Abstract
The calcium-activated protein phosphatase calcineurin plays a critical role in the virulence of Candida albicans. Previous studies demonstrated that calcineurin is not required for the yeast-hypha dimorphic transition, host cell adherence, or host cell injury, which are all established virulence attributes of this organism. Calcineurin is, however, essential for survival in serum and disseminated infection. Here we identify the component of serum that is toxic to calcineurin mutant cells. Proteins, peptides, lipids, and other hydrophobic components were all excluded as essential toxic elements. Upon testing of small molecules present in serum, we discovered that calcineurin protects cells from stress caused by the endogenous levels of calcium ions present in serum. These studies illustrate how calcineurin functions in a calcium homeostatic pathway that enables a common human commensal to survive passage through the hostile environment of the bloodstream to establish deep-seated infections and cause disease.
Calcineurin is a calcium-activated serine-threonine-specific protein phosphatase that is conserved from yeasts to mammals. This phosphatase is composed of a regulatory (B) and a catalytic (A) subunit that form an inactive AB heterodimeric complex; calcium-induced binding by calmodulin elicits conformation changes leading to enzyme activation (1, 23, 28, 43). An influx of calcium ions into the cytoplasm of eukaryotic cells from either the extracellular environment or intracellular stores leads to calmodulin and calcineurin activation (41). In fungal cells, this influx of Ca2+ is often triggered by diverse environmental stresses, such as temperature fluctuation, high extracellular ionic concentrations, and changes in extracellular pH. In Saccharomyces cerevisiae, calcineurin activation leads to downstream events that mediate cellular responses to stress and concomitantly reduce the concentration of Ca2+ within the cytoplasm to basal levels (14).
Recent studies reveal that calcineurin is essential for the virulence of two divergent pathogenic fungi, Cryptococcus neoformans and Candida albicans (2, 7, 38, 44). Although the phenotypes conferred by calcineurin inhibition or mutation share common features in the two fungi, the roles that this phosphatase fulfills in virulence differ. Calcineurin is essential for C. neoformans survival at mammalian physiological body temperatures (37°C and higher) (12, 38), and as a consequence, calcineurin mutants of this pathogen fail to produce lethal infections in animal models of pathogenesis. Calcineurin is not, however, required for the survival of C. albicans at elevated temperatures (7). Instead, C. albicans calcineurin is essential for survival in serum, and as a consequence, mutants lacking the calcineurin A or B subunit are compromised for survival in vivo.
Serum is the liquid component of blood that remains after clotting has occurred and fibrous, insoluble material has been removed. Serum is an undefined, complex medium composed of proteins, lipids, and small molecules, and the interaction of C. albicans with serum has been of long-standing interest in the fungal pathogenesis field. Early work suggested that serum had a lethal effect on C. albicans and that this activity was heat stable and dialyzable (30), but it was later demonstrated that the reduced numbers of CFU measured by these experiments were attributable to increased aggregation of Candida cells rather than to a lethal effect of serum (10). This serum-induced aggregation is related to serum stimulation of the yeast-to-hypha transition, and though the search for the switching stimulus in serum has been one of the most enduring questions in Candida pathogenesis (4, 17, 25, 36, 46), no obvious culprit has been definitively identified yet. Recent studies reveal that serum does become profoundly toxic to C. albicans when calcineurin is either inhibited or mutated (7, 44), findings reminiscent of the early C. albicans-serum work. The requirement of calcineurin for C. albicans survival in serum sparked our interest in searching for serum components that are toxic to calcineurin mutant cells. Here we describe experiments that reveal the component of serum toxic to C. albicans calcineurin mutants.
MATERIALS AND METHODS
Strains and media.
The C. albicans calcineurin mutant strain JRB64 (ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU1/cnb1::ARG4) (11), the complemented calcineurin mutant strain MCC85 (ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::CNB1-HIS1/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU1/cnb1::ARG4) (11), crz1 mutant strains OCC1.1 and OCC3.8 (ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG crz1::UAU1/crz1::ARG4) (40), crz2 mutant strains DAY686 and DAY697 (ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG crz2::UAU1/crz2::ARG4) (generous gifts from Dana Davis prior to publication), strain DAY185, marker-matched to the mutant and complemented strains (URA3/ura3Δ::λimm434 HIS1/his1::hisG ARG4/arg4::hisG) (15), and the wild-type reference strain SC5314 (18) were used in this study. Strains were grown in yeast extract-peptone-dextrose (YPD) rich medium prepared as previously described (45), fetal bovine serum (FBS), murine serum, porcine serum, or ovine serum (all sera were from Sigma). Fresh human serum collected from a healthy volunteer was also used as a growth medium for C. albicans strains. YPD and FBS media were supplemented with FeCl3, ZnSO4, CaCl2, 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and/or EGTA (all chemicals were from Sigma) at the concentrations indicated in the text.
Liquid growth assays.
For all growth experiments, wild-type, calcineurin mutant, or complemented mutant cells were grown overnight at 30°C in liquid YPD medium. Cells were pelleted, washed once in phosphate-buffered saline (PBS), and then counted with a hemocytometer. A total of 5 × 103 cells/ml of each strain were incubated in the indicated media and rotated on a roller drum or incubated in a 96-well culture dish (Corning Inc., NY) at 30°C for the times specified. Aliquots of the cultures were removed at the initial time point (time zero) as well as at the termination of the experiment, and CFU were determined following appropriate dilutions of samples. Fold population change was measured by dividing the CFU at the experimental time point by the CFU measured at the initial time point. Values above 1 indicate growth of the culture, values near 1 indicate culture stasis, and values below 1 indicate cell loss or cell death.
