Abstract
Acanthamoebae produce a painful, sight-threatening corneal infection. The adhesion of parasites to the host cells is a critical first step in the pathogenesis of infection. Subsequent to adhesion, the parasites produce a potent cytopathic effect (CPE) leading to target cell death. Recent studies showing that acanthamoebae express a mannose-binding protein (MBP) and that free α-mannose (α-Man) specifically inhibits the adhesion of parasites to host cells suggest that the MBP plays a key role in the pathogenesis of Acanthamoeba infection by mediating host-parasite interactions. However, direct evidence showing that Acanthamoeba MBP is a virulence protein has been lacking. In this study, we demonstrate that the polyclonal immunoglobulin Y (IgY) antibodies prepared against affinity-purified Acanthamoeba MBP markedly inhibit the adhesion of parasites to host cells. The antibody also inhibited the Acanthamoeba-induced CPE on host cells. In contrast, preimmune IgY did not influence either the adhesion of the parasites to host cells or the amoeba-induced CPE. Using a variety of approaches, including affinity chromatography on an α-Man gel, electrophoresis under native and denaturing conditions, biotinylation of cell surface proteins, and immunostaining, it was conclusively established that Acanthamoeba MBP is located on the surface membranes of the parasites. Neutral-sugar analysis and lectin binding experiments using succinylated concanavalin A, a plant lectin with high affinity for mannose, revealed that Acanthamoeba MBP is itself a mannose-containing glycoprotein. N-Glycanase treatment to remove N-linked oligosaccharides shifted the subunit molecular mass of MBP from 130 kDa to 110 kDa. Hexosamine analysis revealed that Acanthamoeba MBP lacks detectable levels of GalNAc, suggesting the absence of O-linked oligosaccharides. In summary, we have characterized Acanthamoeba MBP and have shown that it is a major virulence protein responsible for host-parasite interactions and the parasite-induced target cell destruction.
Acanthamoebae produce a painful, sight-threatening corneal infection known as Acanthamoeba keratitis (9, 15, 21). While the cornea is the only known susceptible tissue in healthy, immunocompetent individuals, this protozoan parasite produces chronic granulomatous amoebic encephalitis and disseminating infections, including dermatitis and pneumonitis, in immunocompromised individuals (13, 14). It is generally accepted that minor trauma to the corneal epithelium caused by contact lens wear (2, 9, 22) or other noxious agents and exposure to contaminated solutions, including lens care products and tap water (8, 11), are two major predisposing factors in the pathogenesis of the keratitis. Studies aimed at characterization of the molecular mechanism by which the parasite invades the corneal tissue have suggested that a carbohydrate-based recognition system plays a key role in the adhesion of the parasites to the host cells (16, 19, 24) and the amoeba-induced cytopathic effect (CPE) that occurs subsequent to the adhesion (3, 12). Specifically, these studies have shown that (i) acanthamoebae bind to a neoglycoprotein, mannosylated bovine serum albumin (Man-BSA), but not to galactose-BSA (1); (ii) methyl-α-mannopyranoside (α-Man), but not closely related saccharides, including mannitol and glucose (Glc), specifically inhibit the adhesion of acanthamoebae to the surfaces of corneal buttons, as well as to corneal epithelial cells in culture (1, 17, 19, 25); (iii) α-Man, α1-3-d-mannobiose, α1-3,α1-6-d-mannotriose, and α1-3,α1-6,α1-3,α1-6-d-mannopentose, which inhibit the amoeba's binding to Man-BSA, are also potent inhibitors of amoeba-induced CPE on corneal epithelial cells (1); and (iv) acanthamoebae express an ∼400-kDa mannose-binding protein (MBP) that is constituted of multiple 130-kDa subunits (4). While these studies indirectly suggest that the adhesion of Acanthamoeba to the corneal surface is mediated by interactions between a mannose-specific lectin on the surface of the amoeba and mannose residues of glycoproteins of the corneal epithelium, direct evidence demonstrating that the Acanthamoeba MBP is a virulence protein is lacking. In large part, this is due to difficulties in transfection of genes into Acanthamoeba cells. In the present study, using antibodies against affinity-purified Acanthamoeba MBP, we provide direct evidence that the amoeba lectin is a major virulence protein. Clearly, MBP can mediate the adhesion of parasites to host cells only if it is located on the cell surface. Our recent studies of cDNA sequence analysis of MBP have suggested that the lectin is a transmembrane protein (4). In addition, in an early preliminary study using a cell surface biotinylation technique, we showed that a 136-kDa component with affinity for mannose is present on surface membranes of the amoebae (25). In the present study, by immunostaining and electrophoresis of affinity-purified cell surface biotin-labeled MBP under native and denaturing conditions, we conclusively established that Acanthamoeba MBP is indeed a cell surface protein. Finally, we demonstrate here for the first time that Acanthamoeba MBP is itself a mannose-containing glycoprotein.
