Abstract
Mycoplasma arthritidis induces toxicity, arthritis, and dermal necrosis in mice. Virulence factors include a superantigen and membrane adhesins and possibly also a bacteriophage component. Here we compare the biological properties of Triton X-114 extracts derived from avirulent and virulent M. arthritidis strains. Macrophage cell lines and resident peritoneal macrophages were used to assess inflammatory potential as indicated by production of tumor necrosis factor alpha, interleukin-6, and/or nitric oxide. The activity resided exclusively within the hydrophobic detergent phase, was unaffected by heat treatment at 100°C for 30 min, and was resistant to proteinase K digestion, suggesting involvement of a lipopeptide. Contamination of extracts with endotoxin or superantigen was excluded. Extracts of the more virulent strain had higher activity than did those of the avirulent strain. Using CHO cells expressing Toll-like receptor 2 (TLR2) or TLR4, both with transfected CD14, we showed that extracts activated these cells via TLR2 but not by TLR4. Also, macrophages from C57BL/6 TLR2−/− mice failed to respond to the extracts, whereas those from TLR2+/+ cells did respond. The preparations from the virulent strain of M. arthritidis were also more potent in activating dendritic cells, as evidenced by up-regulation of major histocompatibility complex class II, CD40, B7-1, and B7-2. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequent elution of gel slices revealed the presence of three active moieties which corresponded to molecular masses of approximately 24, 28, and 40 kDa. Three active components were also found by reverse-phase chromatography. We suggest that macrophage activation by M. arthritidis could play a significant role in the inflammatory response induced in the host by this organism.
Toll-like molecules are a group of ancient, highly conserved receptors which were originally discovered in Drosophila (2) but are now known to occur in mammals on the surfaces of cells of the innate immune system (27), where they recognize pathogen-associated molecular patterns. This early agent/Toll-like receptor (TLR) interaction stimulates innate immunity, offering rapid protection against infection as well as influencing the later adaptive immune response (26). At least 12 mammalian TLRs are now known, and one or more may be required for recognition of specific agonists (1, 4). The number of microbial products that are now known to directly interact with the innate immune system by signaling through TLRs has been steadily growing in the last few years; these products include lipopolysaccharides, lipoproteins, lipopeptides, peptidoglycan, heat shock proteins, CpG DNA, and single-stranded RNA (1, 13, 14, 39, 42, 49).
In the early 1990s, a macrophage-activating lipoprotein component was isolated from Mycoplasma fermentans (31, 60) and was subsequently characterized, and the active moiety, a lipopeptide, was identified, synthesized, and called macrophage-activating lipopeptide 2 (MALP-2) (32). In contrast to the bacterial lipoproteins which are triacylated and characteristically utilize TLR2 in association with TLR1 (43, 44), MALP-2 is diacylated and uses TLR2 in association with TLR6 (17). A related but distinct component was identified in M. hyorhinis (33), an agent of swine arthritis. A TLR2-utilizing component has also been detected in M. salivarium (18), an organism that has been associated with periodontal disease, as well as in M. hominis (M. R. Peltier et al., unpublished observations), which has been associated with human infections and reproductive disease. Although a number of these moieties are known to be potent macrophage activators, their precise roles in disease pathogenesis are largely unclear at this time due to the lack of good experimental animal models.
The M. arthritidis model of inflammatory disease has been extensively studied in our laboratories (6, 53) and that of others, resulting in the identification of animal strains that differ in their susceptibility to disease as well as the identification and derivation of organisms exhibiting differing degrees of virulence. Mycoplasma arthritidis is a natural pathogen of rodents that can induce an acute or chronic arthritis. Some mouse strains are also highly susceptible to lethal toxic shock (6, 30) and to a necrotizing fasciitis-like syndrome (6, 8). There is evidence that a superantigen, M. arthritidis mitogen (MAM), plays a role in these inflammatory diseases mediated by M. arthritidis, although by itself it does not produce clinical symptoms. We have, however, clearly shown that it is a potent immunomodulator in the murine host (29). There is evidence that the adhesins MAAl and MAA2 (55, 57) also play a role in virulence, since immunization of rats against these molecules protects against arthritis (56). Recently, M. arthritidis was found to harbor a virus, MAV-1 (51), which is now also thought to contribute to disease pathogenesis (48, 51).
In the present study, we searched for evidence that a cell-associated M. arthritidis component(s) other than MAM might contribute or act synergistically with MAM, resulting in the inflammatory syndromes induced by live M. arthritidis. We show here that M. arthritidis possesses a cell-associated moiety(ies), distinct from MAM, that directly activates macrophages through a pathway that is TLR2 dependent. The active moiety(ies) is also present at higher concentrations in virulent than in avirulent strains of M. arthritidis and can induce dendritic cell maturation with increased expression of major histocompatibility complex class II (MHC-II), CD40, B7-1, and B7-2 molecules.
MATERIALS AND METHODS
M. arthritidis strains.
Three M. arthritidis strains were used in the present study. First, strain 158 is a laboratory-maintained avirulent strain (11) that lacks the MAV-1 bacteriophage (51). Second, strain 158L3-1a was derived from 158 by transfection with MAV-1 and has been reported to exhibit increased arthritogenic potential in rodents (51). Third, strain 158p10p9 was derived from 158 by 10 successive passages through rats followed by 9 passages through mice (11); it is highly arthritogenic and toxigenic and possesses MAV-1 (51).
Mouse strains.
Female C57BL/6 (H-2b, Eα−, TLR2+/4+), C3H/HeJ (H-2k, Eα+, TLR2+/4−), C3H/HeSnJ (H-2k, Eα+, TLR2+/4+), and SJL (H-2S, Eα−, TLR2+/4+) mice were purchased from Jackson Laboratory (Bar Harbor, ME). C57BL/6 TLR2 knockout (KO) mice were obtained from Thomas Hawn by courtesy of S. Akira, Osaka University, Japan. Mice were maintained in specific-pathogen-free conditions at the Animal Resource Center (University of Utah Health Science Center) in compliance with the Animal Welfare Act and were used at 8 to 12 weeks of age.
Control stimulants.
Synthetic MALP-2 from Mycoplasma fermentans which utilizes TLR2 was initially received as a gift from Peter Muhlradt (German Research Centre for Biotechnology, Immunobiology Research Group, Braunschweig, Germany) or later purchased from Alexis Biochemicals (San Diego, CA). Lipopolysaccharide (LPS) from Escherichia coli O111:B4 was from DIFCO laboratory (Detroit, MI) and the lipid A moiety of LPS from Sigma (St. Louis, MO). Both LPS and lipid A use TLR4. The superantigen MAM, a product of M. arthritidis, was prepared as detailed previously (3).
