Abstract
Vibrio cholerae O139 emerged in 1992 as a major cause of epidemic cholera. However, the incidence of disease due to this new serogroup subsequently decreased for almost a decade. In April 2002, there was a dramatic resurgence of V. cholerae O139 in Bangladesh. We compared the phenotypic properties of the bacterial isolates and the immunological responses in patients with disease due to V. cholerae O139 during the 2002 epidemic with those dating to the emergence of this disease in 1993 to 1995. Strains isolated from patients in the two time periods were compared with respect to capsular polysaccharide, their resistance to the bactericidal effect of serum, and their capacity to be used as target strains in complement-mediated vibriocidal assays. Phase-contrast microscopy showed that strains isolated in 2002 had less capsular material than those isolated from 1993 to 1995 (P = <0.001), a finding confirmed by electron microscopic studies. Strains isolated in 2002 were more susceptible to the bactericidal activity of serum compared to strains from 1993 to 1995 (P = 0.013). Compared to results using a standard O139 strain, a modified vibriocidal assay utilizing a 2002 strain, CIRS 134, as the target organism detected higher vibriocidal responses in both O139-infected cholera patients as well as O139 vaccine recipients. The vibriocidal assay utilizing the less encapsulated 2002 strain, CIRS 134, is a more sensitive indicator of adaptive immune responses to recent infection with V. cholerae O139. Consequently, this assay may be useful in studies of both O139-infected patients and recipients of O139 vaccines.
Vibrio cholerae is a gram-negative bacillus that causes severe, dehydrating diarrhea in humans (20). V. cholerae is part of the free-living bacterial flora of estuarine environments. In areas where cholera is endemic, cholera exhibits a distinctive seasonal pattern, often coinciding with changes in temperature and water level (25, 34). In addition to its environmental growth, V. cholerae is unique among the diarrheal pathogens because of its ability to cause global pandemics. Since 1817, there have been seven pandemics during which disease has spread across Asia, Europe, and Africa and to the Western Hemisphere.
A large component of the ability of V. cholerae to cause pandemic disease may be the continued differentiation and evolution of the organism. Strains of V. cholerae can be differentiated serologically by the O side chain of the lipopolysaccharide (LPS) component of the outer membrane. The vast majority of strains that produce epidemic cholera belong to serogroup O1 or O139. V. cholerae O1 is divided into two biotypes, classical and El Tor, which differ clinically and biochemically. V. cholerae O1 biotype El Tor is responsible for the current seventh pandemic of cholera; the last classical strain was isolated at the International Centre for Diarrhoeal Disease Research in Bangladesh (ICDDR,B) in 1991. In 1992, a previously unrecognized serogroup of V. cholerae emerged as a cause of cholera in South and Southeast Asia, V. cholerae O139 (3, 33). This new serogroup is nearly identical to the El Tor biotype of V. cholerae O1, except for the substitution of genes encoding the O139 LPS and acquisition of the ability to produce a capsule (6-9, 38). After a period in which V. cholerae O139 caused extensive cholera in Southeast Asia, El Tor V. cholerae O1 returned and now predominates over a smaller number of continuing cases of V. cholerae O139.
V. cholerae O139 induces strong mucosal and systemic antibody responses in patients with dehydrating illness (16, 18, 28, 31, 32). However, the presence of the capsular polysaccharide in V. cholerae O139 makes O139 strains more difficult to use in conventional vibriocidal antibody assays, which is the only currently recognized marker of protective immunity to V. cholerae O1 infection (16). We have previously shown that O139 infection gives rise to significant vibriocidal antibody responses (31); however, these responses could only be determined using capsule-deficient variants of O139 strains and under somewhat different conditions than those used for V. cholerae O1 (31). The immune responses observed in individuals immunized with live oral or killed O139 vaccines are also different from those seen following O1 immunization with regard to magnitude and kinetics of vibriocidal immune responses. In 2002, an epidemic of V. cholerae O139 infection in Bangladesh (11) prompted us to more closely analyze the microbiologic properties and immunologic responses to V. cholerae O139 infection, especially since they were similar to what was observed when O139 first emerged over a decade ago.
(This work was presented in part at the 12th Annual Meeting of the International Centers for Tropical Disease Research Network, Bethesda, MD, May 2003.)
MATERIALS AND METHODS
Study subjects.
Twenty-eight adult patients (14 males and 14 females), aged between 22 to 35 years, with cholera caused by V. cholerae O139 were recruited for the study from March to May 2002 at the ICDDR,B. For comparison, sera and strains collected from 33 patients with V. cholerae O139 infection from November 1993 to May 1995 (31, 32) were also analyzed. Twenty adult individuals (14 males and 6 females) in the same age group as the patients, whose stools tested negative for enteric pathogens and who were of socioeconomic backgrounds similar to those of enrolled patients, were randomly recruited from in and around Dhaka and studied as healthy controls. Informed consent was obtained from study participants. The research was approved by the Ethical Review Committee of the ICDDR,B.
