Abstract
Colonization of the avian respiratory tract with Mycoplasma gallisepticum results in a profound inflammatory response in the trachea, air sacs, conjunctiva, and lungs. A live attenuated M. gallisepticum vaccine strain, GT5, was previously shown to be protective in chickens upon challenge; however, the mechanisms by which this vaccine and others confer protection remain largely unknown. The current study evaluated several potential correlates of GT5 vaccine-mediated immune protection following challenge with the pathogenic M. gallisepticum strain Rlow. GT5-vaccinated chickens developed mild tracheal lesions, consisting of few and scattered, discrete, lymphofollicular aggregates in the lamina propria. In addition, low numbers of aggregated B, CD4+, and CD8+ cells were observed to infiltrate the trachea, in stark contrast to the large numbers infiltrating the tracheas of sham-vaccinated chickens challenged with Rlow. Lymphofollicular aggregates were rarely observed prior to day 12 postchallenge in sham-vaccinated chickens. Instead, they contained an increasingly more cellular inflammatory response characterized by expansion of the lamina propria by lymphoplasmacytic and histiocytic infiltrates. This was due in part to expansion of interfollicular zones by large numbers of infiltrating CD4+ and CD8+ cells and a sizeable population of immunoglobulin A (IgA)- and IgG-secreting plasma cells. GT5-vaccinated chickens also had higher serum IgG concentrations, and significantly higher numbers of M. gallisepticum-specific IgG- and IgA-secreting plasma/B cells within the trachea, than did sham-vaccinated chickens. These responses were observed as early as day 4 postchallenge, indicating the importance of antibody-mediated clearance of mycoplasma in GT5-vaccinated chickens.
Mycoplasma gallisepticum is a highly infectious respiratory pathogen affecting poultry. When present in concert with other respiratory pathogens such as infectious bronchitis virus, Newcastle disease virus, Escherichia coli, or Haemophilus paragallinarum, a condition known as chronic respiratory disease results. The clinical signs associated with M. gallisepticum infection in chickens include respiratory rales, nasal discharge, coughing, and occasionally conjunctivitis (20). The most prominent pathological findings include inflammatory lesions in the trachea, air sacs, lungs, conjunctiva, and other tissues such as the oviduct (25). Significant economic losses from M. gallisepticum infection in poultry occur due to reduced egg production and hatchability, as well as downgrading of carcasses (20). Mycoplasma gallisepticum transmission can occur both horizontally through aerosols and vertically through the egg, leading to a rapid spread within the flock. Control programs for M. gallisepticum infection are based on maintaining a commercial breeding stock that is M. gallisepticum free and rearing them in single-aged, all-in/all-out farms. However, in multiple-age production complexes, administration of suitable antibiotic medication is practiced to reduce production losses and egg transmission (18). Eradication by means of vaccination is also one of the preferred methods of control under such circumstances.
Previously, bacterins and live vaccines have been commercially used for the control of M. gallisepticum infection in chickens (35). Killed whole-cell M. gallisepticum bacterins reduced the severity of lesions and egg production losses but did not completely prevent M. gallisepticum colonization of the chicken respiratory tract upon challenge (14, 37). At the same time, bacterins fail to provide complete protection against heterologous exposure. Over the past decade, the use of bacterins has been supplanted by more effective live attenuated vaccines. Three live attenuated vaccines (ts-11, 6/85, and F-strain) have been commercialized and have shown a high degree of efficacy in controlling the dissemination of disease (1, 6, 15, 35). Little is known regarding the genetic basis of attenuation in these strains, as well as the immune mechanisms by which protection is conferred. It is important to understand both elements of the host-pathogen interactions in order to further refine vaccines and to understand vaccine failures, if and when they occur.
The attenuated Rhigh strain of M. gallisepticum was developed by serial passage of a virulent strain (Rlow) 164 times (17), rendering it avirulent, even when administered at a 10,000-fold-higher dose than a pathogenic dose of the Rlow strain (27). Characterization of mutations in M. gallisepticum Rhigh indicated that three membrane proteins were absent, namely, the cytadhesin molecule GapA, cytadherence-related molecule A (CrmA), and a component of the ABC transport system, HatA (28). Surface expression of GapA is required for cytadherence, as anti-GapA antibodies inhibited attachment of M. gallisepticum to the chicken tracheal epithelium (13). It was further shown that surface coexpression of GapA and CrmA was essential for M. gallisepticum cytadherence and virulence and that a strain obtained by complementation of Rhigh with GapA alone, designated the GT5 strain, did not have cytadherence restored and remained avirulent (26).
The GT5 strain of M. gallisepticum has been shown to be protective in chickens upon challenge with a pathogenic dose of M. gallisepticum Rlow (27). Although serum immunoglobulin G (IgG) levels were significantly higher in GT5-vaccinated than in sham-vaccinated chickens 2 weeks postchallenge, GT5-vaccinated birds produced lower levels of mucosal IgA than those observed in the sham-immunized group. The presence of low levels of mucosal IgA correlated with reduced severity of tracheal lesions and reduced M. gallisepticum colonization (27).
In the current study, we evaluated several potential correlates of GT5 vaccine-mediated immune protection by examining cellular infiltrates and enumerating specific antibody-secreting cells (ASCs) within the tracheas of chickens after challenge with pathogenic M. gallisepticum Rlow. Among the novel results reported here is that protection in GT5-vaccinated chickens is correlated with lower numbers of infiltrating B cells and CD4+ and CD8+ cells along with the appearance of higher numbers of mycoplasma-specific ASCs as early as day 1 postchallenge. It is widely accepted that mucosal antibodies confer protection primarily by blocking M. gallisepticum cytadherence to the epithelial surface (2, 3, 16, 38). The current study confirms and extends these observations by emphasizing the importance of inducing a rapid and vigorous mucosal immune response soon after exposure in order to prevent colonization and progression to a more severe respiratory disease.
(Portions of this work will be published in abstract form in association with the 77th Northeastern Conference on Avian Diseases, Cornell University, Ithaca, N.Y., 15 to 17 June 2005.)
MATERIALS AND METHODS
Strains and media.
M. gallisepticum strain Rlow (15 passages) was provided by Sharon Levisohn (Ministry of Agriculture, Kimron Veterinary Institute, Israel). Growth conditions for GT5 have been described previously (28). All strains were cultured at 37°C in Hayflick's medium supplemented with 10% horse serum and 5% yeast extract (7) or on Hayflick's plates. Gentamicin was added (final concentration of 100 μg/ml) to the GT5 propagation medium.
Animals and immunization.
Five-week-old, specific-pathogen-free, female white Leghorn chickens (SPAFAS, North Franklin, CT) were used for all vaccination/challenge experiments. Nonmedicated feed (Blue Seal, Waltham, MA) and water were provided ad libitum. Chickens were either vaccinated intratracheally with 100 μl of 3 × 108 CFU GT5 vaccine or sham vaccinated with 100 μl phosphate-buffered saline (PBS) on days 0 and 2. Following vaccination, groups of three chickens were sacrificed on days 8 and 16 postvaccination, and selected tissues were harvested. Two remaining groups of GT5- or sham-vaccinated chickens were challenged intratracheally on days 16 and 18 postvaccination with 100 μl of 1 × 107 CFU M. gallisepticum Rlow. Chickens were sacrificed on days 1, 4, 8, 12, and 16 postchallenge (days 19, 22, 26, 30, and 34 postvaccination, respectively). Blood was collected prior to vaccination and at necropsy and serum was stored at −20°C for future analysis.
