Abstract
Interleukin-23 (IL-23) is a heterodimeric cytokine that shares IL-12 p40 but contains a unique p19 subunit similar to IL-12 p35. Previous studies indicate a greater importance for intact IL-12/23 p40 expression than IL-12 p35 for immunity against Mycobacterium tuberculosis, suggesting a role for IL-23 in host defense. The effects of IL-23 on the outcome of pulmonary infection with M. tuberculosis have not been described. Here, we show that local delivery of replication-defective adenovirus vectors encoding IL-23 (AdIL-23) greatly stimulated expression of both gamma interferon (IFN-γ) and IL-17 in lung tissues of otherwise normal mice. When given 72 h prior to infection with M. tuberculosis, AdIL-23 significantly reduced the bacterial burden at 14, 21, and 28 days. Markedly lower levels of lung inflammation were observed at 28 days than in control mice pretreated with control adenovirus (AdNull) or vehicle controls. AdIL-23 pretreatment resulted in increased numbers of CD4+ CD25+ activated T cells in lungs and draining lymph nodes compared to control groups and more CD4+ T cells bearing surface memory markers in lung lymph nodes. IL-23 gene delivery also significantly enhanced host anti-mycobacterial T-cell responses, as shown by elevated levels of IFN-γ and IL-17 secreted in vitro following restimulation with M. tuberculosis purified protein derivative. Overall, our data show that transient IL-23 gene delivery in the lung is well tolerated, and they provide the initial demonstration that this factor controls mycobacterial growth while augmenting early pulmonary T-cell immunity.
The resurgence of Mycobacterium tuberculosis infection linked to the human immunodeficiency virus epidemic highlights the importance of cellular immunity in controlling the growth of this pathogen. Effective host defense against pulmonary infection with M. tuberculosis requires the coordinated actions of both the innate and adaptive immune systems (10). Interleukin-12 (IL-12) is well-established as a cytokine released by antigen-presenting cells early in M. tuberculosis infection, and this cytokine is critical for the generation of a Th1 polarized adaptive immune response and subsequent host defenses. Indeed, the exogenous administration of IL-12 augments M. tuberculosis clearance (6, 11, 25). IL-23 has recently been identified as a member of the IL-12 cytokine family and may also play a role in host defense against this pathogen. IL-23 is a heterodimer that shares an identical p40 subunit with IL-12 but contains a unique p19 chain that closely resembles IL-12 p35 (26). Secreted by dendritic cells and other antigen-presenting cells, IL-23 stimulates the production of gamma interferon (IFN-γ) by activated/memory CD45RO+ T cells but not naïve CD45RA+ cells. In contrast, IL-12 elicits IFN-γ from both subsets. IL-23 also induces the proliferation of activated/memory T cells but not naïve T cells. IL-12 and IL-23 share binding affinity for the IL-12Rβ1 subunit; however, IL-23 binds to a distinct IL-23 receptor (IL-23R), whereas IL-12 utilizes IL-12Rβ2 as its coreceptor (27).
The comparative roles of IL-12 and IL-23 in host defense against a variety of infections are actively under investigation. Through Toll-like receptor 4 signaling, IL-23, but not IL-12, has been shown to play a critical role in stimulating T-cell release of IL-17 following infection with Klebsiella pneumoniae (15), while specific roles for IL-23 in immune defense against the intracellular bacteria Salmonella enteritidis and Francisella tularensis have also been found (9, 21). In regard to M. tuberculosis infections, recent studies have demonstrated a greater sensitivity in IL-12/23 p40−/− mice than in IL-12 p35−/− animals (5, 17). Moreover, macrophages rapidly express IL-23 when exposed to mycobacterial antigens, suggesting an immune-stimulatory role for this cytokine during infection (4, 33).
The evidence of a role for IL-23 in the development of immunity against several intracellular pathogens, together with increasing interest in the use of biologic response modifiers to treat disease, led us to study the effects of localized, transient expression of IL-23 during early pulmonary M. tuberculosis infection. In this report, we show that local IL-23 gene delivery using a replication-defective adenovirus vector (AdIL-23) resulted in markedly decreased mycobacterial burden in the lungs up to 28 days after infection. AdIL-23 treatment significantly decreased lung inflammation in infected animals despite increasing numbers of activated CD4+ T cells in lungs and draining lymph nodes. Cultures of T cells from draining lymph nodes of AdIL-23-treated mice showed enhanced anti-mycobacterial responses to purified protein derivative (PPD).
MATERIALS AND METHODS
Vector construction.
