Abstract
Paneth cells are specialized epithelia in the small bowel that secrete antimicrobial proteins. Paneth cells are vital to the innate immunity of the small bowel in adult mammals, but their role during neonatal infection of the small bowel is not well established. Dithizone selectively damages Paneth cells, and when dithizone-treated newborn rats are infected enterally with Escherichia coli, the numbers of E. coli cells in their jejunal and ileal lavage fluid are significantly increased compared to controls. The data support that Paneth cells are necessary for neonatal antibacterial defense.
Antimicrobial proteins and peptides are key effectors of innate immunity at mucosal surfaces in adult animals (21), but their role in host defense during perinatal intestinal development is not clearly delineated. Paneth cells are specialized epithelia in the crypts of the small bowel that control the intestinal growth of bacterial pathogens through the secretion of antimicrobial proteins and peptides (11). Paneth cells secrete their antimicrobial-rich granules upon exposure to pathogenic bacteria and bacterial products (1, 11, 12), and alpha-defensins account for 70% of the secreted bactericidal activity of Paneth cells (1).
Neonatal necrotizing enterocolitis is a disease mainly of preterm human infants, but its pathogenesis is incompletely understood (8). It is hypothesized that abnormal bacterial colonization of the small bowel plays a role (3). A causative association between Paneth cells and necrotizing enterocolitis has been proposed because preterm infants inadequately express alpha-defensins in the small bowel (10). Additionally, Paneth cells in surgical specimens from human infants with necrotizing enterocolitis have a deficiency in lysozyme (4), a prominent antimicrobial protein in Paneth cell granules (11). The inability of neonatal Paneth cells to control the growth of bacterial pathogens in the lumen of the small bowel is an attractive hypothesis related to the initiation of necrotizing enterocolitis, but there is no in vivo evidence that Paneth cells provide host defense in the neonatal small bowel.
This investigation postulated that ablating Paneth cells in neonatal rats would reduce the ability of the neonatal small bowel to clear an infection caused by enteroinvasive Escherichia coli. To test this hypothesis, dithizone was given systemically to neonatal rats. Since dithizone selectively destroys Paneth cells in adult rats (16), we quantified the effects of dithizone on the Paneth cells of neonatal rats. After dithizone treatment, newborn rats were infected with an intragastric dose of Escherichia coli, and the quantitative clearance of this bacterium from the small bowel lumen was measured.
Effect of dithizone on Paneth cells in the noninfected neonatal small bowel.
For studies that examined the effects of dithizone, specific-pathogen-free Sprague Dawley rats (Harlan, San Diego, CA) were studied between 4 and 5 days of age. The studies described below were approved by the Animal Use Committee of the University of California, Davis.
Dithizone (Sigma-Aldrich, St. Louis, MO) was suspended in 25 mM Li2CO3 buffer, stirred for 2 h at 37°C, and then filtered through preweighed Whatman 1 paper (Whatman Inc., Clifton, NJ). The filter paper was completely dried after filtration and was reweighed to determine the mg/ml of dissolved dithizone. The measurement of dissolved dithizone was used to properly dose administration of the dye.
Three separate litters of 4-day-old pups were given an intraperitoneal (i.p.) injection of either filter-sterilized dithizone (75 mg/kg of body weight) in mM Li2CO3 buffer, 25 mM Li2CO3 buffer alone, or normal saline. Twenty-four hours after i.p. injection, each pup had an illness score assigned (6), followed by euthanasia with i.p. pentobarbital. The distal half of the small bowel was fixed in situ, and vertical sections of the distal ileal crypts (4 μm) were stained with hematoxylin and eosin (13). For each pup, 100 crypts were examined microscopically using oil immersion (magnification, ×1,000). Paneth cells were identified based on their eosinophilic granules and incident light fluorescence (13). The total number of Paneth cells and mitotic figures per 100 crypts, the number of Paneth cells and mitotic figures per crypt, and the distribution of one or more Paneth cells and mitotic figures per crypt were calculated from the results of nine pups randomly selected from each treatment group. Since the eosinophilic granules of Paneth cells contain their antibacterial peptides (12), a Lickert score was developed to grade the numbers and characteristics of the cytoplasmic granules. A score of 3 was assigned to Paneth cells with large, well-rounded, and deeply staining eosinophilic granules that filled the entire cytoplasm, while a score of 1 was applied to Paneth cells that had significantly reduced numbers of poorly staining and pleomorphic eosinophilic granules in their cytoplasm. A score of 2 indicated an intermediate appearance.
