Abstract
Human diabetics frequently suffer delayed wound healing, increased susceptibility to localized and systemic infections, and limb amputations as a consequence of the disease. Lower-limb infections in diabetic patients are most often polymicrobial, involving mixtures of aerobic, facultative anaerobic, and anaerobic bacteria. The purpose of this study is to determine if these organisms contribute to synergy in polymicrobial infections by using diabetic mice as an in vivo model. The model was the obese diabetic mouse strain BKS.Cg-m +/+ Leprdb/J, a model of human type 2 diabetes. Young (5- to 6-week-old) prediabetic mice and aged (23- to 24-week-old) diabetic mice were compared. The mice were injected subcutaneously with mixed cultures containing Escherichia coli, Bacteroides fragilis, and Clostridium perfringens. Progression of the infection (usually abscess formation) was monitored by examining mice for bacterial populations and numbers of white blood cells at 1, 8, and 22 days postinfection. Synergy in the mixed infections was defined as a statistically significant increase in the number of bacteria at the site of injection when coinfected with a second bacterium, compared to when the bacterium was inoculated alone. E. coli provided strong synergy to B. fragilis but not to C. perfringens. C. perfringens and B. fragilis provided moderate synergy to each other but only in young mice. B. fragilis was anergistic (antagonistic) to E. coli in coinfections in young mice at 22 days postinfection. When age-matched nondiabetic mice (C57BLKS/J) were used as controls, the diabetic mice exhibited 5 to 35 times the number of CFU as did the nondiabetic mice, indicating that diabetes was a significant factor in the severity of the polymicrobial infections.
The Centers for Disease Control (CDC) estimates that 18.2 million people in the United States, 6.3% of the population, have diabetes (11). Lower-limb amputations are a common and severe side effect of diabetes. This is shown by statistics from the CDC: more than 60% of nontraumatic lower-limb amputations in the United States occur among people with diabetes. From 2001 to 2002, about 82,000 nontraumatic lower-limb amputations were performed each year among people with diabetes (11). Bacterial infections account for ∼85% of circumstances that require lower-limb amputations in diabetic patients (1).
Diabetes-associated manifestations that contribute to the increased susceptibility to infections include peripheral vascular disease (with accompanying ischemia), neuropathy, and a dysfunctional immune system (27). These syndromes lead to the development of foot ulcers, which then become infected (27). The infections are most often polymicrobial, with mixtures of aerobes, facultative anaerobes, and obligate anaerobes (37, 38). These polymicrobial infections are difficult to cure, since the bacterial species present often have a wide variety of natural and acquired resistance to antibiotics (20). Broad-spectrum antibiotics delivered intravenously, surgical debridement, and limb amputation are the methods most often used to treat the infected diabetic limb (1).
The pathogenic bacteria present in polymicrobial infections exhibit synergistic effects in their ability to cause infections (6). Synergy was originally defined as a significant increase in the number of bacteria in a wound when coinfected with a second bacterium, compared to when the bacterium was inoculated alone. This definition was modified over time to define synergy as the cooperative interaction of two or more bacterial species that produces a result not achieved by the individual bacterium acting alone (3). Synergy has been demonstrated in experiments where the formation of an abscess in a mouse or rat was used as the in vivo assay model (6, 36). For example, Bacteroides fragilis was synergistic for the facultative anaerobe Escherichia coli (5) and the obligate anaerobe Clostridium perfringens (9). The presence of E. coli was also synergistic for C. perfringens and vice versa (9).
Based on clinical and experimental results with animal models, it has been hypothesized that different types of bacteria promote synergy within polymicrobial infections via different mechanisms (36). Aerobes and facultative anaerobes (e.g., E. coli) are thought to enhance the ability of anaerobes to grow by lowering the ambient oxygen concentration and redox potential of the infected tissue (36). Encapsulated bacteria, such as the anaerobe B. fragilis, act as antiphagocytic and proinflammatory agents, increase the likelihood of abscess formation (5), and protect other organisms from being phagocytosed (23). Others, such as the anaerobe C. perfringens, are thought to contribute to synergy by secreting powerful cytotoxins that disrupt the immune system (36).