TX-114 extraction of M. arthritidis.
M. arthritidis was grown in Difco PPLO broth with 15% heat-inactivated horse serum, 1% yeast extract solution (Gibco), 0.005 mg/ml NAD, 0.25% arginine HCl, and 500 U/ml penicillin G. Cultures were grown in lots of 2 to 10 liters on a shaker at 37°C for 48 to 72 h. Late-log-phase cultures were harvested by centrifugation at 10,000 rpm in a Beckman JA-10 rotor, and pellets were washed three times in LPS-free saline. After testing for bacterial contamination, the sediment from each 5 liters of culture was suspended in 10 ml LPS-free saline and treated overnight at 4°C with 200 μl Triton X-114 (TX-114). The mixture was warmed to 37°C, and aqueous and detergent phases were separated by centrifugation at room temperature. The upper aqueous phase was removed followed by the detergent phase, avoiding any contamination at the interface. Both phases were recentrifuged to remove particulate matter. Proteins were then precipitated with ice-cold acetone, air dried, and redissolved in 1 ml saline. Protein determination was done by using a BCA assay kit (Pierce, Rockford, IL) using bovine serum albumin as a standard. Aliquots were stored at −70°C. The aqueous and detergent phases derived from TX-114-treated saline served as negative controls.
Cell lines.
Murine RAW 264.7 macrophages and human monocytic THP-1 cells were from the American Type Culture Collection (Manassas, VA) and were maintained in Dulbecco modified Eagle medium (BioWhittaker) with 5% heated fetal bovine serum (FBS), 100 U penicillin/ml, and 100 U streptomycin/ml (Sigma Chemical Co.) at 37°C in 5% CO2.
To determine TLR usage, we employed Chinese hamster ovary (CHO) cells that were stably transfected with CD14 (a receptor for LPS) and ELAM-CD25 (a promoter and marker for Toll activation) and that expressed either TLR2 or TLR4 (24). The CHO cell lines were provided by Janice Weiss (Department of Pathology, University of Utah) courtesy of D. Golenbock (University of Massachusetts Medical Center, Worcester, MA). The cells were maintained in Ham's F-12 culture medium (BioWhittaker, Walkersville, Maine) supplemented with 10% heat-inactivated FBS (HIFBS) (HyClone, Logan, UT), 10 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin (BioWhittaker) at 37°C under a 5% CO2 in air atmosphere with humidity.
Activation of splenic cells, peritoneal macrophages, and cell lines.
Mouse spleens were harvested, and single-cell suspensions were prepared and suspended in complete RPMI medium containing 1% Nutridoma (Boehringer Mannheim, Indianapolis, IN) instead of FBS as previously described (29). Cells at 107/ml were treated with inducers for 24 h, and supernatants were assayed for interleukin-2 (IL-2) by enzyme-linked immunosorbent assay (eBiosciences, San Diego, CA). Resident adherent peritoneal cells were also collected as described previously (30) and suspended at 106 cells/ml in complete RPMI medium with 10% FBS. After stimulation, supernatants were collected at 18 h and assayed for tumor necrosis factor alpha (TNF-α) or IL-6. For nitric oxide (NO) production, cells were incubated for 48 h. Levels of NO (NO2− plus NO3−) were determined as described previously (30, 31). For activation of RAW 264.7 and THP-1 cells, suspensions were made at 5 × 105/ml in Dulbecco modified Eagle medium with 5% FBS, 100 U of penicillin/ml and 100 U of streptomycin/ml.
Dendritic cells.
The immature murine dendritic cell (DC) line XS52, derived from the epidermis of a newborn BALB/c mouse (61), was a gift from A. Takashima (The University of Texas Southwestern Medical Center, Dallas, Texas). It was maintained in XS complete medium composed of RPMI 1640 medium with 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, nonessential amino acids, 2-mercaptoethanol (all from Sigma), 10% HIFBS (Gibco-BRL, Gaithersburg, MD), 0.5 ng/ml of recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, Minn.), and culture supernatant (10% from the NS47 stromal cell line maintained in RPMI 1640-10% HIFBS) (25). XS52 cells were incubated for 24 h with GM-CSF (2 ng/ml) and IL-4 (2 ng/ml) and after extensive washing were exposed to the TX-114 extracts for an additional 48 h. Control cells received GM-CSF and IL-4 alone. Cells were then harvested and reacted with antibodies specific for MHC-II, CD40, B7-1, and B7-2 cell surface markers. Isotype-matched control antibodies were also used.
Antibodies.
Monoclonal anti-human TLR4 (clone HTA125) and TLR2 (clone TL2.1) and monoclonal anti-mouse TLR4 (clone MTS510) and TLR2 (clone 6C2) were purchased from eBiosciences. Anti-human CD14 (clone M5E2) and anti-mouse CD14 (clone rmC5-3) were from PharMingen (San Diego, CA). They were functional-grade purified for neutralization of biological function or fluorescence conjugated for flow cytometry. Isotype-matched antibodies to irrelevant antigens were used as controls.
Flow cytometry.
XS52 cells were suspended in 2% FBS and 1% glucose. Cells (106 in 100 μl) were stained with fluorescence-conjugated antibodies to CD40, B7-1, B7-2, or MHC-II or with control isotype-matched monoclonal antibodies on ice with 30-min incubations and thorough washes with phosphate-buffered saline (PBS) to remove unbound antibody. The cells were then centrifuged, washed, and resuspended in 0.2 ml of 1% paraformaldehyde PBS. For each sample, at least 10,000 cells were analyzed on a FACScan (Becton Dickinson, Mountain View, CA). Cells were gated according to size and scatter to eliminate dead cells and debris from analysis. Immunofluorescence histograms were analyzed by CELLQuest software (Becton Dickinson) to determine the percentage of positive cells and mean fluorescence intensity of TLR expression on adherent peritoneal cells.
Physical properties of TX-114 extracts.
Polymyxin B sulfate (Sigma-Aldrich, St. Louis, MO), a known inhibitor of LPS activity, was incubated at 10 μg/ml with TX-114 extracts for 1 h at 37°C, after which the reaction mixture was added to RAW 264.7 cells for assay of macrophage activation. Treatment with LPS (100 ng/ml) or lipid A (100 ng/ml) served as a positive control. Stability to heat was tested at 100°C for 5, 10, and 30 min.
Proteinase K (PK) treatment.
Extracts (1 μg/ml) were digested for 1 h at 37°C with soluble PK-5056 (SPK) at 5 μg/ml followed by heating for 15 min at 100°C to deactivate the enzyme. In a second approach, extracts were treated with PK-agarose beads (PKA), and after digestion, beads were removed by centrifugation. Digested extracts were assayed for TNF-α.