RNA from mouse splenocytes was used to synthesize cDNA with gene-specific primers for IL-23 p19 and IL-12/23 p40. The 5′ primer used to generate p19 included Kozak and ATG start sequences for efficient translation initiation. With the assistance of J. Williman (University of Otago, Otago, New Zealand), an encephalomyocarditis virus internal ribosome entry site was ligated between the subunits to form a bicistronic IL-23 construct. This was ligated into plasmid pENTR (Invitrogen) and then shuttled in an exchange reaction using LR Clonase into pAd/CMV/V5 DEST (Invitrogen). The resultant plasmid was purified, and its sequence was confirmed, and then it was transfected into the 293A producer cell line (ViraPower System; Invitrogen) that supplies the E1 proteins necessary to generate adenovirus. AdNull was constructed similarly but without IL-23 genes in pENTR. Purified Ad stocks were derived and purified according to the manufacturer's instructions and titrated by quantitative real-time PCR. The capacity of AdIL-23 to secrete biologically active IL-23 was confirmed by (i) real-time one-step reverse transcription and PCR (RT-PCR) of infected 293A cell monolayers for p19 mRNA, (ii) Western blotting of infected 293A cell lysates using a polyclonal rabbit anti-murine p19 antibody, and (iii) bioassay of cell supernatants overlying virus-infected 293A cell monolayers for the ability to stimulate IL-17 production by naïve mouse splenocytes.
Mice.
Specific-pathogen-free C57BL/6 mice were used at 6 to 8 weeks of age (National Cancer Institute, Frederick, MD). All mice were housed in a biosafety level 3 facility at the Louisiana State University Health Sciences Center and treated in accordance with institutional guidelines. The mice were provided with water and food ad libitum and subjected to 12-h light/dark cycles. All procedures were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee.
Mycobacteriology.
The virulent M. tuberculosis strain H37Rv was obtained from the American Type Culture Collection (Rockville, MD; catalog no. 27294) and was grown in Middlebrook 7H11 broth at 37°C for 14 days. This culture was concentrated by centrifugation, gently sonicated at 95 W for 10 seconds in a cup-horn sonicator, and stored in 0.1-ml aliquots at −80°C. At the time of inoculation, an aliquot was thawed, gently sonicated, and diluted in endotoxin-free phosphate-buffered saline (PBS) to a concentration of 102 organisms/ml. The lung mycobacterial burden at the time of animal sacrifice was determined as follows. Lungs were homogenized, serially diluted, and plated in quadruplicate on Middlebrook 7H10 agar plates. The plates were incubated at 37°C for 17 days. At the end of the incubation period, the number of CFU present on plates containing between 20 and 200 CFU/quadrant was determined and multiplied by the appropriate dilution in order to determine the number of CFU in the initial tissue homogenate. The M. tuberculosis organism burdens in treatment groups were then compared.
Vector delivery and M. tuberculosis infection model.
Mice were anesthetized by intraperitoneal injection of ketamine-xylazine. The neck was opened in sterile fashion, and the trachea was isolated by blunt dissection. After cannulation with a 30-gauge needle, 2 × 108 PFU of AdIL-23 or AdNull or 100 μl PBS vehicle control was injected under direct observation. The mice were allowed to recover and were returned to their cages for 72 h. They were then anesthetized again, and intratracheal injection of approximately 125 CFU of M. tuberculosis was performed in a volume of 100 μl PBS as described above.
Cell preparation and flow cytometry.
At 14, 21, and 28 days after infection, animals were sacrificed by administration of an overdose of ketamine-xylazine followed by opening of the chest cavity. The lungs were removed, finely minced, and incubated at 37°C with 1 mg/ml type I collagenase and 30 μg/ml type I DNase (Worthington Biochemical Corp, Lakewood, NJ) for 90 min on a rotating shaker. Lung-associated lymph node (LALN) cells were obtained by visual dissection and passage through a 40-μm nylon filter. Lung digests and LALN cells were then passed through a 70-μm nylon mesh filter and centrifuged at 400 × g, and the red cells were lysed using NH4Cl. The cells were washed twice with PBS, counted on a hemacytometer, and stained with monoclonal antibodies (clone in parentheses) recognizing CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD25 (PC61.5), CD44 (IM7), and CD62L (MEL-14) (Pharmingen and eBioscience, San Diego, CA). Flow cytometry was performed on the FACScalibur device (BD Biosciences, San Jose, CA) using isotype controls to assist in gating.
Cell culture and immunoassays.
Aliquots of cells from LALN digests (obtained as described above) and isolated splenocytes were resuspended in complete medium (RPMI 1640 [Gibco/Invitrogen, Carlsbad, CA] supplemented with 1 mM sodium pyruvate, 10 mM HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin, 200 mM l-glutamine, 50 μM 2-mercaptoethanol, and 10% fetal calf serum). For IFN-γ enzyme-linked immunospot (ELISPOT) assays, 2 × 105 spleen or LALN cells from each of the three treatment groups (AdIL-23, AdNull, and PBS) were plated in triplicate onto 96-well MultiScreen-IP sterile plates (Millipore, Billerica, MA) which had been coated overnight at 4°C with 5 μg/ml IFN-γ capture antibody AN18 (Mabtech, Mariemont, OH) in PBS. The cells were incubated at 37°C in a 5% CO2 incubator for 22 h with medium (negative control) or 20 μg/ml M. tuberculosis H37Rv PPD (Mycos Research, Loveland, CO). After culture, the plates were washed five times with PBS, the detection antibody R4-6A2 (Mabtech) was added at 1 μg/ml, and the plates were incubated for 2 h at room temperature. The plates were washed five times and incubated with streptavidin-alkaline phosphatase (Mabetch), 1/1,000 dilution in PBS-0.5% fetal calf serum, for 1 h. The plates were washed five more times and incubated with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium substrate solution (Moss Inc., Pasadena, MD) for 20 min. The color reaction was stopped by rinsing the plates with tap water, and the plates were air dried overnight before being read with the AID-ELISPOT counter (AutoImmun Diagnostika GmbH, Strasburg, Germany). For assay of IFN-γ and IL-17 secretion, 2 × 105 LALN cells were plated in triplicate into 48-well plates and cultured with complete medium (negative control) or 20 μg/ml PPD for 72 h. The supernatants were harvested for assay using the Bio-Plex cytokine bead array system (Bio-Rad, Hercules, CA), which has an approximate sensitivity of 10 pg/ml.