MATERIALS AND METHODS
Parasites and epithelial cells.
A clinical isolate of Acanthamoeba derived from an infected human cornea (MEEI 0184; Acanthamoeba castellanii, based on morphological characteristics) was used throughout this study. The parasites were axenically cultured in a proteose peptone-yeast extract-glucose medium (7). Under these conditions, more than 90% of the parasites were recovered as trophozoites. Immortalized cultures of rabbit corneal epithelial cells were prepared as described earlier (1). A colon cancer epithelial cell line (Caco-2) was obtained from the American Type Culture Collection (Manassas, VA). These cell lines were cultured to confluence on culture plates and used for Acanthamoeba binding or CPE assays.
Saccharides and saccharide-conjugated matrices.
A neoglycoprotein, Man-BSA (15 to 25 mol of α-d-mannopyrannoside/mol of BSA), and α-Man gel were purchased from EY Laboratories, Inc. (San Mateo, CA). Methyl-α-d-galactopyrannoside (α-Gal), α-Glc, and α-Man were purchased from Pfanstiehl Laboratories Inc. (Waukegan, IL); N-acetyl-d-galactosamine (GalNac) and mannitol were purchased from Sigma-Aldrich Inc. (St. Louis, MO).
Isolation and characterization of membrane-associated mannose-binding protein of Acanthamoeba.
To determine whether the MBP of Acanthamoeba is cell membrane associated, the parasites were labeled with a membrane-impermeable reagent (EZ-Link Sulfo-NHS-Biotin; Pierce, Rockford, IL) which covalently attaches biotin to proteins on the outer surfaces of intact cells (6). The biotin-labeled proteins were chromatographed on the α-Man affinity column, and the bound fraction eluted with 0.1 M α-Man was electrophoresed and analyzed by staining of the blots with an avidin-biotin-peroxidase reagent. Briefly, the amoebae (109) were washed with ice-cold phosphate-buffered saline (PBS) and incubated with the biotin-labeling reagent (0.5 mg of Sulfo-NHS-Biotin) in 5 ml of PBS for 30 min at room temperature. At the end of the incubation period, the parasites were washed three times with ice-cold PBS to remove the excess biotinylation reagent. Biotin-labeled amoebae were lysed by two freeze-thaw cycles in resuspension buffer (25 mM Tris-HCl [pH 7.2], 100 mM NaCl, 20 mM CaCl2, 1 mM phenylmethylsulfonyl fluoride), and the cell fragments were pelleted and washed by centrifugation (10 min; 1,000 × g) in resuspension buffer. To isolate the MBP, the plasma membrane proteins in the pellet were solubilized in extraction buffer {0.5% 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate [CHAPS] plus 2 mM β-mercaptoethanol in resuspension buffer} and were chromatographed on an α-Man affinity column as described above. The biotinylated proteins eluted from the column using α-Man were electrophoresed and blotted onto nitrocellulose membranes. To visualize the biotinylated proteins, the blots were sequentially incubated with 2% BSA in PBS (overnight; 4°C), a freshly prepared solution of avidin-biotin-peroxidase complex (0.5%; 30 min) (Vector Laboratories, Burlingame, CA), and diaminobenzidine-H2O2 reagent (0.05% diaminobenzidine-0.01% H2O2-0.04% nickel chloride in Tris-HCl buffer, pH 7.2). The sugar binding specificity of the biotinylated MBP was tested by a dot blot assay. For this, a sheet of nitrocellulose paper was coated with a neoglycoprotein, Man-BSA (1 μg/ml; 1 h; 37°C); nonspecific binding sites were blocked with 2% BSA in PBS (overnight; 4°C); and the paper was placed in a dot blot apparatus. The blot was exposed to the affinity-purified biotinylated amoeba MBP (100 ng/well) in the absence or presence of 0.1 M α-Man, mannitol, α-Gal, or GalNAc (30 min; 37°C) and was then developed using a freshly prepared solution of avidin-biotin-peroxidase complex.
To determine whether the MBP itself is mannosylated, duplicate aliquots of isolated Acanthamoeba MBP (300 ng/lane) were electrophoresed on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels (10) and transferred onto nitrocellulose papers using a Trans-Blot cell (Bio-Rad, Hercules, CA) (23). The blots were treated with 2% BSA in PBS (4°C; overnight), incubated with biotin-labeled succinylated concanavalin A (s-ConA) (1 μg/ml; Vector Laboratories) in the absence or presence of 0.1 M α-Man and were then sequentially incubated with a freshly prepared solution of avidin-biotin-peroxidase complex (Vectastain ABC kit [Vector Laboratories, Inc.]; 0.5% in 25 mM Tris-HCl, pH 7.4; 30 min) and a diaminobenzidine-H2O2 reagent (0.05% diaminobenzidine-0.01% H2O2-0.04% nickel chloride in Tris-HCl buffer, pH 7.2).
