Abstract
Neisseria meningitidis is a human specific pathogen that is part of the normal nasopharyngeal flora. Little is known about the metabolic constraints on survival of the meningococcus during colonization of the upper airways. Here we show that glucose and lactate, both carbon energy sources for meningococcal growth, are present in millimolar concentrations within nasopharyngeal tissue. We used a mutant defective for the uptake of lactate (C311ΔlctP) to investigate the contribution of this energy source during colonization. Explants of nasopharyngeal tissue were inoculated with the wild-type strain (C311) and C311ΔlctP; the mutant was recovered at significantly lower levels (P = 0.01) than C311 18 h later. This defect was not due to changes in the expression of adhesins or initial adhesion in C311ΔlctP to epithelial cells. Instead, lactate appears to be important energy source for the bacterium during colonization and is necessary for growth of the bacterium in nasopharyngeal tissue. Studies with other strains defective for the uptake of specific nutrients should provide valuable information about the environment in which N. meningitidis persists during carriage.
Neisseria meningitidis is an important cause of septicemia and meningitis (4, 11). However, in the overwhelming majority of instances infection with this bacterium is entirely asymptomatic (2). N. meningitidis colonizes the human nasopharynx in up to 40% of individuals (5, 17), and this is the sole reservoir of the bacterium.
Little is known about the metabolic requirements of the bacterium during colonization, even though the ability to acquire key nutrients is essential for the successful infection of a host. Neisseria spp. have strict nutritional requirements and can utilize only a limited number of carbon energy sources, including glucose and lactate (7). These are found in combination in many sites in the body, such as in serum, cerebrospinal fluid, and secretions of the upper airways (18), although the levels of lactate and glucose in tissue from the nasopharynx are unknown. We sought to investigate the role of lactate acquisition by N. meningitidis during colonization.
The interaction between N. meningitidis and human epithelial cells is an early step in colonization, and has been studied extensively. The principle adhesin expressed by the meningococcus is the type 4 pilus (Tfp), (9), while other surface proteins, including as Opas and Opc, contribute to the initial adhesion of strains, especially those lacking pili and capsule (1, 25). However, N. meningitidis is also found internal to the epithelial cell layer in the nasopharynx (17), so we used the organ culture model (OCM) to examine the effect of lactate availability on successful colonization by N. meningitidis. In the OCM, explants of nasopharyngeal tissue are maintained with an air interface and can be successfully infected with pathogenic bacteria (13, 15). The OCM has the major advantage that it includes the full repertoire and organization of cell types and extracellular matrix proteins that N. meningitidis encounters during colonization.
We have previously identified the lactate permease of N. meningitidis (3a). NMB0543 (20) is predicted to encode a protein with 14 transmembrane domains located in the inner bacterial membrane, which is consistent with a role as a permease. A mutant lacking lctP was unable to take up lactate and failed to grow with lactate as the sole carbon source, while growth on glucose was equivalent to that of the wild-type strain; complementation restored the ability of the mutant to grow on lactate and its virulence in the infant rat model. Here, we used the lctP mutant to further understand the nutritional requirements of N. meningitidis during nasopharyngeal colonization, a critical stage in the pathogenesis of meningococcal infection.
MATERIALS AND METHODS
Bacterial strains and growth.
N. meningitidis strain C311 is a serogroup B strain isolated from a patient with invasive meningococcal infection (24). C311ΔlctP carries a deletion in NMB0543 (19) that encodes the N. meningitidis lactate permease (3a). The mutation was backcrossed into the parental strain, and multiple colonies were collected to exclude any influence from phase variation and other second site mutations. In C311ΔpilE, pilE has been insertionally inactivated with the gene encoding resistance to kanamycin. N. meningitidis was grown on brain heart infusion medium with 5% Levanthal's supplement. Escherichia coli was propagated on Luria-Bertani media. Kanamycin (Kan) was added at 75 and 25 μg ml−1 for N. meningitidis and E. coli, respectively.