Diabetic mice have been used to study the effects of diabetes on periodontitis (18), urinary tract infections (33), septicemia (25), and lung infections (32). Bessman et al. (2) used a strain of mice, C57B1.Ks-J-db-m (now named BKS.Cg-m +/+ Leprdb/J), which is homozygous for the diabetes spontaneous mutation in the leptin receptor and is a model of type 2 diabetes (see reference 12 and http://jaxmice.jax.org/jaxmice-cgi/jaxmicedb.cgi?objtype=pricedetail&stock=000642 for a description of the strain), to examine abscess formation and bacterial load after subcutaneous inoculation of E. coli, B. fragilis, and enterococcus. In the study by Bessman et al., 9- to 11-week-old BKS.Cg-m +/+ Leprdb/J mice and their nondiabetic littermates were infected with each possible two-organism combination of E. coli, B. fragilis, and enterococcus (2). Bessman et al. demonstrated that abscesses in the diabetic mice were more persistent than and harbored a higher number of CFU than abscesses induced in nondiabetic mice (2). We used the same strain of mice, BKS.Cg-m +/+ Leprdb/J, and the pathogens E. coli, B. fragilis, and C. perfringens in this study to ask two questions. (i) Do these bacteria exhibit synergy towards each other in an abscess model? (ii) Does long-term exposure to the symptoms of diabetes predispose the mice towards lower resistance to controlling the growth and persistence of these bacteria in an abscess model? We used multiple combinations of bacteria to infect young prediabetic mice (5 to 6 weeks old) and aged diabetic mice (23 to 24 weeks old) in an abscess model and found that there are significant differences in synergistic pattern and susceptibility to infection between the young and aged diabetic mice.
MATERIALS AND METHODS
Bacterial strains and growth media.
Three strains of bacteria were used in this study. E. coli strain 360A was obtained from S. Finegold (Veterans Administration Medical Center, Los Angeles, CA) and is a clinical isolate taken from the leg ulcer of a male diabetic patient. Strain 360A was sent to the Pennsylvania State University Gastroenteric Disease Center, Wiley Laboratory, University Park, PA for serotyping and genotyping. The results are shown in Table 1. The strain has O type 6, a nontypeable H antigen, as well as the gene encoding cytotoxic necrotizing factor 1 (CNF1). Also, we detected hemolysis on blood agar plates with strain 360A, and the hlyA gene, encoding hemolysin A (HlyA), was detected using PCR methods (data not shown). Preparations of cell suspensions in India ink showed the presence of a capsule around strain 360A, but this was a non-K1-type capsule (Table 1). B. fragilis NCTC9343 is the type strain for B. fragilis, which was originally isolated from an appendix abscess from a human patient (24). C. perfringens strain 13 was isolated from a human gangrene infection (29).
TABLE 1.
Serotyping and genotyping results for the E. coli strain (360A) used in this studya
| Strain | O type | H type | STa | STb | LT | SLT1 | SLT2 | CNF1 | CNF2 | EAE | K1 | BFP | HlyA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 360A | 6 | + | − | − | − | − | − | + | − | − | − | − | + |
STa and STb, heat stable toxins a and b; LT, heat labile toxin; SLT1 and SLT2, Shiga-like toxins 1 and 2; EAE, intimin; BFP, bundle-forming pili.
E. coli cultures were grown aerobically at 37°C in Luria-Bertani broth. The anaerobes B. fragilis and C. perfringens were grown at 37°C in a Coy anaerobic chamber (Coy Laboratory Products, Grass Lake, MI), with C. perfringens in peptone-glucose-yeast extract medium (30) and B. fragilis in Trypticase-yeast extract-glucose (TYG) medium, which contained, per liter, the following: 10 g Trypticase, 5 g yeast extract, 2 g glucose, 0.04 g vitamin K (menadione), 0.004 g of hemin, 0.001 g of resazurin, 0.004 g of FeSO4 · 7H2O, 0.5 g cysteine (free base), 0.02 g of MgSO4 · 7H2O, 0.4 g NaHCO3, 0.08 g NaCl, 0.008 g CaCl2, 0.1 M KHPO4 (pH 7.2).
Diabetic and nondiabetic control mice.
