Abstract
Vaccine development has been hampered by difficulties in developing new and safe adjuvants, so alternative technologies that offer new avenues forward are urgently needed. The goal of this study was to express a monoclonal recombinant immune complex in a transgenic plant. A recombinant protein consisting of a tetanus toxin C fragment-specific monoclonal antibody fused with the tetanus toxin C fragment was designed and expressed. Immune complex formation occurred between individual fusion proteins to form immune complex-like aggregates that bound C1q and FcγRIIa receptor and could be targeted to antigen-presenting cells. Unlike antigen alone, the recombinant immune fusion complexes were highly immunogenic in mice and did not require coadministration of an adjuvant (when injected subcutaneously). Indeed, these complexes elicited antibody titers that were more than 10,000 times higher than those observed in animals immunized with the antigen alone. Furthermore, animals immunized with only 1 μg of recombinant immune complex without adjuvant were fully protected against lethal challenge. This the first report on the use of a genetic fusion between antigen and antibody to ensure an optimal expression ratio between the two moieties and to obtain fully functional recombinant immune complexes as a new vaccine model.
New reliable and affordable vaccines are urgently needed for the control of infectious (and other) diseases, and the development of vaccines with built-in adjuvanticity is highly desirable. It has been determined that the coadministration of antigen with antibody in the form of immune complexes can markedly enhance the immunogenicity of the antigen (27). This opens the possibility of using immune complexes for vaccination, with the significant advantage that an additional adjuvant may not be required. At present, alum is the only adjuvant licensed for use in humans; that there is only one thus far is a reflection of the technical difficulties inherent in adjuvant development. Immune complexes can enhance immune responsiveness through several mechanisms (23). They promote Fc receptor-mediated recognition by antigen-presenting cells (3, 28). Immune complexes also activate the complement cascade (14, 32). It has also been suggested that through binding to FcR and complement receptors, there is localization of the complex on follicular dendritic cells (DCs) which carry both types of receptor or that the complexes might directly stimulate B cells via their complement receptors (10). Antibody binding to antigen also leads to protection of the antigen from proteolysis extracellularly (13) and intracellularly (22), and this can lead to modulation of antigen processing, as well as antigen presentation (1, 5, 30). However, conventional preparation of immune complexes is not applicable for vaccine development. Indeed, it would require the in vitro mixing of antigen and antibody at an optimum ratio, which is not easily reproducible. Moreover, it is necessary to use either polyclonal antisera or cocktails of monoclonal antibodies (MAbs) to achieve complexing. Thus, the complexity of formulation does not lend itself to pharmaceutical development.
In the present study, we describe for the first time the production of recombinant immune complexes (RICs). Theoretically, any eukaryotic expression system could be used; in this case we have utilized transgenic plants. The 47-kDa tetanus toxin fragment C (TTFC) was used as a model antigen (21). The genetic fusion between antigen and a specific antibody was designed to ensure an optimal expression ratio between the two moieties and to obtain fully functional recombinant immune-complexes as a new vaccine model (see Fig. 1). The complexes utilize a MAb and bind C1q, as well as Fc receptors. They have enhanced immunogenicity in comparison to antigen alone and induced protective immunity in mice.
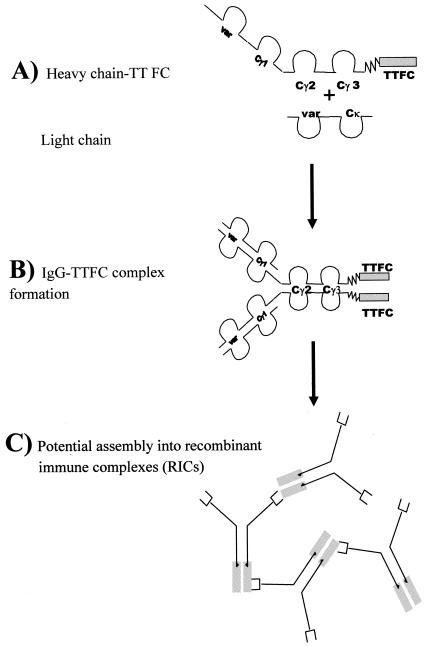
FIG. 1.
Diagrammatic representation of antigen fusion protein expressed in plants (A) and potential assembly arrangements into IgG-TTFC fusion protein (B) and RICs (C).
MATERIALS AND METHODS
Cloning and genetic engineering.
MAb 278.02 is a murine immunoglobulin G2a (IgG2a) that was raised against tetanus toxoid. It binds strongly to TTFC in an enzyme-linked immunosorbent assay (ELISA; data not shown). Total RNA from the TT-specific MAb 278.02 was kindly supplied by C. Koch and N. Kirkby. After reverse transcription with Moloney murine leukemia virus (Stratagene), the cDNA was used as a template for PCR cloning of the antibody light and heavy chains.
Light chain.
An ∼700-bp fragment corresponding to the coding sequence for the murine kappa light chain was generated by PCR by using Pfu polymerase (Stratagene). The amplification was carried out by using the forward primer 5′-GTG GTA CCT CGA GCG AYA TYS WGM TSA CCC ART CT-3′ with a XhoI site and the reverse primer 5′-GGG GAG CTG GTG GTG AAT TCG TCG ACC TTT GTC TCT AAC ACT C-3′ containing an EcoRI site and a stop codon. The PCR product was digested with the appropriate restriction enzymes and cloned into the pBluescript vector (Stratagene).