Organ culture model.
Human nasopharyngeal mucosa explants were derived from adenoids resected from children with noninfective conditions after informed consent from parents or guardians was obtained. The study was approved by the South Sheffield Research Ethics Committee. Tissue was immersed in antibiotic-containing minimal essential medium (MEM; Gibco, Uxbridge, United Kingdom) for 4 h and dissected to produce 3 to 4 mm blocks of mucosa (13, 14, 15). The tissue was then transferred to MEM without antibiotics for 1 h and then supported in a 4-cm petri dish with the epithelial surface projecting above a bed of non-nutrient agar. The technique of culturing explants with an air interface was then used (13, 22). The petri dish with the agar-embedded explant was placed inside a 10-cm petri dish containing a reservoir of MEM that was perfused into the agar with a wick. The OCM was incubated in a humidified atmosphere of 5% CO2 in air at 37°C.
Measurement of meningococcal survival within nasopharyngeal tissue.
To measure meningococcal survival within the tissue, 100 μl of phosphate-buffered saline (PBS) containing ca. 1.5 × 108 CFU of N. meningitidis was placed on the surface of the explant. After 4 h and 18 h of incubation, explants were washed, weighed, and then homogenized in a modified French press (Constant Systems, Warwick, United Kingdom) at 10 lb/in2. Viable bacteria within the homogenized tissue were enumerated by plating onto solid media. Each strain was inoculated into eight OCMs from different individuals.
Measurement of lactate and glucose.
Nasopharyngeal tissue (n = 5) was lysed by using a cell disruptor as described above and then passed through 0.4-μm pore filter. The amount of lactate and glucose in the lysates was measured on an automated Beckman Synchron LX20 analyzer and adjusted for the volume of tissue. Lactate was assayed by its conversion to pyruvate by lactate dehydrogenase and modification of the NAD to NADH coenzyme (monitored by absorbance at 340 nm). Glucose levels were determined by its metabolism by glucose oxidase in the presence of oxygen. Each sample was loaded into a reaction cup containing a Beckman oxygen electrode (Beckman-Coulter, High Wycombe, United Kingdom). The rate of oxygen consumption was measured, and the glucose concentration within the samples was calculated from the standard curve.
Western analysis.
Whole-cell lysates were prepared from overnight cultures of bacteria, and the cell density was adjusted to 1010 CFU ml−1 and mixed with an equal volume of dissociation buffer (50 mM Tris [pH 6.8], 2% sodium dodecyl sulfate [SDS], 0.1% bromophenol blue, 10% glycerol). Samples were denatured by boiling for 5 min prior to fractionation by SDS-16% polyacrylamide gel electrophoresis before transfer to polyvinylidene difluoride membranes (Immobilon-P; Millipore) for 90 min at 70 V. Membranes were washed in 0.5% milk in PBS and then incubated for 2 h at room temperature with monoclonal antibody SM1 (anti-pilin) (23), B306 (anti-Opc) (26), A222 (anti-Opc) (8), or B33 (anti-Opa) (21) at a 1:10,000 dilution, followed by 1 h of incubation with the secondary antibody (Dako) at the same dilution. Cross-reaction was detected by using the ECL chemiluminescence rapid detection kit (Amersham)
Interactions with epithelial cells.
For adhesion to epithelial cells, bacteria were harvested from solid media into PBS, adjusted to the desired concentration, and then added to Chang epithelial cells at a multiplicity of infection of 100:1 (bacteria to epithelial cells). Adhesion to the cells was assayed by standard protocols (27); cells were infected for 3 h and washed three times to remove nonadherent bacteria, and then the adherent bacteria were recovered after lysis of host cells in saponin (0.1%) for 10 min. Assays were carried out in triplicate and on three independent occasions.
Statistical analysis.
