Abstract
Atomic force microscopy (AFM) is a technique used routinely in material science to image substances at a submicron (including nm) scale. We apply this technique to analysis of the fine structure of organic-walled Precambrian fossils, microscopic sphaeromorph acritarchs (cysts of planktonic unicellular protists) permineralized in ≈650-million-year-old cherts of the Chichkan Formation of southern Kazakhstan. AFM images, backed by laser-Raman spectroscopic analysis of individual specimens, demonstrate that the walls of these petrified fossils are composed of stacked arrays of ≈200-nm-sized angular platelets of polycyclic aromatic kerogen. Together, AFM and laser-Raman spectroscopy provide means by which to elucidate the submicron-scale structure of individual microscopic fossils, investigate the geochemical maturation of ancient organic matter, and, potentially, distinguish true fossils from pseudofossils and probe the mechanisms of fossil preservation by silica permineralization.
Traditionally, understanding of the history of life has been based chiefly on studies of the morphology of fossils. Morphology, however, can provide only limited insight about the underlying biochemical and physiological capabilities of ancient organisms, a deficiency particularly detrimental to understanding the earliest, Precambrian, seven-eighths of life's history. Unlike the more recent, shorter, and much more familiar Phanerozoic fossil record, that of the Precambrian was dominated by diverse prokaryotic microorganisms having only a limited range of simple morphologies yet widely divergent metabolic capabilities. Because prokaryotic taxa having more or less identical morphologies can differ greatly in metabolic capability, and because the evolutionary development of these various capabilities had profound effects on the evolution of the Earth's oceans, atmosphere, and surficial environment (1), there is a fundamental need to develop new techniques that by correlating chemistry with morphology in individual Precambrian microscopic fossils can provide insight into their underlying biochemical makeup.
Significant progress toward answering this need has been made by using ion microprobe spectrometry to analyze the carbon isotopic composition of single Precambrian microfossils (2, 3), an approach to understanding the chemistry of such fossils that has recently been extended to a molecular level by laser-Raman spectroscopic imagery of individual Precambrian fossil microbes (4, 5). In principal, information about the structural makeup of such molecular components should be accessible by using atomic force microscopy (AFM), a technique used routinely in material science to elucidate the nm-scale structure of macromolecules such as DNA (6). Here, we use AFM to image the fine structure of the cell walls of Precambrian microfossils, an approach to this problem that coupled with laser-Raman spectroscopy reveals the submicron-scale organization of their kerogenous components. This combination of AFM and laser-Raman spectroscopy provides means not only to elucidate the fine structure of individual microscopic fossils but to investigate the geochemical maturation of ancient organic matter, the mechanisms that underlie fossil preservation by permineralization, and, potentially, to determine whether carbonaceous microscopic fossil-like objects are true fossils rather than pseudofossil “look-alikes.”
Materials and Methods
Specimens Analyzed.
The fossils studied here are permineralized in carbonaceous cherty stromatolites (Conophyton gaubiza) of the ≈650-million-year-old Chichkan Formation from the Maly Karatau Mountains, Ayusakan, of southern Kazakstan [Precambian Paleobiology Research Group sample 1473 (7)].
Sample Preparation for AFM.
Individual microscopic fossils situated at the uppermost surfaces of petrographic thin sections polished with a paste containing 0.25-μm-sized diamonds were located, identified, and photographed in transmitted white light by using a digital video camera connected to a Zeiss Axiovert inverse microscope (Fig. 1). The sections, supported at their ends and inverted to permit loosened detritus to fall away, were then immersed in dilute (5%) hydrofluoric acid, typically for 30–40 min, withdrawn from the acid, immersed sequentially in three baths of distilled water, and allowed to dry in an evacuated desiccator. So as to not dislodge fragile organic structures exposed by the acid etching, throughout this sequence the solutions were unstirred and the sections were manipulated slowly and very gently. After the sections had dried, the fossils were then relocated on the upper surfaces of the etched thin sections by using a low-power binocular microscope having a large working distance and circled with a Sanford extra-fine point permanent Sharpie marker (especially suitable for this purpose because of its fast-drying ink). Sketch maps were then prepared showing the location of the fossils within the circled areas, and the fossils were located by using the video camera connected to the AFM apparatus and digitally photographed in reflected white light (Fig. 2A).