Heavy chain.
An approximately 1.4-Kb fragment corresponding to the coding region for the heavy chain was also generated by Pfu PCR. The amplification was carried out by using the forward primer 5′-GAC TAG TCC ATG GGC CAG GTS MAR CTG SAG TCW G-3′ with 5′ NcoI and SpeI sites and the reverse primer 5′-GGA ATT CAG ATC TGC CAC CGC CTT TAC CCR GAG WCC GGG AGA with a 5′ BglII site (that includes part of a linker sequence for ligation with the antigen). The PCR product was first digested with NcoI and Bgl II and then cloned into Litmus 29 vector (Novagen). The cloned product was then digested with SpeI and Bgl II and purified for ligation with the TTFC sequence.
TTFC.
The plasmid encoding the TTFC open reading frame sequence (pTET tac 215) was used as a template (21). Pfu PCR amplification was carried out by using the forward primer 5′-CGA CTC GAG AAA AAC CTT GAT TGT TGG GTC and the reverse primer 5′-CGA CTC GAG TTA GTC GTT GGT CCA ACC TTC ATC, both with 5′-terminal XhoI restriction sites. The PCR product, a 1.35-Kb fragment was digested with XhoI and cloned into the pBluescript vector.
Heavy chain-TTFC fusion (HC-TTFC).
The forward primer used was 5′-GGC GGA TCC GGT GGA GGC GGT TCA GGC GGA GGT GGC TCT AAA AAC CTT GAT TGT TGG GTC GAC with a BamHI site (including part of the linker sequence for ligation with the antibody heavy chain), whereas the reverse primer was the one used for TTFC alone. The linker amino acid sequence is (GGGGS)3. The PCR product was digested with BamHI and XhoI and cloned into Litmus 29 vector. The vector was then digested with SpeI and BamHI and ligated with the SpeI/BglII heavy-chain-digested product in order to obtain a Litmus 29 vector containing the antibody HC-TTFC fused sequences.
Each construct was confirmed by automatic sequencing and transferred to a binary plant expression vector (pMON530) that contains the 35S cauliflower mosaic virus promoter sequence and a murine immunoglobulin leader sequence for targeting of the transgene product to the plant endomembrane system (29). The recombinant vector was used to transform Escherichia coli DH5α (Gibco-BRL). Plasmid DNA was purified and introduced into Agrobacterium tumefaciens as described previously (6).
Transformation and regeneration of transgenic plants.
Using the gene constructs described above, transgenic plants were prepared that express TTFC, MAb 278-02 kappa chain (K), MAb 278-02 gamma chain fusion with TTFC (HC-TTFC), or MAb 278-02 kappa chain with gamma chain fusion with TTFC.
Leaf disks of Nicotiana tabacum (var. xanthii) were transformed as described previously (12). The disks were incubated on medium supplemented with kanamycin (200 mg/liter) and carbenicillin (500 mg/liter) for regeneration of shoots, and the shoots were transferred to root-inducing medium supplemented with kanamycin (200 mg/liter). As soon as roots were established, plantlets were transplanted into soil. Twenty independent, primary transformants were regenerated for each construct. Each plant was screened for expression of either immunoglobulin chains or TTFC by specific ELISA. Plants expressing HC-TTFC were cross fertilized with those expressing the light chain as described previously (18) and screened by antigen- and antibody-specific ELISAs.
ELISA for detection of IgG2a light and heavy chains, TTFC, HC-TTFC, and immune fusion complexes formed from IgG-TTFC in plant extracts.
Young plant leaves were homogenized on ice in phosphate-buffered saline (PBS) containing 2.5% bovine serum albumin (BSA), 0.1% Tween 20, and leupeptin (10 μg/ml; Roche). After centrifugation at 13,000 × g for 2 min at 4°C, the supernatant was collected and stored at −70°C.
Maxisorp 96-well microplates (Nunc) were coated with anti-mouse kappa chain antiserum (Caltag), anti-mouse IgG gamma chain antiserum (The Binding Site), sheep anti-TT antiserum (Biogenesis), or complement protein C1q (Calbiochem) at 7.5 μg/ml in PBS (pH 7.4) overnight at 4°C. The plates were washed and blocked with PBS-2.5% BSA for 2 h at 37°C. After washing, the plates were incubated with twofold dilutions of plant extract in diluting buffer (PBS, Tween 20 [0.1%], and BSA [2.5%]) overnight at 4°C. After further washing, the plates were incubated with appropriate peroxidase-conjugated secondary antibodies—sheep anti-mouse IgG gamma chain (The Binding Site), sheep anti-mouse IgG2a (The Binding Site), goat anti-mouse immunoglobulin kappa chain (Caltag), or biotinylated anti-TT (Biogenesis) antibody—in diluting buffer, for 2 h at 37°C. When biotinylated antibodies were used, the plates were washed and incubated with peroxidase-labeled streptavidin (Roche) for 1 h at 37°C. Enzyme activity was detected by addition of 100 μl of substrate solution (0.01% 3,3′,5,5′-tetramethylbenzidine and 0.004% hydrogen peroxide in citrate-phosphate buffer [pH 5.0]). After 10 min at room temperature the enzymatic reaction was stopped with 50 μl of 2 M H2SO4, and the optical density at 450 nm was determined.
ELISAs for detection of anti-TTFC antibody in immunized animals.