Fractionation of extracts by SDS-PAGE.
TX-114-extracted lipoproteins were analyzed by monocyte Western blotting as described previously (52). Briefly, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of lipoproteins obtained by TX-114 phase separation was performed in a 10% gel according to the method of Laemmli (23). The gel bands were electroeluted to a 0.45-μm-pore-size nitrocellulose membrane (Bio-Rad). Lanes were cut into 2-mm segments that were subsequently dissolved in 1-ml amounts of dimethyl sulfoxide. The lipoprotein-coated particles were formed by the addition of 3 ml sodium carbonate buffer to the melted membrane, washed three times with normal saline, and incubated with RAW 264.7 cell culture. TNF-α production was assayed as described above.
Reverse-phase chromatography.
In an alternative approach, the detergent phase of TX-114 extraction (0.5 ml) was loaded onto a 1-ml Resource 15 reverse-phase chromatography (RPC) column using high-performance liquid chromatography-grade water (Fisher Scientific) as the loading buffer. After washing with buffer, the column was eluted with a 0 to 100% fast protein liquid chromatography-grade isopropanol gradient over 15 min and 1-ml fractions were collected using an AKTA fast-performance liquid chromatography system (Amersham Biosciences). The fractions were lyophilized, redissolved in 0.2 ml normal saline (NS), and tested for macrophage activation as detected by TNF-α production by RAW 264.7 cells.
Statistical analysis.
Results are shown as means ± standard errors of the mean (SEM). The two-tailed Student t test was calculated using Statview (Abacus Concepts, Inc., Berkeley, CA) for cytokine levels and cell receptor expression. P values of less than 0.05 were considered significant. All experiments were repeated one or two times with similar outcomes.
RESULTS
Macrophage-activating ability of TX-114 extract of M. arthritidis is not due to contamination with MAM superantigen or LPS.
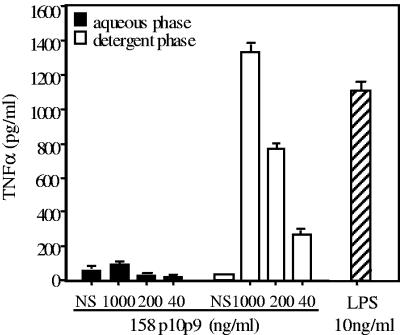
In a preliminary experiment, detergent and aqueous preparations resulting from TX-114 extraction of washed cells of virulent M. arthritidis 158p10p9 were tested for the ability to induce TNF-α and IL-6 after incubation with murine macrophage-like RAW 264.7 cells. Repeated extractions indicated that the detergent phase consistently exhibited the ability to induce TNF-α and did so in a dose-dependent manner. There was negligible activity in the aqueous phase, indicating that the active component(s) is hydrophobic (Fig. 1).
FIG. 1.
TX-114 extraction of M. arthritidis indicates activity is restricted to the detergent phase. Following detergent extraction of washed pellets of virulent strain 158p10p9 (see Materials and Methods), aqueous and detergent phases were assayed for induction of TNF-α by RAW 264.7 cells after 24 h. LPS-free NS was similarly treated with TX-114, and the phases were also tested. LPS (10 ng/ml) was a positive control. Mean values ± SEM of triplicate determinations are shown.
Mycoplasma arthritidis secretes a superantigen, MAM, which is an activator of T cells and macrophages (7, 38). Although LPS is not produced by M. arthritidis (40), it often occurs as a contaminant in the laboratory. Since both molecules have potent effects on immune cells, we needed to exclude their presence from our preparations. TX-114 extracts of M. arthritidis at 1.0 μg/ml failed to induce IL-2 in splenocytes from C3H/HeSnJ mice (<25 pg/ml), whereas the MAM used as a positive control induced high levels of IL-2 (2,200 pg/ml) at doses of 10 ng/ml. We also tested the responses of cells from the SJL mouse which is nonresponsive to MAM due to a lack of the required MHC alleles and T-cell receptors (9). SJL macrophages responded highly to the TX-114 extracts (4,400 pg/ml of IL-6 at 0.2 μg/ml) but failed to respond to MAM and the PBS control (<100 pg/ml; data not shown).
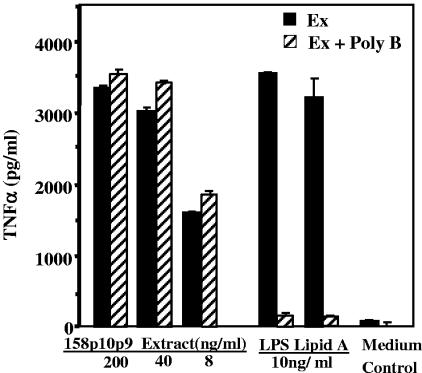
To test for potential LPS contamination, the extracts were examined for the ability to activate macrophages treated with polymyxin B, an inhibitor of LPS-mediated stimulation. Extract activity was not reduced by polymyxin B, even at low extract concentrations, whereas polymyxin B inhibited LPS and lipid A activity by about 95% (Fig. 2). We also compared the abilities of extracts and LPS to induce TNF-α in cultures of peritoneal macrophages from C3H/HeSnJ mice and in C3H/HeJ mice which carry the lpsd mutation, a point mutation in TLR4 that renders them hyporesponsive to LPS. C3H/HeSnJ macrophages produced high levels (>2,000 pg/ml) of IL-6 in response to both the 158p10p9 extract and LPS; although macrophages from C3H/HeJ mice responded similarly to the extract, they failed to respond to LPS (data not shown).
FIG. 2.
Effect of polymyxin B on M. arthritidis extracts. Polymyxin B (10 μg/ml final concentration) was incubated with extract (Ex) dilutions from virulent strain 158p10p9 at 200, 40, and 8 ng/ml for 1 h at 37°C prior to being added to RAW 264.7 cells for induction of TNF-α. LPS (100 ng/ml) and lipid A (100 ng/ml) were used as positive controls to show the inhibitory effects of polymyxin B on activation by these inducers.
Association of macrophage activation with M. arthritidis strain virulence.