Necropsy.
Chickens were euthanized by cervical dislocation. Tracheas from glottis and larynx to the syrinx at the level of the bifurcation were dissected and removed. Annular tissue samples of 5 mm width from the cranial, mid-, and caudal trachea were obtained, snap frozen in OCT compound (Sakura Finetec U.S.A., Inc., Torrance, CA), and stored at −80°C until sectioning. At the same time, samples from the lungs and air sacs (left and right thoracic and abdominal) and duplicate samples of trachea were fixed in 10% neutral buffered formalin and submitted for histological analysis. These tissues were routinely processed, embedded in paraffin, sectioned at 4 μm, mounted on glass slides, and stained with hematoxylin and eosin according to standard histological protocols. Transverse sections of trachea from three different levels were examined microscopically. Tracheal lymphocytic infiltrates were scored using the system of Nunoya et al. (24) with minor modifications (27). The heterophilic infiltrates observed soon after infection in sham-vaccinated, Rlow-challenged birds were scored separately, as follows: 0, no significant finding; 0.5, minimal intraepithelial or lamina proprial infiltrates in a few (one to three) foci; 1, mild intraepithelial or lamina proprial infiltrates, diffuse or ≥4 foci; 2, moderate infiltrates in lumen, epithelium, and lamina propria focally or diffusely; and 3, severe infiltrates in lumen, epithelium, and lamina propria focally or diffusely. A final score was obtained by combining the lymphocytic and heterophilic scores. To collect tracheal washes, 1 milliliter of sterile PBS was gently instilled into the tracheal lumen, allowing it to pass through the length of the trachea, and collected in prelabeled tubes. The tracheal washes were stored at −20°C for subsequent analysis.
Leukocyte isolation from trachea.
The tracheas were cut into pieces (approximately 2 to 5 mm) and placed in a petri dish containing 10 to 15 ml of RPMI 1640 medium. The tissues were washed three to six times in the medium, transferred into a 50-ml Erlenmeyer flask containing 20 ml of RPMI-10/EDTA (RPMI 1640 supplemented with 10% fetal bovine serum, 15 mM HEPES, 100 μg/ml streptomycin sulfate, and 100 U/ml penicillin, pH 7.2, along with 5 mM EDTA, pH 8), and gently stirred for 15 min at room temperature using a magnetic stirrer to remove epithelial cells. The EDTA treatment was repeated two to three times until the supernatant appeared clear. Residual EDTA was then removed by washing in RPMI-10. This was followed by treatment with RPMI-10/collagenase type 2 at 200 U/ml (Worthington Biochemical Corporation, Lakewood, NJ) for 1 h with gentle stirring at room temperature to release lamina proprial lymphocytes. Following collagenase treatment, the contents of the flask were filtered through a sterile nylon screen and the filtrate centrifuged at 850 × g for 10 min. The cell suspension was washed to remove residual collagenase, and the final pellet was resuspended in 5 ml of ice-cold RPMI-10. A 10-μl cell suspension sample was removed for viable cell assessment using trypan blue dye exclusion. All steps were performed under aseptic conditions.
Immunophenotypic analysis of cells isolated from trachea.
Leukocytes isolated from tracheas were incubated with monoclonal antibodies, mouse anti-chicken Bu-1-biotin conjugate at 1 μg per million cells, mouse anti-chicken CD4-fluorescein isothiocyanate conjugate at 1 μg per million cells, and mouse anti-chicken CD8-phycoerythrin conjugate at 0.2 μg per million cells diluted in fluorescence-activated cell sorting (FACS) buffer (PBS, 1% bovine serum albumin [BSA], 0.1% sodium azide) for 45 to 60 min on ice. All antibodies were purchased from Southern Biotechnology Associates, Inc., Birmingham, AL. A blocking step was performed prior to specific staining using rabbit IgG at 100 μg per million cells. For detection of biotin-conjugated anti-chicken Bu-1 antibody, samples were incubated with streptavidin-fluorescein isothiocyanate (Southern Biotechnology, Birmingham, AL) for 45 min on ice. After a final wash with FACS buffer, stained cells were fixed in 1% paraformaldehyde and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). The voltage settings were kept constant for all readings, and samples were analyzed after gating the lymphocyte populations as determined after splenocyte staining. Data collection was performed using CellQuest software (Becton Dickinson, version 3.2.1f1). The proportion of each cell population was obtained as the percentage of fluorescently labeled cells, and the total number of specific cells was determined by multiplying their percentages by the total number of cells isolated per centimeter of trachea.
Immunohistochemical analysis of tracheal sections.
OCT-embedded tracheal samples were sectioned using a cryostat (Leica CM 3050 S; Leica Microsystems, Heidelberger, Germany) at 5 μm and mounted on poly-l-lysine-coated glass slides (Fisher Scientific, Pittsburgh, PA). Sections were immersed in ice-cold acetone for 10 min, air dried at room temperature, and stored at −20°C until staining. After an endogenous peroxidase block (0.03% hydrogen peroxide for 2 min) followed by a block using an avidin-biotin blocking kit (SP-2001; Vector Laboratories, Burlingame, CA) and normal mouse serum (3%), tissue sections were stained to detect CD8+, IgG+, and IgA+ cells by using mouse monoclonal antibodies to chicken CD8 at 1:50, to chicken IgG at 1:100, and to chicken IgA at 1:100. All monoclonal antibodies were biotin conjugated and were purchased from Southern Biotechnology Associates. (Birmingham, AL). After a 45- to 60-min primary antibody incubation followed by a wash using PBS, avidin-biotinylated horseradish peroxidase reagent (ABC-HRP) (PK-6100; Vector Laboratories) was used for 45 min. Color development was achieved by addition of aminoethylcarbazole substrate (SK-4200; Vector Laboratories). Tissue sections were counterstained using hemotoxylin (Vector Laboratories), mounted using an aqueous mounting medium (Biomeda Corp., Foster City, CA), and coverslipped. A conjugate control, using an irrelevant isotype-matched monoclonal antibody (mouse anti-pig CD3; Southern Biotechnology), was used for comparison to tissue sections stained with specific antibody. Double staining was performed to simultaneously detect B cells and CD4+ cells within the same tissue section. Tissue sections were first stained for CD4+ cells by using biotin-conjugated mouse anti-chicken CD4 at 1:50 as described above. Color development was performed using avidin-biotinylated alkaline phosphatase reagent (AK-5000; Vector Laboratories) followed by treatment with alkaline phosphatase substrate (SK-5300; Vector Laboratories). After a PBS wash and endogenous avidin-biotin block, tissue sections were stained to detect B cells within the same section by using biotin-conjugated mouse anti-chicken Bu-1 at 1:50. Detection of B cells was performed using ABC-HRP and aminoethylcarbazole substrate. All antibodies used for immunohistochemical studies were diluted in PBS, and all steps were carried out at room temperature.