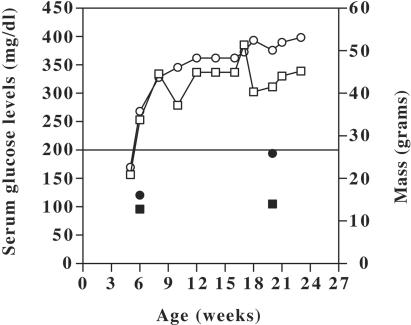
Three- to four-week-old female mice of strain BKS.Cg-m +/+ Leprdb/J were obtained from The Jackson Laboratories (Bar Harbor, Maine). Mice were quarantined for a week in isolator cages with filter tops. Two groups of mice were used in this study: (i) young (5- to 6-week-old), prediabetic mice and (ii) aged (23- to 24-week-old), diabetic mice. Mice were randomly assigned to one age group and were housed in cages of two to three mice until the appropriate age was attained. Mouse feed necessary to maintain the health of the diabetic mice was provided ad libitum. On a biweekly basis, the mice were weighed and blood glucose levels were determined for the assessment of hyperglycemia by using a handheld glucometer. The mean values of mass and blood glucose levels for all of the mice used in this study are shown in Fig. 1. A glucose level of >200 mg/dl indicated hyperglycemia, and this occurred between the ages of 5 and 6 weeks for these mice.
FIG. 1.
Graph showing the mean values of serum glucose levels (open squares) and masses (open circles) with increasing age in diabetic BKS.Cg-m +/+ Leprdb/J mice. Also shown are the serum glucose levels (filled squares) and masses (filled circles) of the young and aged nondiabetic C57BLKS/J mice. Blood glucose levels above 200 mg/dl (horizontal bar) were considered evidence of hyperglycemia.
Nondiabetic mice of strain C57BLKS/J, the parent strain of BKS.Cg-m +/+ Leprdb, were obtained from Jackson Labs at either 4 to 5 weeks of age or 22 to 23 weeks of age. The older mice were from a group of retired breeders. The mice were maintained on the same diet as the diabetic mice and used as age-matched controls for the polymicrobial infections in a manner identical to that for the diabetic mice. These mice did not show signs of hyperglycemia or obesity (Fig. 1).
Polymicrobial infections in mice.
Bacterial cultures were grown overnight and then subcultured the following day into fresh media appropriate for each bacterium. Three milliliters of each culture was pelleted and washed three times with phosphate-buffered saline (PBS) to remove residual medium and toxins. Bacterial suspensions were diluted in PBS to give a final concentration of 2 × 106 to 3 × 107 CFU/ml (as determined by serial dilutions and plating of the PBS suspensions of bacteria). The polymicrobial infections for the young and aged diabetic mice were separated into eight experimental groups, as shown in Table 2. One hundred-microliter samples of each bacterial suspension (2 × 105 to 3 × 106 CFU), singly or in combination, were injected subcutaneously into the inner thighs of the mice, as previously described (7). Each experimental group (Table 2) was comprised of 24 mice. At 1, 8, and 22 days postinjection, eight mice from each experimental group were euthanized by cervical dislocation following the induction of deep anesthesia with 100% CO2 gas. Six mice were used for the determination of the number of CFU in the injection area; the remaining two mice were used to assess the pathology of the injection site. Blood from all mice was removed from the caudal vena cava and/or directly from the heart and placed in blood vials containing EDTA. These samples were sent to a reference laboratory (Ani Lytics, Inc., Gaithersburg, MD) for determining complete blood cell counts.
TABLE 2.
Combinations of bacteria used to infect diabetic micea
| Bacterium | Injection combination for exptl group no.c
|
Controlb | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| E. coli | X | X | X | X | ||||
| B. fragilis | X | X | X | X | ||||
| C. perfringens | X | X | X | X | ||||
The same patterns were used for both the young (5- to 6-week-old) and the aged (23- to 24-week-old) mice.
The control group was injected only with PBS.
An X indicates that the bacterium was injected.
