Abstract
In spite of its many other benefits, DNA vaccine is limited in its application by its insufficient immunogenicity. One promising approach for enhancing its immunogenicity is to maximize its expression in the immunized host. In the current study, we investigated whether codon optimization of the mycobacterial antigen Ag85B gene could enhance the expression and immunogenicity of the Ag85B DNA vaccine. We generated a synthetic humanized Ag85B (hAg85B) gene in which codon usage was optimized for expression in human cells. DNA plasmids with codon-optimized hAg85B increased the level of protein expression in vitro and in vivo. DNA vaccine with hAg85B induced stronger Th1-like and cytotoxic T-cell immune responses in BALB/c mice and generated higher protective immunity in a BALB/c mouse model of Mycobacterium tuberculosis aerosol infection than did the DNA vaccine with wild-type Ag85B. Therefore, our results suggest that codon optimization of mycobacterial antigens (e.g., Ag85B) could improve protein expression and thereby enhance the immunogenicity of DNA vaccines against M. tuberculosis.
Tuberculosis is one of the most lethal infectious diseases in the world, killing two million adults each year (26). Though childhood immunization with Mycobacterium bovis (BCG) has likely reduced several severe childhood diseases (e.g., tuberculous meningitis), its efficacy against adult pulmonary disease is highly variable, with efficacy estimates ranging from 0% to approximately 80%. Since adult pulmonary disease is the main threat posed to public health by tuberculosis (14), an improved vaccine against tuberculosis is urgently needed.
Plasmid DNA encoding antigen Ag85B, one of the Mycobacterium tuberculosis secretory antigens, induced cellular immune responses dominated by antigen-specific Th1-type T cells, thereby affording some protection against subsequent challenge with M. tuberculosis in mice (25, 43). Although the immunogenicity of the DNA vaccine encoding Ag85B in mice was promising, the protective immunity against M. tuberculosis H37Rv was relatively weak compared to that provided by M. bovis BCG (25, 30).
Though DNA vaccines offer many benefits, their usefulness is limited by their insufficient immunogenicity (2, 40, 49). Various attempts have been made to improve DNA vaccines, including coexpression of cytokines (3, 19) or chemokines (13), modification of subcellular localization of expressed antigen (Ag) (29, 38), induction of apoptosis (39) or antiapoptosis (27) of transfected cells, the targeting of Ag to the major histocompatibility complex class I or II processing pathway (37), and the use of self-destructing attenuated intracellular bacteria as carriers for the DNA vaccines (31).
One promising approach for improving the response of naked DNA vaccine is to maximize its expression in mammalian cells (15, 38). Recently, several reports showed that codon optimization or codon usage modification of genes encoding human papillomavirus E7 (7), human immunodeficiency virus gp120 (1, 21), gp160 (48), Gag (8), Plasmodium falciparum EBA-175 (36), Clostridium tetani TetC (44), and Listeria monocytogenes LLO91-99 (34, 47) toward human consensus codon usage significantly increased both the protein expression and the immunogenicity of DNA vaccines.
In the current study, we have attempted to investigate whether codon optimization of the Ag85B gene could enhance the expression and the immunogenicity of the Ag85B DNA vaccine. Although “optimized codons” for protein synthesis may be different defending on species, most mammals including Homo sapiens and Mus musculus have very similar codon usage (35). Therefore, we tested the efficacy of DNA vaccine with humanized codon usage in mice. To this end, we generated a synthetic humanized Ag85B (hAg85B) gene in which codon usage was optimized for expression in human cells. DNA vaccine with codon-optimized Ag85B induced higher immune responses and stronger protective immunity in a BALB/c mouse model of M. tuberculosis infection than did a vaccine with wild-type Ag85B, suggesting that the efficacy of DNA vaccines against M. tuberculosis can be significantly improved by codon optimization.
MATERIALS AND METHODS
Plasmid constructions.
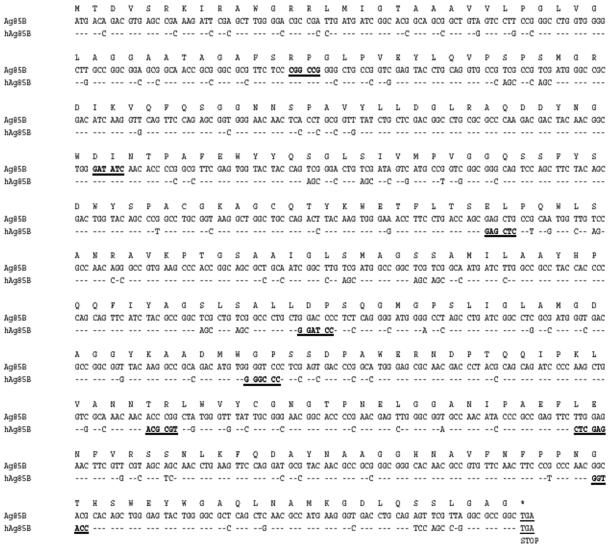
To generate the pCK-Ag85B (pAg85B), Ag85B cDNA was cloned from the genomic DNA of M. tuberculosis H37Rv by using the upstream primer 5′-GAA TTC GCC GCC ACC ATG ACA GAC GTG AGC CGA AAG-3′ and the downstream primer 5′-GCG GCC GCT CAG CCG GCG CCT AAC GAA CTC TG-3′ (restriction sites are underlined). The hAg85B cDNA was synthesized by assembling mutually priming synthetic oligonucleotides (Fig. 1). The gene was divided into nine segments of approximately equal length, and nine pairs of oligonucleotides were synthesized; each pair consisted of two overlapping long oligonucleotides with a short stretch of overlap containing restriction endonuclease recognition sites (Fig. 1, underlined residues), with one oligonucleotide coding for the sense strand and the other coding for the antisense strand. Some deviations from strict adherence to optimized codon usage were made to accommodate the introduction of unique restriction sites into the final gene product at approximately 150-bp intervals (Fig. 1). Each gene segment was amplified by PCR and ligated to a pGEM-T vector (Promega). The correct sequence was confirmed by DNA sequencing (3730 DNA Analyzer; Applied Biosystems). The final product was amplified by PCR, using a pair of oligonucleotides partially complementary to hAg85B cDNA (boldfaced in the sequences presented below) and containing restriction sites EcoRI and NotI (underlined in the sequences presented below) for cloning. The sequence of the upstream primer, which includes a Kozak consensus sequence, was 5′-GAA TTC GCC GCC ACC ATG ACC GAC GTG TCC CGC AAG-3′, and the sequence of the downstream primer was 5′-GCG GCC GCT CAG CCG GCG CCC AGG CTG GAC TGC AGG TC-3′. The resulting hAg85B gene was cloned into pCK (3, 6, 28). The plasmids were electroporated into Escherichia coli Top10F′ and purified from large-scale cultures using a QIAGEN Endofree Giga Kit (QIAGEN, Hilden, Germany).