M. gallisepticum-specific ASCs within trachea.
Enzyme-linked immunospot (ELISPOT) assays, as described by Wu et al. (36), were performed, with some modifications, on leukocytes isolated from the trachea. Ninety-six-well multiscreen-IP plates (Millipore Corporation, Bedford, MA) were coated with 2 μg per well of killed M. gallisepticum Rlow suspended in sodium carbonate coating buffer (pH 9.6). Control wells were coated with 2 μg per well of BSA. Plates were washed using sterile filtered PBS-Tween (0.05%) (PBST) and blocked using 1% BSA for 1 h at room temperature. Cell suspensions from the trachea were added at 105 leukocytes per well and incubated for 14 to 16 h at 37°C in a 5% CO2 humidified incubator. Cells were applied in triplicate to the ELISPOT plate, with all steps performed under aseptic conditions. At the end of the incubation, cells were removed by decanting the supernatant and washing the plate with PBST solution. Rabbit anti-chicken IgG-alkaline phosphatase conjugate (Sigma Chemical Co. St. Louis, MO) at a 1: 40,000 dilution and goat anti-chicken IgA-alkaline phosphatase conjugate (Bethyl Laboratories, Inc., Montgomery, TX) at a 1:1,000 dilution in PBST were used to detect IgG- and IgA-secreting cells, respectively. Plates were incubated for 2 h at room temperature, followed by the addition of BCIP (5-bromo-4-chloro-3-indolylphosphate)/nitroblue tetrazolium substrate (B-5655; Sigma, St. Louis, MO). Each plate was coded, and spots were individually counted using a dissecting microscope (Wild Leesbrugg, Switzerland) by two people, one of whom was blinded to the experimental groups. Antigen-specific spots were calculated by subtracting the background BSA control spots from the M. gallisepticum Rlow spots and the means expressed as specific IgG or IgA ASCs per 106 cells.
Antigen-specific and total antibody concentrations in sera and tracheal washes.
M. gallisepticum-specific sera and tracheal wash IgG, IgM, and IgA concentrations were determined by indirect enzyme-linked immunosorbent assay (ELISA) as described previously (27) using ELISA quantitation kits (Bethyl, Montgomery, TX). Briefly, killed M. gallisepticum Rlow or BSA (control) were applied at 1 μg (100 μl/well) in 0.05 M carbonate buffer, pH 9.6, and stored at 4°C until use. On the day of assay, appropriate capture antibody (goat anti-chicken IgG-Fc/IgA/IgM, affinity purified, depending on the antibody isotype being measured) was applied to wells dedicated for preparation of a standard curve. After incubation for 1 h at room temperature, plates were washed with wash solution (50 mM Tris, 14 M NaCl, 0.05% Tween, pH 8.0) using an EL 403 plate washer (Bio Tek Instruments, Winooski, VT) and then blocked using 1% BSA for 30 min. Diluted samples and standards (with standardized chicken IgG, IgA, and IgM concentrations) were applied to the wells and incubated for 1 h at room temperature. After washing, plates were incubated with the appropriate goat anti-chicken IgG/IgA/IgM-HRP conjugate at 1:50,000 for all wells for 1 h at room temperature. Plates were washed and developed using the TMB microwell peroxidase substrate system (3,3′,5,5′-tetramethyl benzidine and peroxidase; KPL, Inc., Gaithersburg, MD). Color development was stopped using 2 M H2SO4. Absorption was measured using an EL311 microtiter plate reader (Bio Tek Instruments, Winooski, VT) at a λ of 450 nm. To detect the total IgG, IgA, and IgM antibody concentration in the tracheal washes, wells were coated with the appropriate capture antibody (goat anti-chicken IgG-Fc/IgA/IgM, affinity purified). The remaining steps were performed as described above.
Statistical analysis.
Data on Bu-1, CD4+, and CD8+ cell numbers; numbers of antigen-specific IgG- and IgA-forming cells, and antibody concentrations in GT5- and sham-vaccinated chickens at different time points were compared by using the unpaired t test or the Mann-Whitney test (if normality failed). A P value of <0.05 was considered statistically significant. All statistical analyses were performed using the InStat Instant Biostatistics version 3.05 software program (GraphPad, San Diego, CA).
RESULTS
Protection of GT5-vaccinated chickens upon M. gallisepticum Rlow challenge.
GT5-vaccinated chickens had low lesion scores on day 1 post-Rlow challenge, characterized by few, scattered lymphocytic infiltrates without significant heterophilic infiltration. Conversely, sham-vaccinated chickens developed luminal exudates of heterophils (Table 1). A diffuse heterophilic infiltration became more prominent in sham-immunized birds by day 4 postchallenge, with an average lesion score of 1.375 (P < 0.05). The lamina propria was thickened due to mild to moderate, diffuse lymphocytic infiltrates containing some plasma cells, accompanied by deciliation of the epithelium and epithelial degeneration. GT5-vaccinated birds had few, discrete lymphocytic infiltrates without any significant heterophilic infiltration. On day 8 postchallenge, two of the four sham-immunized birds developed moderate to severe lesions with marked thickening of the mucosa due to infiltrates of large numbers of lymphocytes, histiocytes, and plasma cells within the lamina propria accompanied by few heterophils spread diffusely within the epithelium and in luminal exudates. GT5-vaccinated chickens had minimal to mild lymphocytic infiltrates associated with lymphoid follicles. On day 12 postchallenge, focal lymphoid infiltrates in the form of clearly defined follicles were found in GT5-vaccinated chickens. Sham-vaccinated birds developed thickening of the mucosal surface with focal lymphocytic infiltrates reminiscent of lymphoid follicles. By day 16 postchallenge, GT5-vaccinated chickens had minimal to mild lymphoid infiltrates, without any heterophilic infiltration, while three of four sham-vaccinated birds had mucosal thickening due to mild, multifocal lymphoid infiltrates.
TABLE 1.
Protection of GT5-vaccinated chickens upon challenge
| Day postchallenge | Vaccine/challenge | Tracheal lesion scorea | Tracheal mucosal thickness (μm)a |
|---|---|---|---|
| 1 | GT5/Rlow | 0.75 ± 0.15 | 51.7 ± 1.9 |
| PBS/Rlow | 1.62 ± 0.55 | 46.1 ± 6.0 | |
| 4 | GT5/Rlow | 0.88 ± 0.12 | 65.5 ± 9.5 |
| PBS/Rlow | 2.62 ± 0.55* | 111.6 ± 9.3** | |
| 8 | GT5/Rlow | 0.62 ± 0.12 | 37.0 ± 2.8 |
| PBS/Rlow | 1.25 ± 0.43 | 81.0 ± 32.2 | |
| 12 | GT5/Rlow | 1.12 ± 0.24 | 55.9 ± 7.2 |
| PBS/Rlow | 1.12 ± 0.24 | 67.9 ± 7.7 | |
| 16 | GT5/Rlow | 0.75 ± 0.14 | 48.2 ± 5.2 |
| PBS/Rlow | 1.88 ± 0.77 | 86.4 ± 25.4 |
Mean ± standard error of the mean for four chickens per group. *, P < 0.05 between GT5- and PBS-vaccinated chickens at each time point postchallenge; **, P < 0.01.