From six of the mice, the tissue surrounding the area at the site of injection was excised and the spleens were removed. Each sample was homogenized using a tissue homogenizer and resuspended in a final volume of 1 ml of PBS. Appropriate dilutions were plated on a selective medium specific for growth of each of the three organisms used. E. coli-containing samples were plated on eosin-methylene blue agar (Becton Dickinson, Cockeysville, MD) and incubated aerobically at 37°C overnight. B. fragilis-containing samples were plated on Bacteroides bile esculin agar (Becton Dickinson, Cockeysville, MD) and incubated anaerobically at 37°C for approximately 2 days. C. perfringens-containing samples were plated on either tryptose-sulfate cycloserine agar base (EM Science, Gibbstown, NJ) or Shahidi Ferguson Perfringens agar base (Becton Dickinson, Sparks, MD) and incubated anaerobically at 44°C for 8 to 12 h.
The remaining two mice were necropsied, and the area of injection and spleens were examined for pathological changes in the infection. Five-micron-thick sections of the abscess area were stained with hematoxylin and eosin and mounted on microscope slides.
Experimental protocols involving mice were examined and approved by the Virginia Tech Institutional Animal Care and Use Committee.
Statistical methods.
For CFU counts of each species (E. coli, B. fragilis, and C. perfringens) for the diabetic mice alone (Table 3), the SAS system (version 8.2; SAS Institute, Inc., Cary, NC) was used to perform analysis of variance (ANOVA) to test for the main effects of age and challenge with each of the other two organisms as well as all of their two- and three-way interactions. All CFU data were log transformed before analysis to stabilize variances. Model adequacy was assessed using standardized residual plots. Other statistical analyses were done using Instat 3 software (Graphpad, Inc.). For all statistical analyses, P values of <0.05 were considered significant.
TABLE 3.
Factors affecting bacterial populations in polymicrobial infectionsa
| Organism and factor(s) |
P value on:
|
||
|---|---|---|---|
| Day 1 | Day 8 | Day 22 | |
| E. coli | |||
| Age | 0.2875 | <0.0001 | <0.0001 |
| B. fragilis | 0.0352 | 0.8206 | 0.3407 |
| Age + B. fragilis | 0.5910 | 0.9802 | 0.0204 |
| C. perfringens | 0.3825 | 0.3966 | 0.0157 |
| Age + C. perfringens | 0.1676 | 0.3188 | 0.3970 |
| B. fragilis + C. perfringens | 0.6595 | 0.1266 | <0.0001 |
| Age + B. fragilis + C. perfringens | 0.5528 | 0.2091 | <0.0001 |
| B. fragilis | |||
| Age | 0.0007 | 0.0055 | 0.0003 |
| E. coli | <0.0001 | <0.0001 | 0.0013 |
| Age + E. coli | <0.0001 | 0.0079 | 0.0004 |
| C. perfringens | 0.0123 | 0.0012 | 0.0157 |
| Age + C. perfringens | 0.0001 | 0.4214 | 0.0387 |
| E. coli + C. perfringens | 0.8645 | 0.6550 | 0.0122 |
| Age + E. coli + C. perfringens | 0.4973 | 0.0548 | 0.0045 |
| C. perfringens | |||
| Age | <0.0001 | 0.1064 | 0.1152 |
| E. coli | 0.0002 | 0.9333 | 0.1152 |
| Age + E. coli | 0.1044 | 0.9333 | 0.1152 |
| B. fragilis | 0.1044 | 0.9333 | 0.1152 |
| Age + B. fragilis | 0.0002 | 0.9333 | 0.1152 |
| E. coli + B. fragilis | <0.0001 | 0.1064 | 0.1152 |
| Age + E. coli + B. fragilis | <0.0001 | 0.1064 | 0.1152 |
The data illustrated in Fig. 2 and 3 were analyzed by ANOVA. The results are listed here as the probabilities that a factor(s) had a statistically significant effect on the number of CFU of each species of bacteria at the number of days postinfection indicated. P values of <0.05 were considered statistically significant (shown in bold).
RESULTS
Polymicrobial infections in young (prediabetic) mice.