Maxisorp 96-well microplates were coated at 5 μg/ml with recombinant TTFC produced in E. coli overnight at 4°C. They were then washed with water and blocked with PBS containing 2.5% BSA for 2 h at 37°C. After being washed, the plates were incubated with two- or fivefold dilutions (starting from 1/50) of mouse sera in diluting buffer for 2 h at 37°C and washed. The plates were incubated with peroxidase-labeled sheep anti-mouse IgG (The Binding Site) in diluting buffer for 2 h at 37°C. Color development and measurement of the optical density were as described above.
Western blotting.
Plant extracts or purified recombinant proteins were boiled with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (75 mM Tris-HCl [pH 6.8], 2% SDS, with or without 5% β-mercaptoethanol). Boiled samples were separated by SDS-4 to 20% polyacrylamide gel electrophoresis and transferred onto nitrocellulose by using a semidry blotting apparatus (Hoefer). The blot was blocked with 5% nonfat dry milk in PBS for 2 h at room temperature on a shaking table. Detection of proteins bound to the nitrocellulose was by incubation with anti-mouse IgG (H+L), anti-mouse IgG or anti-TT antisera diluted 1:1,000 in diluting buffer, followed by an alkaline phosphatase-labeled donkey anti-goat or sheep secondary antiserum (Jackson Laboratories). Visualization was with nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Bio-Rad).
Purification of plant recombinant proteins.
For the purification of recombinant TTFC and HC-TTFC from plants, tobacco plant leaves were homogenized on ice with extraction buffer (37.5 mM Tris-HCl [pH 7.5], 50 mM NaCl, 15 mM EDTA, 75 mM sodium citrate, 0.2% sodium thiosulfate, 10 μg of leupeptin/ml, and 10 μg of 4-amidinophenylmethanesulfonyl fluoride hydrochloride [APMSF]/ml). The suspension was filtered through a sieve and centrifuged at 17,000 × g for 20 min at 4°C. The supernatant was filtered through Whatman paper No. 54, the pH was reduced to 5.1 by the addition of 8 M acetate-NaOH buffer (pH 3.8), and the solution was centrifuged as before. The pH of the solution was neutralized by addition of 1.5 M Tris-HCl (pH 9.5), and ammonium sulfate was added to 16% saturation. After centrifugation at 17,000 × g for 20 min at 4°C, the precipitate was discarded, ammonium sulfate was added to the supernatant to 40% saturation. After 2 h at 4°C, the solution was centrifuged again, the pellet was resuspended in PBS to 1/5 of original volume, and the final solution was then centrifuged at 25,000 × g for 30 min at 4°C. The protein solution was concentrated by ultrafiltration through a 30-kDa molecular mass cutoff membrane (Amicon) and dialyzed overnight at 4°C against PBS. The concentrated extract was then filtered through a 0.45-μm-pore-size filter and loaded onto an anti-mouse IgG Sepharose column (Sigma) or an anti-TT Sepharose column (prepared with anti-TT antibodies; Biogenesis). Elution was done with 0.1 M glycine-HCl (pH 2.2), and the eluate was neutralized with 1.5 M Tris-HCl (pH 9.5).
For purification of plant IgG-TTFC, the plant extract supernatant (obtained after the first centrifugation at 17,000 × g) was directly incubated with protein A-Sepharose (Sigma) in batch chromatography. After being washed with PBS and centrifugation at 100 × g for 2 min, the IgG-TTFC was eluted from the Sepharose by addition of glycine-HCl (pH 3.0). The solution was neutralized with 1.5 M Tris-HCl (pH 9.5).
Surface plasmon resonance interaction analysis.
A biosensor (Biacore 3000; Biacore AB, St. Albans, United Kingdom) was used to quantify the interaction of monomeric IgG2a and IgG-TTFC immune fusion complexes to an E. coli recombinant FcγRIIa (20). All experiments were performed with HBS-EP (10 mM HEPES [pH 7.4], 150 mM NaCl, 3.4 mM EDTA, 0.005% surfactant P20) as a running buffer. Direct coupling of recombinant FcγRIIa on CM5 sensor chips (BIAcore AB) was performed by using the standard procedure. In brief, buffer flow was maintained at 5 μl/min throughout the immobilization protocol. A total of 30 μl of a mix of N-hydroxysulfosuccinimide sodium salt (NHS) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) (1:1) was injected. When the surface was activated, 20 μl of FcγRIIa (10 μg/ml in 10 mM acetate buffer [pH 5.0]) was added, followed by 30 μl of 1 M ethanolamine. Using this protocol, 1,500 resonance units of receptor were immobilized onto the chip. The association and dissociation rate constants and affinity constants of the interactions between FcγRIIa and monomeric IgG2a or IgG-TTFC were determined over a range of different concentrations with a buffer flow at 15 μl/min. The kinetic parameters were determined from the sensorgrams with BIAanalysis version 3.1 software.
Cellular immunofluorescence assessment of IgG-TTFC uptake.