Neutral-sugar and hexosamine analyses of affinity-purified unlabeled MBP prepared as described earlier (4) were performed at the Glycotechnology Core, University of California, San Diego. To assess, the extent to which the N-linked oligosaccharides contribute to the molecular weight of the MBP, purified Acanthamoeba MBP was incubated with peptide-N-glycosidase F (PNGase F; New England Biolabs, Beverly, MA) for 1 h at 37°C following the manufacturer's instructions. Control reactions were incubated in the enzyme buffer alone. The reaction mixtures were electrophoresed on 10% SDS-polyacrylamide gels, and the electrophoretic mobility of the MBP treated with PNGase was compared with that of control MBP incubated in the enzyme buffer alone. A known N-linked carbohydrate-containing glycoprotein (RNase B) was used as a positive control.
Western blot analysis.
Polyclonal anti-MBP immunoglobulin Y (IgY) antibody was produced in chickens as described in our previous work using affinity-purified MBP (4). Briefly, the IgY was purified from the egg yolk (anti-MBP IgY) using a proprietary method (Aves Labs Inc., Tigard, OR). The purified preparation was >95% IgY as determined by Coomassie staining of SDS-polyacrylamide gel electrophoresis gels. For comparison purposes, preimmune IgY was isolated from the egg yolk of the same hen collected prior to immunization. For Western blot analysis, affinity-purified MBP was electrophoresed in native and in 8% reducing SDS-polyacrylamide gels. Proteins from the gels were transferred onto nitrocellulose filters. The protein blots were treated overnight at room temperature with 5% dry milk in PBS to block nonspecific binding sites and were then sequentially incubated with anti-MBP IgY (20 μg/ml; 37°C; 1 h), horseradish peroxidase-linked goat anti-chicken IgY (Aves Labs Inc.; 0.2 μg/ml; 37°C; 1 h), and a freshly prepared solution of diaminobenzidine-H2O2 reagent (0.05% diaminobenzidine-0.01% H2O2-0.04% nickel chloride in Tris-HCl buffer, pH 7.2). The membranes were washed extensively with PBS containing 0.1% Tween 20 after each incubation step. Control reactions were performed as described above, except that the blots were incubated with preimmune IgY instead of the anti-MBP IgY.
Immunostaining of Acanthamoeba trophozoites by anti-MBP IgY.
Acanthamoeba trophozoites (106 cells) were incubated with purified anti-MBP IgY or preimmune IgY (80 μg/ml in 0.5% BSA in PBS; 30 min) and subsequently with fluorescein-labeled goat anti-chicken IgY (Aves Labs Inc.; 10 μg/ml in 0.5% BSA in PBS; 30 min). After being thoroughly rinsed with PBS, the cells were smeared onto coverslips, fixed in 2.5% paraformaldehyde, rinsed three times, and mounted. The staining was examined using a Leica TCS SP2 confocal microscope.
Analysis of the effect of anti-MBP on the adhesion of Acanthamoeba to Man-BSA and corneal epithelial cells and amoeba-induced cytopathic effect.
Prior to use for the binding or CPE assays, Acanthamoeba parasites (106 cells/ml in PBS; >95% trophozoites) were incubated with anti-MBP IgY or preimmune IgY (0.2 mg/ml; 37°C; 30 min) and rinsed five times with PBS. Incubation of the parasites with the anti-MBP did not influence the apparent health or the morphology of the parasite. To detect the effect of the anti-MBP on the adhesion of parasites to Man-BSA, binding assays were performed as described in published protocols (1). Briefly, wells of microtiter plates were coated with Man-BSA, nonspecific binding sites were blocked with 3% BSA in PBS (1 h; room temperature), a 50-μl aliquot of 35S-labeled Acanthamoebae (5 × 105 cells/ml in PBS; 1 to 2 cpm/parasite; >95% trophozoites) pretreated with either anti-MBP IgY or preimmune IgY was added to each well, and the plates were incubated for 30 min at room temperature. At the end of the incubation period, the plates were rinsed with PBS to remove unbound acanthamoebae, 0.1 ml of 2% SDS was added to each well, and the radioactivity in aliquots of solubilized material was determined in a scintillation counter. For assessing Acanthamoeba binding to epithelial cells, the same procedure was used, except that instead of microtiter wells coated with Man-BSA, confluent cultures of rabbit corneal epithelial cells in 24-well plates were used and the blocking step was omitted.