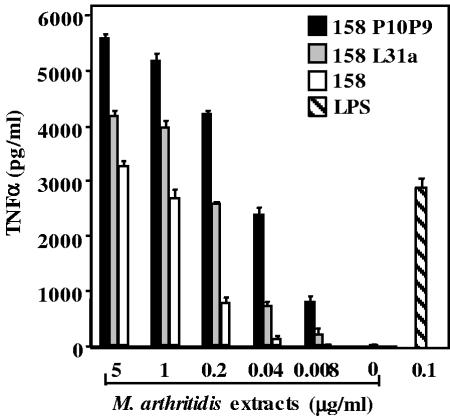
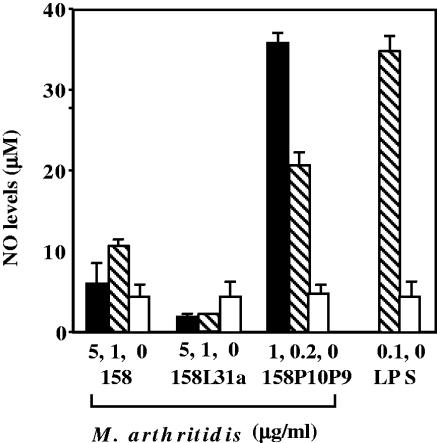
We next compared the activities present in the detergent phases from extracts of the avirulent 158 strain, the MAV-1-infected 158L3-1a strain, and the highly virulent 158p10p9 strain of M. arthritidis. Figure 3 shows that the TX-114 extract of the virulent 158p10p9 strain induced more TNF-α than did extracts from the avirulent 158 strain (P < 0.001) or from the virus-infected 158L3-1a strain (P < 0.002). Also, the 158L3-1a activity was significantly greater (P < 0.02) than that of the virus-free 158 strain. We also compared the abilities of the extracts to induce NO in supernatants of RAW 264.7 cells. Strain 158p10p9 induced high levels of NO, but extracts of strains 158 and 158L3-1a induced minimal or undetectable amounts (P < 0.01) (Fig. 4).
FIG. 3.
Comparative activities of TX-114 extracts of virulent versus avirulent M. arthritidis strains. The detergent phases from TX-114 extraction of avirulent strain 158, MAV-1-infected 158L3-1a, and highly virulent 158p10p9 were compared in a dose-response experiment for TNF-α production by RAW 264.7 cells. Results are expressed as means ± SEM (see text for P values). Three different preparations of strains 158 and 158p10p9 and two of 158L3-1a were tested with similar results.
FIG. 4.
Nitric oxide production by TX-114 extracts from virulent versus avirulent M. arthritidis strains. Extracts from strains 158, 158L3-1a, and 158p10p9 were incubated at various concentrations with RAW 264.7 cells for 48 h, and supernatant NO content was measured after conversion to nitrite and nitrate. Mean results from three experiments are shown (see text for P values).
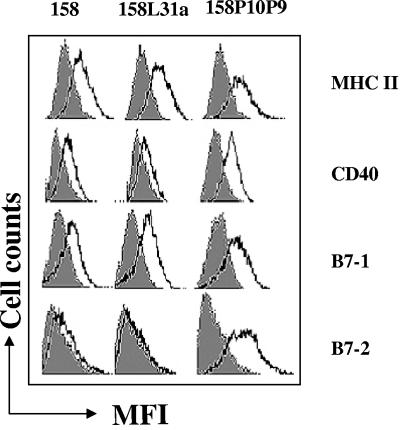
Maturation of XS52 DC by M. arthritidis components from virulent and avirulent strains.
A preliminary experiment established that as for macrophage activation, stimulation of XS52 DC with 158, 158L3-1a, and 158p10p9 extracts resulted in IL-6 production, with 158p10p9 being the most potent (data not shown). Since DC are now known to play a key role in both innate and adaptive immunity, we investigated whether the M. arthritidis preparations induced maturation of DC and up-regulation of the associated cell surface markers which impact on the later adaptive immune response. After treatment of XS52 cells with extract, the cells were reacted with antibodies against cell surface maturation markers or control antibodies and were examined by flow cytometry. All extracts up-regulated class II MHC and B7-1, with no significant differences between the various extracts (Fig. 5). However, levels of CD40 and B7-2 expression were significantly higher (P < 0.0001) in response to 158p10p9 than were the levels seen for 158 and 158L3-1a extracts. The combined results suggest either that the active moiety in the virulent 158p10p9 strain is present at a higher concentration or that the virulent strain contains components that are not present in the less virulent strains.
FIG. 5.
Activation of DC by extracts from virulent versus avirulent strains of M. arthritidis. XS52 cells were treated with 1 μg/ml of extracts from all three strains of M. arthritidis. After 24 h, cells were harvested and stained with fluorescein isothiocyanate-conjugated anti-class II MHC or anti-CD40 antibodies or with phycoerythrin-conjugated anti-B7-1 or -B7-2 antibodies (open histograms). Isotype-matched control antibodies (filled histograms) were included in all experiments (see Materials and Methods). Flow cytometry data from a representative experiment of three are shown. (See text for P values.)
Physical and enzymatic characterization of TX-114 extract activity.
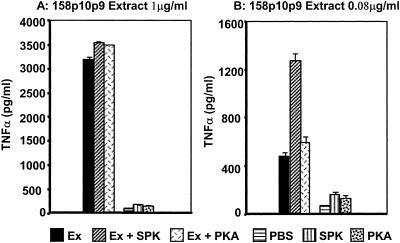
Heat treatment of extracts from all strains of M. arthritidis (1 to 5 μg/ml) at 100°C for up to 30 min either had no effect or slightly increased TNF-α production (data not shown); this contrasts with the heat lability of MAM (7). TX-114 extracts were also treated with two different forms of PK, SPK or PKA, prior to addition to RAW cells. Neither treatment decreased the activity of the extracts; in fact, at low extract concentrations (80 ng/ml) the soluble enzyme resulted in enhanced bioactivity. The enzyme preparations alone without extracts neither increased nor decreased background production of TNF-α by macrophages. The data using virulent strain 158p10p9 are shown in Fig. 6. The results suggest that as for the MALP-404 lipoprotein from M. fermentans, the intact protein moiety(ies) from M. arthritidis is not required for biological activity and may be analogous to MALP-2. To confirm digestion, we demonstrated that SDS-PAGE of proteinase-treated 158p10p9 extract failed to show intact protein bands. Recombinant MAM (22) similarly treated lost the protein band and all of the activity (not shown).
FIG. 6.
Effect of PK on activity of M. arthritidis 158p10p9 extracts. Extracts (Ex) at 1 μg/ml (A) or 0.08 μg/ml (B) were treated for 1 h at 37°C with 5 μg/ml SPK or 5 μg/ml agarose-bound (PKA) enzyme. The soluble enzyme was deactivated by 100°C heat or the agarose-bound enzyme was removed by centrifugation prior to assay for TNF-α on RAW 264.7 cells.
In vitro and in vivo activity of M. arthritidis TX-114 extracts is due to an interaction with TLR2.