The mouse macrophage-like cell line J774.1 (ATCC TIB 67) was seeded (2 × 105 cells/ml) on coverslips and grown to semiconfluence in Dulbecco minimal essential medium containing 10% heat-inactivated fetal calf serum at 37°C in a humidified atmosphere of 5% CO2 in air. The cells were then incubated with 10 μg of plant TTFC, 10 μg of MAb 278.02, 10 μg of plant HC-TTFC, or 10 μg of plant IgG-TTFC/ml for 30 min in serum-free Dulbecco modified Eagle medium medium. Cells were fixed in 4% paraformaldehyde and permeabilized with 1% Triton X-100. Cells were blocked in PBS-2% BSA and then stained with goat anti-mouse heavy chain (Caltag) or goat anti-TT (Biogenesis) antibody (1:100 in PBS-2% BSA-0.1% Tween), washed, and visualized with fluorescein isothiocyanate (FITC)-labeled anti-goat F(ab′)2 antiserum (1:100 in PBS-2% BSA-0.1% Tween) by using an E500 fluorescence microscope (Nikon, Inc.) and the RT SPOT digital camera/software package (Diagnostic Instruments).
Animals and immunization schedules.
Inbred 6- to 7-week-old female BALB/c (H-2d) mice were purchased from Charles Rivers United Kingdom. Mice were immunized at week 0 and boosted at 3 and 8 or 9 weeks by subcutaneous injection at the base of the tail.
For the titration study, the immunizing solutions were 50 μl of PBS, a commercial tetanus toxoid vaccine (Pasteur Merieux Connaught) (equivalent to 1/10 the human dose), or recombinant proteins purified from transgenic plants: TTFC (0.1 and 1 μg) and IgG-TTFC (0.1 and 1 μg), dissolved in PBS. Serum samples were collected at 0 (preimmune), 3, 9, and 14 weeks. Preimmune sera were used as negative control samples in all of the analyses.
For the challenge study, immunizing solutions were 50 μl of PBS containing either purified IgG-TTFC (1 μg) or purified MAb 278.02 (1 μg). The MAb 278.02 was purified from hybridoma cell culture supernatant by Protein G affinity chromatography. For TT challenge, mice were injected subcutaneously 3 weeks after the last boost in the right flank with 0.5 ml of PBS containing 50 50% paralytic doses (PD50) of TT and sacrificed as soon as they developed signs of paralysis.
RESULTS
In order to generate monoclonal immune complexes, chimeric genes were engineered in which DNA encoding TTFC was cloned downstream of the gene encoding the immunoglobulin heavy chain from a TTFC-specific MAb (278.02) (Fig. 1A). The HC-TTFC fusion protein was expressed in transgenic N. tabacum plants that were grown to maturity and cross-fertilized with transgenic N. tabacum expressing the immunoglobulin light (kappa) chain of MAb 278.02.
From the resulting seeds, individual transgenic plants were screened by capture ELISAs for the expression of each of the relevant recombinant proteins (data not shown). These ELISAs were specific for kappa light chain, gamma heavy chain, and HC-TTFC fusion protein as positive controls; the parent anti-TTFC MAb (MAb 278.02) was used for heavy and light chains ELISAs, and a mixture of TTFC antigen with MAb 278.02 was used for HC-TTFC ELISAs. A nontransformed wild-type plant was used as the negative control in each assay. Transgenic plants expressing kappa chain, heavy chain, and HC-TTFC were selected.
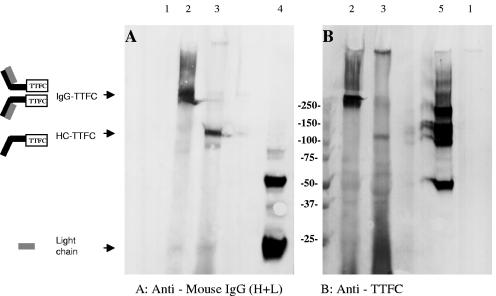
Immunoblotting was used to determine whether assembly of the recombinant immunoglobulin chains had occurred to form an IgG-TTFC protein complex (depicted in Fig. 1B), and the results from a representative plant (that was positive by ELISA for both kappa and HC-TTFC fusion protein) are shown in Fig. 2. Plant extracts or control reagents were electrophoresed on a 4 to 20% gradient SDS-PAGE gel under reducing or nonreducing conditions, transferred to nitrocellulose, and probed with either anti-mouse IgG or anti-TTFC antisera. No immunoreactive bands were observed by using a nontransformed wild-type plant extract (lanes 1). Under nonreducing conditions (lanes 2), a band with an approximate relative molecular weight (Mr) of 250,000 was visualized both with anti-mouse IgG and anti-TT antibodies from the IgG-TTFC plant extract, and this was the expected size for assembled IgG-TTFC molecules. A diagram of the predicted molecular complex is shown. There was some evidence for higher-molecular-mass species, which may correspond to formation of trimers or tetramers. Under reducing conditions, the IgG-TTFC plant extract contains a band at the expected relative molecular mass (Mr = 100,000) for HC-TTFC, which was detected both with anti-mouse IgG and anti-TT specific antibodies, and a faint band (Mr = ∼25,000) corresponding to the antibody light chain that was detected by the anti-mouse IgG antiserum. A smear of small size products is visible in Fig. 2B, lane 3; this finding is probably due to cross-reactivity of the (non-affinity-purified) rabbit antiserum with no specific plant proteins or possibly to degradation of the TTFC molecule. The controls are a monoclonal murine IgG2a antibody to demonstrate the position of heavy and light chains on Western blot (Fig. 2A, lane 4) and E. coli recombinant TTFC (Fig. 2B, lane 5).
FIG. 2.