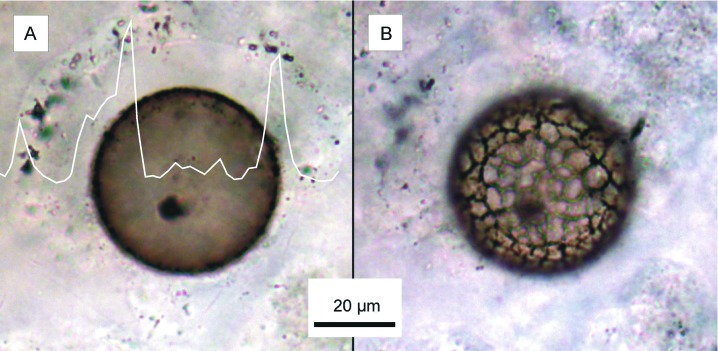
Figure 1.
Optical photomicrographs in transmitted white light showing the representative Chichkan sphaeromorph acritarch imaged by AFM. (A) Specimen transected at its equatorial plane by, and exposed at the upper surface of the polished thin section, superimposed by a plot of an electron microprobe measurement showing that carbon is concentrated in and near its cell wall. (B) The lower half of the specimen, embedded in the chert matrix, showing the characteristic reticulate surface ornamentation of this taxon of Chichkan sphaeromorph.
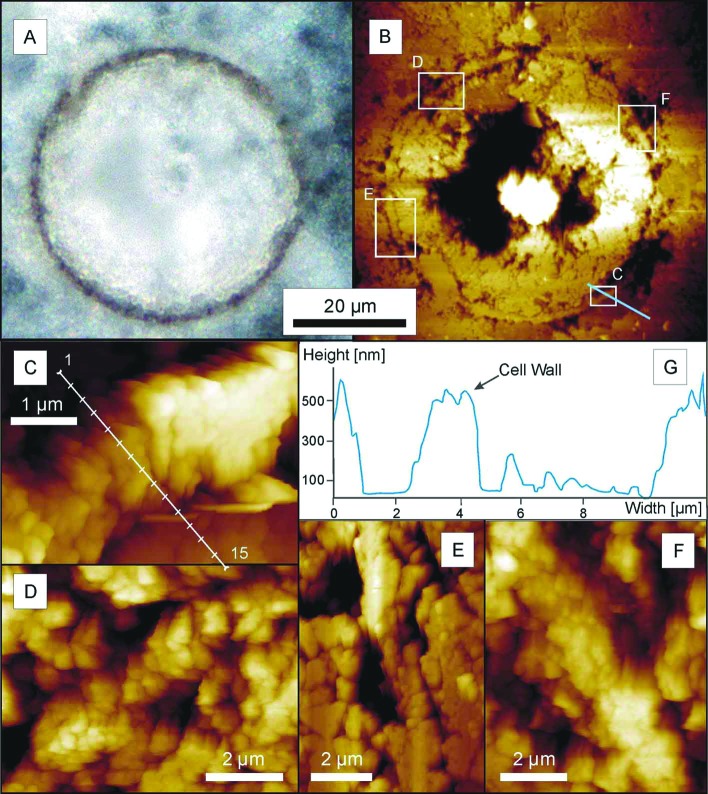
Figure 2.