Data were collected from experiments by using explants from multiple donors. Comparisons between mutant and wild-type bacteria were initially screened by Kruskal-Wallis analysis and, where the null hypothesis was rejected, comparisons between the lctP mutant and wild-type were tested by Mann-Whitney U using the SPSS statistical package (version 11) after adjustment for variation in the inocula. Kruskal-Wallis analysis was used to analyze levels of lactate and glucose in explants.
RESULTS
Measurement of carbon sources in nasopharyngeal tissue.
To evaluate the carbon sources available to N. meningitidis during colonization, levels of glucose and lactate were measured in homogenates of nasopharyngeal tissue. Glucose and lactate were present in all five samples analyzed (Table 1), with levels similar to those present in serum (reference serum levels of 0.6 to 2.4 mmol/liter and 3.9 to 5.8 mmol/liter for lactate and glucose, respectively). The amount of lactate or glucose did not change significantly over time and was not affected by infection either with the wild-type strain or lctP-deficient bacteria.
TABLE 1.
Levels of lactate and glucose in nasopharyngeal tissue
| Strain | Concn(mM)a
|
|||
|---|---|---|---|---|
| Lactate
|
Glucose
|
|||
| 4 h | 18 h | 4 h | 18 h | |
| Uninfected | 1.50 (1.10) | 1.00 (0.68) | 6.84 (2.83) | 6.20 (1.60) |
| C311 | 2.64 (1.21) | 1.00 (1.06) | 7.12 (1.05) | 6.70 (1.85) |
| C311ΔlctP | 1.74 (0.70) | 0.64 (0.47) | 7.14 (2.78) | 5.88 (1.17) |
Results are shown with standard deviations in parentheses. There was no significant difference in concentrations between infected (with either C311 or C311ΔlctP) and noninfected tissues or at the two time points (Kruskal-Wallis analysis).
The lactate permease is required for effective colonization of the organ culture model.
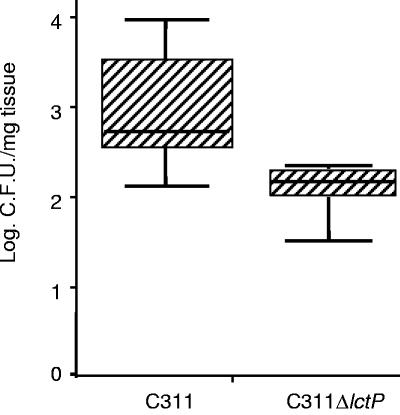
Next, the ability of C311ΔlctP to colonize the OCM was compared to the wild-type strain; both strains were assayed in explants from eight patients. After 18 h of incubation, C311ΔlctP was at a significant disadvantage compared to C311, with the mutant colonizing at approximately a tenth of the level of the wild-type bacterium (Fig. 1, P = 0.01). The organization of open reading frames around lctP indicates that the colonization defect of C311ΔlctP could not have arisen due to polar effects but resulted from the loss of the lactate permease.
FIG. 1.
The lactate permease is required for efficient nasopharyngeal colonization. Nasopharyngeal tissue explants (eight for each strain) received 1.5 × 108 CFU of bacteria, followed by incubation for 18 h. The number of recovered bacteria is shown (the 25th and 75th percentiles are indicated by the shaded box). The wild type is recovered in 10-fold-greater levels than the mutant (Mann-Whitney, P = 0.01).
Loss of the lactate permease does not affect the expression of known adhesins.