Western blot of IgG-TTFC plant extract. Crude plant extract was separated by SDS-PAGE on a 4 to 20% polyacrylamide gel under reducing and nonreducing conditions as indicated. After transfer to nitrocellulose, immunodetection was with either goat anti-mouse IgG (H+L) antiserum (A) or rabbit anti-TT C-fragment antiserum (B), followed by the appropriate alkaline phosphatase-labeled secondary antiserum. Lanes: 1, wild-type plant; 2, IgG-TTFC plant (nonreducing); 3, IgG-TTFC plant (reducing); 4, IgG2a (100 ng); 5, TTFC (200 ng, nonreducing).
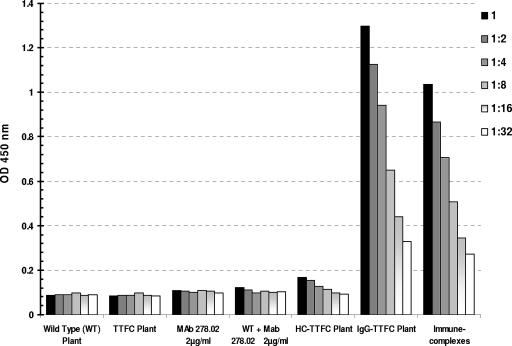
Since the antibody incorporated in the IgG-TTFC fusion molecule is specific for TTFC but the genetic fusion with antigen takes place at the Fc end of the IgG, the two antigen binding sites in each IgG-TT molecule should remain available for binding to antigen (Fig. 1B). Thus, we sought to test the hypothesis that larger immune complexes might form spontaneously in transgenic plants (as depicted in Fig. 1C). Three sets of experiments were carried out. The first was the evaluation of binding to C1q, the first component of the classical pathway of the complement cascade. The C1q ELISA has been commonly used for the detection of immune complexes in biological fluids (31), and the results with transgenic plant extracts are shown in Fig. 3. A number of negative controls were examined, including extracts from nontransformed wild-type plants, transgenic plants expressing TTFC, and transgenic plants expressing HC-TTFC fusion protein. In addition, the anti-TTFC MAb (purified from culture supernatant) was used in the presence or absence of an extract from a wild-type plant. As a positive control, immune complexes were obtained by mixing a goat polyclonal anti-TT antiserum with TTFC and MAb 278.02. The results indicate that wild-type plant extract, TTFC, or MAb 278.02 alone do not bind to C1q. Nor was any binding detected in extracts from plants expressing the HC-TTFC recombinant fusion protein. However, a strong signal was observed in the extract containing IgG-TTFC fusion complex, indicating the formation of assembled immune-complexes. The specificity of this ELISA for antibodies aggregated as immune complexes was confirmed by the binding of in vitro-prepared immune complexes.
FIG. 3.
C1q ELISA. The binding activity of plant extracts and control solutions to immobilized C1q is shown. Detection was done with a peroxidase-labeled anti-mouse IgG antiserum. The data for six dilutions of each sample are shown.
In a second series of experiments we tested the direct interaction of the plant derived complexes with Fc receptor gamma RIIa (FcγRIIa) by surface plasmon resonance. Soluble FcγRIIa was immobilized on a CM5 sensorchip, and the interaction with monomeric IgG2a or IgG-TTFC were determined over a range of five different concentrations (not shown). The kinetic parameters, association (ka) and dissociation (kd) rate constants, and affinity constants (K = ka/kd) were determined from the sensorgrams by using BIAanalysis version 3.1 software (BIAcore). The affinity constant for monomeric IgG2a binding to FcγRIIa was low, i.e., (1.08 ± 0.12) × 106 M−1, and was consistent with previous studies (20), whereas the plant derived immune complexes (IgG-TTFC) displayed a much higher affinity for the same receptor: (8.34 ± 0.38) × 108 M−1, representing an increase of almost 3 logs. This difference can be explained by the higher functional affinity of IgG-TTFC complexes that allow cross-linking of several Fc regions.
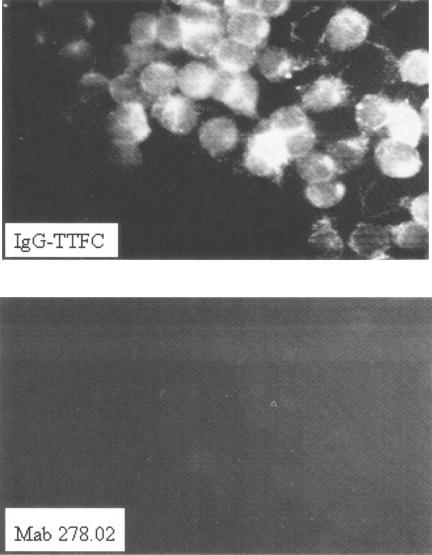
Finally, cellular immunofluorescence assessment of IgG-TTFC uptake by a macrophage-like cell line was carried out. The mouse cell line J774.1 (ATCC TIB 67) was incubated with E. coli recombinant TTFC, MAb 278.02, plant HC-TTFC, or plant IgG-TTFC. The cells were fixed, permeabilized, and blocked and then stained with a goat anti-mouse heavy chain antiserum, followed by an FITC-labeled anti-Goat F(ab′)2 antiserum. As shown in Fig. 4, whereas IgG-TTFC binding and uptake by J774.1 were evident after 30 min of incubation, the uptake of the noncomplexed MAb 278.02 IgG2a alone was negligible. Similarly, no fluorescent signal was detected for TTFC alone or for plant HC-TTFC (data not shown). The same results were obtained with a goat anti-TT antiserum (Biogenesis) in place of the primary goat anti-mouse heavy-chain antiserum for detection (data not shown).