After 24 h, there were no signs of illness and survival was 100% in the noninfected rat pups given either i.p. dithizone or control injections. The numbers of Paneth cells identified in 100 crypts 24 h after i.p. injection of saline, Li2CO3 buffer, or dithizone were 27 ± 3, 26 ± 1, and 19 ± 2, respectively (means ± standard errors of the means; P < 0.05). The average numbers of Paneth cells per crypt were, for saline, 0.33 ± 0.03; for Li2CO3 buffer, 0.30 ± 0.02; and for dithizone, 0.22 ± 0.03 (P < 0.05). The granules in Paneth cells of dithizone-treated pups had a mean Lickert score of 1.6, a median score of 1, and a range of 1 to 2, whereas those from pups given i.p. saline or Li2CO3 buffer had average Lickert scores of 2.5 and 2.2, respectively, a median score of 3, and a range of 2 to 3 (Fig. 1). Analysis of Lickert scores for Paneth cells of dithizone-treated pups showed significant abnormalities compared to controls (by the Kruskal Wallis statistic, H = 8.117, P < 0.017, and post hoc analysis for dithizone versus saline or Li2CO3 buffer, each at the P < 0.01 level) (7).
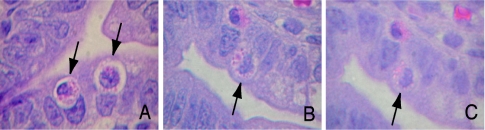
FIG. 1.
The appearance of Paneth cells in the ileal crypts 24 h after newborn rats received either intraperitoneal dithizone (75 mg/kg) in 25 mM lithium carbonate buffer or L2CO3 buffer alone. Photomicrographs are ileal sections stained with hematoxylin and eosin (magnification, ×1,000). The arrows identify individual Paneth cells. Paneth cells were examined for the content and the nature of their eosinophilic granules by using a Lickert score of 1 to 3, with 3 being best. (A) Crypt with two Paneth cells in a rat pup treated with i.p. dithizone; the Paneth cell was assigned a score of 2, while the Paneth cell on the right had a score of 1. (B) Paneth cell of a pup given i.p. L2CO3 buffer; this Paneth cell was given a score of 3. (C) The same Paneth cell as in panel B, except the incident light has been changed to show the autofluorescence of the cytoplasmic granules. Only epithelia with this autofluorescence were judged to be Paneth cells.
The histological appearance of the crypts and villi, however, did not reveal abnormalities among the three groups, and no inflammatory cell response in any group was apparent. The distribution of Paneth cells in crypts (i.e., none, 1, 2, etc.) did not differ among the three groups. The average numbers of mitotic figures per villous crypt were, for saline, 0.60 ± 0.05; for Li2CO3 buffer, 0.61 ± 0.07; and for dithizone, 0.63 ± 0.1 (P = 0.96). The distribution of mitotic figures in the crypts (i.e., none, 1, 2, etc.) also did not differ among the three groups.
A 25 to 30% reduction in Paneth cells after dithizone treatment of neonatal rats is consistent with studies in adult rats (16), but a quantitative assessment of Paneth-cell-related granules was not made in the adult. The altered characteristics of the granules in Paneth cells may have significance beyond that of simply diminished numbers of Paneth cells. An abnormal appearance of Paneth cell granules in human infants with necrotizing enterocolitis has been reported (4).