Probing serum proteins.
FBS was filtered through Centricon filters (Millipore) with 30-kDa, 10-kDa, and finally 3-kDa molecular mass exclusion sizes according to the manufacturer's instructions. The filtrates and retentates were tested for activity against the calcineurin mutant strain in a liquid growth assay following reconstitution of the samples to the original volume with sterilized, distilled water to achieve the original concentrations. The wild-type, calcineurin mutant, and complemented calcineurin mutant strains were tested in the liquid growth assay using boiled, clarified FBS to further assess serum protein activity against calcineurin mutant strains. FBS was heated to boiling temperature for 10 min in a double-boiler system to prevent the serum from scorching. Any insoluble denatured proteins were removed by centrifugation at 3,000 rpm in a Sorvall RT 6000D centrifuge. Following centrifugation, sterile distilled water was added to adjust the solution to the original concentration, and this boiled, clarified serum was tested for activity against a C. albicans calcineurin mutant strain. Finally, serum that had been incubated with 50 μg/ml proteinase K for 16 h at 30°C to digest serum proteins and peptides was tested for activity against the reference, calcineurin mutant, and complemented calcineurin mutant strains. Similarly treated YPD was used to control for any influence the active proteinase K might have on strain growth. Untreated serum and YPD were also incubated at 30°C for 16 h and used for comparison.
Lipids in serum.
To determine if lipids or other hydrophobic serum components contribute to lethal activity against calcineurin mutant strains of C. albicans, the aqueous layer of chloroform-extracted FBS was tested for toxicity. An equal volume of chloroform was added to FBS and vortexed. The FBS-chloroform solution was centrifuged for 15 min at 3,000 rpm in a Sorvall RT 6000D centrifuge, and the aqueous layer was removed and lyophilized. The dried aqueous phase of serum was reconstituted with water and then tested for activity against calcineurin mutant strains.
Ca2+ concentration in serum.
The concentration of calcium ions in FBS and YPD was measured by using a calcium colorimetric assay from Point Scientific (Lincoln Park, MI) according to the manufacturer's guidelines. In the absence of Ca2+, the detection solution is yellow, whereas the presence of Ca2+ yields a purple color. The color intensity is proportional to the sample Ca2+ concentration. A 20-μl volume of each medium was added to the reagents supplied in the kit, and the optical density of these solutions at a wavelength of 570 nm was compared to a Ca2+ standard by using a Beckman DU 640 spectrophotometer.
RESULTS
Serum is lethal to C. albicans in the presence of calcineurin inhibitors.
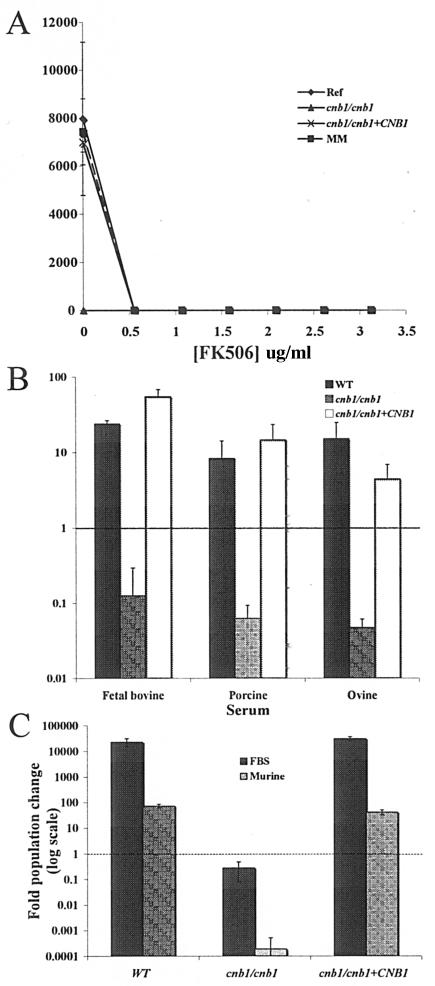
It was previously demonstrated that serum is lethal to strains lacking calcineurin (7). To establish whether this serum lethality is observed in wild-type cells by the addition of a calcineurin inhibitor, we tested the effect of supplementing serum with FK506. The reference (SC5314), calcineurin mutant (JRB64) (cnb1/cnb1), and calcineurin-reconstituted (cnb1/cnb1+CNB1) strains, as well as a strain marker-matched to the calcineurin mutant and reconstituted strains (DAY185) (15), were added to serum containing increasing concentrations of FK506. The calcineurin mutants were unable to survive in serum regardless of FK506 addition, and the reference, marker-matched, and reconstituted strains exhibited partial sensitivity to serum in the presence of 0.04 μg/ml FK506 and complete serum sensitivity in the presence of 0.55 μg/ml FK506 (Fig. 1A and data not shown). We note that in this experiment, as well as in several other experiments, DAY185 and SC5314 exhibited nearly identical phenotypes. To simplify results, SC5314 was used as the reference strain in the remainder of the studies presented here and will henceforth be referred to as the wild-type strain.
FIG. 1.