Sample collection.
After microbiological confirmation of V. cholerae O139 in stools, patients were enrolled into the study (31, 32). Venous blood was collected from consenting patients approximately 2 days after the onset of diarrhea (day 2 [acute stage]) as well as on day 7 and 21, during convalescence. Single blood samples were collected from the 20 healthy subjects. Pooled sera from cholera patients, consisting of serum from five individuals at the convalescent phase of infection (day 7 and/or day 21), as well as pooled sera from 10 healthy control subjects were also used. Serum samples that were collected from patients from 1993 to 1995 had been kept frozen at −70°C. Preimmunization (day 0) and postimmunization (day 21 [7 days after the second dose of the vaccine]) sera from 21 adult male Bangladeshis who had received two doses of the oral bivalent B-subunit O1/O139 whole-cell killed cholera vaccine (17, 27) were also used as patients in the study.
Strains.
V. cholerae O139 strains isolated from patients from March to May 2002 (designated VC-O139/2002) and during the first outbreak in Bangladesh between 1993 and 1995 (designated VC-O139/1993) were studied (31). Strains were stored at −70°C in T1N1 broth containing 20% glycerol and were streaked on blood agar for subsequent vibriocidal assays and on gelatin agar plates for other bactericidal assays (29). Proteolytic soluble hemagglutinin activity was tested in 22 VC-O139/2002 isolates and selected strains of VC-O139/1993 (VC044, VC088, and 4260B) using the azocasein assay with bacteria grown in trypticase soy broth (5, 23). Characterization of VC-O139/2002 strains was also carried out using phage JA1, which is specific for O139 encapsulated strains (1).
India ink staining.
The V. cholerae O139 strains (22 VC-O139/2002 and 15 VC-O139/1993 strains) cultured on gelatin agar plates were tested for the presence of capsule by using negative staining with India ink (22, 30). For staining, one loopful of India ink was placed on a glass slide, and one loopful of sterile saline was added. A portion of a colony of V. cholerae O139 grown on a gelatin agar plate overnight at 37°C was transferred to the slide and mixed with India ink stain. The unstained halo of capsular material around the bacteria was visualized using phase-contrast microscopy (Leica DM microscope) at ×400 magnification. The percentage of the opaque capsular area was estimated as the percentage of total area minus the percentage of stained cellular area using the Leica QWin software version 2.0 computer program (Leica Wetzlar GmbH, Germany).
Electron microscopy.
For ultrastructural studies, VCO139/2002 (CIRS 121, 124, and 134) and VCO139/1993 (VC088) strains were grown on Luria agar plates, resuspended in cacodylate buffer (0.1 M, pH 7.2), labeled with polycationic ferritin (1 mg/ml), and visualized using a Philips 420T transmission electron microscope (15).
Serum bactericidal killing.
Bactericidal killing was carried out with V. cholerae O139 strains grown on gelatin agar overnight at 37°C and then subcultured in Luria broth for 4 h. Pooled normal human sera (ranging from 10% to 95% concentration) and bacteria (∼1 × 103 CFU) were incubated at 37°C for 2 h, and viable counts were determined (30). Results were expressed as the percentage of bacterial cells that were killed by different concentrations of serum compared to that observed in the absence of serum.
Vibriocidal antibody assays.
Vibriocidal assays were carried out using a standard procedure (31) with guinea pig complement (1:10 dilution) and pooled sera from cholera patients or using sera on different study days from individual patients (days 2, 7, and 21 after onset of disease). The assay was carried out using VCO139/1993 and VCO139/2002 strains. For the assay, V. cholerae O139 strain 4260B, the current standard O139 strain used in the vibriocidal assay, was used as the control (26, 31, 32). Strains stored at −70°C were subcultured on blood agar plates for around 18 h at 37°C (19, 31). Opaque colonies that produced only a small zone and halo around it were selected and grown in brain heart infusion (BHI) broth (Difco, Detroit, Mich.) at 37°C for 3 h. After centrifugation at 3,000 × g, the pellet was suspended in physiological saline to an optical density (OD) at 600 nm of 0.2 (approximately 4 × 108 CFU/ml); 20 μl of this suspension (final dilution, 1:150; 2.7 × 106 CFU/ml) was added to 300 μl of guinea pig complement (final concentration, 1:10) and 2.68 ml of physiological saline to make a final volume of 3 ml. The mixture was applied to microtiter plates (25 μl/well; Nunc, Roskilde, Denmark) containing serially twofold-diluted (25 μl/well) serum samples (starting dilution, 1:10), and this mixture was incubated in a shaker incubator (40 rpm) for 1 h at 37°C. After adding 150 μl of BHI broth, the plates were again incubated at 37°C for about 4 h. The ODs were measured at 595 nm. The vibriocidal titer was defined as the reciprocal of the highest serum dilution causing a >50% reduction of the OD at 595 nm when compared with the OD of the control wells without serum. Wells containing only serum, physiological saline, or growth medium were included on each plate to exclude the possibility of bacterial contamination of reagents. Paired serum samples were tested in each case. All plates contained duplicate rows of pooled convalescent-phase sera as internal positive controls.