Petrified Chichkan sphaeromorph. (A) Digital photograph of the equatorial plane of the specimen in reflected light, before AFM analysis. (B) AFM image showing the part of the specimen analyzed and, denoted by the white rectangles, the areas for which higher magnification micrographs are shown (C–F); the irregular black areas are cavities within and adjacent to the cell wall, produced by acid etching; the blue line crossing rectangle C shows the transect through the cell wall along which topography was measured (G). (C–F) Atomic force micrographs showing the substructure of the representative portions of the cell wall shown in the corresponding rectangles in B; the white line in C denotes the transect (locations 1–15, from outside the spheroid to beyond its inner surface) where the adhesion measurements plotted in Fig. 5 were obtained. (G) A topographic transect through a portion of the cell wall, measured from inside the spheroid (Left) to beyond its outer surface (Right) along the blue line that in B crosses rectangle C, demonstrating the characteristic preferential etching of the matrix adjacent to both the inner and outer surfaces of the wall.
Submicron Structure.
AFM images (Figs. 2 B–F and 3 A–D) were obtained by using a Veeco (Santa Barbara, CA) (formerly Topometrix) Explorer equipped with a three-piezo scanner, having a maximum planar range of 130 μm in each of the planar (x, y) directions and a maximum vertical (z) range of 10.5 μm, mounted on the video camera-connected inverse microscope noted above. The images were acquired by using the intermittent contact mode [using Nanosensors (Norderfriedrichskoog, Germany) noncontact high-resolution reflex-coated wafer and Nanosensors super sharp silicon-noncontact high-frequency cantilevers], selected among various AFM modes tested as resulting in the least disruption of the scanned surface and the use of which permitted acquisition of high-resolution (nm scale) topographic data (Fig. 2G).
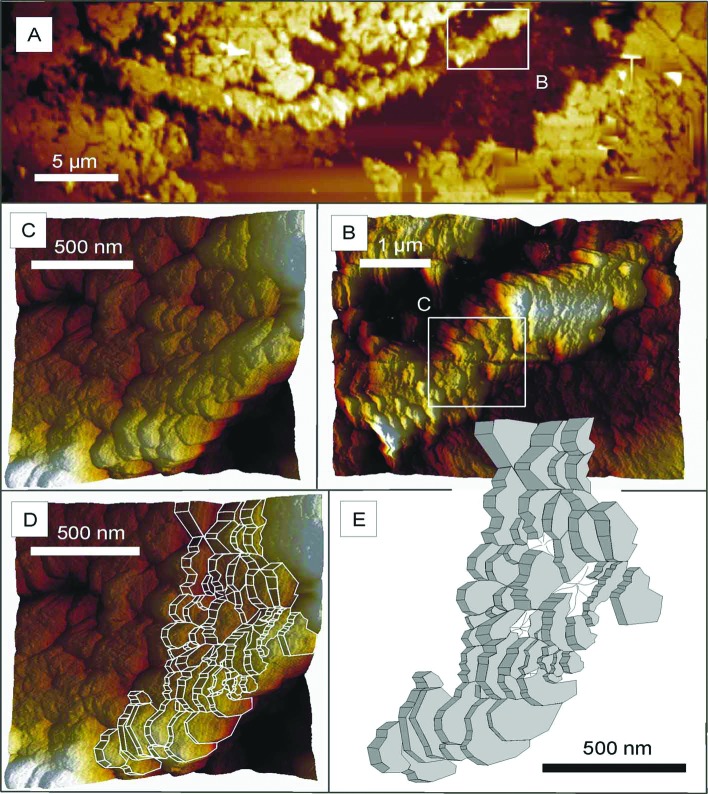
Figure 3.
Atomic force micrographs of a portion of the petrified Chichkan sphaeromorph. (A) The portion of the cell wall imaged in B–D and illustrated in E, a segment of the wall shown in the lowermost part of Fig. 2B. (B) That portion of the wall indicated by the white rectangle in A, shown here at higher magnification. (C) That portion of the wall indicated by the white rectangle in B, shown here at higher magnification. (D) That portion of the wall shown in C with a superimposed computer-generated outline of its component parts. (E) A computer-generated sketch of the platelet-like wall subunits.