Histology.
Lungs were fixed by inflation with 10% formalin to visual total capacity and embedded in paraffin. Lung sections were mounted onto slides and stained with hematoxylin and eosin. The lung sections were inspected with the aid of an eyepiece grid, in which each square encompassed approximately 1 mm2 of tissue at ×100 magnification. The field was judged to be normal or abnormal. An average of 200 fields were evaluated in sections from each mouse. A histological score for each lung was determined according to the following criteria: 0, no lung abnormality; 1, presence of inflammation and granulomata involving 10% of the lung parenchyma; 2, lesions involving 10 to 30% of the lung; 3, lesions involving 30 to 50% of the lung; and 4, lesions involving >50% of the lung as described elsewhere (14). The slides were evaluated by an experienced microscopist without knowledge of the treatment group assignment. Photomicrographs of representative sections were taken.
Real-time RT-PCR.
Total RNA from lung homogenates was isolated using the RNeasy minikit (QIAGEN, Valencia, CA). Total RNA (10 ng) was subjected to RT-PCR using TaqMan linear hydrolysis chemistry on the iCycler thermocycler (Bio-Rad, Hercules, CA). Gene-specific primers and dual-labeled probe sequences for IL-23 p19, IL-17, and 18S rRNA were designed using Beacon Designer 2.12 (Premier Biosoft International, Palo Alto, CA) as follows (primer, primer, probe): IL-17, GCTCCAGAAGGCCCTCAGA, CTTTCCCTCCGCATTGACA, and ACCTCAACCGTTCCACGTCAC; IL-23 p19, TGGCTGTGCCTAGGAGTAGCA, TTCATCCTCTTCTTCTCTTAGTAGATTCATA, and CTCTGCATGCTAGCCTGGAAC; 18S rRNA, ATTCGAACGTCTGCCCTATCA, GTCACCCGTGGTCACCATG, and TCGATGGTAGTCGCCGTGCCTACC. IFN-γ mRNA was assayed using SYBR Green dye and the following gene-specific primers: TCAGCAACAGCAAGGCGAAA and CCGCTTCCTGAGGCTGGAT. A melt curve analysis was performed after all SYBR runs to ensure single PCR product formation, and the expected IFN-γ amplicon size was confirmed by gel electrophoresis of the PCR product. All samples were normalized to the 18S rRNA content. Data are expressed as transcript copy numbers per ng of 18S rRNA, using cRNA standards as a reference.
Statistical analysis.
Comparisons of lung bacterial burdens between treatment groups were made using analysis of variance, with a follow-up Fisher's protected least significant difference test. Differences between treatment groups for ELISPOT and cytokine assays were determined using Student's t test. Significance was accepted at a P value of <0.05.
RESULTS
Expression of IL-23 p19, IL-17, and IFN-γ mRNAs in lungs following local administration of recombinant adenovirus vectors.