In keeping with previous studies, chickens vaccinated with GT5 or PBS alone (without challenge) had either no lesions or minimal multifocal lymphocytic infiltrates when examined at days 8 and 16 postvaccination (data not shown).
B, CD4+, and CD8+ cell numbers in GT5-vaccinated versus sham-vaccinated chickens.
GT5-vaccinated chickens had lower numbers of leukocytes (per centimeter of trachea) than sham-vaccinated chickens on all days examined (Fig. 1A), which was also reflected in the histopathological lesion scores (Table 1). Significantly higher numbers of leukocytes were isolated per centimeter of trachea in sham-immunized chickens on day 4 postchallenge (P = 0.05). By day 8, two of the sham-vaccinated chickens had developed moderate to severe lesions, with leukocyte numbers averaging more than 1.5 million cells/cm, whereas GT5-vaccinated chickens averaged fewer than 0.4 million cells per cm of trachea.
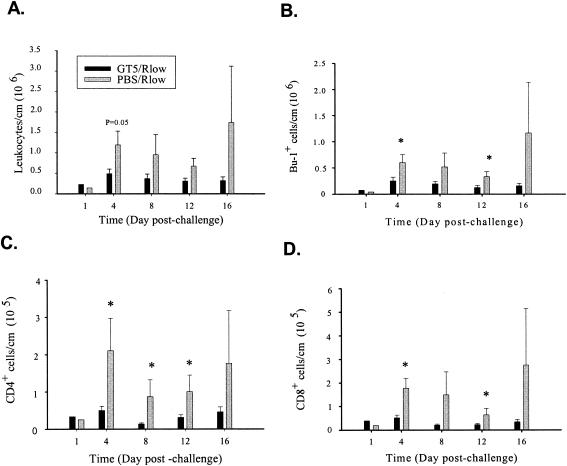
FIG. 1.
Kinetics of leukocyte infiltration/proliferation within the tracheas of GT5 (3 × 108 CFU)- or PBS (sham)-vaccinated chickens challenged with 1 × 107 CFU M. gallisepticum Rlow. Leukocytes were isolated from the chicken trachea and viable cells counted by trypan blue dye exclusion. The cells isolated from tracheas of two birds within the same group were pooled on day 1 postchallenge in order to acquire sufficient cell numbers. Cells were immunophenotyped using antibodies specific to chicken B cells (Bu-1) and CD4+ and CD8+ cells and analyzed by flow cytometry. The percentages of cells falling into each quadrant were multiplied by the total number of cells isolated and plotted at the appropriate time points. A. Total number of leukocytes per centimeter of trachea. B. Number of B cells per centimeter of trachea. C. Number of CD4+ cells per centimeter of trachea. D. Number of CD8+ cell per centimeter of trachea. Bars represent the means ± standard errors of the means for four birds per time point. *, statistical significance at P < 0.05.
Immunophenotypic analysis using monoclonal antibodies against chicken B-cell and CD4 and CD8 T-cell markers revealed that sham-vaccinated chickens had consistently higher numbers of B cells and CD4+ and CD8+ T cells than the GT5-vaccinated group on days 4, 8, and 12 postchallenge. B-cell numbers in sham-vaccinated chickens were two- to threefold higher than those in GT5-vaccinated chickens on days 4, 8, and 12 postchallenge (P < 0.05 on days 4 and 12) (Fig. 1B). CD4+ T-cell numbers were three- to sixfold higher (P < 0.05) (Fig. 1C) and CD8+ T-cell numbers were three- to sevenfold higher (P < 0.05 on days 4 and 12) (Fig. 1D) in sham-vaccinated chickens than in GT5-vaccinated chickens.
Immunohistochemical analysis of B, CD4+, and CD8+ cells and of IgG- and IgA-containing cells.
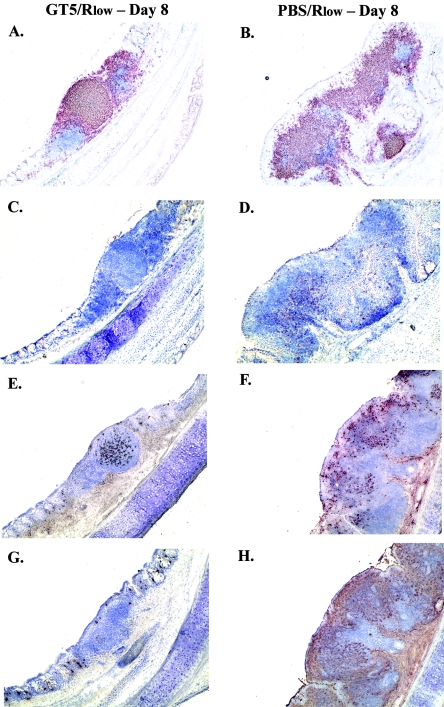
Immunohistochemical analysis of snap-frozen tissues was performed to further characterize the lymphocyte subpopulations (B, CD4+, and CD8+ cells) infiltrating the tracheas of GT5- versus sham-vaccinated chickens after Rlow challenge. Consistent with the flow cytometric results (Fig. 1B), B cells were found to be the major lymphocyte population infiltrating the tracheal lamina propria, beginning as early as day 1 postchallenge in both GT5- and PBS-vaccinated chickens. By day 4 postchallenge, GT5-vaccinated chickens had clusters of B cells surrounded by CD4+ cells, while sham-vaccinated chickens had increased numbers of B cells, both clustered and scattered within the lamina propria, with many interspersed CD4+ cells. CD8+ cells were diffusely scattered throughout the lamina propria, although sham-immunized birds had significantly higher numbers than those vaccinated with GT5. By day 8 postchallenge, the B-cell infiltrates of GT5-vaccinated chickens were organized in a typical follicular pattern, complete with parafollicular CD4+ cells (Fig. 2A), while two of the sham-immunized birds had less well-defined clusters of B cells interspersed with many CD4+ (Fig. 2B and CD8+ cells, resulting in a more generalized expansion of the lamina propria. CD8+ cells were scattered uniformly throughout the lamina propria (Fig. 2D). GT5-vaccinated chickens maintained this follicular architecture until day 16 postchallenge.
FIG. 2.
Immunohistochemical detection of B cells and CD4+ cells (A and B), CD8+ cells (C and D), IgG+ cells (E and F), and IgA+ cells (G and H) cells in tracheas of chickens vaccinated with GT5 (A, C, E, and G) or PBS (B, D, F, and H) on day 8 postchallenge. For A and B, double immunostaining of chicken trachea, detecting B cells (red) and CD4+ cells (blue), was performed. Lymphofollicular aggregates with less thickening of lamina propria were observed in GT5-vaccinated chickens, while large numbers of B, CD4+, and CD8+ cells along with IgG+ and IgA+ B/plasma cells were detected in sham-vaccinated chickens. Magnification, ×100.