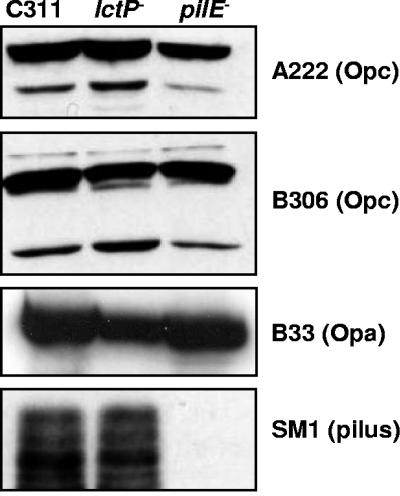
Strains unable to express Tfp are defective for survival in the OCM (12; R. M. Exley, R. B. Sim, L. Goodwin, Y. Li, E. N. Mowe, R. C. Read, and C. M. Tang, Abstr. 14th International Pathogenic Neisseria Conference, p. 275, 2004). To determine whether the diminished colonization capacity of the lctP mutant resulted from alterations in adhesins at the bacterial surface, the expression of Tfp, Opa, and Opc was examined. Western analysis was performed on whole-cell lysates of the wild-type strain, C311ΔlctP, and a pilE mutant. Loss of lctP was not associated with a detectable change in the production of Tfp, Opa, and Opc by N. meningitidis (Fig. 2).
FIG. 2.
Expression of adhesins by the lctP mutant. Whole-cell extracts were obtained from the strains, separated by SDS-16% polyacrylamide gel electrophoresis, and transferred to membranes. The reactivities of extracts with the monoclonal antibodies SM1 (α-pili), B306 (α-Opc), A222 (α-Opc), and B33 (α-Opa) are shown.
Bacteria lacking lctP have enhanced adherence with isolated epithelial cells.
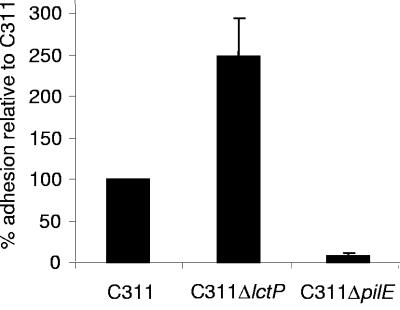
To examine whether lctP might contribute to colonization during the interaction of the bacterium with epithelial cells, Chang conjunctival cells were infected with C311 and C311ΔlctP, and the adhesion of the strains was compared. As a negative control, cells were infected with bacteria unable to express Tfp (C311ΔpilE), the principle meningococcal adhesin. As expected, the pilE mutant strain adhered to Chang cells at reduced rates compared to C311. The lctP mutant did not have a defect for host cell adhesion, but instead exhibited enhanced association with epithelial cells compared to the wild-type strain (Fig. 3). Infected epithelial cells were also examined by immunofluorescence microscopy; there was no evidence that clumping of the lctP mutant that could account for its enhanced recovery in the adhesion assays (not shown).
FIG. 3.
The lactate permease is not defective for adhesion to epithelial cells. Bacteria were incubated with Chang cells at a multiplicity of infection of 100:1 for 3 h, and adherent bacteria were enumerated by plating. C311ΔlctP associates with epithelial cells at higher levels than the wild-type strain (Student t test, P < 0.01; error bars show the standard error of the mean).
The lctP mutant does not exhibit an early colonization defect.
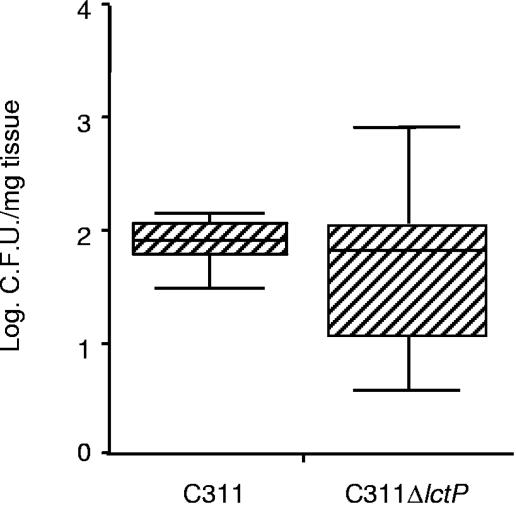
Although the lctP mutant was not defective during interactions with isolated epithelial cells, these cells may not be fully representative of the cells to which N. meningitidis adheres in the OCM during the initial stage of colonization. Therefore, to provide further insights into the role of LctP on meningococcal colonization, OCMs were infected with the wild-type strain or C311ΔlctP, and bacteria were recovered only 4 h later. At this early time point, there was no detectable difference in the level of C311ΔlctP and C311 recovered from the tissue (Fig. 4).