Moreover, the methods used in this study differ from reports in adult rats in that newborn rats received a lower dose of dithizone, namely, 75 mg/kg versus 100 mg/kg (16). Saturated Li2CO3 (>200 mM) was used to dissolve dithizone in adult studies, while a 10-fold lower concentration of Li2CO3 (25 mM) was used in neonatal studies. The dithizone was given to neonatal rats by i.p. injection, while adult rats received dithizone intravenously. These changes were aimed to avoid toxicity caused by dithizone administration. Achieving this goal is supported by an absence of illness in the pups and a lack of alterations in the histologic appearance of the villi and crypts compared to control animals. Furthermore, the mitotic activity of intestinal epithelia adjacent to dithizone-exposed Paneth cells was similar to the mitotic indices of epithelia in control pups, suggesting that epithelial cells adjacent to Paneth cells were not injured. This finding is also consistent with the in vitro treatment of the rat intestinal epithelial cell 6 line whose proliferation did not change in high concentrations of dithizone (17). Thus, a model is described here that uses dithizone to chemically ablate and alter the granular characteristics of newborn rat Paneth cells.
In the control neonatal pups, the number of Paneth cells identified per 100 crypts was <10% of that reported for adult rats (16). This low number of Paneth cells may be one reason why neonatal rats are the preferred animal model for studying the pathogenesis of human necrotizing enterocolitis (8). As described in the next series of experiments, the diminished numbers and the altered granules seen in the Paneth cells of dithizone-treated pups may explain why neonatal pups could not effectively clear a burden of pathogenic Escherichia coli from the small bowel.
Effect of dithizone on the antibacterial activity of neonatal small bowel.
For studies involving the intestinal clearance of E. coli, dithizone was given i.p. before the infection was induced in 4-day-old rat pups, and at least 3 litters and 24 newborn rats (8 pups per litter) were used for each experimental condition. Six hours after pups were given i.p. dithizone (25 mg/kg or 75 mg/kg) versus 25 mM Li2CO3 buffer or saline as controls, a dispersed suspension of E. coli (1 × 1012 CFU/kg of body weight) was inoculated into the stomach by using a catheter. Six hours was selected as the time to initiate infection because this is when a maximal reduction in Paneth cells occurs after dithizone administration in adult rats (16).
E. coli strain Ec5 was used to induce enteral infection because it is an isolate from a human preterm infant and has the following surface determinants associated with virulence, O18:K1:H7 (9). This strain has been used in past reports of enteral infection in neonatal rats and was prepared as described in those studies (6, 18).
Eighteen hours after infection, the number of deaths was recorded and an illness score was assigned in surviving pups (6). After euthanasia, the jejunum and ileum of the surviving pups were aseptically procured for bacteriologic studies. The weight and length of each bowel segment were measured to calculate bowel mass, and each segment was lavaged with sterile saline as previously reported (18). The recovered microliters of lavage fluid were measured, and the percent fractional recovery was calculated from the instilled volume. After the lavage procedure, the jejunum and ileum were separately homogenized in 1 ml of sterile saline. Jejunal and ileal lavage effluents and the homogenized jejunum and ileum were serially diluted in sterile saline, and quantitative cultures were performed using 5% sheep blood agar and MacConkey agar plates. After 48 h of incubation, the E. coli cells were enumerated. The numbers of E. coli cells in intestinal fluid were corrected for fractional recovery of the lavage effluent, and the numbers of E. coli cells in the lavage fluid and bowel wall homogenates were then standardized to bowel mass (18).
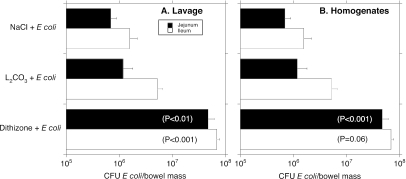
Figure 2 shows the numbers of E. coli cells recovered in the jejunal and ileal lavage fluid of newborn rats given a 75-mg/kg dose of dithizone. E. coli cells present in the small bowel fluid of dithizone-treated pups were significantly higher in numbers than those cultured in controls. This difference was not a result of a disparity in recovered lavage fluid, which ranged from 97 to 111% of the instilled lavage volume (P was not significant among the groups). Mucosa- and bowel-wall-associated numbers of E. coli, determined by cultures of gut wall homogenates, were also significantly higher in the jejuna of dithizone-treated newborn rats versus controls, but the numbers of E. coli cells in the ileal segments did not reach statistical significance among the three groups (P = 0.06 for dithizone versus controls).