Serum is lethal to wild-type cells in the presence of calcineurin inhibitors, and toxicity is not unique to fetal bovine serum. (A) The reference strain (SC5314) (⧫), a marker-matched strain (DAY185) (▪), a cnb1/cnb1 calcineurin B mutant strain (JRB64) (▴), and a calcineurin B-complemented strain (MCC85) (×) were grown overnight in fetal bovine serum supplemented with FK506 at the indicated concentrations. (B and C) The reference strain (SC5314) (WT), the cnb1/cnb1 calcineurin B mutant strain (JRB64), and the cnb1/cnb1+CNB1 calcineurin B-complemented strain (MCC85) were tested for survival in 100% fetal bovine, porcine, ovine (B), or murine (C) sera. Approximately 5,000 cells/ml of each strain were incubated in the indicated media for 9 h (B) or 48 h (C) at 30°C.
Serum from diverse sources is toxic to C. albicans calcineurin mutants.
Fetal bovine serum supports the survival and growth of wild-type C. albicans cells but not of calcineurin mutant cells (7, 44). We sought to identify the factor(s) in serum that is toxic to C. albicans calcineurin mutants in order to provide insight into the role of calcineurin in virulence. However, serum is a complex and undefined medium, complicating this analysis. To rule out the possibility that the killing activity of serum is attributable to a species-specific factor, porcine, ovine (sheep), murine, and human sera were also tested for their toxicity against calcineurin mutant cells. The growth of wild-type cells in porcine and ovine sera was similar to that in FBS, and calcineurin mutant cells were unable to survive in the presence of 100% serum from any of these sources (Fig. 1B). Growth of wild-type C. albicans cells was much slower in murine serum (∼6 population doublings in murine serum versus ∼14.5 population doublings in FBS at 48 h) and fresh human serum (only ∼4.2 population doublings after 24 h), and calcineurin mutants also failed to survive in these media, as in FBS (Fig. 1C and data not shown). We conclude that the factor toxic to C. albicans calcineurin mutant cells is a ubiquitous component of sera.
The lethal factor in serum is not proteinaceous.
A series of experiments was conducted to determine whether the lethal factor in serum is a protein, peptide, lipid, or small molecule. Serum contains approximately 60 to 80 mg/ml protein, and we first tested whether one or more of these proteins might be toxic to C. albicans strains lacking calcineurin. Because separating and testing individual serum proteins would be laborious, and more than one protein could contribute to lethality, we first tested whether the killing activity was retained in serum in which proteins had been denatured and removed, and whether activity would be retained in serum subjected to proteinase K treatment.
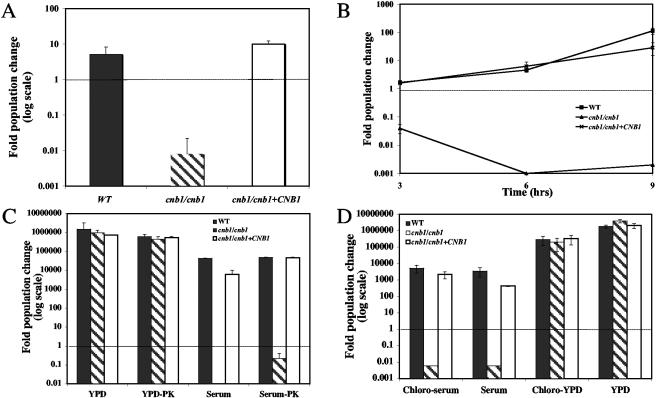
Serum fractions of >30 kDa, 10 to 30 kDa, 3 to 10 kDa, and <3 kDa were obtained by sequential filtration and tested for killing activity against calcineurin mutant cells. The <3-kDa fraction retained killing activity against calcineurin mutant strains, indicating that if the toxic activity is attributable to protein, it would have to be relatively small (<30 amino acids) (Fig. 2A). Serum fractions of >3 kDa did not support growth of the calcineurin mutant strains either, but the toxicity of combined fractions of >3 kDa is much reduced (a fold population change of ∼0.6 at 9 h, more than 73-fold higher than that in the <3-kDa fraction). This moderate toxicity might be due to intrinsic activity or to a small molecule (<3 kDa) bound to one or more proteins in the >3-kDa fraction of serum. Next, serum proteins were denatured by boiling and removed by centrifugation, and the supernatant of the boiled, clarified serum was tested for activity against calcineurin mutant strains. This boiled serum retained toxicity against calcineurin mutant cells, providing evidence that the toxic serum factor is not proteinaceous (Fig. 2B). One caveat is that while most proteins are destroyed by this treatment, small peptides (including antimicrobial peptides) might both pass through a 3-kDa Centricon filter and survive boiling.
FIG. 2.
The lethal factor of serum is not a protein or a lipid. (A) The wild-type strain (SC5314) (solid bar), a cnb1/cnb1 calcineurin B mutant (JRB64) (shaded bar), and a cnb1/cnb1+CNB1 calcineurin B-complemented strain (MCC85) (open bar) were tested for survival at 9 hours in fetal bovine serum that had been filtered through a 3-kDa Centricon filter. (B) The survival of wild-type (▪), cnb1/cnb1 (▴), and cnb1/cnb1+CNB1 (×) strains was measured at the indicated times in the soluble fraction of fetal bovine serum that had been boiled and centrifuged to denature and remove proteins. (C) The survival of wild-type (solid bars), cnb1/cnb1 (shaded bars), and cnb1/cnb1+CNB1 (open bars) strains in YPD, proteinase K-pretreated YPD (YPD-PK), serum, or proteinase K-pretreated serum (serum-PK) was measured at 24 h. (D) Survival of wild-type (solid bars), cnb1/cnb1 (shaded bars), and cnb1/cnb1+CNB1 (open bars) strains was measured at 24 h in chloroform-extracted serum, serum, chloroform-extracted YPD, or YPD that had been lyophilized and reconstituted.