FIG. 4.
The lactate permease is not necessary for the early stage of nasopharyngeal colonization. Bacteria were harvested from nasopharyngeal tissue explants 4 h after receiving 1.5 × 108 CFU of N. meningitidis. The numbers of recovered bacteria are shown (the 25th and 75th percentiles are indicated by the shaded box; Mann-Whitney, P > 0.05).
DISCUSSION
Colonization of the nasopharynx by N. meningitidis is a complex series of events that requires efficient scavenging of vital nutrients from human tissues, evasion of innate immune killing, and tropism to specific host cells. Here, we used a mutant defective for the acquisition of lactate to demonstrate that N. meningitidis must acquire this carbon source to colonize nasopharyngeal tissue successfully. Rather than only studying the interaction of the bacterium with isolated epithelial cells, we utilized the OCM, which provides the broad range of cellular environments and metabolic constraints encountered by the bacterium in the upper airway.
We found that the levels of both glucose and lactate in nasopharyngeal tissue are comparable to those in serum. Both are known to be effective carbon sources for N. meningitidis. However, levels of glucose and lactate did not alter appreciably during infection with the wild-type or lctP mutant bacteria. This may be because N. meningitidis utilizes only a fraction of the total carbon sources available in the upper airway. Alternatively, there might be mechanisms in the tissue that replenish the levels of glucose and lactate.
There are several potential explanations for the colonization defect of the lctP mutant, including (i) enhanced susceptibility to innate immune effectors, (ii) failure to interact with certain host cells, and (iii) inability to acquire nutrients for growth. We have recently shown that the N. meningitidis lctP mutant is susceptible to complement-mediated lysis, a finding consistent with the effect of adding lactate to the gonococcus (10, 18). However, there is little or no proinflammatory response to N. meningitidis in the upper airway, with colonization being asymptomatic in the overwhelming majority of cases. In the absence of active inflammation, complement levels at the mucosal surface are negligible (6), and therefore serum-resistant strains would not have a selective advantage for survival in the OCM. Alternatively, the mutant might be selectively eliminated by other components of the innate immune response such as antimicrobial peptides or during interactions with phagocytic cells. To colonize the nasopharynx effectively, N. meningitidis needs to express adhesins to bind host cells in the upper airways. We found that the expression of Tfp and other adhesins was not affected in the lctP mutant, which actually exhibited enhanced association with epithelial cells. The likeliest reason for this increased adhesion is that the lipopolysaccharide of wild-type bacteria is more extensively sialylated than in an lctP mutant (Exley et al., in press), which may inhibit bacterial association with epithelial cells (3).
We propose that the reason for the attenuation of C311ΔlctP is a failure to replicate at wild-type levels through an inability to utilize available lactate for growth. This is supported by our finding that the lactate permease mutant colonized at wild-type levels early on after infection (at 4 h). If the mutant was excluded either through enhanced susceptibility to complement or failure to adhere to epithelial cells, C311ΔlctP should be at a competitive disadvantage soon after infection. Between 4 and 18 h, the number of wild-type bacteria increased in the OCMs by an order of magnitude, whereas the level of infection with C311ΔlctP remained almost constant. We have shown previously that lactate is utilized more rapidly than glucose in the cerebrospinal fluid and that additional lactate enhances the growth rate of the bacterium in the media containing glucose. Therefore, the inability to utilize lactate will affect the growth of the lctP mutant in an environment with mixed carbon energy sources such as the nasopharynx (Table 1).