FIG. 2.
Effect of dithizone treatment on the numbers of Escherichia coli cells in small bowel specimens recovered from newborn rats. The x axis shows the CFU of E. coli per bowel mass. The y axis identifies the control groups (saline [NaCl] and Li2CO3 buffer) and the treatment group (dithizone). (A) Findings in small bowel lavage fluid; (B) the results seen in small bowel homogenates. The internal legend (A) signifies which studies involve jejunum or ileum. The statistical significance for comparisons between the dithizone group and the saline and Li2CO3 buffer controls is shown within the bars.
Table 1 shows the death rate and illness scores of newborn rats given 75 mg/kg of i.p. dithizone and infected with enteral E. coli compared to the control groups. The higher death rate in dithizone-treated, E. coli-infected pups may be related to the postmortem observation that 25% of the ileal specimens had gross necrosis. The devitalized bowel may explain the lower numbers of E. coli in the ilea of dithizone-treated pups.
TABLE 1.
Effect of dithizone treatment followed by enteral infection with E. coli on the rate of death and illness in neonatal rats
| Parameter |
E. coli plus:
|
P | ||
|---|---|---|---|---|
| Saline | Li2CO3 | Dithizone | ||
| No. of deaths (% of pups) | 2 (8.3%) | 2 (8.3%) | 12 (37.5%) | <.001 |
| Mean and median illness scores | 0.3, 0 | 0.4, 0 | 2.1, 2 | <.001 |
| No. of pups and no. of litters studied | 24, 3 | 24, 3 | 32, 4 | Not significant |
Newborn rats given a lower dose (25 mg/kg) of dithizone before enteral infection with E. coli also had a 2- to 3-log increase of E. coli in the jejunal and ileal lavage fluids and in jejunal homogenates compared to the control groups (P < 0.01); however, their mortality rate (3 of 24 [12.5%]) and their illness scores were lower than those for E. coli-infected pups treated with 75 mg/kg of dithizone. None of the E. coli-infected pups given 25 mg/kg of dithizone had gross necrosis of the distal ileum.
Previous studies of fetal and neonatal Paneth cells in animals and humans have reported their anatomic and biochemical characteristics without a direct linkage to the killing of bacteria (2, 4, 5, 10, 14, 19). The findings reported here show that dithizone-induced alterations in the Paneth cells of newborn rat pups (Fig. 1) are associated with (i) a reduced ability of the small bowel to kill E. coli (Fig. 2) and (ii) an increased morbidity and mortality after infectious challenge of the small intestine (Table 1).
This study is consistent with reports showing that Paneth cells are an important antibacterial defense of the adult intestine. Matrilysin-deficient (MAT−/−) mice lack processed alpha-defensins in Paneth cells, and when MAT−/− mice are infected with intragastric E. coli, this bacterium survives in large numbers in the distal small bowel compared to similarly infected wild-type (MAT+/+) mice (20). Further compelling evidence for Paneth cell involvement in innate host defense is the protection of transgenic mice expressing human defensin 5 from gastrointestinal infection caused by Salmonella enterica serovar Typhimurium (15). Studying small bowel infections in neonatal mice with genetically engineered abnormalities in Paneth-cell-related antimicrobial proteins would be difficult because of their small size. Dithizone-treated newborn rats with generalized abnormalities in Paneth cells represent a new model for studying the function of their antimicrobial proteins in the neonatal small intestine.
In conclusion, compromised Paneth cell function is detrimental to host defense against E. coli infection in the neonatal small intestine. The results of this study, which used dithizone to deplete Paneth cell numbers and decrease their antimicrobial-rich granules, fully support this concept. We suggest that this new model may be useful in future studies of innate intestinal host defense relevant to newborn human infants.
Acknowledgments
This work was supported by a Children's Miracle Network grant to M.P.S. and Public Service grants HD046305 to M.P.S. and AI/DK50843 to C.L.B.