Large numbers of IgG+ and IgA+ B/plasma cells were present in sham-vaccinated, Rlow-challenged chickens in comparison to GT5-vaccinated birds. IgA+ B/plasma cells were absent in both groups on day 1 postchallenge, although GT5-vaccinated chickens had more IgG+ cells than did the sham-vaccinated birds. On day 4, GT5-vaccinated chickens had greater numbers of IgG+ cells. Sham-vaccinated chickens had considerably fewer IgG+ cells, although many IgA+ cells were observed. By day 8 postchallenge, two of the four sham-immunized chickens had very high numbers of IgA+ and IgG+ B/plasma cells dispersed within the lamina propria (Fig. 2F and H), while GT5-vaccinated chickens had few IgA+ yet many diffusely scattered and some clustered IgG+ B/plasma cells within the follicular infiltrate (Fig. 2E and G). The number of IgA+ cells remained high in the sham-vaccinated chickens on day 12 postchallenge, while GT5-vaccinated chickens had only a few diffusely scattered IgA+ cells, with IgG+ cells more frequently localized within the follicular regions.
Immune response to M. gallisepticum infection in GT5- and sham-vaccinated chickens.
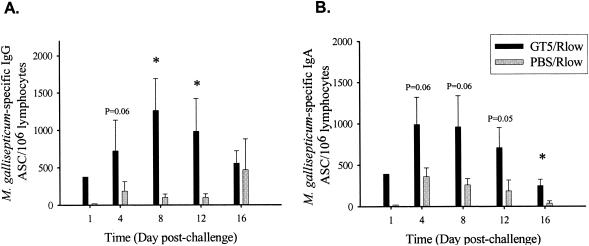
M. gallisepticum-specific IgA or IgG ELISPOT assays revealed significantly higher numbers of antigen-specific ASCs in the tracheas of GT5-vaccinated chickens than in those that were sham vaccinated. Birds vaccinated with PBS alone showed less than 20 (background level) ASCs per million leukocytes. GT5-vaccinated chickens had 8- to 60-fold-higher numbers of M. gallisepticum-specific IgG and IgA ASCs than did sham-vaccinated chickens as early as day 1 postchallenge. GT5-vaccinated chickens had 4-fold-higher IgG ASCs on day 4 (P = 0.06), which further increased to 12-fold on day 8 (P < 0.05), in comparison to sham-immunized chickens (Fig. 3A). GT5-vaccinated chickens also had three- to sevenfold-higher numbers of IgA ASCs on days 4, 8 (P = 0.06), 12 (P = 0.05), and 16 (P < 0.05) postchallenge than did sham-vaccinated animals (Fig. 3B). Although M. gallisepticum-specific IgG and IgA ASCs were comparable within the GT5-vaccinated chickens, sham-vaccinated chickens had twice as many specific IgA ASCs as IgG ASCs.
FIG. 3.
Numbers of M. gallisepticum-specific IgG (A)- and IgA (B)-secreting cells per million leukocytes isolated from the tracheas of GT5- or PBS-vaccinated chickens postchallenge as measured by ELISPOT assay. The cells isolated from tracheas of two birds within the same group were pooled on day 1 postchallenge in order to acquire sufficient cell numbers. GT5-vaccinated chickens had significantly higher numbers of specific IgG- and IgA-secreting B/plasma cells. Bars represent the means ± standard errors of the means for four birds at each time point. *, statistical significance at P < 0.05.
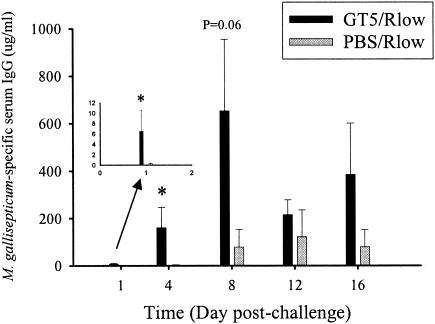
M. gallisepticum-specific serum IgG levels, as well as specific and total IgG and IgA levels in tracheal washes, were measured in GT5- and sham-vaccinated chickens on days 1, 4, 8, 12, and 16 postchallenge. GT5-vaccinated chickens exhibited a 20-fold-higher M. gallisepticum-specific serum IgG concentration on day 1 postchallenge (P = 0.01), increasing to 50-fold higher on day 4 (P = 0.01) and 8-fold higher on day 8 (P = 0.06), than sham-immunized chickens (Fig. 4). There was also a 34-fold increase in serum IgG concentration in GT5-vaccinated birds on day 4 postchallenge over prechallenge levels in birds receiving the GT5 vaccine only (without challenge), which had further increased to 136-fold by day 8. GT5-vaccinated chickens developed very high concentrations of M. gallisepticum-specific serum IgG as early as day 4 and 8 postchallenge, with concentrations of 160 μg/ml and 650 μg/ml, respectively, while sham-immunized chickens achieved a maximum concentration of 120 μg/ml on day 12. There was a similar pattern for M. gallisepticum-specific IgG concentration in the tracheal washes of vaccinated birds upon challenge. GT5-vaccinated birds showed significantly higher (12-fold; P = 0.01) M. gallisepticum-specific IgG levels on day 4 and a 5-fold-higher IgG level on day 8 postchallenge than sham-vaccinated birds (Fig. 5A). Conversely, M. gallisepticum-specific IgA levels in tracheal washes were higher in the sham-immunized than in the GT5-immunized chickens on days 4, 8, and 12 postchallenge, although this difference was not statistically significant (Fig. 5B). Birds vaccinated with PBS alone (without challenge) did not develop any detectable M. gallisepticum-specific antibodies in serum or tracheal washes.
FIG. 4.
M. gallisepticum-specific serum IgG concentration in chickens vaccinated with GT5 or PBS followed by Rlow challenge. An early and vigorous rise in specific serum IgG levels was observed in GT5-vaccinated chickens upon challenge. Bars represent the means ± standard errors of the means for four birds at each time point. *, statistical significance at P < 0.05.
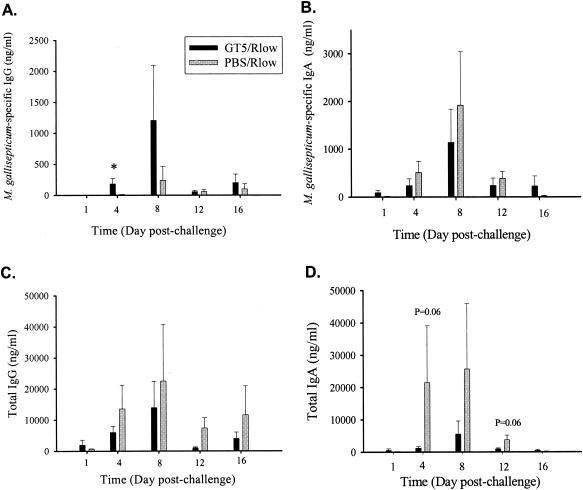
FIG. 5.
M. gallisepticum-specific (A and B) and total (C and D) IgG and IgA concentrations in tracheal washes of GT5- or PBS-vaccinated chickens following Rlow challenge. Significantly higher levels of specific IgG (A) in tracheal washes of GT5-vaccinated chickens were observed, while sham-vaccinated chickens had higher concentrations of specific IgA (B) as well as total IgG (C) and IgA (D). Bars represent the means ± standard errors of the means for four birds at each time point. *, statistical significance at P < 0.05.