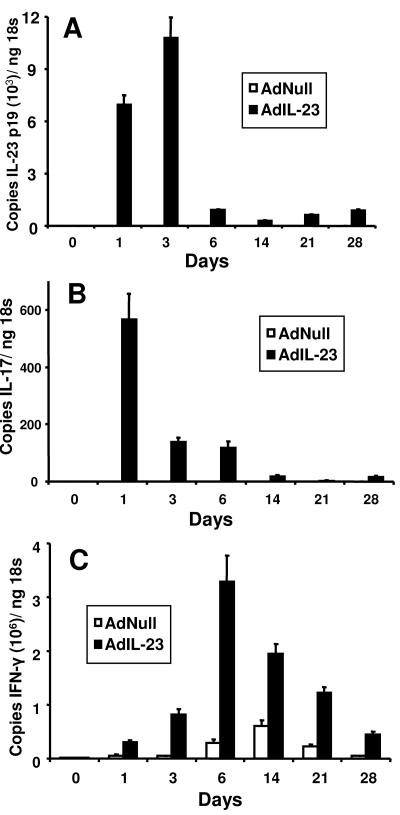
Our initial experiments were performed in naïve mice in order to determine cytokine secretion kinetics following intratracheal delivery of AdIL-23 and also the safety of this approach. A dose of 2 × 108 PFU was chosen, since pulmonary delivery of a similar dose of AdIL-12 has been reported to be effective in a different model of pulmonary bacterial infection (12). Our pilot studies indicated that this dose of AdIL-23 did not cause significant pulmonary inflammation or observable side effects in inoculated mice. IL-23 p19 mRNA was significantly up-regulated in lung homogenates taken 1 day following vector administration and reached peak levels on day 3 (Fig. 1A). Control adenovirus (AdNull) delivery did not induce IL-23 p19 mRNA, suggesting the IL-23 p19 mRNA detected in lungs of AdIL-23-treated animals was the result of IL-23 gene delivery rather than a primary host immune response to the virus. Since IL-23 induces IL-17 expression by T cells (1, 15), we also sought to determine if AdIL-23 delivery could induce pulmonary IL-17 expression. IL-17 mRNA expression was observed in AdIL-23- but not AdNull-treated animals (Fig. 1B). The kinetics of IL-17 induction in these animals resembled that of p19 mRNA, suggesting that airway delivery of IL-23 rapidly stimulates a lung T-cell IL-17 response, and this was confirmed by measurement of IL-17 protein in lung homogenates (data not shown). Since IL-23 stimulates human natural killer and memory T-cell expression of IFN-γ, we next determined the ability of AdIL-23 treatment to induce IFN-γ in the lungs. IFN-γ mRNA induction peaked on day 6 following AdIL-23 delivery but differed from IL-17 expression in that IFN-γ transcripts persisted to day 28 (Fig. 1C). AdNull control virus induced much lower levels of IFN-γ, which most likely represents a primary immune response to the Ad vector. These data show that local IL-23 gene delivery in replication-defective Ad vectors induces short-term IL-17 production and more sustained local secretion of IFN-γ in the lungs. Both AdIL-23 and AdNull vectors were well tolerated in all mice, with no outward signs of ill health and similar mild airway inflammation induced by both constructs.
FIG. 1.
Lung cytokine expression following pulmonary inoculation with 2 × 108 PFU replication-incompetent adenovirus encoding IL-23 (AdIL-23) or control vector (AdNull). Whole lung tissue (A) IL-23 p19, (B) IL-17, and (C) IFN-γ mRNA expression are shown. n = 4 to 6 mice per group. The error bars indicate 1 standard deviation.
Pulmonary M. tuberculosis burden following IL-23 gene delivery.
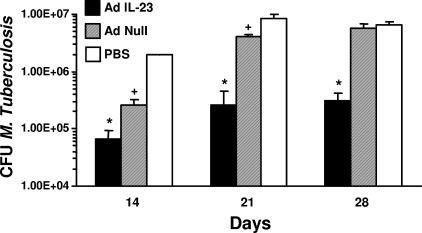
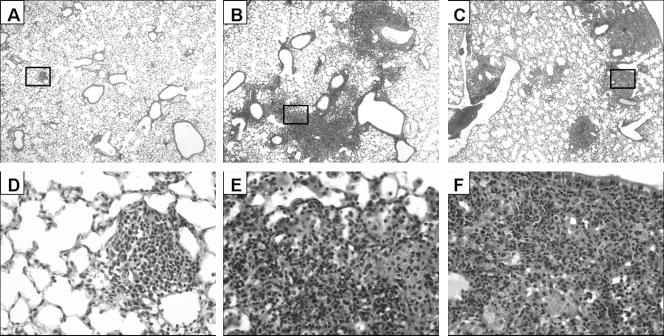
Having established the safety and efficacy of Ad-mediated delivery of IL-23 to the lung, we then sought to determine the effects of transient IL-23 overexpression on the outcome of pulmonary infection with M. tuberculosis. Mice were given intratracheal injections of 2 × 108 PFU AdIL-23, AdNull, or sterile PBS 72 h prior to mycobacterial infection. Pretreatment with AdIL-23 resulted in a 30-fold reduction in the recovery of viable mycobacteria 14 days postinfection, compared to an 8-fold reduction in mice given AdNull (Fig. 2). The effects of IL-23 gene delivery were even more apparent by 21 days postinfection, at which time AdIL-23-treated animals maintained a 30-fold reduction in bacterial burden compared to the PBS control group, while AdNull pretreatment was associated with only a 2-fold decrease in CFU. A 20-fold reduction in bacterial recovery relative to controls was still apparent at 28 days postinfection, by which time AdNull pretreatment appeared to have no inhibitory effect on bacterial growth. At each of these time points, lungs were harvested for histological assessment (Fig. 3). There was minimal inflammation until day 28, at which time evidence of a granulomatous response was seen in the AdNull (Fig. 3B and E; average histologic score, 3) and PBS (Fig. 3C and F; average score, 3) control groups, with a far less intense response seen in animals pretreated with AdIL-23 (Fig. 3A and D; average score, 0.5). This granulomatous inflammation predominantly consisted of monocytic phagocytes and lymphocytes, with smaller numbers of plasma cells and neutrophils. Fibrosis and hyperplastic changes were minimal. The cellular constituency of inflammatory infiltrates seen in the lungs of AdIL-23-pretreated animals was similar to that in controls but these were minimal, involving markedly less lung tissue. Thus, pulmonary IL-23 gene delivery prior to infection with M. tuberculosis markedly inhibits local bacterial growth and inflammation.