Modified vibriocidal assay for V. cholerae O139.
The vibriocidal assay described above was also modified to make it more compatible with the assay that is used for V. cholerae O1 (31) using VCO139/2002 strain CIRS 134. After screening 28 VCO139/2002 strains in the vibriocidal assay described above, VCO139/2002 strain CIRS 134 was chosen as a type strain for use in a modified vibriocidal assay, since consistently higher titers of vibriocidal antibody responses could be obtained when CIRS 134 was used as the target bacterium. For the modified assay, CIRS 134 was grown in BHI broth as described above and adjusted to an OD at 600 nm of 0.3 (approximately 2 × 109 to 3 × 109 CFU/ml), and 150 μl of the suspension (final dilution of 1:20) containing 1 × 108 CFU/ml was used in a volume of 3 ml of the assay mixture. The assay was completed after incubation for 2 h at 37°C with CIRS 134 as the target bacterium. For vibriocidal antibody responses using V. cholerae O1 Ogawa and Inaba as target bacteria, previously described procedures were used (31). For calculations, a titer of 5 was assigned in cases in which no vibriocidal activity was observed. A fourfold or greater increase in titer from the onset of cholera or vaccination from that in follow-up were considered to represent seroconversion.
Phagocytosis.
Killing by polymorphonuclear neutrophils (PMNs) was carried out using PMNs and pooled human sera isolated from healthy volunteers (2, 30). Blood was collected from healthy adult individuals, and PMNs were separated by density gradient centrifugation using Ficoll-Hypaque followed by dextran sedimentation. Residual erythrocytes were removed by hypotonic lysis in deionized water. PMNs were washed twice in Hanks balanced salt solution and titrated to a density of 1 × 107 cells/ml. Killing of bacterial cells by PMNs was determined by mixing 0.1 ml of bacterial suspension (approximately 1 × 105 CFU/ml), 0.4 ml of PMNs, and 0.1 ml of pooled serum in siliconized glass tubes. Hanks balanced salt solution was substituted for PMNs in the controls. Tubes were incubated at 37°C for 2 h, after which 8 ml of cold deionized water was added to lyse PMNs. Samples were plated for viable counts on gelatin agar medium (30).
Statistical analyses.
The Wilcoxon signed-rank test and the Mann-Whitney U test were used where applicable for statistical analyses. A two-tailed P value of ≤0.05 was the criterion for a significant difference. Analyses were carried out using the statistical software SigmaStat version 2.01 (Jandel Scientific, San Rafael, CA). Data are expressed as median values with 25 and 75 centiles or as geometric means ± 1 standard errors of the means (SEM).
RESULTS
Clinical characteristics of V. cholerae O139-infected patients.
The patients enrolled in the spring of 2002 were similar in age (Table 1) to those recruited from November 1993 to May 1995. However, compared to the initial study population, the 2002 cohort included women. Most patients enrolled in the study from both epidemic periods suffered from severe dehydration and were given intravenous fluid rehydration. Patients in 2002 mainly received doxycycline, while those in 1993 to 1995 received tetracycline or erythromycin.
TABLE 1.
Characteristics of V. cholerae O139 strains and cholera patients studied from 1993 to 1995 and in 2002
| Parameter | Period of recruitment
|
|
|---|---|---|
| 1993-1995 | 2002 | |
| No. of patients studied | 33 | 28 |
| Age, median yra (range) | 30 (21-45) | 30 (22.5-35) |
| Gender (male:female) | 33:0b | 14:14 |
| Dehydration status (severe) (%) | 67c | 88 |
| Antibiotic used (no. of patients/total no. [%]) | Erythromycin (13/33 [39]), tetracycline (20/33 [61]) | Erythromycin (3/28 [10]), doxycycline (25/28 [90]) |
| Blood group (%) | O (71), A (15), B (14), AB (0) | O (46), A (18), B (29), AB (7) |
| Antibioticd susceptibility pattern of isolated strains | Tet, S; Strep, R; SXT, R; Nal, S | Tet, S; Strep, S; SXT, S; Nal, R |
| Lysis by phage JA1e (no. of patients/total no.) | 28/28 | 27/28 |
Median and range (25th to 75th centile) are shown. Clinical features were monitored at the acute phase of infection.
Only adult males could be enrolled in the study from 1993 to 1995.
Statistical significance (P < 0.001) was determined by χ2 test.
All strains isolated from patients were susceptible (S) to tetracycline (Tet), streptomycin (Strep), trimethoprim, and sulfamethoxazole (SXT) as well as other antibiotics (12). Only strains isolated in 2002 were resistant (R) to nalidixic acid (Nal).