Chemical Composition.
Measurements showing the concentration of carbon in the cell walls of the specimens analyzed (e.g., Fig. 1A) were performed with a Cameca (Paris) SX50 (WDX) electron microprobe. Laser-Raman spectra showing the molecular structure of the Chichkan sphaeromorphs (Fig. 4) were acquired by using a Dilor XY (formerly Instruments SA, now JY Horiba, Edison, NJ) 0.8-m triple-stage system having macrofocal, microfocal, and confocal line-scan imaging options and using the instrumental configuration and analytical procedures specified by Schopf et al. (5). For measurement of surface adhesion, an index of the carbon content of the cell walls relative to that of their encompassing quartz matrices (Fig. 5), a Veeco (formerly Park Scientific) Bioprobe AFM was used in the tapping mode for imaging and in the contact mode for force-distance measurements [using Silicon-MDT (Moscow) Ultrasharp NSC 11 cantilevers having a spring constant of 3 N/m].
Figure 4.
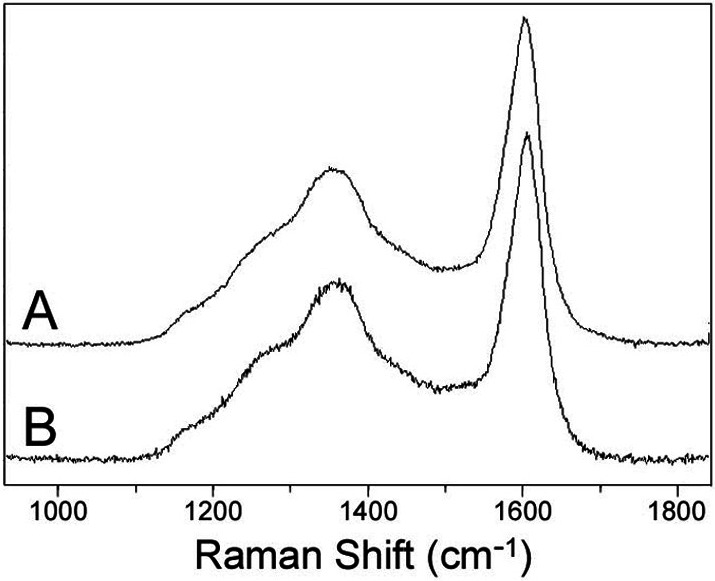
Laser-Raman point spectra of the carbonaceous walls of two Chichkan sphaeromorph specimens of the same taxon as the acritarch illustrated in Fig. 1, showing the prominent vibrational bands at ≈1,350 cm−1 and ≈1,600 cm−1 characteristic of polycyclic aromatic kerogenous organic matter. (A) Specimen C-19. (B) Specimen C-2.
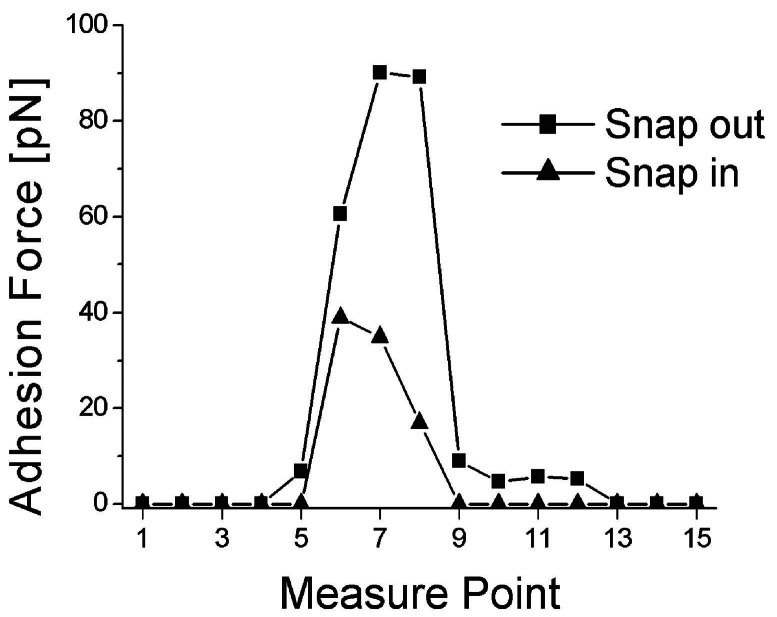
Figure 5.