FIG. 2.
M. tuberculosis burden in whole lung tissue homogenates 14, 21, and 28 days after intratracheal infection with 125 CFU virulent bacteria. * and + indicate significant differences (P < 0.05) versus other groups at that time point. The data are representative of two separate infectivity experiments. n = 4 mice per group. The error bars indicate 1 standard deviation.
FIG. 3.
Lung histology 28 days following M. tuberculosis infection. Sections of representative fields are shown at ×40 and ×400 magnifications from mice pretreated with AdIL-23 (A and D, respectively), AdNull (B and E, respectively), or PBS (C and F, respectively).
T-cell activation following pulmonary M. tuberculosis infection.
CD4+ T-cell activation, defined as surface expression of the low-affinity IL-2 receptor (CD25), was also measured in both lung and LALN cell isolates 14 and 28 days after mycobacterial infection. Pooled lung and LALN samples from animals pretreated with AdIL-23 had sharply increased numbers of CD25+ CD4 T cells on day 14 post-M. tuberculosis infection (Table 1). These differences were no longer evident by 28 days.
TABLE 1.
Activated CD25+ CD4+ T-cell numbers (105) and percentages after M. tuberculosis infection
| Treatment | No. (%) T cells in:a
|
|||
|---|---|---|---|---|
| Lung
|
LALN
|
|||
| Day 14 | Day 28 | Day 14 | Day 28 | |
| AdIL-23 | 7.90 (4.5) | 3.02 (2.2) | 5.86 (10.7) | 3.68 (11.0) |
| AdNull | 2.23 (2.1) | 1.74 (1.5) | 1.75 (11.8) | 3.79 (6.3) |
| PBS | 0.89 (1.3) | 2.98 (0.7) | 1.81 (8.3) | 2.37 (7.1) |
Lung and lung associated lymph node (LALN) cells were isolated 14 and 28 days after intratracheal M. tuberculosis infection. Absolute CD25+ CD4+ T-cell numbers and percentages of total CD4+ T cells were determined by fluorescence-activated cell sorting. The data represent pooled cell populations harvested from three or four animals per treatment group per time point.
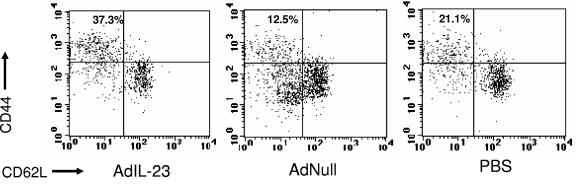
Since IL-23 may preferentially stimulate the expansion of memory T cells (26), we examined LALN isolates for differences in CD4+ T-cell populations bearing surface memory markers (CD44bright/CD62Ldim) 21 days after M. tuberculosis infection. This was the earliest time point at which we observed differences in bacterial burdens of at least 1 log10 unit between IL-23-treated animals and our two control groups. A greater percentage of LALN CD4+ T cells stained CD44bright/CD62Ldim in the AdIL-23 group (37.3%) than in the AdNull and PBS treatment groups (12.5% and 21.1%, respectively) (Fig. 4). In terms of absolute cell counts, greater numbers of CD44bright/CD62Ldim CD4+ T cells were found in LALN cells of AdIL-23-treated animals (3.9 × 106) compared to AdNull- (1.2 × 106) and PBS-treated (0.9 × 106) groups.
FIG. 4.
CD4+ T cells bearing surface memory markers in LALN 21 days after infection with M. tuberculosis. The results shown were obtained from pooled LALN cells from AdIL-23, AdNull, and PBS treatment groups. n = 4 mice per treatment group.
Effects of IL-23 gene delivery on local and systemic M. tuberculosis-specific T-cell responses.