To further eliminate proteins as a potential lethal factor, C. albicans cells were incubated in serum pretreated with 50 μg/ml (1.5 U/ml) proteinase K for 16 h at 30°C. Calcineurin mutant strains were still killed by the protease-treated serum, whereas the growth of wild-type cells was unaffected (Fig. 2C). Proteinase K was not inactivated in this experiment, and to determine whether the protease activity itself influenced calcineurin mutant survival, wild-type, calcineurin mutant, and complemented calcineurin mutant strains were tested for survival in proteinase K-treated YPD. Proteinase K treatment of YPD did not affect the growth of the calcineurin mutant or wild-type strains of C. albicans (Fig. 2C). We conclude that the toxic component of serum is not a protein or a peptide.
The lethal factor of serum is not a lipid.
Calcineurin is essential when the membrane of C. albicans is stressed, such as following inhibition of ergosterol biosynthesis inhibitors or after treatment with sodium dodecyl sulfate (11). The incorporation of mammalian lipids or cholesterol from serum into the membrane of C. albicans might evoke a similar stress, requiring calcineurin signaling for survival. Lipids and other hydrophobic molecules typically found in the cell membrane, such as sterols, are abundant in serum (4 to 8 mg/ml). Incorporation of cholesterol (the mammalian equivalent of the fungal sterol ergosterol) by a related species, Candida glabrata, has been observed (35), and thus uptake of sterols and lipids by C. albicans might occur in the lipid-rich environment of serum.
To determine whether lipids or other hydrophobic molecules contribute to serum lethality, serum and YPD were chloroform extracted, and the aqueous supernatant was tested for activity against calcineurin mutant and wild-type cells. The presence of even trace amounts of chloroform was lethal to C. albicans cells (data not shown), and so the hydrophobic phase of the extraction could not be tested for toxicity against calcineurin mutant cells. The aqueous phase of the chloroform extraction was lyophilized and reconstituted with sterile distilled water prior to testing. Assays were performed with both extracted YPD and serum as well as unextracted media to control for any effects that lyophilization might have on survival. Chloroform extraction and lyophilization had no effect on calcineurin-dependent survival of the C. albicans strains tested, and chloroform-extracted serum retained activity against calcineurin mutant cells at a level comparable to that of native serum (Fig. 2D). Furthermore, the addition of 0.25 M cholesterol to PBS (∼12 to 24 times the amount present in serum) did not affect the survival of calcineurin mutant or wild-type cells (data not shown). We conclude that lipids, sterols, and other hydrophobic molecules present in serum are not the components toxic to cells lacking calcineurin activity.
Fe3+ and Zn2+ have no calcineurin-dependent antifungal activity.
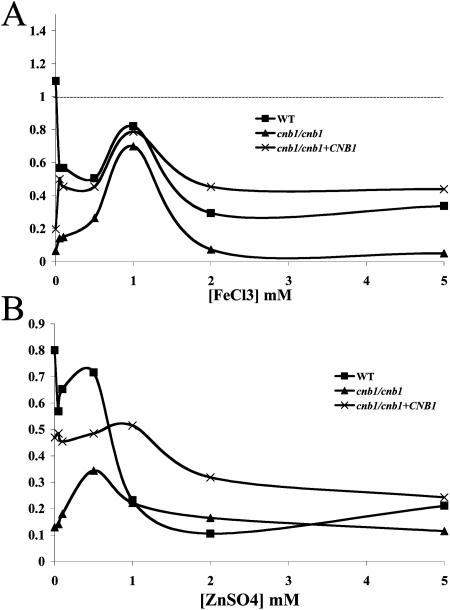
Although these studies exclude proteins, peptides, and lipids as toxic factors, myriad hydrophilic small-molecule components of serum remain that could affect the survival of C. albicans calcineurin mutant cells. These include vitamins (which were discussed in a previous publication [7]) and ions, including the metal ions iron and zinc. Iron is essential for life, and host chelation of iron in blood has driven microorganisms to develop methods to scavenge this essential cofactor from chelating proteins and peptides (for a review, see reference 21). However, high levels of iron can be lethal to cells via reactions with oxygen that produce toxic free radicals (20, 48). FeCl3 was tested for any calcineurin-dependent lethal role it might play in serum toxicity. The addition of 1 mM Fe3+ to PBS enhanced the survival of calcineurin mutants over that in PBS alone, but at higher concentrations of Fe3+ (2 to 5 mM) this survival benefit was lost, and survival rates returned to levels equivalent to those in PBS alone (Fig. 3A). The survival of wild-type and complemented strains was reduced by the addition of FeCl3 to PBS from survival in PBS without FeCl3 (Fig. 3A). Like iron, zinc is also required at low concentrations for cell survival but can be toxic at higher levels. This antimicrobial activity is used clinically to treat topical fungal infections (16). Also as with iron, the addition of 1 mM ZnSO4 enhanced the survival of calcineurin mutants in PBS, but 2 to 5 mM ZnSO4 did not exhibit calcineurin-dependent toxicity (Fig. 3B). Thus, these essential but potentially toxic metals do not appear to play a role in the toxicity of serum against C. albicans cells lacking calcineurin function.