FIG. 1.
Nucleotide sequences of the Ag85B cDNA and the deduced amino acid sequences. Above each codon is the single-letter designation for the amino acid. The humanized codons introduced in the hAg85B sequence are shown below the substituted nucleotide of Ag85B. The sites of the restriction enzymes used to assemble extended pairs of oligonucleotides are underlined and in boldface.
Analysis of codon usage.
The most commonly used codons for H. sapiens and M. tuberculosis were determined using the Codon Usage Database on the GenBank website (www.kazusa.or.jp/codon/).
Mice and immunization.
Female BALB/c mice (Charles River, Biogenomics, Seoul, Republic of Korea) were kept at 22°C and 55% relative humidity on a 12-h day-night rhythm and given free access to food and water. The mice were housed in germ-free conditions at the Laboratory Animal Center of Seoul National University until use. Mice used in the studies were from 6 to 8 weeks of age. Prior to experimental manipulation, mice were anesthetized with an intraperitoneal injection of ketamine-xylazine. Each of the BALB/c mice was twice intramuscularly injected, at 2-week intervals, in the right and left tibialis anterior muscles with 100 μg of plasmid DNA in 100 μl of phosphate-buffered saline (PBS) using a 30-gauge insulin syringe. The negative control group was injected with the same volume (100 μg/mouse) of an empty pCK vector.
For protection assays, specific pathogen-free 6-week-old female BALB/c mice were purchased from Japan SLC, Inc. (Shijuoka, Japan), maintained under barrier conditions in a BL-3 biohazard animal room at Yonsei University Medical Research Center, and fed a sterile commercial mouse diet and water ad libitum. All experiments were done in compliance with relevant laws and institutional guidelines.
Peptides.
The sequence of the p11 peptide used in the assays is LTSELPQWLSANRAVKPTGS (amino acids 141 to ∼160) from Ag85B (GenBank accession no. AAO62005). p11 was an H2d-restricted Th1 T-cell epitope peptide of Ag85B, which was known to induce the production of interleukin-2 (IL-2) and gamma interferon (IFN-γ) in response to restimulation of spleen cells from BALB/c mice vaccinated with plasmid DNA encoding Ag85B or infected with M. tuberculosis (12).
In vitro transfection studies and Western blotting.
COS-7 cells were transiently transfected with plasmids by Effectene (QIAGEN), and cells were harvested 48 h posttransfection. Total cell lysates were prepared by using CytoBuster Protein Extraction Reagent (Novagen), which was supplemented with a complete protease inhibitor cocktail (Roche). Equal amounts of cell lysates (400 μg of total protein) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes (BioTrace NT; Pall Corporation, Ann Arbor, MI). Blots were blocked for 1 h at room temperature in PBS containing 5% nonfat dried milk and 0.1% Tween 20 under gentle shaking. Membranes were incubated overnight with Ag85B-specific polyclonal antibody that was purified from sera of BALB/c mice immunized with recombinant Ag85B protein and adjuvants. Then, membranes were incubated with a 1:2,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; Sigma, St. Louis, MO). The liquid diaminobenzidine substrate-chromogen system (DakoCytomation, Fort Collins, CO) was used for Western blotting. The intensity of each band was read by using a ChemiImager 4400 densitometer (Alpha Innotech, San Leandro, Calif.) and calculated by AlphaEase version 5.5 software.
Luciferase assay.
The pAg85B- and phAg85B-luciferase plasmids were constructed by using the luciferase gene of the pGL3-Enhancer (Promega, Madison, WI), and luciferase activity was measured in duplicate using a Dual Luciferase Assay kit (Promega), following the manufacturer's recommendations, along with a Luminoskan Ascent luminometer (Thermo Labsystems USA, Franklin, MA). Luciferase activity was normalized by β-galactosidase activity. For the in vivo expression assay, 200 μg of luciferase-expressing plasmids was intramuscularly injected into the right tibialis anterior muscle of BALB/c mice. Fourteen days later, muscles around the site of injection were removed and homogenized. After lysis, 20 μl of cell lysates (0.5 mg/ml) was added into each well, along with 100 μl of the luciferin buffer. Light emission was registered immediately in the luminometer as relative light units (RLU) (20-s integration and 1-s settle time).
RT-PCR.
To assess the mRNA transcription level in the cells transfected with plasmids, the constructed plasmid was transfected into CT26 cells and COS-7 cells as described above, and after 48 h, total RNA was isolated using an RNeasy mini kit (QIAGEN), as directed in the user's manual. For digestion of the DNA plasmid, DNaseI (RNase-free) (Takara, Shiga, Japan) was treated following the manufacturer's protocol. For reverse transcription PCR (RT-PCR), 2.5 μg of total RNA was reverse transcribed into cDNA by oligo(dT) priming using superscript II RT (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Sense and antisense primers used in the PCR were as follows: for Ag85B, 5′-CAG CAG TTC ATC TAC GCC-3′ and 5′-GAA GTT GAA CAC GGC GTT GTG-3′; for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TTA GCA CCC CTG GCC AAG G-3′ and 5′-CTT ACT CCT TGG AGG CCA TG-3′. Equal aliquots of RT-PCR product for Ag85B (348 bp) and GAPDH (500 bp) were separated on 1.5% agarose gels and stained with ethidium bromide. Agarose gels were scanned by densitometer to measure the densities of bands. The ratio of Ag85B to GAPDH was calculated and used to obtain the ratio of Ag85B transcripts (Ag85B:hAg85B).
Enzyme-linked immunosorbent assay (ELISA) for Ag85B-specific Ab.
To obtain recombinant Ag85B protein, Ag85B cDNA was cloned into pET28a(+) (Novagen, Darmstadt, Germany) and expressed in E. coli BL21(DE3). Recombinant Ag85B protein was further purified by Ni-nitrilotriacetic acid agarose (QIAGEN, Valencia, CA) following the manufacturer's protocol. Sera from immunized mice were collected by orbital bleeding 3 weeks after the final immunization. Alkaline phosphatase-conjugated goat anti-mouse IgG (Pierce, Illinois), IgG1, or IgG2a antibody (Ab) (Southern Biotechnology, Alabama) was used to detect Ag85B-specific Ab. The titer of the sera was determined by using the reciprocal of the last dilution yielding an absorbance twice above the background, which had been established using saline-injected mouse serum diluted 1:200.