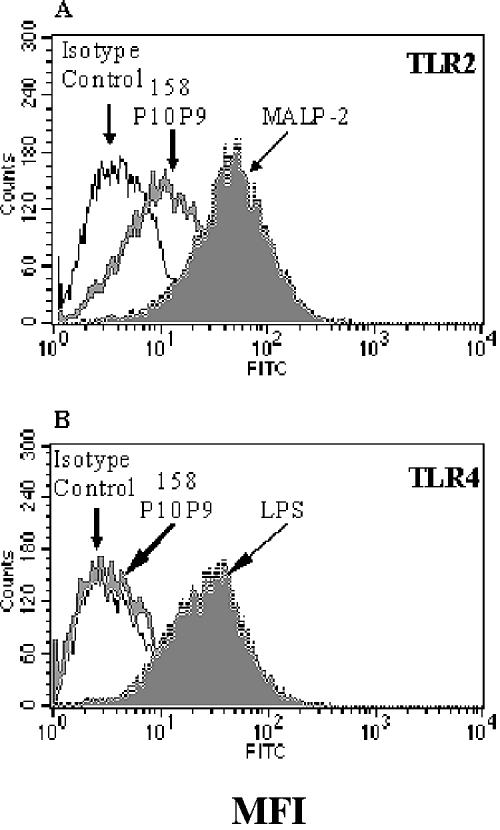
Microbial products which interact with and activate cells of the innate immune system characteristically do so via one or more Toll-like receptors. To test whether TLRs are involved in macrophage activation induced by virulent M. arthritidis, we first tested whether the extracts activated CHO cells transfected with either TLR2 or TLR4 in combination with CD14, a coreceptor for LPS; CD25 expression was used as a marker for TLR activation. The data (Fig. 7) show that CHO cells expressing TLR2 were activated following treatment with both MALP-2 and 158p10p9 extracts. In contrast, CHO cells expressing TLR4 were strongly activated by LPS but not by extracts of M. arthritidis. The MAM superantigen failed to activate either cell line (data not shown), presumably due to the absence of class II MHC, which is required for MAM binding to macrophages and other antigen-presenting cells.
FIG. 7.
Up-regulation of CD25 by CHO cells transfected with TLR2 or TLR4 in response to 158p10p9 extracts. CHO cells with the TLR2 or TLR4 transgenes together with CD14 were exposed to M. arthritidis 158p10p9 extracts for 18 h at 37°C. CD25 up-regulation, a measure of TLR activation, was quantitated by flow cytometry. MALP-2 was used as a positive control for activation of TLR2, and LPS was used as a control for activation of TLR4. FITC, fluorescein isothiocyanate.
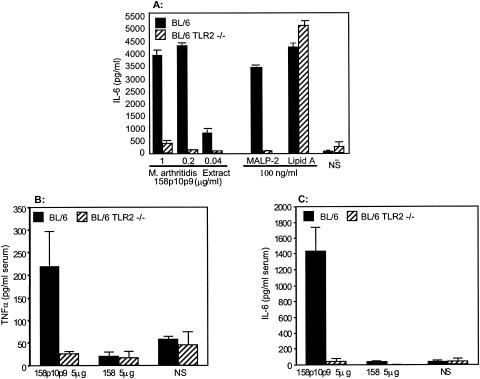
Next, we compared peritoneal macrophages from wild-type C57BL/6 mice (TLR2+/4+) with cells from C57BL/6 TLR2 KO mice (TLR2−/4+) for the ability to produce IL-6 in response to stimulation with TX-114 extracts, MALP-2, or lipid A. The results in Fig. 8A show that macrophages from C57BL/6 mice produced high levels of IL-6 in response to all inducers. In contrast, cells from mice lacking TLR2 but which express TLR4 failed to respond to the extracts and to MALP-2 but still responded well to lipid A (which utilizes TLR4). Similar findings were seen for TNF-α induction.
FIG. 8.
IL-6 production in vitro and in vivo induced by M. arthritidis extracts in macrophages from TLR2+/+ and TLR2−/− mice. (A) Macrophages from C57BL/6 and C57BL/6 TLR2 KO mice. Resident peritoneal macrophages were harvested from individual mice, and suspensions were treated in duplicate with various doses of 158p10p9 extracts, MALP-2 (100 ng/ml), lipid A (100 ng/ml), or NS alone. Supernatants were harvested at 18 h and assayed in triplicate for IL-6 production. Mean values ± SEM are shown. (B and C) Three mice each of strains C57BL/6 and C57BL/6 TLR2 KO received an intravenous injection of 158 or 158p10p9 extracts (5 μg/mouse) and were exsanguinated 90 min later, and the resulting sera were tested for (B) IL-6 and (C) TNF-α. Mean results from three mice with triplicate assays ± SEM are shown.
We next tested the ability of 158 versus 158p10p9 extracts to induce an early in vivo cytokine response and determined whether this also involved an interaction with TLR2. M. arthritidis 158p10p9 extracts induced TNF-α (Fig. 8B) and IL-6 (Fig. 8C) 90 min after intravenous injection (5 μg/mouse) into C57BL/6 mice, whereas mice that received injections of 158 extracts failed to evoke a serum cytokine response. Neither extract induced cytokines in sera from C57BL/6 TLR2 KO mice. The results again indicate that the more virulent M. arthritidis strain exhibits a greater ability to induce an inflammatory response in vitro and in vivo than do the less virulent strains and that this in vivo response is also dependent upon TLR2.
Role of CD14 in macrophage activation.
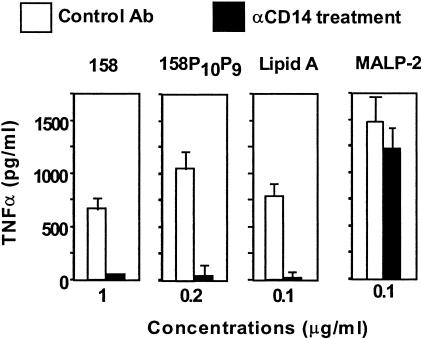
Some microbial macrophage activators, such as LPS, require CD14, which is believed to function as a coreceptor together with TLR molecules (5). To determine whether CD14 is required for macrophage activation by M. arthritidis extracts, we examined the effect of antibody against CD14 on TNF-α production by THP-1 monocytic cells. The data in Fig. 9 show that anti-human CD14 antibody totally inhibits TNF-α induction by both 158 and 158p10p9 extracts in comparison with the activities seen with cells treated with an isotype-matched control antibody. As expected, lipid A was also inhibited. In contrast, the activity of MALP-2 was unaffected by treatment with anti-CD14 antibody.
FIG. 9.