Meningococcal infection and disease show a pronounced seasonal variation, with the highest rates observed during winter. There is also an association between influenza and invasive meningococcal infection (16, 28). This could be explained by viral infection increasing the presence of activated phagocytic cells at the epithelial surface, increasing local lactate concentrations. The lactate could then be used as a substrate for meningococcal growth and promote resistance to complement-mediated lysis, favoring survival of the bacterium in the bloodstream, and increasing the likelihood that infection will lead to disease.
Colonization of the nasopharynx is a prerequisite for meningococcal disease, and therefore detailed knowledge of the growth requirements and metabolic state of the bacterium in this location will have important implications on the design novel interventions for protecting individuals against this pathogen. The approach of evaluating mutants with specific defects in the acquisition of key nutrients in the OCM could be used to further understand the role of other aspects of microbial physiology during colonization and provide insights into the microenvironment where N. meningitidis survives and replicates during this critical stage in pathogenesis.
Acknowledgments
This study was supported by the Meningitis Research Foundation.
We thank Martin Loxley of Sheffield Teaching Hospital Foundation Trust (STHFT) for help with the lactate and glucose measurements, Peter Bull and coworkers of the Department of Otolaryngology, and the Theater Sisters and Staff of ENT Theaters, STHFT.
Editor: V. J. DiRita
REFERENCES
- 1.Aho, E. L., J. A. Dempsey, M. M. Hobbs, D. G. Klapper, and J. G. Cannon. 1991. Characterization of the opa (class 5) gene family of Neisseria meningitidis. Mol. Microbiol. 5:1429-1437. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries, F. P., A. van Der Ende, J. P. van Putten, and J. Dankert. 1996. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Exley, R. M., J. Shaw, E. Mowe, Y. H. Sun, N. P. West, M. Williamson, M. Botto, H. Smith, and C. M. Tang. 2005. Available carbon source influences the resistance of Neisseria meningitidis against complement. J. Exp. Med. 201:1637-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feavers, I. M. 2000. ABC of meningococcal diversity. Nature 404:451-452. [DOI] [PubMed] [Google Scholar]
- 5.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. Miller, K. A. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 6.Lai a Fat, R. F., D. Suurmond, and R. van Furth. 1973. In vitro synthesis of immunoglobulins, secretory component and complement in normal and pathological skin and the adjacent mucous membranes. Clin. Exp. Immunol. 14:377-395. [PMC free article] [PubMed] [Google Scholar]
- 7.Leighton, M. P., D. J. Kelly, M. P. Williamson, and J. G. Shaw. 2001. An NMR and enzyme study of the carbon metabolism of Neisseria meningitidis. Microbiology 147:1473-1482. [DOI] [PubMed] [Google Scholar]
- 8.Merker, P., J. Tommassen, B. Kusecek, M. Virji, D. Sesardic, and M. Achtman. 1997. Two-dimensional structure of the Opc invasion from Neisseria meningitidis. Mol. Microbiol. 23:281-293. [DOI] [PubMed] [Google Scholar]
- 9.Nassif, X., M. Marceau, C. Pujol, B. Bron, J. L. Beretti, and M. K. Taha. 1997. Type-4 pili and meningococcal adhesiveness. Gene 192:149-153. [DOI] [PubMed] [Google Scholar]
- 10.Parsons, N. J., G. J. Boons, P. R. Ashton, P. D. Redfern, P. Quirk, Y. Gao, C. Constantinidou, J. Patel, J. Bramley, J. A. Cole, and H. Smith. 1996. Lactic acid is the factor in blood cell extracts which enhances the ability of CMP-NANA to sialylate gonococcal lipopolysaccharide and induce serum resistance. Microb. Pathog. 20:87-100. [DOI] [PubMed] [Google Scholar]