Force distance spectra recorded at equidistant points along the transect through the cell wall denoted in Fig. 2C. “Snap out” values demonstrate that the carbonaceous wall of the sphaeromorph is decidedly more sticky, having more adhesion to the AFM probe (by >90 pN), than the surrounding quartz matrix. The hysteresis from “snap in” to snap out, as great as 50 pN and occurring exclusively at the cell wall, shows that the carbonaceous wall has greater plasticity than the encompassing quartz.
Results
This study demonstrates a successful attempt to image the submicron-scale wall structure of permineralized (petrified) fossils by AFM. Of the ≈40 permineralized acritarchs (“resting cell” cysts of planktonic single-celled protists) we have analyzed from cherts of the Chichkan Formation by AFM and/or laser-Raman spectroscopy, the representative specimen illustrated here is a spheroid ≈50 μm in diameter (Figs. 1 and 2 A and B) enclosed by a well-defined carbonaceous cell wall ≈1–2 μm thick (Figs. 1A and 2), a sphaeromorph taxon distinctive in the assemblage because of its characteristically reticulate surface ornamentation (Fig. 1B).
As shown in Fig. 2G, the embedding quartz matrix etched especially deeply immediately adjacent to both the inner and outer surfaces of the wall of the fossil, a pattern of differential etching that reflects a phase change from the more or less pure quartz of the encompassing matrix to the quartz-kerogen mixture of the cell wall and that in part is due also to active etching at the arcuate margins of quartz grains that impinge on, but do not penetrate through, the sphaeromorph wall. The presence of this phase change is shown also by adhesion measurements along transects perpendicular to the wall (Fig. 5), which establish that the quartz-kerogen wall is decidedly “stickier,” that it adheres to the AFM probe with appreciably greater force (measured to be >90 pN), than quartz grains devoid of kerogen situated on the outside or inside of the permineralized wall where the adhesion force is almost zero. High-resolution AFM images show this wall to be composed of platy subunits oriented approximately parallel to radii through the spheroid (Fig. 2 C–F) that where especially well defined are made up of stacked arrays of angular tile-like components ≈200 nm in size (Fig. 3 B–E).
The carbonaceous composition of the cell wall of the representative Chichkan fossil illustrated here is shown by its brown color and typically kerogenous granular texture (Figs. l and 2A), electron microprobe analyses (Fig. 1A), and laser-Raman spectroscopy of other Chichkan specimens of this distinctively reticulate-surfaced sphaeromorph taxon (Fig. 4), which show them to be composed of geochemically relatively little altered aggregates of disordered predominantly polycyclic aromatic kerogen molecules (5).
Taken together, the results reported here establish that the wall of this Precambrian microscopic fossil is composed of submicron-sized platy tiles of carbon-rich polycyclic aromatic compounds.