Splenocyte proliferation assay.
Splenocytes were prepared from spleens of immunized mice and erythrocytes were depleted. Cells were plated at 5 × 105 spleen cells per well in 96-well round-bottom microtiter plates (Nunc) with complete culture medium. Cells were cultured for 3 days with 10 μg/ml of p11 or alone. After 72 h of incubation, including a final 22-h pulse with [3H]thymidine (1 μCi per well), the cultured splenocytes were harvested using an automatic harvester (Skatron, Sterling, VA), and incorporation of the label was measured using a liquid scintillation counter (Wallac, Turku, Finland).
ELISA for IFN-γ.
Immunized mice were sacrificed 2 weeks after the last immunization, and spleens were removed aseptically. Spleen cells were plated at 8 × 106 cells per well and cultured for 72 h in the presence of 10 μg/ml of peptide p11 of Ag85B or alone. Culture supernatants were tested for the presence of IFN-γ by sandwich ELISA. For IFN-γ sandwich ELISA, we used purified rat anti-mouse IFN-γ monoclonal Ab (clone R4-6A2), biotinylated rat anti-mouse IFN-γ monoclonal Ab (clone XMG1.2) (both from BD Pharmingen, San Diego, CA), and recombinant IFN-γ.
ELISPOT assay.
The enzyme-linked immunospot (ELISPOT) assay (32) was modified to detect Ag85B-p11-specific IFN-γ-secreting cells. Briefly, 96-well multiscreen filter plates (Mutiscreen MAHA; Millipore, France) were coated with 10 μg/ml rat anti-mouse IFN-γ antibody (clone R4-6A2; BD PharMingen, San Diego, CA) in 50 μl of PBS. After overnight incubation at 4°C, the wells were washed and blocked for 2 h with 200 μl of RPMI medium-10% fetal bovine serum (FBS; all from Bio Whittaker, MD). Splenocytes were resuspended to 2 × 107 cells/ml, placed in duplicates, and serially diluted (1 × 107, 0.5 × 107, and 0.25 × 107 cells/well). Cells were incubated at 37°C for 24 h either with or without 1 μg/ml peptide p11. After culture, the plate was washed six times with PBS containing 1% Tween 20 and then incubated 2 h at room temperature with 5 μg/ml biotinylated rat anti-mouse IFN-γ antibody (clone XMG1.2; BD PharMingen, San Diego, CA) in 50 μl of PBS-10% FBS. After six washes with PBS, 1.25 μg/ml peroxidase (Sigma) in 50 μl of PBS-10% FBS was added and incubated 1 h at room temperature. Spots were developed by adding 50 μl of a 5-bromo-4-chloro-3,3-indolyl phosphate-nitroblue tetrazolium solution (Boehringer Mannhem, Indianapolis, IN) and incubated at room temperature for 10 min. Spots were counted electronically using a Zeiss KS-ELISPOT, version 4.5.21.
51Cr release assay.
To prepare the target cell line (CT26-Ag85B) for cytotoxic assay, mycobacterial Ag85B cDNA was cloned into the mammalian expression vector pDON-AI (kindly provided by S. Y. Kim, Seoul National University, Republic of Korea) and cotransfected into the CT26 cell line with pGagPol and pEnv plasmids (kindly provided by S. Y. Kim, Seoul National University, Republic of Korea). Two weeks after the third injection, spleen cells from immunized mice were prepared and restimulated by coculturing with CT26-Ag85B cells (1 × 107 cells per mouse) that had been treated with mitomycin C at 37°C for 30 min. After 6 days of culture, restimulated cells were harvested and used as effector cells in a 4-h 51Cr release assay. 51Cr-labeled 1 × 104 CT26-Ag85B target cells were added to the effector cells. After incubation, a 100-μl aliquot was removed from each well for counting in a Wallac 1470 Wizard automatic gamma counter. The values were expressed as the percentage of specific lysis. Percent specific lysis was calculated as [(experimental release − background release)/(maximal release − background release)] × 100. Background release was defined as 51Cr release by target cells in the absence of effector cells, and the maximal release was determined after lysing target cells with 1% Triton X-100.
Protection assay.
Mice were immunized intramuscularly three times at 3-week intervals with 100 μg of pAg85B, phAg85B, or mock vector. In addition, one group of mice was immunized with BCG (1 × 105 CFU) twice at weeks 3 and 6. Four weeks after final immunization, mice were exposed to 500 CFU of M. tuberculosis H37Rv (ATCC 27294) via aerosol for 60 min in the inhalation chamber of an airborne infection apparatus (Glas-Col, Terre Haute, Ind.). After 4 weeks, mice were euthanized with CO2, and lungs were homogenized in 0.04% Tween 80 and saline. The tissue suspension was diluted 10-fold, and 100 μl of each dilution was plated in duplicate onto Middlebrook 7H11 agar plates and incubated at 37°C for 3 to 4 weeks. M. tuberculosis colonies on each plate were counted, and the results are expressed as the log10 of CFU per organ. Data are expressed as the means of individual determinants, plus or minus standard deviations.
Statistical analysis.
The values of P for the experiments were determined using a Student's t test and the SigmaPlot 5.0 program (SPSS Inc.).
RESULTS
Construction of an hAg85B gene based on optimal codon usage.
In order to test the hypothesis that codon optimization would improve the protein expression and the immunogenicity of Ag85B DNA plasmid (pAg85B), we constructed an hAg85B DNA plasmid (phAg85B) using the codons which most frequently appear in humans, without altering amino acid sequences of pAg85B-encoded protein (Fig. 1). The synthetic hAg85B gene was inserted into the mammalian cDNA expression vector pCK under the control of the human cytomegalovirus (HCMV) immediate-early (IE) enhancer and promoter. pCK contains not only the full-length IE promoter of HCMV but also its entire 5′ untranslated region consisting of the entire exon 1 and intron a and of a part of exon 2 (28). Furthermore, the Kozak consensus sequence, GCCGCCACC, was introduced immediately preceding the insertion of the AUG initiator codon, which was intended to coincide with the ATG codon of the original IE gene of HCMV.
Enhanced expression of Ag85B by codon optimization.