FIG. 4.
IgG-TTFC immune complex targeting to a mouse macrophage-like cell line. Uptake of 10 μg of MAb 278.02 or 10 μg of plant IgG-TTFC/ml by the murine cell line J774.1 cell line was examined by immunofluorescence microscopy. Detection was done with a goat anti-mouse heavy-chain antiserum and visualized with FITC-labeled anti-goat F(ab′)2 antiserum.
Taken together, we conclude that these findings provide evidence for the formation of high-molecular-size complexes of IgG-TTFC fusion molecule in transgenic plants. Moreover, this complexing is sufficient to promote C1q binding, binding to Fcγ RIIa, and uptake by a macrophage-like cell line in vitro. We next extended our studies to in vivo immunization in order to examine the immunogenicity of the antigen presented in this form and to determine whether the recombinant monoclonal immune fusion complexes did not require the addition of an adjuvant to generate a protective immune response.
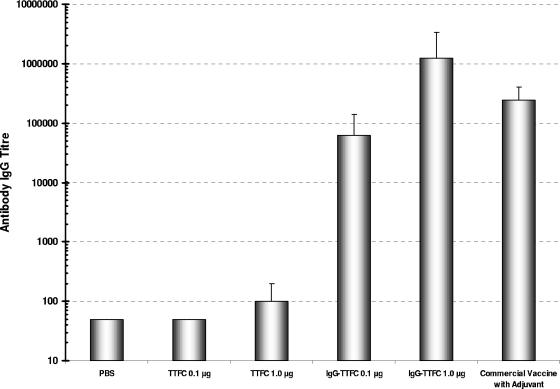
An immunogenicity study using purified antigens without adjuvant was designed, and five groups of five mice were immunized three times with saline, TTFC, and IgG-TTFC at different doses. As a positive control, a sixth group received 50 μl of commercial TT commercial vaccine containing alum as adjuvant (Pasteur Merieux Connaught). Immunizations were at 0, 3, and 9 weeks. Serum samples were taken at various points with a final bleed at 14 weeks, and the results of the anti-TTFC titers are presented in Fig. 5. Plant-derived TTFC alone (without adjuvant) was poorly immunogenic. At a dose of 1 μg, the specific IgG titer in the final bleed was approximately log10 2.0. In comparison, 0.1 μg of IgG-TTFC without adjuvant induced similar IgG titers of log10 4.90, and at a dose of 1 μg of IgG-TTFC without adjuvant the IgG titer was log10 6.08. These titers compare favorably with the group of mice immunized with the commercial TT vaccine (which contains alum), with an IgG titer log10 of 5.38.
FIG. 5.
Serum antibody responses in immunized mice. IgG antibody responses were determined by an antigen-specific ELISA, and the titration points determined in the final bleed for each experimental group. The data are presented as mean antibody titers ± the standard deviation for each group of mice.
In a second immunization study, we investigated whether the induced antibody response was sufficient to protect against TT challenge. Two groups of five mice were immunized with either 1 μg of purified IgG-TTFC or 1 μg of purified MAb 278.02 without adjuvant on weeks 0, 3, and 8. At week 10, the antibody titers in the IgG-TTFC group were similar to those observed previously, but no anti-TTFC antibodies were detectable in the group immunized with MAb 278.02. At week 11, the mice were challenged subcutaneously with 50 PD50 of TT (a gift from Thea Sesardic, NIBSC). The mice were monitored over 5 days for signs of paralysis, which would indicate a nonimmune status. All five mice receiving MAb 278.02 alone exhibited signs of tetanus paralysis on day 2 that necessitated sacrifice. In contrast, all five mice immunized with the plant-derived IgG-TTFC survived TT challenge completely. These results demonstrate that the plant recombinant monoclonal immune fusion complexes induce a protective antibody response in mice. By including MAb 278.02 as the control in this experiment, we excluded the possibility that the protection might be due to passive protection from the antibody moiety of the fusion complex. No evidence for an anti-murine IgG response (detecting IgG1, IgG2b, or lambda binding), induced by immunization with IgG-TTFC was detected in any of the mice that received IgG-TTFC preparations (data not shown).
DISCUSSION
In this study, we designed and expressed a novel form of immunogen that auto-assembles into complexes and has properties akin to conventional immune complexes. These immunogens differ from conventional immune complexes by virtue of the genetic fusion between the antigen and antibody moieties and because they incorporate a MAb. Nevertheless, the RICs have secondary effector functions (C1q binding, binding to Fcγ receptor, and uptake by antigen-presenting cells), and most importantly they appear to be potent immunogens. Thus, they may have important applications in the field of vaccine design, where it would be highly desirable to avoid the need for an additional adjuvant.