Editor: A. D. O'Brien
REFERENCES
- 1.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C Parkes, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 2.Bjerknes, M., and H. Cheng. 1981. The stem-cell zone of the small intestinal epithelium. II. Evidence from Paneth cells in the newborn mouse. Am. J. Anat. 160:65-75. [DOI] [PubMed] [Google Scholar]
- 3.Claud, E. C., and W. A. Walker. 2001. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 15:1398-1403. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho, H. B., H. C. da Mota, V. B. Coutinho, T. I. Robalinho, A. F. Furtado, E. Walker, G. King, Y. R. Mahida, H. F. Sewell, and D. Wakelin. 1998. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J. Clin. Pathol. 51:512-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darmoul, D., D. Brown, M. E. Selsted, and A. J. Ouellette. 1997. Cryptdin gene expression in developing mouse small intestine. Am. J. Physiol. 272:G197-G206. [DOI] [PubMed] [Google Scholar]
- 6.Edde, L., R. B. Hipolito, F. F. Y. Hwang, D. R. Headon, R. A. Shalwitz, and M. P. Sherman. 2001. Lactoferrin protects neonatal rats from gut-related systemic infection. Am. J. Physiol. Gastroenterol. Liver Physiol. 281:G1140-G1150. [DOI] [PubMed] [Google Scholar]
- 7.Glantz, S. A. 2002. Primer of biostatistics. CD-ROM version 5.0. McGraw-Hill, New York, N.Y.
- 8.Hsueh, W., M. S. Caplan, X. W. Qu, X. D. Tan, I. G. De Plaen, and F. Gonzalez-Cruzzi. 2003. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr. Dev. Pathol. 6:6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, K. S., H. Itabashi, P. Genski, J. Sadoff, R. L. Warren, and A. S. Cross. 1992. The K1 capsule is the critical determinant in the development of E. coli meningitis in the rat. J. Clin. Investig. 90:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallow, E. B., A. Harris, N. Salzman, J. P. Russell, R. J. DeBerardinis, E. Ruchelli, and C. L. Bevins. 1996. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 271:4038-4045. [DOI] [PubMed] [Google Scholar]
- 11.Porter, E. M., C. L. Bevins, D. Ghosh, and T. Ganz. 2002. The multifaceted Paneth cell. Cell. Mol. Life Sci. 59:156-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu, X.-D., K. C. Lloyd, J. H. Walsh, and R. I. Lehrer. 1996. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect. Immun. 64:5161-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio, C. A., and G. Nesi. 2003. A simple method to demonstrate normal and metaplastic Paneth cells in tissue sections. In Vivo 17:67-72. [PubMed] [Google Scholar]
- 14.Salzman, N., R. A. Polin, M. C. Harris, E. Ruchelli, A. Hebra, S. Zirin-Butler, A. Jawad, E. Porter Martin, and C. L. Bevins. 1998. Enteric defensin expression in necrotizing enterocolitis. Pediatr. Res. 44:20-26. [DOI] [PubMed] [Google Scholar]
- 15.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 16.Sawada, M., K. Takahashi, S. Sawada, and O. Midorikawa. 1991. Selective killing of Paneth cells by intravenous administration of dithizone in rats. Int. J. Exp. Pathol. 72:407-421. [PMC free article] [PubMed] [Google Scholar]
- 17.Seno, H., M. Sawada, H. Fukuzawa, Y. Morita-Fujisawa, S. Takaishi, H. Hiai, and T. Chiba. 2002. Involvement of tumor necrosis factor alpha in intestinal epithelial cell proliferation following Paneth cell destruction. Scand. J. Gastroenterol. 37:154-160. [DOI] [PubMed] [Google Scholar]
- 18.Sherman, M. P., S. H. Bennett, F. F. Y. Hwang, and C. Yu. 2004. Neonatal small bowel epithelia: enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals 17:285-289. [DOI] [PubMed] [Google Scholar]
- 19.Tan, X., W. Hsueh, and F. Gonzalez-Crussi. 1993. Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF expression by Paneth cells, intestinal eosinophils, and macrophages. Am. J. Pathol. 142:1858-1865. [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 21.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]