FIG. 3.
Calcineurin does not mediate responses to iron or zinc toxicity. Wild-type (SC5314) (▪), cnb1/cnb1 (JRB64) (▴), and cnb1/cnb1+CNB1 (MCC85) (×) strains were tested for their abilities to persist in PBS with increasing concentrations of FeCl3 (A) or ZnSO4 (B). Cells were incubated for 24 h at 30°C.
Calcium is the toxic component in serum.
In previous studies investigating the role of the calcineurin A catalytic subunit in C. albicans virulence, calcineurin mutant cells were reported to be hypersensitive to salt stress compared to wild-type controls (2). This included hypersensitivity to 1 mM NaCl, 300 mM LiCl, and 10 mM MnSO4, phenotypes shared with S. cerevisiae and C. neoformans, but it also included hypersensitivity to 300 mM CaCl2, a phenotype not shared with these fungi (34, 38). In fact, calcineurin mutant strains of S. cerevisiae display increased tolerance to high concentrations of calcium (13). Based on this observation, we investigated whether Ca2+ levels in serum might contribute to serum toxicity against calcineurin mutant cells of C. albicans.
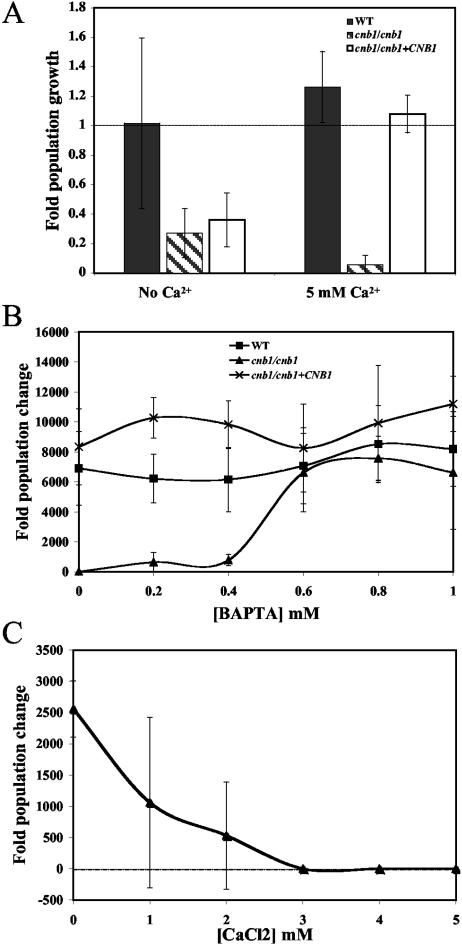
Prior to testing of the toxicity of Ca2+ in serum, the concentrations of Ca2+ in FBS and YPD medium were measured. The concentration of Ca2+ in 100% FBS ranged from 3.5 to 4 mM, more than 30-fold greater than the concentration of Ca2+ in unsupplemented YPD medium (data not shown) but 100-fold less than the concentration tested in the study of Bader et al. (2). Indeed, the addition of 5 mM Ca2+ to YPD did not affect the growth of the calcineurin mutant strain (data not shown). However, PBS supplemented with 5 mM CaCl2 was unable to support the survival of calcineurin mutant strains, whereas both wild-type and complemented calcineurin mutant strains survived (Fig. 4A). Moreover, PBS supplemented with 5 mM CaCl2 and 1.07 μg/ml FK506 did not support the survival of the wild-type strain (data not shown). To further explore these findings, serum Ca2+ was chelated by the addition of the extracellular Ca2+ chelator BAPTA. If serum Ca2+ levels are toxic to C. albicans calcineurin mutant cells, removal of Ca2+ should restore growth to this strain. The addition of BAPTA (∼0.8 mM) to serum rescued the growth of calcineurin mutant cells (Fig. 4B). BAPTA chelates 1 molecule of calcium per BAPTA molecule. Thus, the concentration of BAPTA necessary to rescue the growth of calcineurin mutant cells in serum is ∼4-fold lower than the Ca2+ concentration in serum. This observation suggests that not all Ca2+ needs to be sequestered to restore cnb1/cnb1 mutant survival in serum and that the MIC for growth inhibition by Ca2+ may be in the range of 2.5 to 3 mM. In addition, other calcium chelators may contribute to sequestering Ca2+ under these conditions. Interestingly, the addition of as little as 3 mM Ca2+ to BAPTA-chelated serum was sufficient to restore robust serum killing activity against calcineurin mutant cells, and lower concentrations of Ca2+ (1 or 2 mM) also restored toxicity, although in these cases there was higher variability in replicate assays (Fig. 4C). These findings support the conclusion that calcineurin is necessary to mediate C. albicans survival in response to stress induced by the levels of Ca2+ normally present in serum.
FIG. 4.
Calcineurin is important for the response to stress induced by Ca2+ in serum. (A) The abilities of wild-type (SC5314) (solid bars), cnb1/cnb1 (JRB64) (shaded bars), and cnb1/cnb1+CNB1 (MCC85) (open bars) strains to persist in PBS were measured with or without 5 mM Ca2+. (B) Wild-type (▪), cnb1/cnb1 (▴), and cnb1/cnb1+CNB1 (×) strains were tested for their abilities to survive and proliferate in serum alone or in serum supplemented with the indicated concentrations of BAPTA. (C) The survival of the cnb1/cnb1 calcineurin mutant strain was measured in serum with 0.8 mM BAPTA and supplemented with the indicated concentrations of Ca2+. Cells were incubated at 30°C with (A) or without (B and C) agitation. The experiments for which results are shown in panels B and C were performed in 96-well plates.