The production of RICs utilizes transgenic plant biotechnology and builds upon antibody expression technology that has been in development in plants for the last 15 years. A key requirement is the assembly of the tetrameric IgG-like complex by coexpression of the light and heavy chains. This is a chaperone-mediated process that for IgG is performed very efficiently by plants (7, 24). Our findings suggest that the addition of an Mr 50,000 protein as a C terminus fusion to the immunoglobulin heavy chain (thereby doubling the size of the heavy chain) does not affect the assembly process. Nor does it affect the effector functions associated with the Fc region. The complement component C1q is known to bind to IgG immune complexes but not to monomeric IgG. Indeed, binding to immune complexes with Mrs of <600,000 is usually undetectable (15). In the present study we demonstrated that the IgG-TTFC fusion complexes were efficiently recognized by C1q, whereas the monomeric MAb or HC-TTFC did not bind. Furthermore, we have demonstrated high-affinity binding of IgG-TTFC to soluble FcγRIIa receptor, which is consistent with the formation of immune complexes. The biological activity of IgG-TTFC complexes was also confirmed by the immunofluorescent uptake assay in a mouse macrophage-like cell line. This showed that the IgG-TTFC complexes can be captured by antigen-presenting cells. It has been suggested that plant glycosylation of antibodies might adversely affect their effector functions. Our results demonstrate that this is not the case and that murine antibodies decorated with plant glycans can bind complement and Fc receptors in a manner similar to that of their mammalian counterparts.
The immunization studies demonstrated that RICs are extremely efficient at eliciting serum antibody responses, leading to protective immunity. Moreover, we have demonstrated that these responses can be induced without additional adjuvant, which is consistent with our original hypothesis that immune fusion complexes might represent attractive vaccine candidates. The design of the RIC fusion molecule results in the expression of antigen with antigen binding site at the 1:1 ratio that is optimal for complex formation, but the intracellular molecular assembly requirement restricts this application to eukaryotic expression systems, such as plants.
The level of expression of the IgG-TT RICs in crude plant extract was estimated at a minimum of 0.8% of total soluble protein. At this stage, we have not attempted to control the size of the immune complexes, and larger complexes are likely to have been lost during the centrifugation steps, thus affecting the purification yield, which we have estimated to be ca. 50%.
We believe that it may also be possible to extend the use of RICs to diseases requiring cytotoxic-T-cell responses. Fcγ RIIa receptors, as well as novel Fcγ receptors, are found on non-antigen-presenting cells, such as endothelial cells, which can mediate internalization of IgG (8, 9). However, there is also mounting evidence that FcR targeting of antigen by immune complexes to DCs can result in cross priming of CD8 cytotoxic-T-cell responses. The phenomenon was first observed in mice primed with cells that expressed foreign minor HC antigens but not host major histocompatibility complex (2). More recently there have been a number of reports of cross-presentation of tumor and model antigens using immune complexes (4), which result in tumor immunity and protection (25). The mechanism involves uptake of antigen through Fc receptors on DCs (19, 26), leading to DC maturation, augmented CD4 priming, and preferential Th1 polarization of the immune response, and there is not only an enhanced antibody response but also the induction of potent CD8 effector immunity. Thus, there is compelling evidence that FcγR targeting by immune complexes has the potential to accomplish several important features of vaccine design, which include efficient antigen uptake and antigen processing for both major histocompatibility complex class I and II presentation for priming of CD4 and CD8 cells.
The use of plant cells to process and assemble multimeric complexes has progressed from IgG antibodies (11) to secretory IgA antibodies (16, 17) and now to immune complexes. This places plants in a virtually unique position as an expression system for complex proteins and opens the way for many more “designer” proteins. For example, we envisage the use of IgA RICs for mucosal immunization or of RICs with multiple antigens or immunomodulatory molecules. The use of plants also opens the way for production of these molecules at a large scale and hopefully at an affordable cost.
Acknowledgments
The European Union Framework IV (FAIR) Programme and The Wellcome Trust have contributed to the funding of this study. This study was partly performed at Guy's Hospital Dental School, London, United Kingdom.
We thank Claus Koch and Nikolai Kirkby for MAb 278-02, John G. Raynes for valuable assistance with the surface plasmon resonance studies, and Peter Sondermann for providing the soluble FcγRIIa receptor.
Editor: A. D. O'Brien
REFERENCES
- 1.Antoniou, A. N., and C. Watts. 2002. Antibody modulation of antigen presentation: positive and negative effects on presentation of the tetanus toxin antigen via the murine B-cell isoform of FcγRII. Eur. J. Immunol. 32:530-540. [DOI] [PubMed] [Google Scholar]
- 2.Bevan, M. J. 1976. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 117:2233-2238. [PubMed] [Google Scholar]
- 3.Celis, E., and T. W. Chang. 1984. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science 224:297-299. [DOI] [PubMed] [Google Scholar]