Number positive for lysis/total number studied.
Characteristics of V. cholerae O139 strains.
All strains were smooth and agglutinated with monoclonal antibody specific for the lipopolysaccharide of V. cholerae O139. VCO139/1993 strains were resistant to streptomycin, trimethoprim, and sulfamethoxazole but susceptible to nalidixic acid; VCO139/2002 strains were resistant to nalidixic acid but susceptible to other antibiotics. Most strains were also susceptible to lysis by phage JA1 (27/28 of the 2002 isolates) (Table 1). Strains isolated during the two periods showed similar protease activities. Only 4260B, the control strain used for vibriocidal assays, showed an approximately 10-fold lower protease activity as has been previously reported (19, 31).
Phase-contrast and electron microscopy for visualization of capsular content.
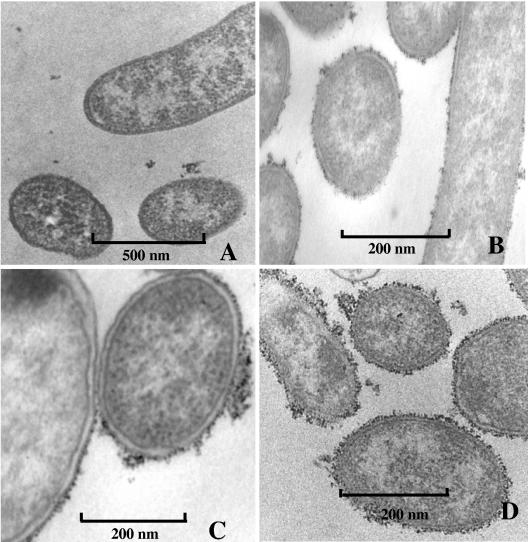
India ink staining showed that strains isolated in 2002 had an average of 40% less capsular content than those that had been isolated earlier (Table 2). Electron microscopy studies using ultrathin sections of V. cholerae O139 strains labeled with polycationic ferritin revealed that representative strains isolated in 2002 (Fig. 1A to C) had less capsular material than a representative strain, VC088, from the 1993-to-1995 period (Fig. 1D). The amount of capsular material varied in the three strains from 2002 that were studied. Strain CIRS 134 had little capsular material (Figure A), while strains CIRS 121 (Fig. 1B) and CIRS 124 (Fig. 1C) had more capsular material.
TABLE 2.
Quantification of capsular polysaccharide using phase-contrast microscopy of India ink-stained V. cholerae O139 strains isolated from 1993 to 1995 and in 2002a
| V. cholerae O139 isolated in | % Capsular contenta (median) |
|---|---|
| 1993-1995 (n = 15) | 85 (82-89)b |
| 2002 (n = 22) | 45 (31-54)b |
Phase-contrast microscopy was used for visualization (magnification, ×400); strains isolated from 1993 to 1995 and in 2002 were studied. Percent capsular content was calculated as the percentage of total area minus percentage of stained cellular area.
Median (25 to 75 centiles).
FIG. 1.
Ultrathin sections of ferritin-labeled V. cholerae O139 strains isolated in 2002, (A) CIRS 134, (B) CIRS 121, and (C) CIRS 124 and a strain isolated in 1993, (D) VC 088. Ferritin particles are visible in different densities around the cell periphery, indicating the presence of capsule. Various amounts of particles are visible in the different strains. CIRS 134 has the least amount of capsular material.
Susceptibility of V. cholerae O139 to killing by normal human serum.
As shown in Table 3, strains isolated in 2002 were more susceptible to relatively lower concentrations of serum in bactericidal assays compared with what was observed using strains isolated from 1993 to 1995.
TABLE 3.
Killing of strains of V. cholerae O139 by normal human seruma
| Yr(s) of isolation | No. of susceptible strains/total no. (%)
|
||
|---|---|---|---|
| 30-40% serum | 50-80% serum | >90% serum | |
| 1993-95 | 2/28 (7) | 22/28 (79) | 22/28 (79) |
| 2002 | 19/28 (68) | 28/28 (100) | 28/28 (100) |
Bactericidal activity was determined after incubation in different concentrations of serum for 120 minutes. Results show lowest serum concentrations producing bactericidal activity for the different strains.
Effect of variation of strains on the vibriocidal response.