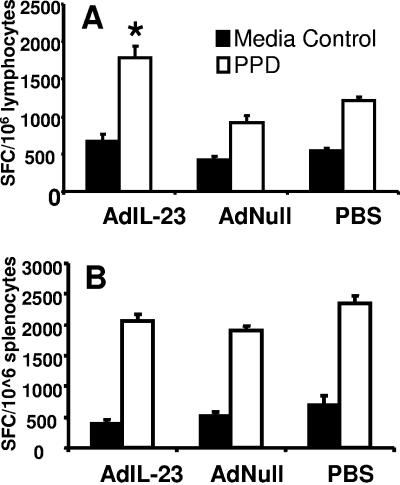
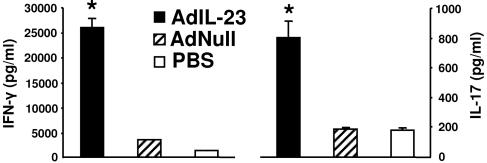
Frequencies of mycobacterium-specific pulmonary and systemic T cells were determined by ELISPOT assay of PPD-stimulated LALN and spleen cells harvested from mice 28 days after mycobacterial infection. As expected, mice in all groups produced IFN-γ in response to PPD stimulation. However, as shown in Fig. 5A, AdIL-23 gene delivery significantly (P < 0.05) enhanced the number of PPD-specific IFN-γ spot-forming cells (SFC) in pulmonary lymph nodes of M. tuberculosis infected mice compared to AdNull- and PBS-treated control animals. Similar enhancement of PPD-specific IFN-γ SFC responses following pulmonary AdIL-23 treatment was not seen in the spleen (Fig. 5B), indicating that pulmonary IL-23 gene delivery enhances the local, but not the systemic, antigen-specific T-cell response to the pathogen. Assay of culture supernatants from LALN cells confirmed the increased expression of M. tuberculosis-specific IFN-γ, and also IL-17, by LALN cells taken from AdIL-23-treated mice compared to control groups (Fig. 6).
FIG. 5.
IFN-γ ELISPOT responses from (A) LALN cells and (B) splenocytes harvested from PPD-stimulated LALN cells isolated from animals 28 days after M. tuberculosis infection. ELISPOT data are shown as mean SFC counts per 106 cells in triplicate cultures using LALN cells or splenocytes pooled within each treatment group. *, P < 0.05 versus AdNull and PBS groups. n = 4 mice per treatment group. Error bars indicate 1 standard deviation in triplicate cultures.
FIG. 6.
IFN-γ and IL-17 levels in supernatants from LALN cell cultures harvested 28 days after M. tuberculosis infection and restimulated in vitro with PPD. Cells from AdIL-23, AdNull, and PBS treatment groups at four mice per group were pooled prior to culture, and cytokines were measured in triplicate using the BioPlex system. *, P < 0.05 versus AdNull and PBS groups. The error bars indicate 1 standard deviation.
DISCUSSION
We set out to determine the effects of local IL-23 gene delivery on the outcome of pulmonary infection with M. tuberculosis in a murine model. This cytokine is a member of the IL-12 cytokine family with specific activity on activated/memory T cells and may therefore have a role in immune defense against intracellular pathogens. Initially, we found that IL-23 gene transfer to naïve mice via replication-defective adenovirus vectors was apparently safe and led to high local levels of expression of both IFN-γ and IL-17. When given 72 h prior to infection with M. tuberculosis, AdIL-23 significantly reduced the bacterial burden for at least 28 days and resulted in markedly less lung inflammation than in control groups. IL-23-treated animals had increased numbers of CD4+ CD25+ activated T cells and CD4+ CD44bright/CD62Ldim T cells, particularly in lymph nodes draining the lung. Local, but not systemic, antimycobacterial T-cell responses were significantly enhanced by IL-23 gene delivery, as shown by elevated IFN-γ SFC numbers in LALN but not in spleen cells. Our data provide the initial demonstration that IL-23 expression controls the growth of M. tuberculosis while augmenting early pulmonary T-cell immunity.
It is now well established that induction of specific CD4+ Th1 cells capable of expressing IFN-γ is critical for resistance to tuberculosis. The precise mechanism(s) underlying the antimycobacterial effects of local IL-23 overexpression in our study are unclear, but the inhibition of bacterial growth following IL-23 gene transfer correlates with sharply increased numbers of activated CD4+ CD25+ pulmonary T cells and enhanced local Th1-type T-cell responses against M. tuberculosis, as shown in the IFN-γ ELISPOT assay. It is likely, therefore, that vector-directed IL-23 expression increases local T-cell numbers (potentially through increased cellular trafficking, local cellular expansion, and/or decreased apoptosis) and augments the production of IFN-γ, which subsequently enhances phagocyte function and microbial killing. It is noteworthy that AdNull pretreatment (which induces a low level of IFN-γ expression in uninfected mice [Fig. 1C]) also decreased the bacterial burden in the lungs 14 and 21 days after M. tuberculosis infection, although the protective effect was significantly less than that mediated by AdIL-23. Thus, the host IFN-γ response to the AdNull vector itself is likely to mediate this antimycobacterial effect. More sustained induction of IFN-γ in AdIL-23-treated mice could account for the control of mycobacterial growth in these animals. Recent in vivo studies have demonstrated physiologically important CD4+ T-cell IFN-γ responses following M. tuberculosis infection in the absence of IL-12, IL-18, and IL-23 activity, evidence of the complex regulatory mechanisms underlying IFN-γ production in response to this pathogen (18).
IL-23 could also facilitate control of mycobacterial growth in the lung via a direct stimulatory effect on phagocytic cells, since coexpression of IL-12Rβ1 and IL-23R on bone marrow-derived macrophages has been reported (27). Indeed, intraperitoneal administration of IL-23 has been shown to induce IFN-γ-independent expression of tumor necrosis factor alpha and IL-1β, cytokines instrumental in early clearance of mycobacteria (3, 8), by local macrophages (7).