Sham-vaccinated birds exhibited higher concentrations of total IgG and IgA in tracheal washes than did GT5-vaccinated birds on days 4, 8, and 12 postchallenge. Sham-vaccinated chickens had 1.5- to 7-fold-higher total IgG (7-fold on day 12; P = 0.01) and 3- to 18-fold-higher total IgA concentrations (18-fold on day 4; P = 0.06) than GT5-vaccinated chickens (Fig. 5C and D). When expressed as a percentage of the total IgG, the M. gallisepticum-specific IgG in the tracheal washes was significantly higher on days 4, 8, and 12 postchallenge in GT5-vaccinated chickens than in sham-vaccinated chickens (3.2, 6.8, and 5.2 versus 0.2, 1.2, and 0.8, respectively; P < 0.01). Similarly, percentages of M. gallisepticum-specific IgA in tracheal washes were two- to threefold higher on days 4, 8, and 12 postchallenge (P < 0.05 on days 4 and 8) in GT5-vaccinated chickens (Table 2).
TABLE 2.
GT5-vaccinated chickens have significantly higher proportions of M. gallisepticum-specific IgG and IgA in the tracheal washes than sham-vaccinated chickens after Rlow challenge
| Day postchallenge | Vaccine/challenge | M. gallisepticum-specific IgG (% of total IgG)a | M. gallisepticum-specific IgA (% of total IgA)a |
|---|---|---|---|
| 4 | GT5/Rlow | 3.2 ± 0.8** | 17.2 ± 3.9* |
| PBS/Rlow | 0.2 ± 0.06 | 5.3 ± 2.4 | |
| 8 | GT5/Rlow | 6.8 ± 1.1** | 30.3 ± 5.2* |
| PBS/Rlow | 1.2 ± 0.7 | 14.7 ± 4.0 | |
| 12 | GT5/Rlow | 5.2 ± 1.4** | 25.4 ± 10.1 |
| PBS/Rlow | 0.8 ± 0.07 | 10.5 ± 1.8 | |
| 16 | GT5/Rlow | 4.6 ± 1.7 | 23.6 ± 13.9 |
| PBS/Rlow | 2.0 ± 1.0 | 18.6 ± 6.7 |
Mean ± standard error of the mean for four chickens per group. *, P < 0.05 between GT5- and PBS-vaccinated chickens at each time point postchallenge; **, P < 0.01.
DISCUSSION
Vaccination is one of the preferred methods for controlling M. gallisepticum infections, and killed as well as live attenuated vaccines have been used commercially for this purpose. Although several efficacious live attenuated vaccines have been developed (1, 6, 15, 18, 35), the mechanisms by which they confer immune protection remain largely unknown. In a previous study (27), a live attenuated M. gallisepticum vaccine, GT5, protected chickens against challenge with a pathogenic dose of M. gallisepticum Rlow at 2 and 4 weeks postchallenge. The current study was designed to identify immunological correlates associated with protection in GT5-vaccinated chickens upon challenge and to compare these correlates with the immune mechanisms employed by nonvaccinated chickens in response to M. gallisepticum infection.
Many studies have emphasized the importance of mycoplasmal adhesion to epithelial surfaces of respiratory and urogenital tracts as a prerequisite to colonization and disease, and several avirulent mycoplasmas have been found to be lacking in the ability to adhere to the epithelium (4, 29). The GT5 strain of M. gallisepticum is attenuated in its ability to cytadhere despite the presence of GapA (major cytadhesin), along with other molecules such as LP64 (10) and vlhA (5) that also are believed to play roles in the adhesion process. The lack of cytadhesion of the GT5 strain was attributed to the absence of the cytadherence-related molecule CrmA, which subsequent complementation studies confirmed as a requirement for effective cytadherence of M. gallisepticum (26). The intimate association of adhering mycoplasmas to host cells causes numerous changes in the epithelium. These include the release of mucus granules from goblet cells and mucus glands, deciliation, detachment and rounding of epithelial cells, and infiltration of the respiratory mucosa by heterophils, histiocytes, and lymphocytes. The infiltration and proliferation of inflammatory cells leads to a dramatic increase in the thickness of the mucosa, primarily due to expansion of the lamina propria (9).
In the current study, we compared tracheal tissue of GT5- and sham-vaccinated chickens for immunological and immunopathological changes upon exposure to M. gallisepticum. The trachea was chosen due to its characteristic and well-described lesions (18). Protection of GT5-vaccinated chickens from challenge was clearly evident from the tracheal lesion scores and leukocyte cell numbers within the trachea (Table 1 and Fig. 1A). Histopathologic findings in tracheas of sham-immunized chickens revealed low to moderate infiltration of heterophils on days 1 and 4 postchallenge, an indication of an acute response, while GT5-vaccinated chickens had little or no heterophilic infiltration. Immunophenotypic analysis of leukocytes isolated from the trachea revealed significant differences in the numbers of B, CD4+, and CD8+ cells between GT5- and sham-immunized chickens after challenge (Fig. 1B to D). Sham-immunized birds had a two- to sevenfold increase in numbers of these cells on days 4, 8, and 12 postchallenge compared to GT5-vaccinated chickens, illustrating a progressive lymphocytic inflammatory response to infection with M. gallisepticum. The major lymphocyte population observed in the tracheas of both GT5- and sham-vaccinated birds was B cells, and previous studies have shown that bursal-dependent lymphoid cells are important in mediating resistance to M. gallisepticum infection (2, 16).
The current study documents three- and sevenfold increases in CD8+ cell numbers within the tracheas of sham-immunized chickens compared to GT5-vaccinated birds on days 4 and 8 postchallenge, respectively (Fig. 1D). Although others have suggested that these cells play a role in limiting the acute stages of respiratory disease (11, 30), their low numbers in vaccine-protected birds and increased numbers in inflamed tissues of sham-vaccinated birds raise questions regarding their role in disease pathogenesis. Many IgG+ and IgA+ B/plasma cells were also observed within the lamina propria of sham-vaccinated birds on days 8 (Fig. 2E to H) and 12 (data not shown) postchallenge compared to GT5-vaccinated birds. Thus, sham-vaccinated birds had a vigorous immune response upon Rlow challenge that was characterized by the infiltration of large numbers of B, CD4+, and CD8+ cells and a sizeable population of dispersed IgG+ and IgA+ B/plasma cells, while GT5-vaccinated chickens developed lymphofollicular aggregates reminiscent of secondary lymphoid follicles (Fig. 2A).
Since cytadherence of mycoplasmas to the host epithelial cell surface is considered a major virulence factor, we analyzed tracheal tissue for M. gallisepticum colonization by immunohistochemisty. As anticipated, M. gallisepticum Rlow colonized the tracheal epithelia of sham-immunized chickens on days 1, 4, 8, and 12 postchallenge (data not shown). The persistence of the organism, in spite of the vigorous host immune response in sham-vaccinated chickens, may be attributed to immune evasion by means of antigenic variation via altered expression of vlhA (12, 22) and other immunogenic proteins (19). Tracheas of chickens vaccinated with GT5 did not reveal any M. gallisepticum colonization by immunohistochemical staining at any of the time points examined, with the exception of one bird which exhibited small, focal areas of M. gallisepticum colonization on day 1 postchallenge (data not shown). It appears that GT5-vaccinated chickens are protected upon challenge by preventing M. gallisepticum attachment/colonization followed by rapid clearance of the organism.