Role of CD14 in macrophage activation by M. arthritidis 158p10p9 TX-114 extracts. THP-1 cells were pretreated with anti-human CD14 (10 μg/ml) for 1 h at 37°C, after which extracts at 1 and 0.2 μg/ml (final concentration) were added. After incubation for a further 18 h at 37°C, culture supernatants were harvested and analyzed for TNF-α. Lipid A and MALP-2, both at 0.1 μg/ml, were also tested. Results are means ± SEM.
Partial characterization of macrophage-activating components.
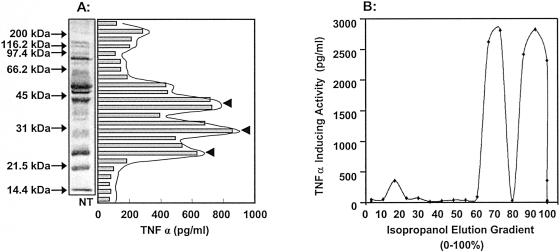
Preliminary studies were initiated to purify the macrophage-activating moieties present in M. arthritidis cells. Proteins extracted by TX-114 were separated by SDS-PAGE and blotted to nitrocellulose, and eluted strips were tested for the ability to induce TNF-α on RAW 264.7 cells. There were three bands of activity within strain 158p10p9 extracts corresponding to molecular mass ranges of 22 to 25 kDa, 25 to 32 kDa, and 36 to 43 kDa (Fig. 10A). The alternative approach of reverse-phase chromatography was also used, employing a 15 RPC column with a gradient of 0 to 100% isopropanol. Assay of fractions again revealed three peaks of TNF-α-inducing activity, and the two most active peaks occurred under highly hydrophobic conditions (Fig. 10B).
FIG. 10.
(A) Activity of TX-114 detergent extracts separated by SDS-PAGE. After PAGE, (lipo)proteins were transferred to a nitrocellulose membrane, and after extraction of 2-mm strips in dimethyl sulfoxide followed by precipitation with sodium carbonate buffer, they were assayed for induction of TNF-α production by RAW 264.7 cells. A region of the gel which contained no extract was also transferred and used as a negative control (NT). There were at least three potent macrophage-activating components (arrowheads). (B) Reverse-phase gradient chromatography of M. arthritidis 158p10p9 extracts. Detergent-phase extracts from TX-114 fractionation were loaded onto a 15 RPC column and eluted with a 0 to 100% isopropanol gradient. Fractions were assayed for TNF-α production by RAW 264.7 cells. Duplicate suspensions were assayed for each fraction, and triplicate wells were tested during the enzyme-linked immunosorbent assay.
DISCUSSION
The present studies have demonstrated that Mycoplasma arthritidis possesses a TLR2-dependent, cell-associated moiety(ies) that is a potent activator of macrophages and DC and that extracts from an animal-passaged virulent strain are more potent in inducing macrophage and DC activation than are extracts from a laboratory-maintained avirulent strain. We documented that the activity was not due to contamination with either LPS or the soluble M. arthritidis-derived superantigen MAM, which are both potent activators of macrophages (6, 37), with LPS utilizing TLR4 (5) and MAM utilizing TLR4 and TLR2 (28).
Our first goal was to determine whether macrophage activation was associated with strain virulence, since this property might play a role in disease pathogenesis. Significantly higher levels of TNF-α were induced by extracts from the virulent 158p10p9 strain of M. arthritidis than were induced by the avirulent 158 strain. Interestingly, extracts from the virus-infected 158L3-1a strain were more potent than those from the nonlysogenized 158 parent strain, possibly explaining the increased arthritogenicity of the 158L3-1a lysogen (48, 51). The 158p10p9 strain also possesses MAV-1 (51, 54), which it presumably acquired during animal passage, perhaps by selective replacement of strain 158 with a preexisting more virulent MAV-1-positive strain. However, 158p10p9 extracts were still more potent than those from 158L3-1a, and 158p10p9 is much more arthritogenic and toxic in mice than is 158L3-1a (B. C. Cole et al., unpublished data). This would suggest the involvement of other virulence factors, possibly the well-characterized MAA1 or MAA2 adhesins (55, 57) or the MAM superantigen (6), or a better ability of the organism to survive in the host. Importantly, strain 158p10p9 extracts exhibited other properties not seen in those from strains 158 or 158L3-1a, namely, in vitro induction of NO, in vivo production of serum TNF-α, and a differential effect on DC, all of which might impact on host responses. The role of these macrophage-activating, cell-bound components in M. arthritidis-mediated disease remains to be established.
Dendritic cells, now known to be important in development of innate immunity and in directing the subsequent adaptive immune response (19, 35), were also activated to various degrees by M. arthritidis extracts. All preparations induced up-regulation of class II MHC and B7-1 expression, both of which are essential for antigen recognition and development of immunity (50). In contrast, strain 158p10p9 was more effective in up-regulating CD40, a molecule that is known to be associated with production of IL-12 (20), which in turn promotes inflammatory Th1 responses (41). The up-regulation of B7-2, a promoter of Th2 cytokines and protective immunity, may be due to the origin of the XS52 DC, which was the BALB/c mouse (61) that preferentially produces type 2 cytokines (12) and which is known to be relatively resistant to M. arthritidis-induced disease (10, 29).
In the past few years it has become apparent that microbial and endogenous interactions of ligands with innate immunity via diverse TLRs play a key role in driving signaling pathways that lead to inflammation (21, 36), that protect against infectious disease (59), and that are now believed to be associated with autoimmunity (34, 47). In particular, microbial LPS can interact with either TLR2 or TLR4, depending upon its bacterial origin (15), and a substantial number of microbial lipoproteins which interact with TLR2 have been described (1). We have shown here that macrophage activation by M. arthritidis is mediated exclusively through an interaction with TLR2 by demonstrating the total failure of macrophages from C57BL/6 TLR2 KO mice to respond to the extracts while still responding normally to the lipid A component of LPS.
Our preliminary studies to characterize the active component(s) in M. arthritidis using TX-114 extraction indicated that the activity separated solely to the detergent phase, and SDS-PAGE indicated the presence of three active extractable bands. Reverse-phase chromatography indicated two active components that were strongly hydrophobic. The finding that activity was not decreased by heat treatment or by digestion with PK indicated lipopeptides or glycolipids might be involved. More recent studies, however, indicate that a lipid is involved, since the activity of PK-treated extracts separated by SDS-PAGE comigrates to a distinct Coomassie blue-positive band that runs just above the dye front (A. Hasebe et al., unpublished observations). The component(s) responsible may well be analogous to the diacylated MALP-2 lipopeptide (32) derived from the MALP-404 lipoprotein (60) that was isolated from Mycoplasma fermentans. Both MALP-2 (45, 58) and the M. arthritidis moiety(ies) activate both macrophages and DC by a TLR2-dependent mechanism. However, there is evidence that these entities might be significantly different; whereas the M. arthritidis component(s) was found to be dependent upon CD14, a coreceptor for LPS (5), we also showed that MALP-2 was independent of CD14. Recent studies by Hoebe et al. (16) have demonstrated that the diacylated MALP-2 is dependent upon CD36 as a facilitator or coreceptor for activation through TLR2/TLR6, therefore serving a function analogous to that of CD14. Clearly, work is now required to determine whether the M. arthritidis lipopeptide(s) is diacylated and requires CD36 or whether CD14 is required. Also to be addressed is whether M. arthritidis requires a complex of TLR2 and TLR6, as for MALP-2 (17) and other bacterial diacylated lipopeptides (43, 46), or an association with TLR1, as for triacylated lipopeptides. These potential differences in coreceptor usage may result in differential signaling pathways and thus elicit different responses in the host.