When VCO139/2002 strains were tested for activity in the vibriocidal antibody assay, we found that 25/28 strains could be used as target bacteria for the assay (Fig. 2); this compares to only 3/28 VCO139/1993 strains that could be used as target bacteria using the standard vibriocidal method and pooled convalescent-phase sera from cholera patients (31). The vibriocidal titers against the 2002 strains ranged from 5 (n = 3) to 640. Of these strains, the antibody titer was highest in 11 VCO139/2002 strains (titer of 640). The titers obtained using the 2002 strains were significantly higher than those measured using the strains from 1993 to 1995 (P = 0.001). Based on these data, we chose four VCO139/2002 strains for further studies. One strain of VCO139/2002, namely, CIRS 134, consistently gave the highest vibriocidal titer (titer of 2,560) with pooled convalescent-phase sera. This strain was used to establish a modified vibriocidal assay, comparable to the vibriocidal assay in which strains of V. cholerae O1 are used as target bacteria. The resultant assay required less time to complete and gave consistently higher vibriocidal antibody titers using either pooled convalescent-phase sera or individual sera from patients collected at convalescence.
FIG. 2.
Vibriocidal antibody titers obtained with V. cholerae O139 strains isolated from 1993 to 1995 and in 2002, using the same pool of convalescent-phase sera collected from 1993 to 1995 from cholera patients (31). The closed symbols indicate titers when strains 4260B (▪) or CIRS 121, 124, 126, or 134 (⧫) were used as target bacteria. Points indicate individual titers, and bars indicate geometric means of titers.
Comparison of the immune responses in O139 patients infected in 2002 and those of patients infected from 1993 to 1995.
We used strains CIRS 134 and 4260B to analyze the vibriocidal antibody responses in O139-infected cholera patients (Table 4). Compared to the responses seen with 4260B, results using strain CIRS 134 as the target organism yielded a fourfold higher mean antibody titer on day 7 after onset of infection both in patients recruited in 2002 (P < 0.001) and in patients from 1993 (P < 0.033), with individual patient responses ranging from 2- to 32-fold higher using strain CIRS 134.
TABLE 4.
Comparison of vibriocidal antibody responses in sera in O139 patients recruited from 1993 to 1995 and in 2002
| Day after onset | Vibriocidala titer (GM)b
|
|||
|---|---|---|---|---|
| 1993-1995 strain
|
2002 strain
|
|||
| 4260B | CIRS134 | 4260B | CIRS134 | |
| 2 | 11 (8-14) | 8 (6-11) | 16 (10-24) | 30 (21-41) |
| 7 | 137 (83-226) | 682 (457-1,018)c | 496 (353-674) | 1,779 (1,236-2,559)c |
| 21 | 54 (28-107) | 103 (48-220) | 370 (262-503) | 692 (501-955) |
Vibriocidal responses were studied using strain 4260B (isolated in 1993) utilizing conventional methods (31) or strain CIRS 134 utilizing a modified method as described in the text.
GM, geometric mean plus range (±1 SEM).
Statistical significance (P < 0.001 [2002]); P < 0.033 [1993 to 1995]) was determined by using the Wilcoxon signed-rank test.
The vibriocidal antibody response rates were also higher in patients recruited in 2002 than those from 1993 to 1995, when either strain 4260B (P < 0.001) or CIRS 134 (P < 0.001) was used as target bacterium (Table 5). Even at the onset of disease, more patients recruited in 2002 had baseline vibriocidal titers over 100 (P < 0.001) when either of the strains was used in the assay. A similar trend was seen at day 7, when more patients from 2002 had titers over 1,000 (P < 0.001) (Table 5).
TABLE 5.
Comparison of response rates in V. cholerae O139 patients during the periods of 1993 to 1995 and 2002
| Yr(s) of isolation | Response at day 2 (titer, >100)
|
Response at day 7 (titer, >1,000)
|
Response at day 21 (titer, >1,000)
|
|||
|---|---|---|---|---|---|---|
| 4260B | CIRS134 | 4260B | CIRS134 | 4260B | CIRS134 | |
| 1993-1995 (n = 33) | 3% | 3% | 21% | 45% | 21% | 27% |
| 2002 (n = 28) | 25% | 22% | 51% | 85% | 31% | 47% |
| P valuea | <0.001 | <0.001 | <0.001 | <0.001 | 0.107 | 0.003 |
Comparison of difference in proportion of responses in cholera patients was carried out using the χ2 test.
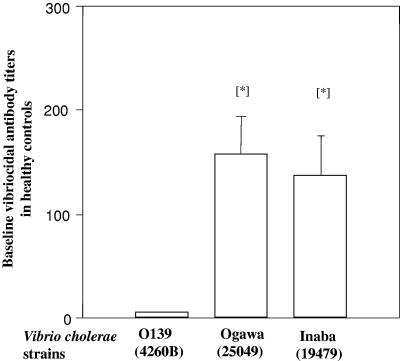
The baseline vibriocidal antibody titers in sera of 20 healthy Bangladeshi control subjects recruited in 2002 were low against V. cholerae O139 (median titer of 5) using either strain 4260B (Fig. 3) or CIRS 134 (median titer of 5); baseline titers were higher against V. cholerae O1 Ogawa and Inaba strains (P < 0.001).
FIG. 3.