A CPE assay described in our published study (1) was used to assess the effect of anti-MBP on the amoeba-induced destruction of epithelial cell monolayers. Briefly, the parasites (>95% trophozoites) were rinsed three times in a serum-free medium supplemented with 0.4% BSA, and aliquots of the parasite suspension (106 parasites/ml; 100 μl/well for 48-well plates) pretreated with either anti-MBP IgY or preimmune IgY were added to wells of confluent cultures of corneal or intestinal (Caco-2) epithelial cells that had been rinsed and preincubated in the serum-free medium for 2 h (n = 4/group). The plates were then incubated at 37°C for 18 h in a CO2 incubator and examined under a phase-contrast microscope for the presence of cell-free plaques in the monolayer. At the end of the incubation period, the cultures were stained with Giemsa (Diff-Quik; Dade Diagnostic Inc., Aguada, PR), and the approximate cell density in each well was estimated by using the Plot Density Tool of Quantity One software (Bio-Rad, Hercules, CA).
RESULTS
Acanthamoeba MBP is membrane associated.
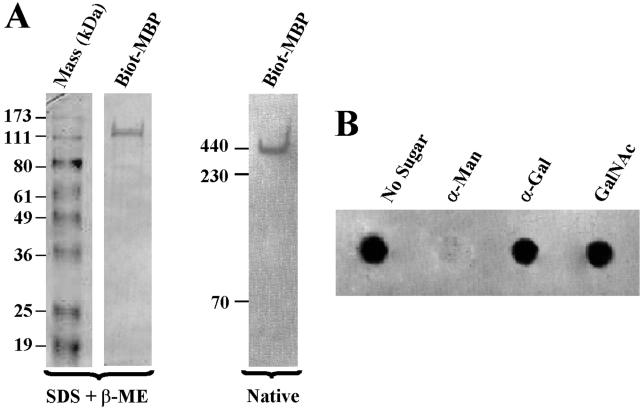
In an early preliminary study using a cell surface biotinylation technique, we showed that a 136-kDa component with affinity for mannose is present on surface membranes of amoebae (25). To conclusively establish that the amoeba MBP is a membrane protein, cell surface proteins of Acanthamoeba were biotinylated, and detergent extracts of biotinylated parasites were chromatographed on an α-Man affinity column. Proteins bound to the column were eluted with 0.1 M α-Man and electrophoresed under denaturing and native conditions, and the protein blots were developed using a freshly prepared solution of avidin-biotin-peroxidase complex. Under denaturing-reducing conditions, a biotin-labeled 130-kDa component was detected (Fig. 1A, left), whereas under native conditions (nonreducing-nondenaturing), a biotin-labeled ∼400-kDa component was detected (Fig. 1A, right). This pattern is identical to that of affinity-purified MBP prepared from unlabeled parasites (data not shown and reference 4). The yield of biotinylated MBP was 10 μg/109 parasites. To assess the sugar binding specificity of the cell surface-labeled MBP, a dot blot assay was used. Among the saccharides tested, α-Man, but not a number of other sugars, including α-Gal and GalNAc (Fig. 1B) and mannitol (not shown), inhibited binding of the biotinylated MBP to Man-BSA.
FIG. 1.
Acanthamoeba MBP is membrane associated. (A) Cell surface proteins of Acanthamoeba were biotinylated (Biot), and detergent extracts of biotinylated parasites were chromatographed on an α-Man affinity column. The amoeba MBP was eluted with 0.1 M α-Man and electrophoresed under denaturing or native conditions. Proteins from the gels were transferred onto a nitrocellulose paper, and the blots were developed using a freshly prepared solution of avidin-biotin-peroxidase complex. Note that under native nondenaturing conditions, a component migrating close to 440-kDa ferritin was detected, whereas under denaturing conditions, a 130-kDa component was detected. (B) Biotinylated MBP has affinity for α-Man. A sheet of nitrocellulose paper was incubated with a neoglycoprotein, Man-BSA, and blocked with BSA, and the paper was placed in a dot blot apparatus. The paper was then exposed to affinity-purified biotinylated MBP in the absence or presence of 0.1 M α-Man, α-Gal, or GalNAc, and the blot was developed using a freshly prepared solution of avidin-biotin-peroxidase complex. Note that the biotinylated component bound to Man-BSA, and this binding was inhibited by α-Man but not by other sugars.