Although the MALP-2 lipopeptide from M. fermentans has been well studied in terms of its physical structure and its interaction with macrophages, its role in disease is questionable at this time due to the lack of a suitable animal model. For this reason, our finding that M. arthritidis, a naturally occurring, well-characterized pathogen of mice and rats, also possesses a macrophage-activating component(s) which utilizes TLR2 provides a very useful model to determine the role of TLR2-ligand interactions in mycoplasma-mediated inflammatory disease. The mechanisms of pathogenesis of M. arthritidis-mediated inflammatory disease are no less complex than those of many other microbial diseases (6, 53). In addition to the TLR2-dependent macrophage-activating moiety found here, we recently showed that the MAM superantigen not only displays typically superantigenic interactions with specific MHC molecules and T-cell receptors (7) but also uniquely activates macrophages through an interaction with both TLR2 and TLR4. In addition, the observed down-regulation of TLR2 function by the MAM/TLR4 interaction potentially provides a homeostatic anti-inflammatory pathway (28). Studies are now required to determine whether the macrophage-activating, cell-associated components might be related to the adhesins and the MAV-1 phage or whether they may work in concert with the adhesins as well as the MAM superantigen to promote the various disease manifestations of this organism in the murine host. Fortunately, mouse strains which exhibit differing responses to these potential virulence factors, and different M. arthritidis strains of various degrees of virulence and differentially possessing these factors, are available for this work.
Acknowledgments
This work was supported by a grant from the Nora Eccles Treadwell Foundation and by National Institutes of Health grant AR02255. B.C.C. is the recipient of the Nora Eccles Harrison Chair in Rheumatology.
We thank Michael Taylor and Chris Li for excellent technical assistance. We thank A. Takashima (The University of Texas Southwestern Medical Center, Dallas, Texas) for the generous gift of XS52 dendritic cells and D. Golenbock (University of Massachusetts Medical School, Worcester, MA) for the TLR2- and TLR4-transfected CHO cells. C57BL/6 TLR2 KO mice were received by permission of Shizuo Akira (Research Institute for Microbial Diseases, Osaka University, Japan) and by courtesy of Thomas Hawn.
Editor: J. L. Flynn
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. V., G. Jurgens, and C. Nusslein-Volhard. 1985. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42:779-789. [DOI] [PubMed] [Google Scholar]
- 3.Atkin, C. L., S. Wei, and B. C. Cole. 1994. The Mycoplasma arthritidis superantigen MAM: purification and identification of an active peptide. Infect. Immun. 62:5367-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257-263. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Cole, B., A. Sawitzke, and H. Mu. 2000. Mycoplasma arthritidis and its superantigen M. arthritidis mitogen as a model for inflammatory and autoimmune disease, p. 93-107. In M. W. Cunningham and R. S. Fujinami (ed.), Effects of microbes on the immune system. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 7.Cole, B. C., and C. L. Atkin. 1991. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol. Today 12:271-276. [DOI] [PubMed] [Google Scholar]
- 8.Cole, B. C., M. W. Piepkorn, and E. C. Wright. 1985. Influence of genes of the major histocompatibility complex on ulcerative dermal necrosis induced in mice by Mycoplasma arthritidis. J. Investig. Dermatol. 85:357-361. [DOI] [PubMed] [Google Scholar]
- 9.Cole, B. C., A. D. Sawitzke, E. A. Ahmed, C. L. Atkin, and C. S. David. 1997. Allelic polymorphisms at the H-2A and HLA-DQ loci influence the response of murine lymphocytes to the Mycoplasma arthritidis superantigen MAM. Infect. Immun. 65:4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, B. C., J. R. Ward, and L. Golightly-Rowland. 1973. Factors influencing the susceptibility of mice to Mycoplasma arthritidis. Infect. Immun. 7:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golightly-Rowland, L., B. C. Cole, J. R. Ward, and B. B. Wiley. 1970. Effect of animal passage on arthritogenic and biological properties of Mycoplasma arthritidis. Infect. Immun. 1:538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guler, M. L., N. G. Jacobson, U. Gubler, and K. M. Murphy. 1997. T cell genetic background determines maintenance of IL-12 signaling: effects on BALB/c and B10.D2 T helper cell type 1 phenotype development. J. Immunol. 159:1767-1774. [PubMed] [Google Scholar]
- 13.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 15.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoebe, K., P. Georgel, S. Rutschmann, X. Du, S. Mudd, K. Crozat, S. Sovath, L. Shamel, T. Hartung, U. Zahringer, and B. Beutler. 2005. CD36 is a sensor of diacylglycerides. Nature 433:523-527. [DOI] [PubMed] [Google Scholar]
- 17.Into, T., K. Kiura, M. Yasuda, H. Kataoka, N. Inoue, A. Hasebe, K. Takeda, S. Akira, and K. Shibata. 2004. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappa B activation. Cell. Microbiol. 6:187-199. [DOI] [PubMed] [Google Scholar]
- 18.Into, T., Y. Nodasaka, A. Hasebe, T. Okuzawa, J. Nakamura, N. Ohata, and K. Shibata. 2002. Mycoplasmal lipoproteins induce toll-like receptor 2- and caspases-mediated cell death in lymphocytes and monocytes. Microbiol. Immunol. 46:265-276. [DOI] [PubMed] [Google Scholar]
- 19.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 20.Kelsall, B. L., E. Stuber, M. Neurath, and W. Strober. 1996. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann. N. Y. Acad. Sci. 795:116-126. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J., M. T. Ochoa, S. R. Krutzik, O. Takeuchi, S. Uematsu, A. J. Legaspi, H. D. Brightbill, D. Holland, W. J. Cunliffe, S. Akira, P. A. Sieling, P. J. Godowski, and R. L. Modlin. 2002. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 169:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudtson, K. L., M. Manohar, D. E. Joyner, E. A. Ahmed, and B. C. Cole. 1997. Expression of the superantigen Mycoplasma arthritidis mitogen in Escherichia coli and characterization of the recombinant protein. Infect. Immun. 65:4965-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 25.Lu, M., M. Zhang, R. L. Kitchens, S. Fosmire, A. Takashima, and R. S. Munford. 2003. Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J. Exp. Med. 197:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 28.Mu, H. H., N. D. Pennock, J. Humphreys, C. J. Kirschning, and B. C. Cole. 2005. Engagement of Toll-like receptors by mycoplasmal superantigen: downregulation of TLR2 by MAM/TLR4 interaction. Cell. Microbiol. 7:789-797. [DOI] [PubMed] [Google Scholar]