- 4.Dhodapkar, K. M., J. Krasovsky, B. Williamson, and M. V. Dhodapkar. 2002. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberg, R. J., D. Long, L. Pereira, B. Hampar, M. Zweig, and G. H. Cohen. 1982. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J. Virol. 41:478-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraley, R. T., S. G. Rogers, R. B. Horsch, P. R. Sanders, J. S. Flick, S. P. Adams, M. L. Bittner, L. A. Brand, C. L. Fink, J. S. Fry, G. R. Galluppi, S. B. Goldberg, N. L. Hoffmann, and S. C. Woo. 1983. Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA 80:4803-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frigerio, L., N. D. Vine, E. Pedrazzini, M. B. Hein, F. Wang, J. K. Ma, and A. Vitale. 2000. Assembly, secretion, and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol. 123:1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gafencu, A., C. Heltianu, A. Burlacu, W. Hunziker, and M. Simionescu. 2003. Investigation of IgG receptors expressed on the surface of human placental endothelial cells. Placenta 24:664-676. [DOI] [PubMed] [Google Scholar]
- 9.Groger, M., G. Sarmay, E. Fiebiger, K. Wolff, and P. Petzelbauer. 1996. Dermal microvascular endothelial cells express CD32 receptors in vivo and in vitro. J. Immunol. 156:1549-1556. [PubMed] [Google Scholar]
- 10.Heyman, B. 1990. The immune complex: possible ways of regulating the antibody response. Immunol. Today 11:310-313. [DOI] [PubMed] [Google Scholar]
- 11.Hiatt, A., R. Cafferkey, and K. Bowdish. 1989. Production of antibodies in transgenic plants. Nature 342:76-78. [DOI] [PubMed] [Google Scholar]
- 12.Horsch, R. B., S. G. Rogers, and R. T. Fraley. 1985. Transgenic plants. Cold Spring Harbor Symp. Quant. Biol. 50:433-437. [DOI] [PubMed] [Google Scholar]
- 13.Jemmerson, R., and Y. Paterson. 1986. Mapping epitopes on a protein antigen by the proteolysis of antigen-antibody complexes. Science 232:1001-1004. [DOI] [PubMed] [Google Scholar]
- 14.Klaus, G. G. 1978. The generation of memory cells. II. Generation of B memory cells with preformed antigen-antibody complexes. Immunology 34:643-652. [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger, G., M. Kneba, I. Bolz, P. Volling, J. Wessels, and G. A. Nagel. 1985. Binding characteristics of three complement-dependent assays for the detection of immune complexes in human serum. J. Clin. Lab. Immunol. 18:129-134. [PubMed] [Google Scholar]
- 16.Ma, J. K., A. Hiatt, M. Hein, N. D. Vine, F. Wang, P. Stabila, C. van Dolleweerd, K. Mostov, and T. Lehner. 1995. Generation and assembly of secretory antibodies in plants. Science 268:716-719. [DOI] [PubMed] [Google Scholar]
- 17.Ma, J. K., B. Y. Hikmat, K. Wycoff, N. D. Vine, D. Chargelegue, L. Yu, M. B. Hein, and T. Lehner. 1998. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 4:601-606. [DOI] [PubMed] [Google Scholar]
- 18.Ma, J. K., T. Lehner, P. Stabila, C. I. Fux, and A. Hiatt. 1994. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur. J. Immunol. 24:131-138. [DOI] [PubMed] [Google Scholar]
- 19.Machy, P., K. Serre, and L. Leserman. 2000. Class I-restricted presentation of exogenous antigen acquired by Fcγ receptor-mediated endocytosis is regulated in dendritic cells. Eur. J. Immunol. 30:848-857. [DOI] [PubMed] [Google Scholar]
- 20.Maenaka, K., P. A. van der Merwe, D. I. Stuart, E. Y. Jones, and P. Sondermann. 2001. The human low affinity Fcγ receptors IIa, IIb, and III bind IgG with fast kinetics and distinct thermodynamic properties. J. Biol. Chem. 276:44898-44904. [DOI] [PubMed] [Google Scholar]
- 21.Makoff, A. J., M. D. Oxer, M. A. Romanos, N. F. Fairweather, and S. Ballantine. 1989. Expression of tetanus toxin fragment C in E. coli: high-level expression by removing rare codons. Nucleic Acids Res. 17:10191-10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manca, F., D. Fenoglio, A. Kunkl, C. Cambiaggi, M. Sasso, and F. Celada. 1988. Differential activation of T-cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J. Immunol. 140:2893-2898. [PubMed] [Google Scholar]
- 23.Marusic-Galesic, S., M. Marusic, and B. Pokric. 1992. Cellular immune response to the antigen administered as an immune complex in vivo. Immunology 75:325-329. [PMC free article] [PubMed] [Google Scholar]
- 24.Nuttall, J., N. Vine, J. L. Hadlington, P. Drake, L. Frigerio, and J. K. Ma. 2002. ER-resident chaperone interactions with recombinant antibodies in transgenic plants. Eur. J. Biochem. 269:6042-6051. [DOI] [PubMed] [Google Scholar]
- 25.Rafiq, K., A. Bergtold, and R. Clynes. 2002. Immune complex-mediated antigen presentation induces tumor immunity. J. Clin. Investig. 110:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roosnek, E., and A. Lanzavecchia. 1991. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J. Exp. Med. 173:487-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schalke, B. C., W. E. Klinkert, H. Wekerle, and D. S. Dwyer. 1985. Enhanced activation of a T-cell line specific for acetylcholine receptor (AChR) by using anti-AChR monoclonal antibodies plus receptors. J. Immunol. 134:3643-3648. [PubMed] [Google Scholar]
- 29.Shusta, E. V., R. T. Raines, A. Pluckthun, and K. D. Wittrup. 1998. Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 16:773-777. [DOI] [PubMed] [Google Scholar]
- 30.Simitsek, P. D., D. G. Campbell, A. Lanzavecchia, N. Fairweather, and C. Watts. 1995. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J. Exp. Med. 181:1957-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanilova, S. A., and E. S. Slavov. 2001. Comparative study of circulating immune complexes quantity detection by three assays: CIF-ELISA, C1q-ELISA, and anti-C3 ELISA. J. Immunol. Methods 253:13-21. [DOI] [PubMed] [Google Scholar]
- 32.Wiersma, E. J., P. G. Coulie, and B. Heyman. 1989. Dual immunoregulatory effects of monoclonal IgG-antibodies: suppression and enhancement of the antibody response. Scand. J. Immunol. 29:439-448. [DOI] [PubMed] [Google Scholar]