- 11.Ramsay, M., E. Kaczmarski, M. Rush, R. Mallard, P. Farrington, and J. White. 1997. Changing patterns of case ascertainment and trends in meningococcal disease in England and Wales. Commun. Dis. Rep. CDR Rev. 7:R49-R54. [PubMed] [Google Scholar]
- 12.Rayner, C. F., A. Dewar, E. R. Moxon, M. Virji, and R. Wilson. 1995. The effect of variations in the expression of pili on the interaction of Neisseria meningitidis with human nasopharyngeal epithelium. J. Infect. Dis. 171:113-121. [DOI] [PubMed] [Google Scholar]
- 13.Read, R. C., and L. Goodwin. 2001. Experimental nasopharyngeal colonization using explant organ culture, p. 621-635. In A. J. Pollard and M. C. Maiden (ed.), Methods in molecular medicine: meningococcal disease. Humana Press, Inc., Toronto, Ontario, Canada. [DOI] [PubMed]
- 14.Read, R. C., L. Goodwin, M. A. Parsons, P. Silcocks, E. B. Kaczmarski, A. Parker, and T. J. Baldwin. 1999. Coinfection with influenza B virus does not affect association of Neisseria meningitidis with human nasopharyngeal mucosa in organ culture. Infect. Immun. 67:3082-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read, R. C., R. Wilson, A. Rutman, V. Lund, H. C. Todd, A. P. Brain, P. K. Jeffery, and P. J. Cole. 1991. Interaction of nontypeable Haemophilus influenzae with human respiratory mucosa in vitro. J. Infect. Dis. 163:549-558. [DOI] [PubMed] [Google Scholar]
- 16.Reilly, S., and P. N. Gaunt. 1991. Influenza A and meningococcal disease. Lancet 338:1143-1144. [DOI] [PubMed] [Google Scholar]
- 17.Sim, R. J., M. M. Harrison, E. R. Moxon, and C. M. Tang. 2000. Underestimation of meningococci in tonsillar tissue by nasopharyngeal swabbing. Lancet 356:1653-1654. [DOI] [PubMed] [Google Scholar]
- 18.Smith, H., E. A. Yates, J. A. Cole, and N. J. Parsons. 2001. Lactate stimulation of gonococcal metabolism in media containing glucose: mechanism, impact on pathogenicity, and wider implications for other pathogens. Infect. Immun. 69:6565-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 20.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 21.Toleman, M., E. Aho, and M. Virji. 2001. Expression of pathogen-like Opa adhesins in commensal Neisseria: genetic and functional analysis. Cell. Microbiol. 3:33-44. [DOI] [PubMed] [Google Scholar]
- 22.Townsend, R., L. Goodwin, T. M. Stevanin, P. B. Silcocks, A. Parker, M. C. Maiden, and R. C. Read. 2002. Invasion by Neisseria meningitidis varies widely between clones and among nasopharyngeal mucosae derived from adult human hosts. Microbiology 148:1467-1474. [DOI] [PubMed] [Google Scholar]
- 23.Virji, M., J. E. Heckels, W. J. Potts, C. A. Hart, and J. R. Saunders. 1989. Identification of epitopes recognized by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp. J. Gen. Microbiol. 135:3239-3251. [DOI] [PubMed] [Google Scholar]
- 24.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 25.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, and E. R. Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 26.Virji, M., K. Makepeace, and E. R. Moxon. 1994. Distinct mechanisms of interactions of Opc-expressing meningococci at apical and basolateral surfaces of human endothelial cells; the role of integrins in apical interactions. Mol. Microbiol. 14:173-184. [DOI] [PubMed] [Google Scholar]
- 27.Virji, M., K. Makepeace, I. Peak, G. Payne, J. R. Saunders, D. J. Ferguson, and E. R. Moxon. 1995. Functional implications of the expression of PilC proteins in meningococci. Mol. Microbiol. 16:1087-1097. [DOI] [PubMed] [Google Scholar]
- 28.Young, L. S., F. M. LaForce, J. J. Head, J. C. Feeley, and J. V. Bennett. 1972. A simultaneous outbreak of meningococcal and influenza infections. N. Engl. J. Med. 287:5-9. [DOI] [PubMed] [Google Scholar]