- 29.Mu, H.-H., A. D. Sawitzke, and B. C. Cole. 2000. Modulation of cytokine profiles by the mycoplasma superantigen Mycoplasma arthritidis mitogen parallels susceptibility to arthritis induced by M. arthritidis. Infect. Immun. 68:1142-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu, H.-H., A. D. Sawitzke, and B. C. Cole. 2001. Presence of Lpsd mutation influences cytokine regulation in vivo by the Mycoplasma arthritidis mitogen superantigen and lethal toxicity in mice infected with M. arthritidis. Infect. Immun. 69:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlradt, P. F., and M. Frisch. 1994. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect. Immun. 62:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1998. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun. 66:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasare, C., and R. Medzhitov. 2003. Toll-like receptors: balancing host resistance with immune tolerance. Curr. Opin. Immunol. 15:677-682. [DOI] [PubMed] [Google Scholar]
- 35.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 36.Revets, H., G. Pynaert, J. Grooten, and P. De Baetselier. 2005. Lipoprotein I, a TLR2/4 ligand modulates Th2-driven allergic immune responses. J. Immunol. 174:1097-1103. [DOI] [PubMed] [Google Scholar]
- 37.Rink, L., and H. Kirchner. 1992. Mycoplasma arthritidis-derived superantigen. Chem. Immunol. 55:137-145. [PubMed] [Google Scholar]
- 38.Rink, L., W. Nicklas, J. Luhm, R. Kruse, and H. Kirchner. 1996. Induction of a proinflammatory cytokine network by Mycoplasma arthritidis-derived superantigen (MAS). J. Interferon Cytokine Res. 16:861-868. [DOI] [PubMed] [Google Scholar]
- 39.Sieling, P. A., and R. L. Modlin. 2001. Activation of toll-like receptors by microbial lipoproteins. Scand. J. Infect. Dis. 33:97-100. [DOI] [PubMed] [Google Scholar]
- 40.Smith, P. F. 1984. Lipoglycans from mycoplasmas. Crit. Rev. Microbiol. 11:157-186. [DOI] [PubMed] [Google Scholar]
- 41.Stuber, E., W. Strober, and M. Neurath. 1996. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J. Exp. Med. 183:693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 43.Takeda, K., O. Takeuchi, and S. Akira. 2002. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin Res. 8:459-463. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, O., and S. Akira. 2001. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 1:625-635. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 47.Toubi, E., and Y. Shoenfeld. 2004. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity 37:183-188. [DOI] [PubMed] [Google Scholar]
- 48.Tu, A. H., J. R. Lindsey, T. R. Schoeb, A. Elgavish, H. Yu, and K. Dybvig. 2002. Role of bacteriophage MAV1 as a mycoplasmal virulence factor for the development of arthritis in mice and rats. J. Infect. Dis. 186:432-435. [DOI] [PubMed] [Google Scholar]
- 49.Vabulas, R. M., H. Wagner, and H. Schild. 2002. Heat shock proteins as ligands of toll-like receptors. Curr. Top. Microbiol. Immunol. 270:169-184. [DOI] [PubMed] [Google Scholar]
- 50.van der Merwe, P. A., and S. J. Davis. 2003. Molecular interactions mediating T cell antigen recognition. Annu. Rev. Immunol. 21:659-684. [DOI] [PubMed] [Google Scholar]
- 51.Voelker, L., K. Weaver, L. Ehle, and L. Washburn. 1995. Association of lysogenic bacteriophage MAV1 with virulence of Mycoplasma arthritidis. Infect. Immun. 63:4016-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallis, R. S., M. Amir-Tahmasseb, and J. J. Ellner. 1990. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc. Natl. Acad. Sci. USA 87:3348-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Washburn, L., and B. Cole. 2002. Mycoplasma arthritidis pathogenicity: membrane, MAM and MAV1, p. 473-489. In S. Razin and R. Herrmann (ed.), Molecular biology and pathogenicity of mycoplasmas. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 54.Washburn, L. R., E. J. Miller, S. Mukherjee, and D. Dannenbring. 2004. Mycoplasma arthritidis bacteriophage MAV1 prophage integration, deletions, and strain-related polymorphisms. Plasmid 52:31-47. [DOI] [PubMed] [Google Scholar]
- 55.Washburn, L. R., E. J. Miller, and K. E. Weaver. 2000. Molecular characterization of Mycoplasma arthritidis membrane lipoprotein MAA1. Infect. Immun. 68:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Washburn, L. R., and E. J. Weaver. 1997. Protection of rats against Mycoplasma arthritidis-induced arthritis by active and passive immunizations with two surface antigens. Clin. Diagn. Lab. Immunol. 4:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Washburn, L. R., K. E. Weaver, E. J. Weaver, W. Donelan, and S. Al-Sheboul. 1998. Molecular characterization of Mycoplasma arthritidis variable surface protein MAA2. Infect. Immun. 66:2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weigt, H., P. F. Muhlradt, A. Emmendorffer, N. Krug, and A. Braun. 2003. Synthetic mycoplasma-derived lipopeptide MALP-2 induces maturation and function of dendritic cells. Immunobiology 207:223-233. [DOI] [PubMed] [Google Scholar]
- 59.Wetzler, L. M. 2003. The role of Toll-like receptor 2 in microbial disease and immunity. Vaccine 21(Suppl. 2):S55-S60. [DOI] [PubMed] [Google Scholar]
- 60.Wise, K. S., M. F. Kim, P. M. Theiss, and S. C. Lo. 1993. A family of strain-variant surface lipoproteins of Mycoplasma fermentans. Infect. Immun. 61:3327-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, S., K. Ariizumi, G. Caceres-Dittmar, D. Edelbaum, K. Hashimoto, P. R. Bergstresser, and A. Takashima. 1995. Successive generation of antigen-presenting, dendritic cell lines from murine epidermis. J. Immunol. 154:2697-2705. [PubMed] [Google Scholar]