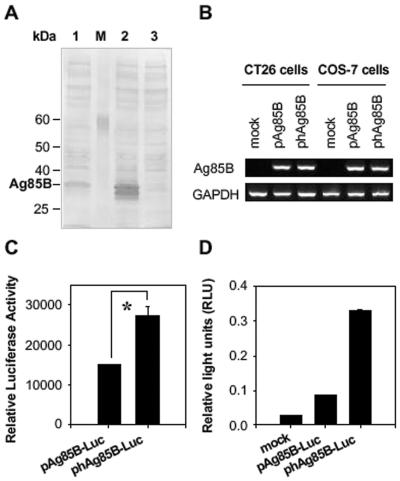
We first checked whether the codon optimization of Ag85B could improve protein expression. To evaluate the relative potency of the wild-type and humanized Ag85B genes, we compared Ag85B protein expression by in vitro and in vivo transfection of the DNA plasmids, pAg85B and phAg85B. At first, the protein levels expressed by transfection of COS-7 cells with DNA vaccine plasmid pAg85B, phAg85B, or mock vector as a negative control were determined by Western blotting (Fig. 2A). Codon optimization of Ag85B increased the level of protein expression approximately sixfold over that of wild-type Ag85B as the intensity of each band having a molecular mass of ∼30 kDa was measured by using densitometer. In the lane of phAg85B-transfected COS-7 cells, there were two major bands around 30 kDa. We compared the bands of high molecular mass, although we assumed that the lower-molecular-mass band might be a truncated form of Ag85B.
FIG. 2.
Increased expression of Ag85B by codon optimization. (A) Comparative Ag85B expression in COS-7 cells transfected with pAg85B (lane 1) and phAg85B (lane 2) constructs. As a control, COS-7 cells were transfected with mock plasmid (lane 3). Cell lysates (400 μg of total protein) were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and analyzed by immunoblotting with Ag85B-specific polyclonal antibody. The arrow at the right indicates the position of the Ag85B protein. The sizes of protein standards (lane M) are indicated in kilodaltons (kDa) at the left. (B) Total RNA was extracted and analyzed for relative levels of Ag85B mRNA by RT-PCR. Agarose gels were scanned by densitometer to measure the densities of bands. The ratio of Ag85B to GAPDH was calculated and used to obtain the ratio of Ag85B transcripts (Ag85B:hAg85B). Data are representative of at least two independent experiments. (C) Comparison of translational efficacy of pAg85B-Luc and phAg85B-Luc. COS-7 cells were transiently transfected with each plasmid, and 48 h after the transfection, cells were lysed, and luciferase activities were assayed. Data represent relative luciferase activities, with transfection efficiencies normalized by β-galactosidase activity. One representative of at least three performed experiments is shown. *, P < 0.03. (D) For the in vivo expression assay, 200 μg of luciferase-expressing plasmids was intramuscularly injected into the right tibialis anterior muscle of BALB/c mice (2 mice per group), and RLU were determined 14 days later. Data are representative of at least three independent experiments.
The protein expression level was also assessed by luciferase assay. We constructed pCK-Ag85B-luciferase (pAg85B-Luc) and codon-substituted pCK-hAg85B-luciferase (phAg85B-Luc) fusion genes and transiently transfected them into COS-7 and CT26 cells. The relative luciferase activities of phAg85B-Luc were higher than those of pAg85B-Luc in both COS-7 cells (Fig. 2C) and CT-26 cells (data not shown). Furthermore, we analyzed the in vivo expression of pAg85B-Luc and phAg85B-Luc in the muscle cells of mice. For in vivo expression, 200 μg of luciferase-expressing plasmids was intramuscularly injected, and then 14 days later RLU were determined from the muscle tissue of the injected site. Injection of phAg85B-Luc resulted in greater luciferase activity than did pAg85B-Luc (Fig. 2D).
To test whether increased Ag85B mRNA transcripts enhanced protein expression levels of phAg85B, we checked the levels of mRNA expression by pAg85B and phAg85B. At 48 h posttransfection of CT26 and COS-7 cells with DNA plasmids, total RNAs were prepared, and Ag85B mRNA levels were assessed by RT-PCR. The densities of bands on the agarose gels were measured by a densitometer. The ratio of each Ag85B transcript to GAPDH was calculated and used to obtain the transcriptional ratio of Ag85B to hAg85B. It was 1:0.93 in CT26 cells and 1:1.02 in COS-7 cells. Therefore, enhanced protein expression of the hAg85B gene can be ascribed to increased Ag85B mRNA translation rather than to increased Ag85B gene transcription (Fig. 2B).
Effects of codon-optimized DNA vaccine on humoral immune responses.
Next, we analyzed Ag85B-specific Ab production by DNA vaccines. Although the level of IgG produced by pAg85B or phAg85B immunization was a bit low, it was significantly increased compared with that of the mock vector-injected group (Table 1). However, DNA immunization of BALB/c mice with codon-optimized phAg85B did not enhance Ag85B-specific IgG Ab responses quantitatively compared with those immunized with pAg85B. On the other hand, the IgG2a/IgG1 ratio showed indirectly that the immune response by phAg85B was slightly skewed toward Th1 responses.
TABLE 1.
Effect of phAg85B immunization on humoral responses against Ag85B
| Immunizationa | Log2 ELISA titerb
|
IgG2a/IgG1 ratio | ||
|---|---|---|---|---|
| Total IgG | IgG1 | IgG2a | ||
| pAg85B | 10.64 ± 0.38c | 11.64 ± 0.53c | 12.50 ± 0.34c | 2.64 ± 0.99 |
| phAg85B | 10.52 ± 0.58c | 10.48 ± 0.79c | 12.48 ± 0.83c | 4.67 ± 1.16 |
| Mock vector | 6.27 ± 0.18 | <5.64 | 6.93 ± 0.29 | |
Mice were immunized with 100 μg of pAg85B or phAg85B intramuscularly three times at 3-week intervals. The titers of Ag85B-specific IgG, IgG1, and IgG2a antibodies were determined by ELISA with sera recovered 2 weeks after the final immunization, and the ratios of IgG2a to IgG1 were calculated.
The results are log2(titer ± SE) of endpoint titers for six to eight mice per group.
P < 0.005 versus mock vector injected group.
phAg85B immunization enhanced T-cell activation.
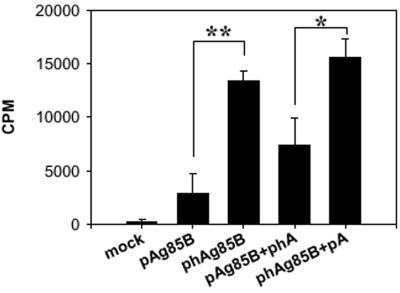
Next, we explored whether immunization with pAg85B or codon-optimized phAg85B would induce cellular immune responses against Ag85B in BALB/c mice. As shown in Fig. 3, T -cell proliferative responses to the Th1 T-cell epitope peptide p11 were only weakly enhanced (2,938 ± 1,811 cpm) after DNA immunization with pAg85B but increased fivefold (13,377 ± 921 cpm) after DNA immunization with phAg85B. Similarly, splenocyte proliferative responses toward purified recombinant Ag85B or culture filtrate protein were also increased by codon optimization (data not shown).