Crz1 and Crz2 have no individual roles in serum sensitivity.
In S. cerevisiae, the Crz1/Tcn1 transcription factor is responsible for a majority of the transcriptional response to calcineurin-dependent activation in response to cell stress (33, 47), and two Crz1 homologs, Crz1 and Crz2, have recently been identified in C. albicans (5, 40). Crz1 plays a modest role in mediating azole resistance downstream of calcineurin, but from genetic analysis, it is clear that other downstream targets of calcineurin also participate in azole resistance (40). By contrast, Crz1 appears to play no role downstream of calcineurin in responding to salt-induced stress (40), whereas Crz2 is regulated by Rim101 at a high pH (pH 8) (5). Because the S. cerevisiae homolog Crz1 plays a critical role in mediating calcineurin responses, we tested whether these proteins play a role in the sensitivity of C. albicans calcineurin mutants to serum.
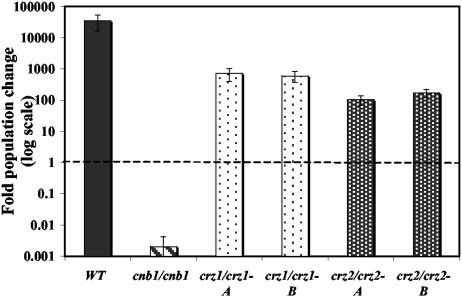
The abilities of crz1/crz1 and crz2/crz2 mutant strains to survive in 100% FBS were compared to those of the wild-type strain and cnb1/cnb1 mutant cells. As originally observed, the wild-type strain readily proliferates in serum, while this medium is toxic to cnb1/cnb1 mutant cells. Both the crz2/crz2 and crz1/crz1 mutant strains survived and proliferated in serum, albeit not quite to the wild-type level (∼9 population doublings for crz1/crz1, ∼7 for crz2/crz2, and ∼15 for the wild-type strain) (Fig. 5). Thus, these putative transcription factors are unlikely to independently play any significant role downstream of calcineurin in responses to serum, suggesting that other calcineurin targets will be important in mediating C. albicans responses to serum stress and virulence.
FIG. 5.
Crz1 and Crz2 are not required for serum survival. Wild-type (SC5314) (WT), cnb1/cnb1 (JRB64), crz1/crz1 (OCC1.1 [bar A] and OCC3.8 [bar B]), and crz2Δ/crz2Δ (DAY686 [bar A] and DAY697 [bar B]) strains were tested for their abilities to survive and proliferate in FBS. Cells were incubated at 30°C for 24 h.
DISCUSSION
Calcineurin is essential for the survival of C. albicans in serum, and we have shown here that calcineurin protects C. albicans from stress induced by endogenous levels of Ca2+ in this medium. Our original hypothesis was that a protein or antimicrobial peptide might be responsible for the killing of calcineurin mutant strains by serum, but the heat stability, small size, and proteinase K resistance of the killing activity in serum excludes this model. The finding that calcium mediates the lethal effect of serum against calcineurin mutants of C. albicans is surprising, because calcineurin mutants of S. cerevisiae are actually more resistant than wild-type cells to calcium stress (13). It has been suggested that the sensitivity of wild-type S. cerevisiae cells to calcium is due to a toxic effect of constant calcineurin activation, which is lacking in calcineurin mutant cells. It appears that C. albicans lacks this toxic effect of sustained calcineurin activity, perhaps because prolonged calcineurin signaling is common in the natural environment of C. albicans to which it has adapted. The results of Bader et al. (2) showing that calcineurin is important for C. albicans to survive calcium stress in YPD medium provided the first clue that Ca2+ levels in serum might contribute to serum lethality.
Interestingly, addition of Ca2+ to YPD at levels similar to those in serum or PBS had no effect on calcineurin mutant survival. YPD may contain natural calcium chelators that reduce the effective Ca2+ concentration in the medium, or the differing metabolic rates of cells in a rich growth medium versus serum may alter physiological responses to Ca2+ ions. Alternatively, additional components in serum may contribute to the lethal effect on calcineurin mutant strains in conjunction with Ca2+. Our assays were designed to detect the major component of serum contributing to its toxic activity against the calcineurin mutants, and minor contributing components may have thus far escaped detection. Any additional toxic serum component(s), however, remains hypothetical at present.