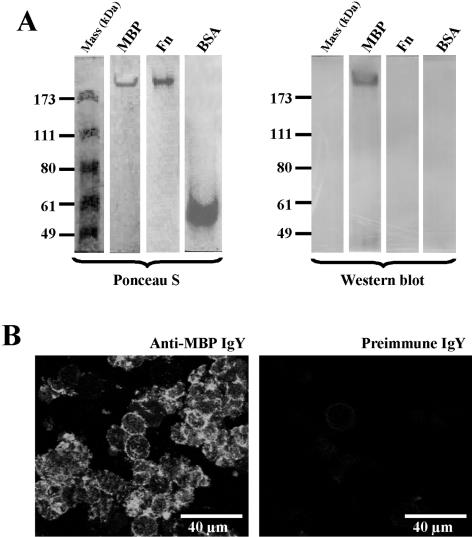
To further confirm that Acanthamoeba MBP is a cell surface protein, the parasites were immunostained using the purified polyclonal antibody produced in chickens against affinity-purified MBP (anti-MBP IgY). We have previously shown that this antibody is highly specific for the 130-kDa component seen in the reducing SDS-polyacrylamide gel electrophoresis gels of the affinity-purified MBP and that it does not react with numerous components present in the total extract of the parasite (4). Also, the antibody binds to recombinant MBP (4). Prior to using the antibody for the immunostaining of parasites, we first performed Western blot analysis to ensure that the antibody also binds the ∼400-kDa native MBP. Indeed, the antibody reacted strongly with the affinity-purified ∼400-kDa MBP seen in the electrophoresis blot of the native gel but not with a number of other proteins, including ferritin (Fn), BSA, and molecular mass markers (Fig. 2A, right), or catalase and lactoferrin (not shown). Also, the MBP did not react with preimmune IgY (not shown). Cell membranes of Acanthamoeba stained intensely with anti-MBP IgY (Fig. 2B, left). In contrast, the preimmune IgY either reacted weakly or did not react with the amoeba cell membranes (Fig. 2B, right). Thus, the IgY antibody, which binds specifically to MBP, has the ability to recognize this lectin on the amoeba cell surface.
FIG. 2.
Western blot analysis and immunohistochemical localization of Acanthamoeba MBP. (A) Affinity-purified amoeba MBP was electrophoresed under native conditions. After electrophoresis, the proteins were transferred onto nitrocellulose membranes; the protein blots were stained with Ponceau S and then processed for immunostaining with anti-MBP IgY. The IgY antibody reacted with the MBP, but not with ferritin (Fn), BSA, or molecular mass markers. Also the MBP did not react with preimmune IgY (not shown). (B) Trophozoites (106 cells) were immunostained in cell suspension using anti-MBP IgY (left) or preimmune IgY (right) and FITC-labeled goat anti-chicken IgY. After being immunostained, the cells were smeared onto glass slides, fixed with 2.5% paraformaldehyde, and visualized under the confocal microscope.
Acanthamoeba MBP is a mannose-containing glycoprotein.
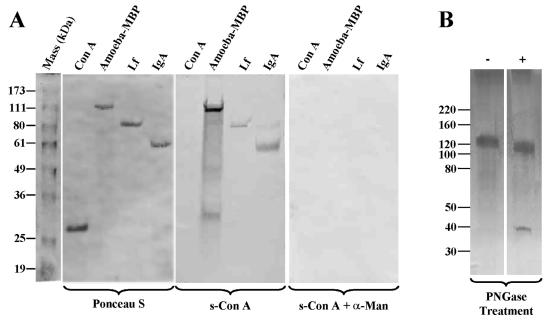
To determine if MBP itself is mannosylated, electrophoresis blots of the affinity-purified MBP were tested for reactivity with biotinylated s-ConA, a lectin with high affinity for α-Man. The amoeba MBP reacted strongly with s-ConA (Fig. 3A, middle). The binding of the MBP to s-ConA was completely inhibited by free α-Man (Fig. 3A, right). Lactoferrin and human IgA served as positive controls for mannose-containing glycoproteins, whereas ConA, which lacks mannose, served as a negative control (Fig. 3A). Neutral-sugar analysis revealed that α-Man and galactose are the major neutral sugars present in the MBP. Together, these two sugars constituted 94% of total neutral sugars of the lectin (α-Man, 27%; galactose, 67%). Fucose was detected only in trace amounts (1.9%). A small amount of glucose detected in the MBP (3.7%) is likely to be an environmental contaminant. Hexosamine analysis indicated that the MBP contains N-acetyl-d-glucosamine (GlcNAc) but lacks GalNac at detectable levels (data not shown), indicating that the MBP contains largely N-linked oligosaccharides. After treatment with PNGase F to remove N-linked oligosaccharides, the electrophoretic mobility of the MBP on the SDS gels shifted from ∼130 kDa to ∼110 kDa (Fig. 3B). As expected, PNGase F itself migrated as a 37-kDa component (Fig. 3B).