FIG. 3.
Increased proliferative responses after phAg85B immunization. Proliferative responses of spleen-derived lymphocytes from five mice immunized intramuscularly twice at 2-week intervals with 100 μg of pAg85B, phAg85B, or the mock vector. To compensate for the difference in CpG contents between the pAg85B and phAg85B sequences, a combination of 100 μg (each) of pAg85B and pGEM-T-hAg85B (pAg85B+phA) or phAg85B and pGEM-T-Ag85B (phAg85B+pA) was injected. Two weeks after the final immunization, the incorporation of [3H]thymidine in response to 10 μg/ml p11 was measured as described in Materials and Methods. Spleens were from five mice tested individually, and results are expressed as the means ± standard errors (cpm). The statistical significance of the difference in the proliferative response was determined by a Student's t test. *, P < 0.05; **, P < 0.001.
Since the CpG content of phAg85B was slightly changed after codon optimization, we addressed whether the change in CpG contents would affect T-cell activation.
To compensate for the differences in the CpG contents of pAg85B and phAg85B sequences, mice were immunized intramuscularly with 100 μg (each) of pAg85B and pGEM-T-hAg85B (pAg85B+phA) or phAg85B and pGEM-T-Ag85B (phAg85B+pA). Since the pGEM-T vector was unable to be expressed in mammalian cells, pGEM-T-Ag85B (pA) and pGEM-T-hAg85B (phA) were coadministered with phAg85B and pAg85B, respectively, to control for differences in the CpG contents between the two genes. Immunization with phAg85B+pA increased antigen-specific proliferation significantly more than did pAg85B+phA immunization, demonstrating that the difference in immunogenicity was not the consequence of differences in CpG content (Fig. 3). Collectively, these results indicate that DNA immunization of BALB/c mice with codon-optimized phAg85B increases the Ag85B-specific T-cell proliferative responses significantly more than does pAg85B, regardless of differences in the CpG contents of DNA vaccines.
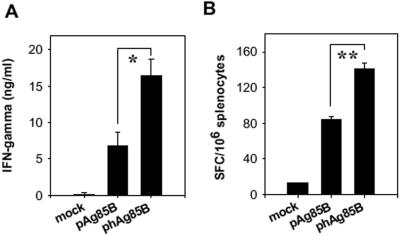
phAg85B DNA immunization enhances IFN-γ expression.
The IFN-γ production of pAg85B-immunized and phAg85B-immunized spleen cells was measured using ELISA and ELISPOT assays. As shown in Fig. 4A, the phAg85B-immunized spleen cells (16,481 ± 2,260 pg/ml) produced much higher levels of IFN-γ than did pAg85B-immunized splenocytes (6,783 ± 1,893 pg/ml). IFN-γ was hardly detected in the culture supernatants of spleen cells taken from mice immunized with a mock vector (Fig. 4A). Consistent with this observation, the number of antigen-specific IFN-γ-secreting cells detected in splenocytes from mice immunized with phAg85B was twice (P < 0.01) that observed in pAg85B-immunized mice (Fig. 4B). Therefore, immunization with phAg85B increased antigen-specific IFN-γ secretion of Th1 cells.
FIG. 4.
phAg85B immunization increased IFN-γ secretion. (A) IFN-γ secreted by p11 peptide-stimulated spleen cells from pAg85B-immunized or phAg85B-immunized mice. BALB/c mice were immunized intramuscularly with pAg85B, phAg85B, or mock vector twice at 2-week intervals. Spleen cells from immunized mice were harvested 2 weeks after the last immunization and stimulated by culturing in the presence or absence of 10 μg/ml of p11 peptide for 3 days. The concentration of IFN-γ in the culture supernatants was determined by sandwich ELISA. Splenocytes from mice immunized with a mock vector were used as negative control. Results are expressed as the mean ± standard error. A statistically significant difference was observed in levels of IFN-γ secreted by spleen cells immunized with pAg85B and those with phAg85B. *, P < 0.03. (B) ELISPOT estimates of the frequencies of cells that produced IFN-γ in response to 10 μg/ml p11 peptide for 24 h. The results are expressed as the mean ± standard error of spot-forming cells (SFC) per 106 splenocytes. There was a remarkable difference in the levels of IFN-γ-secreting cells detected in the groups immunized with pAg85B and those with phAg85B. **, P < 0.01.
We also used a sandwich ELISA to measure the IL-4 production of splenocytes from immunized BALB/c mice. However, IL-4 production was not detected in any of the mice examined (data not shown).
Immunization with phAg85B DNA increased CTL activities.
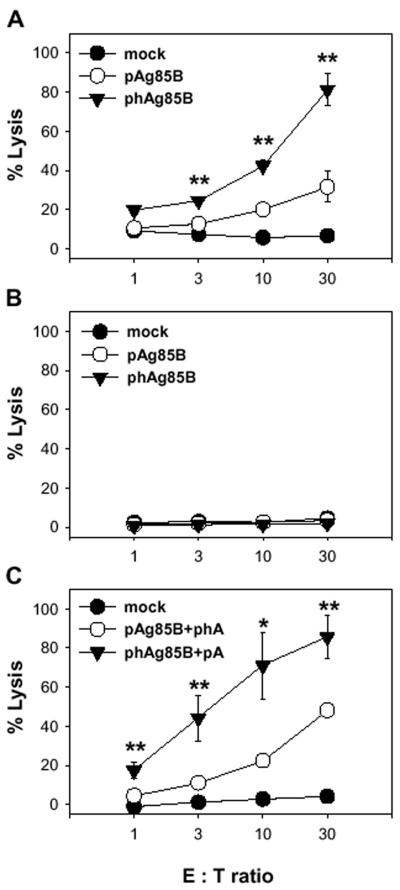
We examined how DNA immunization using the codon-optimized Ag85B gene affected the induction of specific cytotoxic T lymphocytes. As shown in Fig. 5A, splenocytes from BALB/c mice immunized twice at 2-week intervals with phAg85B more strongly lysed CT26-Ag85B cells than did pAg85B-immunized splenocytes after in vitro restimulation by the CT26-Ag85B cells treated with mitomycin C for 6 days. In contrast, neither pAg85B nor phAg85B immunization induced the specific lysis of CT26 cells (Fig. 5B).