Why is calcineurin required to survive stress caused by Ca2+ in serum? Based on the finding that mutants deficient in Crz1 or Crz2 (homologs of the transcription factor Crz1/Tcn1, which is responsible for the transcriptional response downstream of calcineurin in S. cerevisiae) are not as sensitive to serum as calcineurin mutant cells, this response may not be transcriptional. Isolation and analysis of a crz1/crz1 crz2/crz2 double-mutant strain will be required to exclude models in which the two might play a redundant role in serum survival. An alternative model is that the role of calcineurin in serum survival may be mediated by posttranslational regulation of downstream targets. Attractive candidate calcineurin targets in this setting include homologs of S. cerevisiae Hph1, recently reported as a direct calcineurin target (22), and Ca2+ pumps on the cell surface and in intracellular organelles (such as Cch1-Mid1 on the plasma membrane, Pmc1 in the vacuole, and Pmr1 in the Golgi apparatus), which are known to be controlled by calcineurin in S. cerevisiae (14). The integral membrane protein Hph1 is an attractive downstream candidate because, in combination with its close homolog Hph2, it is essential for cell survival of stress induced by high NaCl concentrations, alkaline pHs, and cell wall perturbation (22). However, exhaustive database searches of the C. albicans genomic sequencing project at Stanford University reveal no clear Hph1 or Hph2 homolog in C. albicans (J. Reedy, J. R. Blankenship, C. Onyewu, and J. Heitman, unpublished results). The ionic pumps Cch1-Mid1, Pmc1, and Pmr1 are attractive downstream targets because they modulate the Ca2+ concentration in the cytoplasm and in organelles where calcium can play an essential role as a cofactor (in the endoplasmic reticulum, for example, as a cofactor for protein chaperones). Misregulation of these pumps could lead to an accumulation of toxic levels of Ca2+ in the cytoplasm, leading to aberrant calcium signaling, perhaps through calmodulin, or to a loss of calcium in organelles that require Ca2+ for proper functioning. These possibilities are currently being investigated.
The natural niche of C. albicans is the mucosal surfaces of the gastrointestinal tract of mammals. C. albicans typically lives as a commensal within its host, and until the introduction of immunosuppressive therapy in the last century, serious systemic infections were uncommon (37). With the advent of immunosuppressive therapy for cancer chemotherapy and as antirejection therapy for organ transplant recipients, systemic Candida infections have become increasingly common. To make the transition from a commensal organism to a systemic pathogen, C. albicans must first enter the bloodstream. It can do so by taking advantage of medical devices, such as intravenous catheters, to enter the bloodstream directly (reviewed in reference 29), or it can cross intact or damaged gastrointestinal mucosae into the bloodstream (9). The bloodstream is a hostile environment, and not only does C. albicans face a radical change in nutrient availability; it is also exposed to innate immune defenses, including plasma, macrophages, and neutrophils. Although free-flowing C. albicans appears to be rapidly cleared from the blood (3, 42), the fungus does persist in the bloodstream (∼10% in an experimental rabbit infection model) (3). This persistent bloodstream infection likely contributes to dissemination, but whether candidemia has any additional role in disease progression besides acting as a vehicle for rapid dissemination is not known. It is also unclear how long C. albicans remains exposed to the bloodstream when attached to the endothelium of blood vessels before it can successfully invade to colonize host tissues and organs. Calcineurin is part of the arsenal that C. albicans deploys to survive the hostile bloodstream environment in addition to germ tube formation, host cell adherence, and host cell injury.
Although the Ca2+-sensitive phenotype of calcineurin mutant strains of C. albicans in serum derived from whole blood is described here, it is likely that calcineurin is also important for mediating survival of the fungus in the interstitial fluids present in organs such as the kidney, where free Ca2+ concentrations can be quite high. In the standard American adult diet, approximately 1 g of calcium is ingested daily, and ∼200 mg of this Ca2+ is renally excreted (8). The concentration of Ca2+ in the kidney is highest in the nephrons, but surrounding cells may be exposed to elevated levels as well, and calcineurin might enable C. albicans to survive these conditions. Indeed, C. albicans calcineurin mutants colonize the kidney poorly (7), and this could be due in part to the Ca2+ levels present in the interstitial fluids of this organ.
The risk of death in immunocompromised patients diagnosed with disseminated candidiasis is high, even with current therapy. Although treatment options have increased with the recent introduction of voriconazole and caspofungin, the armamentarium of antifungal drugs to combat candidiasis remains limited. Calcineurin is an attractive drug target: it is essential for C. albicans virulence, and drug treatment should halt dissemination of the infection. Furthermore, calcineurin-targeting drugs already exist, and the crystal structure of the interaction of the drugs with calcineurin has been solved (19, 24, 26, 27), potentially enabling rational design of analogs that will impair fungal calcineurin-dependent virulence but spare calcineurin-dependent signaling events critical for immune responses in the host. Furthermore, several studies have documented a potent fungicidal synergism between calcineurin-inhibiting drugs and drugs that inhibit the ergosterol biosynthetic pathway (6, 11, 31, 32, 39), making calcineurin an even more attractive antifungal-drug target.
Acknowledgments
We thank Dana Davis for the generous gift of strains DAY686 and DAY697 prior to publication. We also thank Stu Levitz for suggesting that we test Zn2+ ions and Chiatogu Onyewu, Jennifer Reedy, Yong-Sun Bahn, John Perfect, and John McCusker for comments on the manuscript.
These studies were supported by RO1 grant AI-50438 from NIAID (to J.H.), and PO1 grant AI-44975 to the Duke University Mycology Research Unit. J.H. is an associate investigator of the Howard Hughes Medical Institute and was a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology.