The early stimulation of local IL-17 expression by vector-encoded IL-23 may also have contributed to host antimycobacterial activity in our system. Both CD4+ and CD8+ T cells respond to IL-23 stimulation by releasing IL-17, a factor that we and others have shown to induce neutrophilia (1, 15, 19). The IL-17 receptor has a wide tissue distribution, including epithelium, endothelium, and fibroblasts. Upon ligation, IL-17 stimulates induction of granulocyte colony-stimulating factor, IL-6, and the neutrophil chemotactic factors keratinocyte-derived chemokine and macrophage inflammatory protein 2 (1, 15, 19). Thus, IL-17 could potentially mediate antimycobacterial activity through increased nonspecific phagocytosis, as neutrophils have been shown to play both antibacterial and immune-modulatory roles in early defense against both tuberculosis and nontuberculous mycobacterial infections (2, 29). The fact that mycobacterial challenge stimulates IL-23 and IL-17 expression also suggests participation of this cytokine axis in the immune response to mycobacteria (5, 22).
The recent description of novel cytokines with structural homology to IL-12 has created interest in characterizing the functional roles of members of this IL-12 “cytokine family” (20, 32). Previous work has shown that the IL-12 p40 subunit, also shared with IL-23, is critical for development of protective immunity against mycobacterial infection (5, 17), while exogenous administration of recombinant IL-12 protein augments clearance of M. tuberculosis (6, 11, 25). Our present results clearly show that transient IL-23 overexpression also controls mycobacterial growth. Recent studies have also indicated specific roles for this factor in immune defense against other intracellular bacteria, including Salmonella and Francisella (9, 21). Given the similarities between IL-12 and IL-23, including the overlapping nature of their subunits and receptor utilization, it is possible that the biologic significance of IL-23 may, in some cases, have been mistakenly ascribed to IL-12, particularly in studies employing neutralizing strategies against, or animals deficient in, these shared components. For instance, IL-23, rather than IL-12, has recently been shown to be critical for pathogenesis of autoimmune inflammatory processes, including collagen-induced arthritis (24) and experimental autoimmune encephalitis (7), and for stimulating T-cell release of IL-17 following infection with Klebsiella pneumoniae (15). IL-27, another recently described member of this cytokine family, up-regulates expression of the IL-12Rβ2 coreceptor and thus T-cell responsiveness to IL-12 p70 signaling (23, 31). Interestingly, however, IL-27 appears to be a negative regulator of cellular responses, as its expression during M. tuberculosis infection appears to be detrimental to immune control of the bacterium (28).
In the present study, we performed AdIL-23 gene transfer 72 h prior to challenge with M. tuberculosis, since our focus was to study the effects of localized, transient expression of IL-23 during early pulmonary M. tuberculosis infection. Since many cases of tuberculosis are due to reactivation of latent infections, IL-23 may also be a useful biologic response modifier in the early treatment of reactivation disease to augment the expansion of memory T-cell populations. In this context, a clear distinction between the activities of IL-12 and IL-23 is that the latter has greater stimulatory properties on activated/memory T cells (26), and this is likely the result of differential receptor expression patterns for IL-12/IL-23 on naïve and memory T cells. Although both IL-12 and IL-23 require IL-12Rβ1 for binding, IL-23 signaling requires the unique IL-23R, while IL-12Rβ2 mediates IL-12 signal transduction (27). While IL-12Rβ1 expression occurs on both naïve and activated T cells, expression of IL-12Rβ2 is preferentially seen in naïve T cells, and IL-23R expression is seen only in activated/memory T cells. The fact that memory T cells are preferentially expanded during reactivation of tuberculosis infection advocates for further study of IL-23 in this setting (13).
Our study demonstrates that single-dose vector-mediated pulmonary IL-23 gene delivery was both safe and effective in controlling the growth of M. tuberculosis in the lungs. The localized delivery of this factor to the airways was clearly effective in stimulating antimycobacterial Th1-type T-cell responses in lung-associated lymph nodes and may have served to limit toxicity compared to systemic immunotherapy. Furthermore, it is noteworthy that IL-23, when delivered to the lung, does not appear to induce a significant local inflammatory response in uninfected mice. Indeed, our experience with cytokine gene therapy has shown that vector-mediated gene delivery results in localized expression of these factors with profound immunologic sequelae and minimal toxicity (16, 30). Further delineation of the role of IL-23 in stimulating antimycobacterial cellular immunity may advance the development of improved prophylaxis and therapies for tuberculosis.
Acknowledgments
We acknowledge the expert assistance of the Morphology and Imaging Core, Gene Therapy Program, LSU Health Sciences Center.
This work was supported by Public Health Service Grants K08AA15163 (K.I.H.), R01AA11760 (C.M.M.), R01AI58810 (A.J.R.), and P01HL076100 (A.J.R. and C.M.M.).