ELISPOT analysis indicated 8- to 60-fold-higher numbers of mycoplasma-specific ASCs (plasma cells/memory B cells) within the trachea in GT5-vaccinated chickens compared to sham-vaccinated chickens as early as day 1 postchallenge (Fig. 3A and B). Furthermore, there was a rapid increase in M. gallisepticum-specific serum IgG levels as early as day 4 postchallenge in the GT5-vaccinated chickens (up to 50-fold higher; P < 0.05) when compared to sham-vaccinated chickens (Fig. 4). Antigen-specific serum immunoglobulin has been shown to confer protection against air sac lesions when high-titer M. gallisepticum-specific antiserum was passively transferred to naive chickens (21). There is also evidence of transudation of serum antibodies onto mucosal surfaces (33, 34). The similar kinetics of specific IgG in the tracheal washes (Fig. 5A), although at very low concentration, and specific serum IgG concentration over time also supports the notion that transudation of antibodies occurs from blood plasma to mucosal tissue. At the same time, ELISPOT data and immunohistochemical staining of IgG+ and IgA+ cells also suggest that there is local secretion of antibodies onto the mucosal surface. The relative importance of locally secreted versus transudated IgG, as well as their relative contributions to the total pool of mucosal antibodies, is unknown at this time. These local antibody responses have long been thought to play a major role in preventing mycoplasmal attachment to respiratory epithelia (3, 38).
In contrast to specific serum and mucosal IgG responses, and consistent with our previous findings (27), the specific IgA levels in tracheal washes of sham-immunized chickens were higher than those observed in GT5-vaccinated birds (Fig. 5B). The persistence of mycoplasmas in sham-vaccinated birds, even in the presence of a vigorous M. gallisepticum-specific IgA response, suggests that secretory IgA might play an important role in limiting initial attachment/colonization by the organism rather than clearing an established infection. At the same time, the rapid induction of high levels of IgG in serum and tracheal secretions in GT5-vaccinated chickens, within 24 to 72 h of initial infection, may facilitate rapid clearance of the organism. In addition, the low numbers of organisms that manage to traverse the epithelium may be cleared by antibody-mediated phagocytosis and complement activation (23).
GT5-vaccinated chickens had a significantly higher proportion of M. gallisepticum-specific IgG and IgA in the tracheal washes than did sham-vaccinated chickens (Table 2). The low percentage of specific antibody in tracheal washes of sham-vaccinated chickens was due to a high total antibody concentration (Fig. 5C and D). This increase in total antibody concentration observed in sham-vaccinated chickens after Rlow challenge might be due to nonspecific, polyclonal B-cell activation or might be a result of a vigorous response to phase-variant epitopes expressed on M. gallisepticum that are not represented in the organisms used as coating antigen in the ELISA and ELISPOT assay (and thereby missed). Certain mycoplasmas, such as M. fermentans, M. arthritidis, M. pneumoniae, and M. gallisepticum, were found to be mitogenic for mouse lymphocytes in vitro (8). Polyclonal antibody production induced by mycoplasmas has also been reported in vivo (29, 31, 32). The capacity to activate lymphocytes in a nonspecific, polyclonal manner might contribute to M. gallisepticum pathogenicity, leading to lesion development and modulation of immunity, thus enabling it to evade host immune defense mechanisms and at the same time establishing chronic disease. For example, polyclonal B-cell activation may lead to a high degree of Fc receptor occupancy by nonspecific antibodies, thereby reducing the likelihood of granulocytic or monocytic phagocytosis of M. gallisepticum. However, nonspecific stimulation of lymphocytes by M. gallisepticum needs to be more thoroughly investigated in vivo in future studies.
By examining early time points postchallenge, the current study documents an unanticipated rapid and vigorous immune response to M. gallisepticum infection following GT5 vaccination. This was characterized by a dramatic increase in both IgG and IgA ASCs in the tracheal lamina propria (observed at both days 1 and 4) and a concomitant rise in serum anti-M. gallisepticum IgG. This work provides additional evidence for the importance of a locally induced humoral immunological memory response to prevent the establishment and progression of mycoplasmal disease, ostensibly by reducing or preventing cytadherence and colonization. This was achieved by vaccination with the live attenuated strain GT5, which was previously shown to be protective against challenge with a pathogenic dose of M. gallisepticum Rlow. Future studies will focus on the analysis of various chemokines and cytokines that are secreted in response to M. gallisepticum infection in vivo and their role in disease pathogenesis.
Acknowledgments
We thank Kevin Kavanagh, Roger Zemek, and Don Bessette for animal care; Vickie Weidig for technical assistance with necropsy; Ione Jackman and Denise Long Woodward for tissue processing and histological preparations; and S. Shafiuddin for assistance with ELISPOT assays.
This work was supported by USDA grant 58-1940-0-007.