Editor: T. R. Kozel
REFERENCES
- 1.Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell Regul. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 2.Bader, T., B. Bodendorfer, K. Schroppel, and J. Morschhauser. 2003. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 71:5344-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baine, W. B., M. G. Koenig, and J. S. Goodman. 1974. Clearance of Candida albicans from the bloodstream of rabbits. Infect. Immun. 10:1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow, A. J., T. Aldersley, and F. W. Chattaway. 1974. Factors present in serum and seminal plasma which promote germ-tube formation and mycelial growth of Candida albicans. J. Gen. Microbiol. 82:261-272. [DOI] [PubMed] [Google Scholar]
- 5.Bensen, E. S., S. J. Martin, M. Li, J. Berman, and D. A. Davis. 2004. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol. 54:1335-1351. [DOI] [PubMed] [Google Scholar]
- 6.Blankenship, J. R., W. J. Steinbach, J. R. Perfect, and J. Heitman. 2003. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4:192-199. [PubMed] [Google Scholar]
- 7.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner, B. M., and F. Rector. 2004. Brenner & Rector's the kidney, 7th ed. Saunders, Philadelphia, Pa.
- 9.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 10.Chilgren, R. A., R. Hong, and P. G. Quie. 1968. Human serum interactions with Candida albicans. J. Immunol. 101:128-132. [PubMed] [Google Scholar]
- 11.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz, M. C., R. A. L. Sia, M. Olson, G. M. Cox, and J. Heitman. 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 68:982-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham, K. W., and G. R. Fink. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143-1150. [DOI] [PubMed] [Google Scholar]
- 15.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faergemann, J. 2000. Management of seborrheic dermatitis and pityriasis versicolor. Am. J. Clin. Dermatol. 1:75-80. [DOI] [PubMed] [Google Scholar]
- 17.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 19.Griffith, J. P., J. L. Kim, E. E. Kim, M. D. Sintchak, J. A. Thomson, M. J. Fitzgibbon, M. A. Felming, P. R. Caron, K. Hsiao, and M. A. Navia. 1995. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell 82:507-522. [DOI] [PubMed] [Google Scholar]
- 20.Gutteridge, J. M., and B. Halliwell. 2000. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann. N. Y. Acad. Sci. 899:136-147. [DOI] [PubMed] [Google Scholar]
- 21.Haas, H. 2003. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 62:316-330. [DOI] [PubMed] [Google Scholar]
- 22.Heath, V. L., S. L. Shaw, S. Roy, and M. S. Cyert. 2004. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemenway, C. S., and J. Heitman. 1999. Calcineurin: structure, function, and inhibition. Cell Biochem. Biophys. 30:115-151. [DOI] [PubMed] [Google Scholar]
- 24.Huai, Q., H. Y. Kim, Y. Liu, Y. Zhao, A. Mondragon, J. O. Liu, and H. Ke. 2002. Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes. Proc. Natl. Acad. Sci. USA 99:12037-12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson, D. A., Q. L. Sciascia, R. J. Sanders, G. E. Norris, P. J. Edwards, P. A. Sullivan, and P. C. Farley. 2004. Identification of the dialysable serum inducer of germ-tube formation in Candida albicans. Microbiology 150:3041-3049. [DOI] [PubMed] [Google Scholar]
- 26.Jin, L., and S. C. Harrison. 2002. Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin. Proc. Natl. Acad. Sci. USA 99:13522-13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kissinger, C. R., H. E. Parge, D. R. Knighton, C. T. Lewis, L. A. Pelletier, A. Tempczyk, V. J. Kalish, K. D. Tucker, R. E. Showalter, E. W. Moomaw, L. N. Gastinel, N. Habuka, X. Chen, F. Maldonado, J. E. Barker, R. Bacquet, and J. E. Villafranca. 1995. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature 378:641-644. [DOI] [PubMed] [Google Scholar]
- 28.Klee, C., H. Ren, and X. Wang. 1998. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273:13367-13370. [DOI] [PubMed] [Google Scholar]
- 29.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louria, D. B., and R. G. Brayton. 1964. A substance in blood lethal for Candida albicans. Nature 201:309. [DOI] [PubMed] [Google Scholar]
- 31.Maesaki, S., P. Marichal, M. A. Hossain, D. Sanglard, H. V. Bossche, and S. Kohno. 1998. Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 42:747-753. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura, T., Y. Liu, D. Hirata, H. Namba, S.-I. Harada, T. Hirokawa, and T. Miyakawa. 1993. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 12:4063-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama, H., M. Izuta, N. Nakayama, M. Arisawa, and Y. Aoki. 2000. Depletion of the squalene synthase (ERG9) gene does not impair growth of Candida glabrata in mice. Antimicrob. Agents Chemother. 44:2411-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noverr, M. C., and G. B. Huffnagle. 2004. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 72:6206-6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odds, F. C. 1988. Candida and candidosis: a review and bibliography, 2nd ed. Bailliere Tindall, London, United Kingdom.
- 38.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onyewu, C., F. L. Wormley, Jr., J. R. Perfect, and J. Heitman. 2004. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 72:7330-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrino, B. A., L. Y. Ng, and T. R. Soderling. 1995. Calcium regulation of calcineurin phosphatase activity by its B subunit calmodulin. J. Biol. Chem. 270:340-346. [DOI] [PubMed] [Google Scholar]
- 42.Rogers, T., and E. Balish. 1976. Experimental Candida albicans infection in conventional mice and germfree rats. Infect. Immun. 14:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 80:1483-1521. [DOI] [PubMed] [Google Scholar]
- 44.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 45.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 46.Smith, J. K., and D. B. Louria. 1972. Anti-Candida factors in serum and their inhibitors. II. Identification of a Candida-clumping factor and the influence of the immune response on the morphology of Candida and on anti-Candida activity of serum in rabbits. J. Infect. Dis. 125:115-122. [DOI] [PubMed] [Google Scholar]
- 47.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]