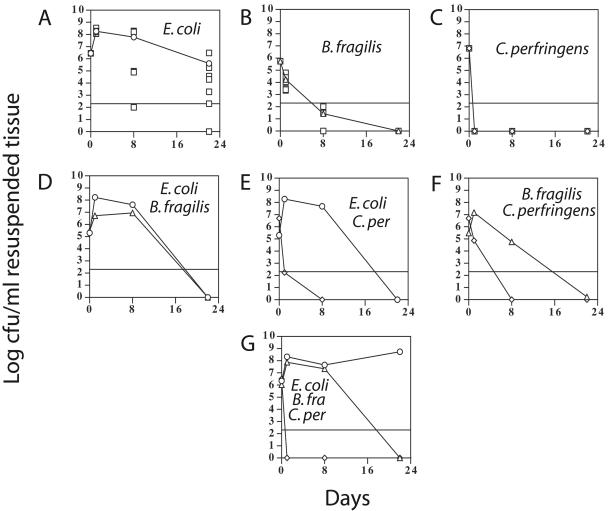
Mice were infected with the combinations of bacteria (or PBS for the control mice) listed in Table 2. At 1, 8, and 22 days postinfection, the abscesses (or infected area if no abscess was present) were excised and the number of CFU determined for each bacterium. We chose these time points to represent early, middle, and late stages of abscess formation. The infected mice formed abscesses in 2 to 3 days. The abscesses then matured in one of two ways during the final 19 days they were monitored: either they were retained by the mice or they penetrated the skin and drained. The mean numbers of CFU in the abscesses formed in the young (5- to 6-week-old) mice are shown in Fig. 2. Some mice succumbed to systemic infection, and all of these cases involved mice infected with E. coli. In experimental groups 1, 5, and 7, 12%, 4%, and 39% of the mice died, respectively, almost always by 2 to 3 days postinfection. Apparently, the injection of all three bacteria together leads to the highest rate of mortality. E. coli-dependent lethal systemic infections have been previously reported for this strain of mouse (2).
FIG. 2.
Number of CFU isolated from abscesses in young (5- to 6-week-old), prediabetic BKS.Cg-m +/+ Leprdb/J mice. One hundred-microliter samples of bacterial suspensions (2 × 105 to 3 × 106 CFU), singly or in combination, were injected subcutaneously into the inner thighs of the mice, as described in Materials and Methods. Control mice (data not shown) were injected with PBS alone. Panels A to G correspond to the infection protocols listed in Table 2 as experimental groups 1 to 7, respectively. For panels A to C, the numbers of CFU for individual mice (squares) are shown along with the mean values for E. coli (panel A, circles), B. fragilis (panel B, triangles), and C. perfringens (panel C, diamonds). In panels D to G, only the mean values are shown, with the same symbols used in panels A to C. The horizontal lines represent the lower limit of detection; for some panels, levels less than the lower limit are shown to illustrate the mean calculated values. B. fra, B. fragilis; C. per, C. perfringens.
With single infections, the E. coli strain exhibited the highest number of CFU over the course of each experiment (Fig. 2). The B. fragilis strain that was used, NCTC9343, has been shown to cause intra-abdominal abscesses in a mouse model when 1 × 106 CFU was injected intraperitoneally along with sterile rat fecal contents (14, 15). This strain did not establish visible abscesses when injected subcutaneously and by itself at the dosage used in these experiments (1 × 106 CFU) in the absence of sterile rat fecal contents (data not shown). However, this was a deliberate part of our experimental design in which we wanted to observe synergistic effects between the bacteria in the absence of adjuvants such as the sterile rat fecal contents. High numbers of CFU in the abscesses formed by injecting the single bacterium would have made synergistic effects difficult to observe.
With the experiments in which multiple bacteria were injected, distinct synergy was observed. In particular, E. coli was synergistic towards B. fragilis in the experiments where they were coinjected, providing about a 6 log increase in B. fragilis CFU at 8 days postinfection (Fig. 2, compare panels B, D, and G). B. fragilis provided a moderate level of synergy to C. perfringens, but at day 1 only, while C. perfringens provided moderate synergy to B. fragilis at days 1 and 8 (Fig. 2, compare panels B, C, and F).
Young mice infected with E. coli exhibited an unusual effect at 22 days postinfection, where the presence of B. fragilis and/or C. perfringens led to a decrease in the number of CFU of E. coli (Fig. 2, compare the day 22 time points in panel A to those in panels D and E). This negative synergy, or “anergy,” was seen only in the young mice late in the infection.