Baseline vibriocidal antibody titers of healthy Bangladeshi subjects when V. cholerae O1 Ogawa, O1 Inaba, or O139 (strain 4260B) was used in assays. Asterisks indicate statistically significant differences between responses using O139 compared to those using O1 Ogawa or O1 Inaba strains (P < 0.001). Bars show geometric means; lines show SEM.
Vibriocidal antibody responses in recipients of the oral whole-cell killed O1/O139 bivalent vaccine.
About 50% of the Bangladeshi vaccinees who received two doses of the oral bivalent cholera vaccine responded with fourfold increases in vibriocidal antibodies when strain CIRS 134 was used in the assay; with strain 4260B, only 9% of patients showed a significant response (Table 6). Thus, both the magnitude of the vibriocidal responses and the proportion of vaccine responders were higher when CIRS 134 was used in the modified vibriocidal assay (P < 0.005).
TABLE 6.
Comparison of serum vibriocidal antibody responses in vaccinees challenged with the bivalent cholera toxin B-subunit O1/O139 whole-cell cholera vaccine by using 4260B and CIRS134 as target bacteria
| Parameter | Strain used for vibriocidal assay
|
|
|---|---|---|
| 4260B | CIRS134 | |
| Fold differencea | 1 | 3.0 |
| GMb (range) | 8.5 (6-11) | 59 (39.5-87) |
| Responder frequencyc | 2/21 (9.5) | 10/20 (50) |
Postimmunization titer (geometric mean) compared to preimmunization titer.
Geometric mean (GM) plus range (±1 SEM) are shown.
A ≥4-fold increase in vibriocidal titer between pre- and postimmunization specimen was shown to signify seroconversion.
DISCUSSION
Although V. cholerae O139 emerged in Bangladesh in 1992, causing epidemics of acute dehydrating illness, the incidence of cholera caused by the O139 serogroup fell and subsequently remained low for about a decade. The sudden reemergence of O139 in 2002 in Bangladesh was associated with an epidemic affecting approximately 30,000 patients (11). Studies have shown that isolates of V. cholerae O139 have undergone more rapid genetic changes in the last decade than the O1 serogroup (12-14). Studies have also shown that isolates of V. cholerae O139 in 2002 have undergone changes in ribotype and antibiotic susceptibility patterns compared to those isolated from 1993 to 1995. However, limited information is available on the evolution of phenotypic differences in the new epidemic strain, particularly in relation to capsule and its impact on resistance to killing by immune or nonimmune sera.
An important surrogate marker for protection from cholera is the vibriocidal antibody assay, which has been used successfully to predict protection from natural infection with V. cholerae O1 and efficacy of O1 cholera vaccines. The vibriocidal assay has been less useful in assessing protection from infection by V. cholerae O139 and in demonstrating responses in O139 vaccinees (4, 10, 21, 24, 31, 35, 36). For example, a recent prospective study in a population of exposed household contacts of patients with cholera in Bangladesh failed to demonstrate any correlation between the baseline vibriocidal antibody titer and protection from subsequent infection with V. cholerae O139 (35). The presence of a capsule in O139 organisms contributes to the decreased utility of the vibriocidal assay since the presence of capsular material interferes with both the binding of vibriocidal antibodies to LPS and other cell surface antigens and the attachment of complement to the bacterial surface. Previous O139-based vibriocidal assays were only feasible when capsule-deficient strains of V. cholerae O139 were used.
We tested wild-type vibriocidal O139 strains isolated in 2002 to see if they had undergone changes in capsular content that might directly alter the capacity of the bacteria to be used as target organisms in the vibriocidal assay. Interestingly, the majority of strains isolated in 2002 contained less capsular material on the cell surface than strains isolated from 1993 to 1995. Strain CIRS 134, which appeared to be almost devoid of capsule by electron microscopy, was the strain that gave the highest vibriocidal antibody responses when used as a target organism. Since almost all VC-O139/2002 strains including CIRS 134 (27/28) were susceptible to lysis by phage JA1, a phage specific for the capsular polysaccharide of V. cholerae O139, we surmise that the strains isolated in 2002 do contain capsular material, although in a lower quantity that permits these strains to be sensitive to lysis by complement and antibody. We found that almost 90% of V. cholerae O139 strains isolated in 2002 could be used in vibriocidal assays, in contrast to only 10% of O139 strains isolated from patients from 1993 to 1995. All 2002 strains were agglutinated by ICL12 (26), a monoclonal antibody specific for the O-antigenic polysaccharide of the LPS of V. cholerae O139, suggesting accessibility of LPS when these strains are used as targets in vibriocidal assays. Using CIRS 134 as the target bacterial strain, a significantly higher vibriocidal antibody response was seen in patients than when the previous vibriocidal O139 type strain, 4260B, was used.