FIG. 3.
Acanthamoeba MBP is a mannose-containing glycoprotein. (A) Duplicate aliquots of purified Acanthamoeba MBP were electrophoresed on denaturing 10% SDS-polyacrylamide gels, and proteins from the gels were transferred onto a nitrocellulose paper. The blots were stained with Ponceau S (left) and were subsequently reacted with biotinylated s-ConA in the absence (middle) or presence (right) of 0.1 M α-Man. The blots were developed using a freshly prepared solution of avidin-biotin-peroxidase complex. Note that the amoeba MBP reacted strongly with s-ConA. The binding of s-ConA to MBP was abolished by α-Man. Lactoferrin (Lf) and human IgA (IgA) served as positive controls for mannose-containing glycoproteins, whereas ConA, which lacks mannose, served as a negative control. (B) Affinity-purified MBP was incubated in the absence (−) and presence (+) of PNGase F (1 h; 37°C). Aliquots from the reaction mixtures were electrophoresed on SDS-polyacrylamide gels. Note that PNGase F treatment resulted in an electrophoretic mobility shift of the MBP from ∼130 kDa to ∼110 kDa, showing that the protein contains N-linked oligosaccharides.
Antibodies to MBP inhibit the adhesion of Acanthamoeba to Man-BSA and to epithelial cells, and Acanthamoeba-induced cytopathic effect.
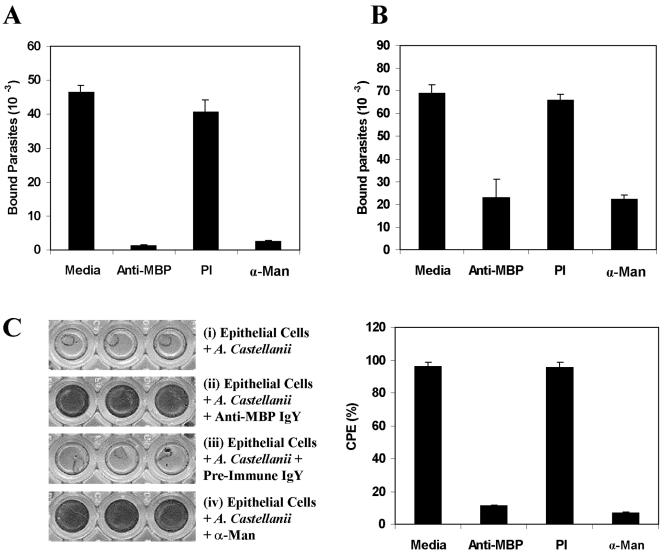
To determine if the MBP plays a role in the mannose-mediated binding of Acanthamoeba to mannose-containing glycoproteins and to epithelial cells, binding was performed using parasites which had been preincubated with purified anti-MBP. The antibody markedly inhibited the adhesion of parasites to both Man-BSA (73.7% ± 0.6%; n = 12; P < 0.0005) (Fig. 4A) and epithelial cells (52% ± 1.6%; n = 12; P < 0.0005) (Fig. 4B). In contrast, preimmune IgY was a poor inhibitor, exhibiting 23.2% ± 1.4% (n = 12; P < 0.0005) and 20% ± 3.2% (n = 12; P < 0.0005) inhibition of amoeba binding to Man-BSA and to corneal epithelial cells, respectively (Fig. 4A and B). Similar results were obtained regardless of whether the adhesion assays were performed using corneal or intestinal (Caco-2) epithelial cells.
FIG. 4.
Polyclonal anti-MBP IgY inhibits (i) adhesion of Acanthamoeba to Man-BSA and to epithelial cells and (ii) amoeba-induced cytopathic effect. Wells of microtiter plates coated with Man-BSA (A) or monolayer cultures of epithelial cells (B) were incubated with 35S-labeled amoebae that had been pretreated with medium alone (Media), anti-MBP IgY (Anti-MBP), preimmune IgY (PI), or 100 mM α-Man. After being washed with PBS, the numbers of bound parasites were estimated as the radioactivity recovered from the wells. The means plus standard deviations of three experiments are shown (n = 12 for each group). (C) Anti-Acanthamoeba MBP inhibits amoeba-induced CPE. Epithelial cells were cultured to confluence on 96-well plates and then incubated for 18 h with acanthamoebae that had been preincubated with either anti-MBP IgY, preimmune IgY, or 100 mM α-Man. After being washed, the cells were fixed, stained with Giemsa, and photographed (left). The approximate cell density in each well was estimated by using the Plot Density tool of Quantity One software (Bio-Rad, Hercules, CA). Clear, unstained regions indicate loss of cells (i and iii). Dark, stained areas indicate the presence of cells (ii and iv). Note that the amoeba-induced CPE of host cells was completely inhibited when the assays were performed using anti-MBP IgY-treated parasites (ii). Similar results were obtained regardless of whether the experiments were performed with rabbit corneal epithelial cells or Caco-2 cells.