FIG. 5.
Codon usage effects of DNA vaccine on CTL induction. (A and B) BALB/c mice were immunized intramuscularly with 100 μg of mock vector, pAg85B, or phAg85B twice at 2-week intervals. (C) To compensate for the differences in CpG contents of the pAg85B and phAg85B sequences, mice were injected with a combination of 100 μg each of pAg85B and pGEM-T-hAg85B (pAg85B+phA) or with phAg85B and pGEM-T-Ag85B (phAg85B+pA). Spleen cells from mice immunized with 200 μg of mock vector were used as a negative control. Each of the splenocytes from the immunized mice (5 mice/group) was harvested 2 weeks after the last immunization and stimulated with mitomycin C-treated CT26-Ag85B cells for 6 days in vitro. Cytotoxicity of splenocytes was evaluated in a 4-h 51Cr-release assay against CT26-Ag85B cells (A and C). Nonspecific lysis was evaluated by using CT26 as target cells (B). The values were expressed as the mean percentage ± standard error of specific lysis from individual mice. The significance of the difference between pAg85B and phAg85B was calculated by a Student's t test. *, P < 0.03; **, P < 0.01.
In order to assess the influence of the CpG motif on the high CTL activity of phAg85B-immunized mice, groups of BALB/c mice were first immunized with pAg85B+phA or phAg85B+pA and then tested for CTL responses. In these experiments, the specific lysis of phAg85B+pA-immunized mice was 85.65% ± 11.17% and that of pAg85B+phA-immunized mice was 48.24% ± 2.50% at an effector/target ratio of 30 (Fig. 5C). These data strongly indicate that the differences in the CpG contents of pAg85B and phAg85B sequences did not significantly contribute to the capacity of phAg85B to induce higher Ag85B-specific CTL responses.
DNA immunization with phAg85B enhances protective efficacy.
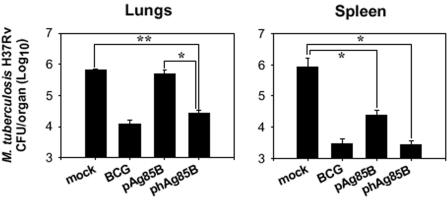
Finally, we investigated whether optimizing the codon usage of the Ag85B DNA vaccine would enhance its protective efficacy against airborne M. tuberculosis H37Rv infection in the BALB/c mouse model. Mice were immunized intramuscularly three times at 3-week intervals with 100 μg of pAg85B, phAg85B, or mock vector. Four weeks after the final immunization, mice were exposed to 500 CFU of M. tuberculosis H37Rv via aerosol for 60 min, and bacterial numbers in organs were determined 4 weeks after infection. DNA immunization with phAg85B provided a significant level of protection against M. tuberculosis, compared with mock immunization, in both lungs and spleens, where protections were comparable to that afforded by BCG (Fig. 6). In contrast, the protective efficacy of pAg85B immunization in the lungs was variable, and the protection by pAg85B immunization was not significant compared to immunization with mock vector alone (Fig. 6). Interestingly, immunization with phAg85B achieved a more consistent and significant level of protection against M. tuberculosis infection in the lungs than did pAg85B immunization (P < 0.05) (Fig. 6). In addition, the number of CFU in spleens of phAg85B-immunized mice was lower than that seen in mice injected with pAg85B (Fig. 6), although there was no significant difference in CFU (P = 0.065). These results demonstrate that codon-optimized pAg85B induced a higher degree of protective immunity in a BALB/c mouse model of M. tuberculosis aerosol infection than did wild-type Ag85B. In particular, the enhanced protection in the primary infection site, the lungs, is meaningful.
FIG. 6.
Enhanced protective efficacy after immunization with codon-optimized phAg85B. Protection against M. tuberculosis in mice immunized intramuscularly three times at 3-week intervals with 100 μg of pAg85B, phAg85B, or mock vector. Mice were challenged with 500 CFU of M. tuberculosis H37Rv by inhalation and bacterial counts in lungs and spleen were determined 4 weeks after infection. Data are the means ± standard deviations of three mice. *, P < 0.05; **, P < 0.01.
DISCUSSION
Although vaccination with plasmid DNA encoding mycobacterial Ag85B has been shown to induce protective immunity against M. tuberculosis through immune responses mediated by CD4+ Th1 cells and CD8+ T cells (9, 23), the extent of protective efficacy was relatively weak compared to that of M. bovis BCG (11, 25, 30, 46). Recently, DNA immunization followed by boosting with protein vaccine (4) or modified vaccinia virus Ankara (16) was shown to increase the protective immunity against M. tuberculosis H37Rv challenge to a level comparable to that afforded by BCG. However, the efficacy of the DNA vaccine itself needs to be improved. In the current study, we showed that DNA immunization with codon-optimized phAg85B in mice significantly enhanced the Ag85B-specific Th1-type immune responses and CTL activities and provided protective immunity against M. tuberculosis.
The relative importance of CD4+ Th1 cells and CD8+ T cells in antituberculosis immunity remains a subject of debate (5, 10, 33). However, Th1 responses seem to be important for diseases requiring cellular immunity such as Leishmania major, M. tuberculosis, and malaria (17). In this regard, a recent report showed that CD8+ T cells may have an important role in maintaining the frequency of CD4+ IFN-γ-producing T cells (18). In addition, another previous study suggested that the amount of antigen expression by plasmid DNA is important for efficient T-cell priming (38). In particular, the appropriate CD4+ T-cell immune responses appear to require repeated Ag exposure and a sufficient amount of Ag (4, 22, 38). Furthermore, CD4+ T-cell help is essential for CTL priming and memory CTL formation (24, 42). Therefore, if the amount of antigen expressed by a DNA vaccine were increased, the CD4+ Th1 cell-mediated immune responses could be enhanced, thereby also improving CD8+ T-cell immunity.
In this study, we constructed an hAg85B DNA vaccine plasmid (phAg85B) with human codon usage to improve the expression of a pAg85B DNA vaccine in mammalian cells. We used the most prevalent codon in humans as the optimized codon of the final gene product. In wild-type mycobacterial Ag85B, many of the Ser and Pro amino acid residues are encoded by triplets rarely seen in human genes. By substituting high-usage codons for these low-usage codons without altering the amino acid sequences, we could increase the level of Ag85B protein expression approximately sixfold in mammalian cells. Because the G+C content of mycobacterial Ag85B is very high (64.2%), the G+C content was raised to approximately 68.5% in the optimized sequence. In comparison, the overall genome G+C contents of vertebrates were 52.31% (in Mus musculus) and 52.59% (in Homo sapiens) (35). Therefore, we tested whether the high G+C content would affect mRNA transcription in mammalian cells. Interestingly, the enhanced protein expression of the hAg85B gene was due to the increased Ag85B mRNA translation rather than to the increased Ag85B gene transcription.