The use of O139 CIRS 134 as the target organism in the vibriocidal assay also allowed detection of more prominent responses in individuals vaccinated with a V. cholerae O139-containing vaccine. Using V. cholerae strain 4260B (17, 37) or AI1837 (36), it has previously been difficult to show prominent vibriocidal responses in O139 vaccinees. Even when an unencapsulated mutant strain of V. cholerae O139 (MO10) was used, low responder frequencies and magnitudes of responses were seen in vaccinees (37). Thus, the use of CIRS 134 and the procedural modifications described above should contribute to an improved utility of the vibriocidal assay for evaluating immune responses in individuals infected with V. cholerae O139 or vaccinated with anti-O139-based vaccines.
Acknowledgments
This work was supported by funds from the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), the National Institute of Allergy and Infectious Diseases (grant UO1 AI58935 to S.B.C.), and the Swedish Agency for Research Cooperation with Developing Countries (Sida-SAREC grant 2002 to F.Q. and A.-M.S.). J.B.H. is an NICHD Fellow of the Pediatric Scientist Development Program (NICHD grant award K12-HD00850).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Albert, M. J. 1996. Epidemiology & molecular biology of Vibrio cholerae O139 Bengal. Indian J. Med. Res. 104:14-27. [PubMed] [Google Scholar]
- 2.Albert, M. J., F. Qadri, N. A. Bhuiyan, S. M. Ahmad, M. Ansaruzzaman, and A. Weintraub. 1999. Phagocytosis of Vibrio cholerae O139 Bengal by human polymorphonuclear leukocytes. Clin. Diagn. Lab. Immunol. 6:276-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert, M. J., A. K. Siddique, M. S. Islam, A. S. Faruque, M. Ansaruzzaman, S. M. Faruque, and R. B. Sack. 1993. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet 341:704. [DOI] [PubMed] [Google Scholar]
- 4.Attridge, S. R., F. Qadri, M. J. Albert, and P. A. Manning. 2000. Susceptibility of Vibrio cholerae O139 to antibody-dependent, complement-mediated bacteriolysis. Clin. Diagn. Lab. Immunol. 7:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benitez, J. A., A. J. Silva, and R. A. Finkelstein. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect. Immun. 69:6549-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bik, E. M., A. E. Bunschoten, R. J. Willems, A. C. Chang, and F. R. Mooi. 1996. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol. Microbiol. 20:799-811. [DOI] [PubMed] [Google Scholar]
- 7.Calia, K. E., M. Murtagh, M. J. Ferraro, and S. B. Calderwood. 1994. Comparison of Vibrio cholerae O139 with V. cholerae O1 classical and El Tor biotypes. Infect. Immun. 62:1504-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock, L. E., J. A. Johnson, J. M. Michalski, J. G. Morris, Jr., and J. B. Kaper. 1996. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol. Microbiol. 19:815-826. [DOI] [PubMed] [Google Scholar]
- 9.Comstock, L. E., D. Maneval, Jr., P. Panigrahi, A. Joseph, M. M. Levine, J. B. Kaper, J. G. Morris, Jr., and J. A. Johnson. 1995. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect. Immun. 63:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coster, T. S., K. P. Killeen, M. K. Waldor, D. T. Beattie, D. R. Spriggs, J. R. Kenner, A. Trofa, J. C. Sadoff, J. J. Mekalanos, and D. N. Taylor. 1995. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet 345:949-952. [DOI] [PubMed] [Google Scholar]
- 11.Faruque, S. M., N. Chowdhury, M. Kamruzzaman, Q. S. Ahmad, A. S. Faruque, M. A. Salam, T. Ramamurthy, G. B. Nair, A. Weintraub, and D. A. Sack. 2003. Reemergence of epidemic Vibrio cholerae O139, Bangladesh. Emerg. Infect. Dis. 9:1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faruque, S. M., D. A. Sack, R. B. Sack, R. R. Colwell, Y. Takeda, and G. B. Nair. 2003. Emergence and evolution of Vibrio cholerae O139. Proc. Natl. Acad. Sci. USA 100:1304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faruque, S. M., M. N. Saha, Asadulghani, P. K. Bag, R. K. Bhadra, S. K. Bhattacharya, R. B. Sack, Y. Takeda, and G. B. Nair. 2000. Genomic diversity among Vibrio cholerae O139 strains isolated in Bangladesh and India between 1992 and 1998. FEMS Microbiol. Lett. 184:279-284. [DOI] [PubMed] [Google Scholar]
- 14.Faruque, S. M., M. N. Saha, Asadulghani, D. A. Sack, R. B. Sack, Y. Takeda, and G. B. Nair. 2000. The O139 serogroup of Vibrio cholerae comprises diverse clones of epidemic and nonepidemic strains derived from multiple V. cholerae O1 or non-O1 progenitors. J. Infect. Dis. 182:1161-1168. [DOI] [PubMed] [Google Scholar]