We previously established that subsequent to adhesion to the host cells, the amoebae produce a potent CPE characterized by the destruction of monolayer epithelial cells in culture (1). To determine whether the anti-MBP inhibits the amoeba-induced CPE, the CPE assays were performed using the parasites that had been preincubated with either purified anti-MBP IgY or preimmune IgY. Control cultures incubated with untreated (Fig. 4C, i) or preimmune-IgY-treated (Fig. 4C, iii) parasites were completely destroyed within 18 h after exposure to the parasites. The amoeba-induced CPE of host cells was almost completely inhibited when the CPE assays were performed using anti-MBP IgY-treated parasites (Fig. 4C, ii). Also, as we have shown before (1), free mannose was a potent inhibitor of the amoeba-induced CPE (Fig. 4C, iv). Reproducible results were obtained in three independent CPE assays, as well as adhesion assays.
DISCUSSION
The observations presented here corroborate our recent studies showing that the Acanthamoeba MBP is a major virulence protein. In addition, we show that the MBP is itself a mannose-containing glycoprotein and conclusively establish that the lectin is located on the surface membranes of parasites.
Neutral-sugar analyses and lectin binding experiments using s-ConA, a plant lectin with high affinity for α-Man, revealed that Acanthamoeba MBP is itself a mannose-containing glycoprotein. This is important because the putative carbohydrate-based intramolecular interactions may substantially influence the folding, conformation, and secondary structure of the MBP. In this respect, it is well established that oligosaccharides linked to protein backbones can significantly influence folding of glycosylated proteins (5, 18, 20). Since the electrophoretic mobility of the MBP shifted from 130 kDa to 110 kDa after treatment with PNGase F to remove N-linked oligosaccharides, it appears that ∼15% of the apparent mass of MBP is carbohydrate. Our finding that the MBP lacks detectable levels of GalNAc, a saccharide that links O-linked oligosaccharides to the protein backbone, suggests that the O-linked oligosaccharides do not contribute significantly to the mass of the MBP.
In the present study, we conclusively established that Acanthamoeba MBP is indeed a cell surface protein. This is important because the MBP can mediate the adhesion of parasites to host cells only if it is located on the cell surface. Since the MBP isolated from the unlabeled parasite extracts is a 400-kDa protein that is constituted of 130-kDa subunits (4), we first demonstrated that biotinylation of parasites labels a protein that migrates as a 400-kDa component in nonreducing gels and as a 130-kDa component on reducing gels. Second, we confirmed that the 400-kDa biotin-labeled component that bound α-Man gel is indeed a mannose-binding protein by showing that in dot blot assays, it bound to Man-BSA, and that this binding was inhibited by α-Man but not by a number of other sugars. Third, antibodies to Acanthamoeba MBP robustly stained the cell membranes of the parasites. These data are consistent with our recent study of cloning and sequencing of the Acanthamoeba MBP, which revealed that the amoeba lectin is a transmembrane protein with characteristics of a typical cell surface receptor (4).
The first step in the pathogenesis of infection is the adhesion of parasites to the host cells. Subsequent to adhesion, the parasites induce a potent CPE. As described above, in earlier studies, we showed that free mannose specifically inhibits the adhesion of amoebae to host cells and the amoeba-induced CPE (1). Therefore, it was of interest to determine whether the antibody prepared against the purified MBP has the potential to inhibit Acanthamoeba binding to host cells and the subsequent CPE. Indeed, we demonstrated that polyclonal anti-MBP prepared in chickens was a potent inhibitor of Acanthamoeba adhesion to epithelial cells and the Acanthamoeba-induced CPE. These data provide direct evidence that Acanthamoeba MBP is a major virulence protein responsible for interaction with host tissues and subsequent CPE. In this respect, in a recent study, we found that oral immunization with recombinant MBP protects against Acanthamoeba keratitis in a hamster animal model and that this protection correlates with the appearance of MBP-specific IgA in the tears of immunized animals (M. Garate, unpublished data). The tear-specific IgA is likely to provide protection by preventing the parasite from binding to the corneal epithelium. Characterization of the biological role of the MBP is an important step in understanding the mechanism of Acanthamoeba keratitis and lays a foundation for future studies to develop better therapeutic strategies for the management of the disease.