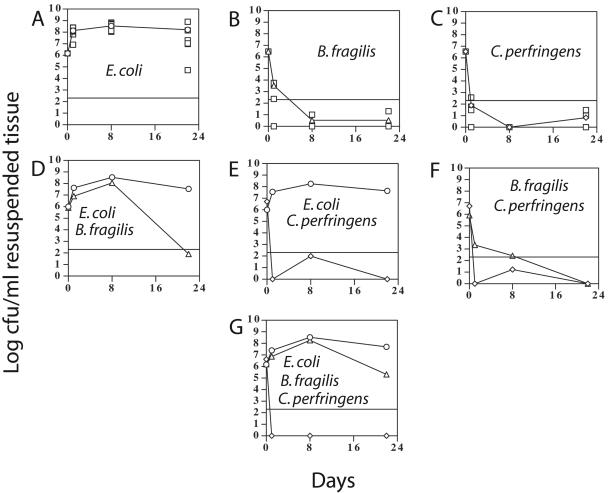
Polymicrobial infections in aged (long-term diabetic) mice.
The mean numbers of CFU in the abscesses formed in the aged (23- to 24-week-old) diabetic mice are shown in Fig. 3. The formation and progression of abscesses were difficult to measure with these mice due to the presence of thick layers of subcutaneous adipose tissue which masked the swelling seen in abscesses formed in the young mice. However, no abscesses were seen to drain through the skin, suggesting that the abscesses (if formed) in the aged mice did not migrate to the skin surface as they did in the young mice.
FIG. 3.
Number of CFU isolated from abscesses in aged (23- to 24-week-old), diabetic BKS.Cg-m +/+ Leprdb/J mice. The symbols used are described in the legend for Fig. 2.
Some of the aged mice died 2 to 3 days postinfection due to systemic infection, and all of these deaths involved mice infected with E. coli. In experimental groups 1, 5, and 7, 7%, 15%, and 14% of the mice died, respectively. For the single infections, E. coli was found at much higher levels than was B. fragilis or C. perfringens over the course of the experiments. The aged mice infected with E. coli alone exhibited higher numbers of CFU at 8 and 22 days postinfection than did the young mice (Fig. 2A and 3A; Table 3). For the mixed infections, the highest level of synergy was provided by E. coli to B. fragilis (Fig. 3, compare panels B, D, and G) at day 8, as was seen with the young mice. For the aged mice, however, there did not appear to be synergistic effects between B. fragilis and C. perfringens, as were observed with the young mice (Fig. 2 and 3, compare panels B, C, and F).
Synergy and age-dependent factors in the bacterial infections.
To determine whether synergistic effects and/or age-dependent effects were responsible for changes in CFU for each bacterium during the course of the experiment, all of the CFU results were compared using ANOVA. For each bacterium, the results at each day postinfection (i.e., day 1, 8, or 22) were analyzed to determine if age or the presence of other bacteria had a significant effect on the number of CFU we observed at that time. A summary of the results from this analysis are shown in Table 3. The complete statistical analyses are attached as supplemental material. For C. perfringens, the large majority of samples from days 8 and 22 did not contain statistically significant numbers of CFU (Fig. 2 and 3), so comparisons with those data are less robust than with the rest of the experimental data. Therefore, statistical analyses from these times were not analyzed further. When age was tested as a variable, sometimes the aged mice had higher numbers of CFU than did the young mice and at other times the reverse was true. The direction of the age-dependent effects can be seen in the supplemental material by examining the sign (positive or negative) of the mean difference between each group.
In all cases except one, E. coli at day 1, age was a statistically significant source of variability for the number of CFU observed (Table 3). For E. coli, the number of CFU was affected only by B. fragilis at day 1, age at day 8, and all effects except (i) B. fragilis and (ii) age plus C. perfringens at day 22 (Table 3). B. fragilis was the bacterium most affected by synergistic and age-dependent effects, in particular at day 22 postinfection (Table 3). As described above, B. fragilis provided synergy in an age-dependent manner to C. perfringens at day 1 (Table 3) and received synergy from C. perfringens at all three times postinfection.
To determine if one of the species used could inhibit the growth of others in vitro, all possible pairs of bacteria were cross-streaked on TYG agar and incubated anaerobically at 37°C, and the growth patterns at the streak junctions were examined. TYG agar was used because all three organisms exhibited normal growth rates in this medium. Examination of the cross streaks revealed that none of the bacteria tested showed any growth inhibition on the other species.