Discussion
Given current knowledge of the chemistry of fossil organic matter (8), it is not surprising that the Chichkan acritarchs are composed of angular platelets of aromatic kerogen. What is notable is that AFM combined with laser-Raman spectroscopy provides means to investigate the makeup of individual microscopic fossils. Not only can this combination of techniques elucidate the fine structure of single fossil microorganisms, such as the one illustrated here, but it provides a powerful way to investigate the products and processes of the geochemical maturation of ancient organic matter, and to thereby determine whether, and to what extent, the chemistry and submicron-scale structure of permineralized fossils reflects their original makeup. Moreover, application of the techniques in tandem holds promise for discrimination between true fossils and pseudofossil look-alikes, a problem that has plagued Precambrian paleobiology since its inception (9–11) and that remains pressing even today (5, 12). For example, by using AFM to determine the orientation of Raman-established aromatic subunits, true fossils, such as the one illustrated here in which the subunits are aligned parallel to the radii of the fossil (Fig. 2 C–F), could be distinguished from such nonbiologic particles as spheroidal carbon black in which the aromatic subunits are aligned parallel to the spheroid's circumference (13). And because AFM has the capability to elucidate the fine-scale physical relationships between individual permineralized fossils and their encompassing mineral matrix, use of this technique, new to paleobiology, combined with routine optical petrologic studies, provides means to probe the products and infer the processes of fossil permineralization.
Clearly, AFM provides a powerful way to investigate the submicron-scale physical structure of ancient life.
Acknowledgments
Help with electron microprobe analyses was provided by G. Magel of the Institute of Petrology, Geochemistry, and Applied Mineralogy, Ludwig-Maximilians-Universität München (Prof. B. Dingwell). Financial support from Deutscher Akademischer Austausch Dienst (to W.M.H.) and the National Science Foundation (to J.W.S.) is gratefully acknowledged.
Abbreviation
- AFM
atomic force microscopy
References
- 1.Schopf J W. Cradle of Life, The Discovery of Earth's Earliest Fossils. Princeton: Princeton Univ. Press; 1999. [Google Scholar]
- 2.House C H, Schopf J W, McKeegan K D, Coath C D, Harrison T M, Stetter K O. Geology. 2000;28:707–710. doi: 10.1130/0091-7613(2000)28<707:cicoip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Ueno Y, Isozaki Y, Yuimoto H, Maruyama S. Int Geol Rev. 2001;43:196–212. [Google Scholar]
- 4.Kudryavtsev A B, Schopf J W, Agresti D G, Wdowiak T J. Proc Natl Acad Sci USA. 2001;98:823–826. doi: 10.1073/pnas.98.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schopf J W, Kudryavtsev A B, Agresti D G, Wdowiak T J, Czaja A D. Nature (London) 2002;416:73–76. doi: 10.1038/416073a. [DOI] [PubMed] [Google Scholar]
- 6.Heckl W M. Thin Solid Films. 1992;210/211:640–647. [Google Scholar]
- 7.Moore T B, Schopf J W. In: The Proterozoic Biosphere, A Multidisciplinary Study. Schopf J W, Klein C, editors. New York: Cambridge Univ. Press; 1992. pp. 603–693. [Google Scholar]
- 8.Durand B, editor. Kerogen, Insoluble Organic Matter from Sedimentary Rocks. Paris: Éditions Technip; 1980. [Google Scholar]
- 9.Schopf J W, Walter M R. In: Earth's Earliest Biosphere, Its Origin and Evolution. Schopf J W, editor. Princeton: Princeton Univ. Press; 1983. pp. 214–239. [Google Scholar]
- 10.Schopf J W. In: The Proterozoic Biosphere, A Multidisciplinary Study. Schopf J W, Klein C, editors. New York: Cambridge Univ. Press; 1992. pp. 25–39. [Google Scholar]
- 11.Schopf J W. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- 12.Brasier M D, Green O R, Jephcoat A P, Kleppe A K, Van Kranendonk M J, Lindsay J F, Steele A, Grassineau N V. Nature (London) 2002;416:76–81. doi: 10.1038/416076a. [DOI] [PubMed] [Google Scholar]
- 13.Oberlin A, Boulmier J L, Villey M. In: Kerogen, Insoluble Organic Matter from Sedimentary Rocks. Durand B, editor. Paris: Éditions Technip; 1980. pp. 191–231. [Google Scholar]