Acknowledgments
This work was supported by NIH grants EY09349 (N.P.) and EY08706 and a core grant (EYP30-13078) for vision research, by the Massachusetts Lions Eye Research Fund, and by the New England Corneal Transplant Research Fund.
We thank Zhiyi Cao and Mary Visciano for helpful comments and editorial assistance, respectively.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Cao, Z., D. M. Jefferson, and N. Panjwani. 1998. Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J. Biol. Chem. 273:15838-15845. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, E. J., J. C. Fulton, C. J. Hoffman, C. J. Rapuano, and P. R. Laibson. 1996. Trends in contact lens-associated corneal ulcers. Cornea 15:566-570. [PubMed] [Google Scholar]
- 3.De Jonckheere, J. F. 1980. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol. 39:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garate, M., Z. Cao, E. Bateman, and N. Panjwani. 2004. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J. Biol. Chem. 279:29849-29856. [DOI] [PubMed] [Google Scholar]
- 5.Helenius, A. 1994. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol. Biol. Cell 5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurley, W. L., E. Finkelstein, and B. D. Holst. 1985. Identification of surface proteins on bovine leukocytes by a biotin-avidin protein blotting technique. J. Immunol. Methods 85:195-202. [DOI] [PubMed] [Google Scholar]
- 7.Jensen, T., W. G. Barnes, and D. Meyers. 1970. Axenic cultivation of large populations of Acanthamoeba Castellanii (JBM). J. Parasitol. 56:904-906. [PubMed] [Google Scholar]
- 8.Kilvington, S., T. Gray, J. Dart, N. Morlet, J. R. Beeching, D. G. Frazer, and M. Matheson. 2004. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Investig. Ophthalmol. Vis. Sci. 45:165-169. [DOI] [PubMed] [Google Scholar]
- 9.Kumar, R., and D. Lloyd. 2002. Recent advances in the treatment of Acanthamoeba keratitis. Clin. Infect. Dis. 35:434-441. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Larkin, D. F., S. Kilvington, and D. L. Easty. 1990. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br. J. Ophthalmol. 74:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin, D. F., M. Berry, and D. L. Easty. 1991. In vitro corneal pathogenicity of Acanthamoeba. Eye 5:560-568. [DOI] [PubMed] [Google Scholar]
- 13.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marciano-Cabral, F., R. Puffenbarger, and G. A. Cabral. 2000. The increasing importance of Acanthamoeba infections. J. Eukaryot. Microbiol. 47:29-36. [DOI] [PubMed] [Google Scholar]
- 15.McCulley, J. P., H. Alizadeh, and J. Y. Niederkorn. 2000. The diagnosis and management of Acanthamoeba keratitis. CLAO J. 26:47-51. [PubMed] [Google Scholar]
- 16.Moore, M. B., J. E. Ubelaker, J. H. Martin, R. Silvany, J. M. Dougherty, D. R. Meyer, and J. P. McCulley. 1991. In vitro penetration of human corneal epithelium by Acanthamoeba castellanii: a scanning and transmission electron microscopy study. Cornea 10:291-298. [DOI] [PubMed] [Google Scholar]
- 17.Morton, L. D., G. L. McLaughlin, and H. E. Whiteley. 1991. Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro. Infect. Immun. 59:3819-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou, W. J., P. H. Cameron, D. Y. Thomas, and J. J. Bergeron. 1993. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature 364:771. [DOI] [PubMed] [Google Scholar]
- 19.Panjwani, N., Z. Zhao, J. Baum, L. D. Hazlett, and Z. Yang. 1997. Acanthamoebae bind to rabbit corneal epithelium in vitro. Investig. Ophthalmol. Vis. Sci. 38:1858-1864. [PubMed] [Google Scholar]
- 20.Parodi, A. J. 2000. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem. J. 348:1. [PMC free article] [PubMed] [Google Scholar]
- 21.Radford, C. F., D. C. Minassian, and J. K. G. Dart. 2002. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br. J. Ophthalmol. 86:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stehr-Green, J. K., T. M. Bailey, and G. S. Visvesvara. 1989. The epidemiology of Acanthamoeba keratitis in the United States. Am. J. Ophthalmol. 107:331-336. [DOI] [PubMed] [Google Scholar]
- 23.Timmons, T. M., and B. S. Dunbar. 1990. Protein blotting and immunodetection. Methods Enzymol. 182:679-688. [DOI] [PubMed] [Google Scholar]
- 24.van Klink, F., H. Alizadeh, G. L. Stewart, M. S. Pidherney, R. E. Silvany, Y. He, et al. 1992. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr. Eye Res. 11:1207-1220. [DOI] [PubMed] [Google Scholar]
- 25.Yang, Z., Z. Cao, and N. Panjwani. 1997. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect. Immun. 65:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]