M. T. Hines, B. A. Byrne, D. C. Alperin, L. K. Norton, and S. A. Hines. 2003. Rhodococcus equi secreted antigens are immunogenic and stimulate a type 1 recall response in the lungs of horses immune to R. equi infection. Infect. Immun. 71:6329-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens, J. G., R. J. Martens, and H. W. Renshaw. 1988. Rhodococcus (Corynebacterium) equi: bactericidal capacity of neutrophils from neonatal and adult horses. Am. J. Vet. Res. 49:295-299. [PubMed] [Google Scholar]
- 11.Moore, T. A., M. W. Newstead, R. M. Strieter, B. Mehrad, B. L. Beaman, and T. J. Standiford. 2000. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides pneumonia. J. Immunol. 164:908-915. [DOI] [PubMed] [Google Scholar]
- 12.Pedrosa, J., B. M. Saunder, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott, J. F. 1991. Rhodococcus equi: an animal and human pathogen. Clin. Microbiol. Rev. 4:20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiler, P., A. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and H. E. Kauffman. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacteria tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 15.Takai, S., Y. Sasaki, and S. Tsubaki. 1992. Influence of inoculation route on virulence of Rhodococcus equi. Microbiol. Immunol. 36:895-898. [DOI] [PubMed] [Google Scholar]