We also investigated whether immune responses elicited by DNA vaccines could be affected by changes in the nucleotide composition of DNA sequences such as alterations in CpG content. It has previously been demonstrated that the codon optimization of the gp120 gene introduced additional CpG motifs into the sequence, perhaps thereby increasing immunogenicity, as these motifs are known to stimulate innate immunity (1, 21). In contrast, the codon optimization of the human immunodeficiency virus type 1 gag gene results in increased immunogenicity due to enhanced gag expression, not to the accumulation of CpG motifs (8). Furthermore, the codon optimization of DNA encoding fragment C of tetanus toxin (TetC) has been shown to increase anti-TetC antibodies in the sera of immunized mice, even when the CpG contents of synthetic and native genes were made uniform (44). In our study, we codelivered a control vector to compensate for the effect of the increased numbers of CpG motifs in the humanized Ag85B gene. Even when the impact of the elevated CpG motifs was thus neutralized, T-cell proliferation and CTL activity remained high, showing that the increase in Ag85B-specific immune responses was not due to the increase in CpG motifs. Instead, we ascribed the increased immunogenicity of phAg85B to the increased expression of Ag85B in the host.
However, humoral responses elicited by DNA vaccines were relatively weak, with no significant differences detected between mice immunized with pAg85B and phAg85B. Since both pAg85B and phAg85B lacked a mammalian signal sequence such as human Ig(κ) signal leader or the tissue plasminogen activator signal sequence in their transcription units, most of the Ag85B expressed by the DNA vaccine was not secreted out of cells. Instead, the enhanced Ag85B expression induced by codon optimization occurred mainly inside transfected cells and so did not significantly affect humoral responses. During apoptosis or cell damage, Ag85B might be released and become available for B cells and dendritic cells. Presumably, these processes, which are required following protein expression, could substantially limit the level of humoral responses induced by DNA vaccines.
Although Ag85B is a promising vaccine candidate, it has been reported that the production of Ag85B decreased during chronic infection of M. tuberculosis in the mouse lungs (41). It has also been reported that the Ag85A epitopes are frequently expressed on the surface of the infected macrophages during early phase but are downregulated at the late phase (45). Because the levels of expression of Ag85B were similar to those of Ag85A at corresponding times of infection (41), we just assessed the protective efficacy of Ag85B-specific immune responses during early phase. For the protection of both early and late phases of mycobacterial infection, development of a multigene DNA vaccine composed of hAg85B and a different antigen which is expressed at least during the late stage of infection may be needed.
In summary, we demonstrated that the optimization of the M. tuberculosis Ag85B coding sequences to those of highly expressed mammalian genes resulted in substantial improvement in protein expression by mammalian cells. In addition, DNA vaccine with codon-optimized hAg85B induced stronger Ag85B-specific Th1-like and cytotoxic T-cell immune responses in BALB/c mice and induced higher protective immunity in a mouse model of M. tuberculosis aerosol infection than did the DNA vaccine with wild-type Ag85B. Therefore, our results suggest that the optimization of codon usage of mycobacterial antigens could improve protein expression and consequently enhance the immunogenicity of DNA vaccines against M. tuberculosis. Our approach to improving the immunogenicity of Ag85B DNA vaccine via enhanced antigen expression included the optimization of codon usage of the mycobacterial Ag85B gene. This was the first attempt to assess the codon optimization of a mycobacterial antigen on protein expression in the mouse model. Surprisingly, DNA immunization of BALB/c mice with codon-optimized phAg85B alone induced protective immunity comparable to that afforded by BCG. Furthermore, the strategy of combining DNA vaccines of codon-optimized mycobacterial antigens with other promising approaches could facilitate the design of improved antituberculosis vaccines for clinical applications.
Next, we will construct a bicistronic vector expressing codon-optimized hAg85B and another mycobacterial antigen such as PstS-3 or ESAT-6 for preclinical study in mice and nonhuman primates. Recently, multigene DNA vaccination combined simultaneously with chemotherapy was proposed as one of the efficient strategies for patients undergoing tuberculosis chemotherapy to prevent exogenous reinfection as well as endogenous reactivation (20). For the clinical use of this strategy, the optimization of codon usage of a mycobacterial antigen of a DNA vaccine may be essential.
Acknowledgments
This study was supported by NITR/Korea FDA grant 04092LIF-007-2 from Korea Food and Drug Administration.
Editor: J. L. Flynn
REFERENCES
- 1.André, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autran, B., G. Carcelain, B. Combadiere, and P. Debre. 2004. Therapeutic vaccines for chronic infections. Science 305:205-208. [DOI] [PubMed] [Google Scholar]
- 3.Baek, K. M., S. Y. Ko, M. Lee, J. S. Lee, J. O. Kim, H. J. Ko, J. W. Lee, S. H. Lee, S. N. Cho, and C. Y. Kang. 2003. Comparative analysis of effects of cytokine gene adjuvants on DNA vaccination against Mycobacterium tuberculosis heat shock protein 65. Vaccine 21:3684-3689. [DOI] [PubMed] [Google Scholar]
- 4.Bajénoff, M., O. Wurtz, and S. Guerder. 2002. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4+ T cells. J. Immunol. 168:1723-1729. [DOI] [PubMed] [Google Scholar]
- 5.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. Immunology 162:5407-5416. [PubMed] [Google Scholar]
- 6.Chang, S. Y., K. C. Lee, S. Y. Ko, H. J. Ko, and C. Y. Kang. 2004. Enhanced efficacy of DNA vaccination against Her-2/neu tumor antigen by genetic adjuvants. Int. J. Cancer 111:86-95. [DOI] [PubMed] [Google Scholar]
- 7.Cid-Arregui, A., V. Juarez, and H. zur Hausen. 2003. A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J. Virol. 77:4928-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deml, L., A. Bojak, S. Steck, M. Graf, J. Wild, R. Schirmbeck, H. Wolf, and R. Wagner. 2001. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J. Virol. 75:10991-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T. P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Souza, S., O. Denis, T. Scorza, F. Nzabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza, S., V. Rosseels, O. Denis, A. Tanghe, N. De Smet, F. Jurion, K. Palfliet, N. Castiglioni, A. Vanonckelen, C. Wheeler, and K. Huygen. 2002. Improved tuberculosis DNA vaccines by formulation in cationic lipids. Infect. Immun. 70:3681-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza, S., V. Rosseels, M. Romano, A. Tanghe, O. Denis, F. Jurion, N. Castiglione, A. Vanonckelen, K. Palfliet, and K. Huygen. 2003. Mapping of murine Th1 helper T-cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 71:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eo, S. K., S. Lee, S. Chun, and B. T. Rouse. 2001. Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J. Virol. 75:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine, P. E. M., I. A. M. Carneiro, J. B. Milstien, and C. J. Clements. 1999. Issues relating to the use of BCG in immunization programmes: a discussion document. WHO Department of Vaccines and Biologicals, document 99.23. WHO, Geneva, Switzerland.