Diabetic mice have higher levels of bacteria in abscesses than comparably aged nondiabetic mice.
Age was a common effect leading to changes in the number of CFU in the abscesses of diabetic mice (Table 3). However, the comparison between the young and the aged diabetic mice was actually composed of two separate variables: age and exposure to diabetes. To compensate for age-dependent factors, we tested 5- to 6-week-old and 23- to 24-week-old mice of strain C57BLKS/J, the parent strain of BKS.Cg-m +/+ Leprdb/J. The C57BLKS/J mice were used instead of the heterozygous Leprdb/+ mice because the heterozygotes exhibit increased metabolic efficiency and survive fasting longer than controls (http://jaxmice.jax.org/jaxmice-cgi/jaxmicedb.cgi?objtype=pricedetail&stock=000642). The C57BLKS/J mice were infected with all three pathogens for 8 days, and the numbers of CFU in the injection area were determined. These results were compared by ANOVA to those from the young and aged diabetic mice that had been infected in the same manner (see experimental group no. 7 in Table 2). There was no statistically significant difference between the young and aged mice within the control or diabetic groups, but there were differences between the control and diabetic-mouse groups (Table 4). For E. coli, the young and aged diabetic mice had, on average, 5-fold- and 27-fold-higher levels of CFU, respectively, than did the comparably aged control mice (Table 4). For B. fragilis, the young and aged diabetic mice had, on average, 20-fold- and 35-fold-higher levels of CFU, respectively, than did the comparably aged control mice (Table 4). C. perfringens cells were not detected at a statistically significant level for any mice examined.
TABLE 4.
Comparison of the numbers of CFU recovered from non-diabetic C57BLKS/J mice, the parent strain of the diabetic BKS.Cg-m +/+ Leprdb/J micea
| Mouse strain(s) | Inoculum | Mean no. of CFU (range) recovered from:
|
||
|---|---|---|---|---|
| 5- to 6-wk-old mice | 23- to 24-wk-old mice | |||
| C57BLKS/J | E. coli | 9.5 × 106 (6.0 × 102-5.6 × 107) | 1.2 × 107 (2.6 × 104-5.2 × 107) | |
| B. fragilis | 1.1 × 106 (0-6.2 × 106) | 5.1 × 106 (1.5 × 102-3.6 × 107) | ||
| BKS.Cg-m +/+ Leprdb/Jb | E. coli | 4.5 × 107 (2.1 × 105-1.6 × 108) | 3.3 × 108 (1.8 × 108-5.6 × 108) | |
| B. fragilis | 2.2 × 107 (1.4 × 106-1.1 × 108) | 1.8 × 108 (8.2 × 107-3.1 × 108) | ||
| Ratio of mean CFU
|
||||
| BKS.Cg-m +/+ Leprdb/J and C57BLKS/J | E. coli | 5 | 27 | |
| B. fragilis | 20 | 35 | ||
For the nondiabetic mice, young or aged mice were infected with E. coli, B. fragilis, and C. perfringens in the abscess model. At 8 days postinfection, the numbers of CFU in the abscesses were determined. No significant levels of C. perfringens were recovered from either age group.
The values for the diabetic BKS.Cg-m +/+ Leprdb/J mice at 8 days postinfection for experimental group 7 (from Table 2) are also shown for comparison.
Inflammatory responses to polymicrobial infections.
The systemic inflammatory responses to the bacterial infections were evaluated by measuring the white blood cell (WBC) counts of all mice. The WBC counts of the mice infected with the bacteria were compared to those of the mock (PBS)-infected controls. For the mock-infected young mice, the mean WBC counts were 5,700, 10,700, and 13,100 per mm3 at 1, 8, and 22 days postinjection, respectively. For the aged mice, the mean WBC counts were 5,500, 3,500, and 3,400 per mm3 at 1, 8, and 22 days postinjection, respectively. These values suggest that, for unknown reasons, the young mice exhibited a consistently higher level of inflammation during the course of the experiment than did the aged mice. The WBC counts from the infected mice were compared to those from the mock-infected mice by using ANOVA. There was a statistically significant difference between some of the age-matched infected and mock-infected groups at the corresponding postinfection times, and these are listed in Table 5. For B. fragilis- and C. perfringens-infected young mice, there was a significant decrease in the WBC counts in comparison to those for the mock-infected control mice (Table 5). For the aged mice, five different infections led to a significant difference in WBC counts, and in all cases the WBC counts increased when E. coli was part of the infectious group (Table 5). Therefore, the young and aged mice exhibited a clear difference in their responses to the bacterial challenge: the young mice exhibited a decreased inflammatory response, while the aged mice showed an increased inflammatory response.