- 15.Garg, S., A. N. Ghosh, A. K. Mukhopadhyay, and G. B. Nair. 1996. Electron microscopic studies on Vibrio cholerae O139. Indian J. Med. Res. 104:134-138. [PubMed] [Google Scholar]
- 16.Glass, R. I., A. M. Svennerholm, M. R. Khan, S. Huda, M. I. Huq, and J. Holmgren. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236-242. [DOI] [PubMed] [Google Scholar]
- 17.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1996. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine 14:1459-1465. [DOI] [PubMed] [Google Scholar]
- 18.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonson, G., J. Osek, A. M. Svennerholm, and J. Holmgren. 1996. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect. Immun. 64:3778-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losonsky, G. A., Y. Lim, P. Motamedi, L. E. Comstock, J. A. Johnson, J. G. Morris, Jr., C. O. Tacket, J. B. Kaper, and M. M. Levine. 1997. Vibriocidal antibody responses in North American volunteers exposed to wild-type or vaccine Vibrio cholerae O139: specificity and relevance to immunity. Clin. Diagn. Lab. Immunol. 4:264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews, K. R., B. M. Jayarao, A. J. Guidry, E. F. Erbe, W. P. Wergin, and S. P. Oliver. 1994. Encapsulation of Streptococcus uberis: influence of storage and cultural conditions. Vet. Microbiol. 39:361-367. [DOI] [PubMed] [Google Scholar]
- 23.Mel, S. F., K. J. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris, J. G., Jr., G. E. Losonsky, J. A. Johnson, C. O. Tacket, J. P. Nataro, P. Panigrahi, and M. M. Levin. 1995. Clinical and immunologic characteristics of Vibrio cholerae O139 Bengal infection in North American volunteers. J. Infect. Dis. 171:903-908. [DOI] [PubMed] [Google Scholar]
- 25.Pascual, M., X. Rodo, S. P. Ellner, R. Colwell, and M. J. Bouma. 2000. Cholera dynamics and El Nino-Southern Oscillation. Science 289:1766-1769. [DOI] [PubMed] [Google Scholar]
- 26.Qadri, F., T. Ahmed, M. A. Wahed, F. Ahmed, N. A. Bhuiyan, A. S. Rahman, J. D. Clemens, R. E. Black, and M. J. Albert. 2004. Suppressive effect of zinc on antibody response to cholera toxin in children given the killed, B subunit-whole cell, oral cholera vaccine. Vaccine 22:416-421. [DOI] [PubMed] [Google Scholar]
- 27.Qadri, F., M. Asaduzzaman, C. Wenneras, G. Mohi, M. J. Albert, M. A. Salam, R. B. Sack, M. Jertborn, J. R. McGhee, D. A. Sack, and J. Holmgren. 2000. Enterotoxin-specific immunoglobulin E responses in humans after infection or vaccination with diarrhea-causing enteropathogens. Infect. Immun. 68:6077-6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri, F., T. R. Bhuiyan, K. K. Dutta, R. Raqib, M. S. Alam, N. H. Alam, A. M. Svennerholm, and M. M. Mathan. 2004. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 53:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qadri, F., A. Chowdhury, J. Hossain, K. Chowdhury, T. Azim, T. Shimada, K. M. Islam, R. B. Sack, and M. J. Albert. 1994. Development and evaluation of rapid monoclonal antibody-based coagglutination test for direct detection of Vibrio cholerae O139 synonym Bengal in stool samples. J. Clin. Microbiol. 32:1589-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qadri, F., M. A. Haque, A. Hossain, T. Azim, K. Alam, and M. J. Albert. 1993. Role of Shigella dysenteriae type 1 slime polysaccharide in resistance to serum killing and phagocytosis. Microb. Pathog. 14:441-449. [DOI] [PubMed] [Google Scholar]
- 31.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karasawa, H. Kurazano, A. Pal, et al. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 34.Rodo, X., M. Pascual, G. Fuchs, and A. S. Faruque. 2002. ENSO and cholera: a nonstationary link related to climate change? Proc. Natl. Acad. Sci. USA 99:12901-12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha, D., R. C. LaRocque, A. I. Khan, J. B. Harris, Y. A. Begum, S. M. Akramuzzaman, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318-2322. [DOI] [PubMed] [Google Scholar]
- 36.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172:883-886. [DOI] [PubMed] [Google Scholar]
- 37.Trach, D. D., P. D. Cam, N. T. Ke, M. R. Rao, D. Dinh, P. V. Hang, N. V. Hung, D. G. Canh, V. D. Thiem, A. Naficy, B. Ivanoff, A. M. Svennerholm, J. Holmgren, and J. D. Clemens. 2002. Investigations into the safety and immunogenicity of a killed oral cholera vaccine developed in Viet Nam. Bull. W. H. O. 80:2-8. [PMC free article] [PubMed] [Google Scholar]
- 38.Waldor, M. K., R. Colwell, and J. J. Mekalanos. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. USA 91:11388-11392. [DOI] [PMC free article] [PubMed] [Google Scholar]