Editor: J. D. Clements
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelberg, R., A. G. Castro, J. Pedrosa, R. A. Silva, I. M. Orme, and P. Minoprio. 1994. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect. Immun. 62:3962-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begum, N. A., K. Ishii, M. Kurita-Taniguchi, M. Tanabe, M. Kobayashi, Y. Moriwaki, M. Matsumoto, Y. Fukumori, I. Azuma, K. Toyoshima, and T. Seya. 2004. Mycobacterium bovis BCG cell wall-specific differentially expressed genes identified by differential display and cDNA subtraction in human macrophages. Infect. Immun. 72:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, A. M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I. M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 168:1322-1327. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84:423-432. [PMC free article] [PubMed] [Google Scholar]
- 7.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744-748. [DOI] [PubMed] [Google Scholar]
- 8.Denis, M., and E. Ghadirian. 1994. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect. Immun. 62:457-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis 84:93-101. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 12.Greenberger, M. J., S. L. Kunkel, R. M. Strieter, N. W. Lukacs, J. Bramson, J. Gauldie, F. L. Graham, M. Hitt, J. M. Danforth, and T. J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 157:3006-3012. [PubMed] [Google Scholar]
- 13.Griffin, J. P., and I. M. Orme. 1994. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect. Immun. 62:1683-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudmundsson, G., and G. W. Hunninghake. 1997. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J. Clin. Investig. 99:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Happel, K. I., M. Zheng, E. Young, L. J. Quinton, E. Lockhart, A. J. Ramsay, J. E. Shellito, J. R. Schurr, G. J. Bagby, S. Nelson, and J. K. Kolls. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan, S. P., P. S. Foster, X. Tan, and A. J. Ramsay. 1998. Mucosal IL-12 gene delivery inhibits allergic airways disease and restores local antiviral immunity. Eur. J. Immunol. 28:413-423. [DOI] [PubMed] [Google Scholar]
- 17.Holscher, C., R. A. Atkinson, B. Arendse, N. Brown, E. Myburgh, G. Alber, and F. Brombacher. 2001. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 167:6957-6966. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami, K., Y. Kinjo, K. Uezu, K. Miyagi, T. Kinjo, S. Yara, Y. Koguchi, A. Miyazato, K. Shibuya, Y. Iwakura, K. Takeda, S. Akira, and A. Saito. 2004. Interferon-gamma production and host protective response against Mycobacterium tuberculosis in mice lacking both IL-12p40 and IL-18. Microbes Infect. 6:339-349. [DOI] [PubMed] [Google Scholar]
- 19.Kolls, J. K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21:467-476. [DOI] [PubMed] [Google Scholar]
- 20.Langrish, C. L., B. S. McKenzie, N. J. Wilson, M. R. de Waal, R. A. Kastelein, and D. J. Cua. 2004. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202:96-105. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann, J., S. Bellmann, C. Werner, R. Schroder, N. Schutze, and G. Alber. 2001. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J. Immunol. 167:5304-5315. [DOI] [PubMed] [Google Scholar]
- 22.Lenarczyk, A., J. Helsloot, K. Farmer, L. Peters, A. Sturgess, and B. Kirkham. 2000. Antigen-induced IL-17 response in the peripheral blood mononuclear cells (PBMC) of healthy controls. Clin. Exp. Immunol. 122:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas, S., N. Ghilardi, J. Li, and F. J. de Sauvage. 2003. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 100:15047-15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy, C. A., C. L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R. A. Kastelein, J. D. Sedgwick, and D. J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolt, D., and J. L. Flynn. 2004. Interleukin-12 therapy reduces the number of immune cells and pathology in lungs of mice infected with Mycobacterium tuberculosis. Infect. Immun. 72:2976-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 27.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K. P. Singh, F. Vega, W. To, J. Wagner, A. M. O'Farrell, T. McClanahan, S. Zurawski, C. Hannum, D. Gorman, D. M. Rennick, R. A. Kastelein, M. R. de Waal, and K. W. Moore. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699-5708. [DOI] [PubMed] [Google Scholar]
- 28.Pearl, J. E., S. A. Khader, A. Solache, L. Gilmartin, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2004. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J. Immunol. 173:7490-7496. [DOI] [PubMed] [Google Scholar]
- 29.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsay, A. J., S. J. Kent, R. A. Strugnell, A. Suhrbier, S. A. Thomson, and I. A. Ramshaw. 1999. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol. Rev. 171:27-44. [DOI] [PubMed] [Google Scholar]
- 31.Takeda, A., S. Hamano, A. Yamanaka, T. Hanada, T. Ishibashi, T. W. Mak, A. Yoshimura, and H. Yoshida. 2003. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 170:4886-4890. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri, G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19:641-644. [DOI] [PubMed] [Google Scholar]
- 33.Verreck, F. A., T. de Boer, D. M. Langenberg, M. A. Hoeve, M. Kramer, E. Vaisberg, R. Kastelein, A. Kolk, R. Waal-Malefyt, and T. H. Ottenhoff. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 101:4560-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]