- 15.Garmory, H. S., K. A. Brown, and R. W. Titball. 2003. DNA vaccines: improving expression of antigens. Genet. Vaccines Ther. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171:1602-1609. [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 18.Gurunathan, S., L. Stobie, C. Prussin, D. L. Sacks, N. Glaichenhaus, A. Iwasaki, D. J. Fowell, R. M. Locksley, J. T. Chang, C. Y. Wu, and R. A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915-924. [DOI] [PubMed] [Google Scholar]
- 19.Ha, S. J., B. Y. Jeon, S. C. Kim, D. J. Kim, M. K. Song, Y. C. Sung, and S. N. Cho. 2003. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 10:1592-1599. [DOI] [PubMed] [Google Scholar]
- 20.Ha, S. J., B. Y. Jeon, J. I. Youn, S. C. Kim, S. N. Cho, and Y. C. Sung. 2005. Protective effect of DNA vaccine during chemotherapy on reactivation and reinfection of Mycobacterium tuberculosis. Gene Ther. 12:634-638. [DOI] [PubMed] [Google Scholar]
- 21.Haas, J., E. C. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 22.Homann, D., L. Teyton, and M. B. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 23.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 24.Jyh-Chiang, E., W. M. Livingstone, and A. M. Livingstone. 2003. CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J. Immunol. 171:6339-6343. [DOI] [PubMed] [Google Scholar]
- 25.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 27.Kim, T. W., C. F. Hung, J. Juang, L. He, J. M. Hardwick, and T. C. Wu. 2004. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene Ther. 11:336-342. [DOI] [PubMed] [Google Scholar]
- 28.Lee, Y., E. J. Park, S. S. Yu, D. K. Kim, and S. Kim. 2000. Improved expression of vascular endothelial growth factor by naked DNA in mouse skeletal muscles: implication for gene therapy of ischemic diseases. Biochem. Biophys. Res. Commun. 272:230-235. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, P. J., S. van Drunen Littel-van den Hurk, and L. A. Babiuk. 1999. Altering the cellular location of an antigen expressed by a DNA-based vaccine modulates the immune response. J. Virol. 73:10214-10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozes, E., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vandenbussche, J. P. Van Vooren, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 31.Miki, K., T. Nagata, T. Tanaka, Y. H. Kim, M. Uchijima, N. Ohara, S. Nakamura, M. Okada, and Y. Koide. 2004. Induction of protective cellular immunity against Mycobacterium tuberculosis by recombinant attenuated self-destructing Listeria monocytogenes strains harboring eukaryotic expression plasmids for antigen 85 complex and MPB/MPT51. Infect. Immun. 72:2014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyahira, Y., K. Murata, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. M. Rodrigues, and F. Zavala. 1995. Quantification of antigen-specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods 181:45-54. [DOI] [PubMed] [Google Scholar]
- 33.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata, T., M. Uchijima, A. Yoshida, M. Kawashima, and Y. Koide. 1999. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem. Biophys. Res. Commun. 261:445-451. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura, Y., T. Gojobori, and T. Ikemura. 2000. Codon usage tabulated from international DNA sequence databases: status for the year. Nucleic Acids Res. 28:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narum, D. L., S. Kumar, W. O. Rogers, S. R. Fuhrmann, H. Liang, M. Oakley, A. Taye, B. K. Sim, and S. L. Hoffman. 2001. Codon optimization of gene fragments encoding Plasmodium falciparum merzoite proteins enhances DNA vaccine protein expression and immunogenicity in mice. Infect. Immun. 69:7250-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez, F., S. Harkins, J. M. Redwine, J. M. de Pereda, and J. L. Whitton. 2001. CD4+ T cells induced by a DNA vaccine: immunological consequences of epitope-specific lysosomal targeting. J. Virol. 75:10421-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rush, C., T. Mitchell, and P. Garside. 2002. Efficient priming of CD4+ and CD8+ T cells by DNA vaccination depends on appropriate targeting of sufficient levels of immunologically relevant antigen to appropriate processing pathways. J. Immunol. 169:4951-4960. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki, S., R. R. Amara, A. E. Oran, J. M. Smith, and H. L. Robinson. 2001. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 19:543-547. [DOI] [PubMed] [Google Scholar]
- 40.Scheerlinck, J. Y. 2001. Genetic adjuvants for DNA vaccines. Vaccine 19:2647-2656. [DOI] [PubMed] [Google Scholar]
- 41.Shi, L., Y.-J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, C. M., N. S. Wilson, J. Waithman, J. A. Villadangos, F. R. Carbone, W. R. Heath, and G. T. Belz. 2004. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat. Immunol. 5:1143-1148. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen, A. L., S. Nagal, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stratford, R., G. Douce, L. Zhang-Barber, N. Fairweather, J. Eskola, and G. Dougan. 2000. Influence of codon usage on the immunogenicity of a DNA vaccine against tetanus. Vaccine 19:810-815. [DOI] [PubMed] [Google Scholar]
- 45.Tanghe, A., P. Lefevre, O. Denis, S. D'Souza, M. Braibant, E. Lozes, M. Singh, D. Montgomery, J. Content, and K. Huygen. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113-1119. [PubMed] [Google Scholar]
- 46.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchijima, M., A. Yoshida, T. Nagata, and Y. Koide. 1998. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J. Immunol. 161:5594-5599. [PubMed] [Google Scholar]
- 48.Vinner, L., H. V. Nielsen, K. Bryder, S. Corbet, C. Nielsen, and A. Fomsgaard. 1999. Gene gun DNA vaccination with Rev-independent synthetic HIV-1 gp160 envelope gene using mammalian codons. Vaccine 17:2166-2175. [DOI] [PubMed] [Google Scholar]
- 49.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]