Editor: J. D. Clements
REFERENCES
- 1.Abd-el-Motelib, T. Y., and S. H. Kleven. 1993. A comparative study of Mycoplasma gallisepticum vaccines in young chickens. Avian Dis. 37:981-987. [PubMed] [Google Scholar]
- 2.Adler, H. E., B. J. Bryant, D. R. Cordy, M. Shifrine, and A. J. DaMassa. 1973. Immunity and mortality in chickens infected with Mycoplasma gallisepticum: influence of the bursa of fabricus. J. Infect. Dis. 127:61-68. [DOI] [PubMed] [Google Scholar]
- 3.Avakian, A. P., and D. H. Ley. 1993. Protective immune response to Mycoplasma gallisepticum demonstrated in respiratory-tract washings from M. gallisepticum-infected chickens. Avian Dis. 37:697-705. [PubMed] [Google Scholar]
- 4.Banai, M., I. Kahane, S. Razin, and W. Bredt. 1978. Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect. Immun. 2:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boguslavsky, S., D. Menaker, I. Lysnyansky, T. Liu, S. Levisohn, R. Rosengarten, M. Garcia, and D. Yogev. 2000. Molecular characterization of the Mycoplasma gallisepticum pvpA gene, which encodes a putative variable cytadhesin protein. Infect. Immun. 68:3956-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter, T. E., E. T. Mallinson, K. F. Miller, R. F. Gentry, and L. D. Schwartz. 1980. Vaccination with F-strain Mycoplasma gallisepticum to reduce production losses in layer chickens. Avian Dis. 25:404-411. [PubMed] [Google Scholar]
- 7.Chancock, R. M., L. Hayflick, and M. F. Barile. 1961. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc. Natl. Acad. Sci. USA 48:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, B. C., K. E. Aldridge, and J. R. Ward. 1977. Mycoplasma-dependent activation of normal lymphocytes: mitogenic potential of mycoplasmas for mouse lymphocytes. Infect. Immun. 18:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dykstra, M. J., S. Levisohn, O. J. Fletcher, and S. H. Kleven. 1985. Evaluation of cytopathologic changes induced in chicken tracheal epithelium by Mycoplasma gallisepticum in vivo and in vitro. Am. J. Vet. Res. 46:116-122. [PubMed] [Google Scholar]
- 10.Forsyth, M. H., M. E. Tourtellotte, and S. J. Geary. 1992. Localization of an immunodominant 64 kDa lipoprotein (LP64) in the membrane of Mycoplasma gallisepticum and its role in cytadherence. Mol. Microbiol. 6:2099-2106. [DOI] [PubMed] [Google Scholar]
- 11.Gaunson, J. E., C. J. Philip, K. G. Whithear, and G. F. Browning. 2000. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology 146:1223-1299. [DOI] [PubMed] [Google Scholar]
- 12.Glew, M. D., G. F. Browning, P. F. Markham, and I. D. Walker. 2000. pMGA phenotypic variation in Mycoplasma gallisepticum occurs in vivo and is mediated by trinucleotide repeat length variation. Infect. Immun. 68:6027-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh, M. S., T. S. Gorton, M. H. Forsyth, K. E. Troy, and S. J. Geary. 1998. Molecular and biochemical analysis of a 105 kDa Mycoplasma gallisepticum cytadhesin (GapA). Microbiology 144:2971-2978. [DOI] [PubMed] [Google Scholar]
- 14.Kleven, S. H. 1986. Tracheal populations of Mycoplasma gallisepticum after challenge of bacterin-vaccinated chickens. Avian Dis. 29:1013-1017. [PubMed] [Google Scholar]
- 15.Kleven, S. H. 1997. Changing expectations in the control of Mycoplasma gallisepticum. Acta Vet, Hung. 45:299-305. [PubMed] [Google Scholar]
- 16.Lam, K. M., and W. Lin. 1984. Resistance of chickens immunized against Mycoplasma gallisepticum is mediated by bursal dependent lymphoid cells. Vet. Microbiol. 9:509-514. [DOI] [PubMed] [Google Scholar]
- 17.Levisohn, S., M. J. Dykstra, M. Y. Lin, and S. H. Kleven. 1986. Comparison of in vivo and in vitro methods of pathogenicity evaluation for Mycoplasma gallisepticum in respiratory infection. Avian Pathol. 15:233-246. [DOI] [PubMed] [Google Scholar]
- 18.Levisohn, S., and S. H. Kleven. 2000. Avian mycoplasmosis (Mycoplasma gallisepticum). Rev. Sci. Tech. 19:425-442. [PubMed] [Google Scholar]
- 19.Levisohn, S., R. Rosengarten, and D. Yogev. 1995. In vivo variation of Mycoplasma gallisepticum antigen expression in experimentally infected chickens. Vet. Microbiol. 45:219-231. [DOI] [PubMed] [Google Scholar]
- 20.Ley, D. H. 2003. Mycoplasma gallisepticum infection, p. 722-744. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry. Iowa State Press, Ames.
- 21.Lin, M. Y., and S. H. Kleven. 1984. Transferred humoral immunity in chickens to Mycoplasma gallisepticum. Avian Dis. 28:79-87. [PubMed] [Google Scholar]
- 22.Markham, P. F., M. D. Glew, G. F. Browning, K. G. Whithear, and I. D. Walker. 1998. Expression of two members of the pMGA gene family of Mycoplasma gallisepticum oscillates and is influenced by pMGA-specific antibodies. Infect. Immun. 66:2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall, A. J., R. J. Miles, and L. Richards. 1995. The phagocytosis of mycoplasmas. J. Med. Microbiol. 43:239-250. [DOI] [PubMed] [Google Scholar]
- 24.Nunoya, T., M. Tajima, T. Yagihashi, and S. Sannai. 1987. Evaluation of respiratory lesions in chickens induced by Mycoplasma gallisepticum. Jpn. J. Vet. Sci. 49:621-629. [DOI] [PubMed] [Google Scholar]
- 25.Nunoya, T., K. Kanai, T. Yagihashi, S. Hoshi, K. Shibuya, and M. Tajima. 1997. Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens. Avian Pathol. 26:391-398. [DOI] [PubMed] [Google Scholar]
- 26.Papazisi, L., S. Jr. Frasca, M. Gladd, X. Liao, D. Yogev, and S. J. Geary. 2002. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum in chickens. Infect. Immun. 70:6839-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papazisi, L., L. K. Silbart, S. Frasca Jr., D. Rood, X. Liao, M. Gladd, M. A. Javed, and S. J. Geary. 2002. A modified live Mycoplasma gallisepticum vaccine to protect chickens from respiratory disease. Vaccine 20:3709-3719. [DOI] [PubMed] [Google Scholar]
- 28.Papazisi, L., K. E. Troy, T. S. Gorton, X. Liao, and S. J. Geary. 2000. Analysis of cytadherence-deficient, GapA-negative Mycoplasma gallisepticum strain R. Infect. Immun. 68:6643-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy, S. K., P. Singh, A. Silim, and J. A. Newman. 1998. Lymphoproliferative responses of specific-pathogen-free chickens to Mycoplasma gallisepticum strain PG31. Avian Pathol. 27:277-283. [DOI] [PubMed] [Google Scholar]
- 31.Ruuth, E., and F. Praz. 1989. Interactions between mycoplasmas and the immune system. Immunol. Rev. 112:133-160. [DOI] [PubMed] [Google Scholar]
- 32.Simecka, J. W., S. E. Ross, G. H. Cassell, and J. K. Davis. 1993. Interactions of mycoplasmas with B cells: antibody production and nonspecific effects. Clin. Infect. Dis. 17:176-182. [DOI] [PubMed] [Google Scholar]
- 33.Suresh, P., and L. H. Arp. 1995. A time-course study of the transfer of immunoglobulin G from blood to tracheal and lacrimal secretions in young turkeys. Avian Dis. 39:349-354. [PubMed] [Google Scholar]
- 34.Toro, H., P. Lavaud, P. Vallejos, and A. Ferreira. 1993. Transfer of IgG from serum to lachrymal fluid in chickens. Avian Dis. 37:60-66. [PubMed] [Google Scholar]
- 35.Whithear, K. G. 1996. Control of avian mycoplasmoses by vaccination. Rev. Sci. Tech. Off. Int. Epiz. 15:1527-1553. [DOI] [PubMed] [Google Scholar]
- 36.Wu, C. C., D. Thiagarajan, and T. L. Lin. 1998. ELISPOT assay for detection of antibody secreting cells to infectious bursal disease virus in chickens. Poultry Sci. 77:662-665. [DOI] [PubMed] [Google Scholar]
- 37.Yagihashi, T., T. Nunoya, and M. Tajima. 1986. Immunity induced with aluminum hydroxide-adsorbed Mycoplasma gallisepticum bacterin in chickens. Avian Dis. 31:149-155. [PubMed] [Google Scholar]
- 38.Yagihashi, T., and M. Tajima. 1986. Antibody responses in sera and respiratory secretions from chickens infected with Mycoplasma gallisepticum. Avian Dis. 30:543-550. [PubMed] [Google Scholar]