TABLE 5.
Infections which led to a statistically significant difference in total WBC count in infected mice compared to that of the mock (PBS)-infected controla
| Inoculumb | Mouse age (wk) | Days postinfection | Mean WBC count
|
P value | Mean difference | 95% confidence level (range) | |
|---|---|---|---|---|---|---|---|
| Mock-infected mice | Treated mice | ||||||
| B. fragilis | 5-6 | 8 | 10,700 | 5,100 | <0.01 | −5,600 | −9,700 to −1,600 |
| C. perfringens | 5-6 | 22 | 13,100 | 5,000 | <0.001 | −8,100 | −12,300 to −3,800 |
| E. coli | 23-24 | 8 | 3,500 | 8,800 | <0.05 | 5,200 | 780-9,600 |
| Ec-Bf-Cp | 23-24 | 8 | 3,500 | 12,100 | <0.001 | 8,600 | 4,000-13,200 |
| E. coli | 23-24 | 22 | 3,400 | 18,800 | <0.001 | 15,400 | 7,900-22,900 |
| Ec-Bf | 23-24 | 22 | 3,400 | 10,300 | <0.05 | 6,900 | 250-13,600 |
| Ec-Bf-Cp | 23-24 | 22 | 3,400 | 10,200 | <0.05 | 6,800 | 150-13,500 |
All other infections did not lead to a difference between the infected mice and mock-infected controls. The number of mice in each sample group ranged from five to eight. The statistical analysis was done using one-way ANOVA, with the Tukey-Kramer posttest.
Ec, E. coli; Bf, B. fragilis; Cp, C. perfringens.
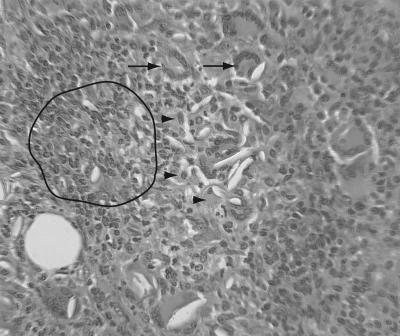
The local inflammatory responses to the infections were measured using tissue sections prepared from the injection sites of 85 animals. This represented a sampling of 33 young mice and 52 aged mice. Injection sites were evaluated blindly by a veterinary pathologist. Lesions were scored on a semiquantitative scale, noting incidence and severity (minimal-mild-moderate-marked-severe). No significant acute or chronic inflammatory lesions were noted in PBS-injected control mice. Infected mice showed formation of microabscesses or acute steatitis and cellulitis at injection sites as acute lesions, or they developed mixed inflammatory cell lesions with fibrosis. In general, more fibrosis was seen with older lesions. Higher percentages of inflammatory lesions at injection sites were seen associated with E. coli infection and with mixed bacterial infections containing E. coli than with other single or combination treatments. For several mice, granuloma formation was noted, with typical infiltrating populations of macrophages, epithelioid cell formation, and giant cells (Fig. 4).
FIG. 4.
Representative image showing a lesion with granulomatous characteristics from an infected diabetic mouse. A 5-μm-thick section from an abscess area of a BKS.Cg-m +/+ Leprdb/J mouse was stained with hematoxylin and eosin. The young (5- to 6-week-old) mouse was infected with a combination of E. coli and B. fragilis and euthanized 22 days postinfection, when the area around the lesion was removed and processed to obtain the section shown. Prominent in the figure are giant cells (arrows), epithelioid cells (arrowheads), and high levels of infiltrating polymorphonuclear leukocytes, macrophages, and lymphoid cells (area inside the black boundary line).
DISCUSSION
We undertook the present study to determine if E. coli, B. fragilis, and C. perfringens exhibited synergy to each other in a mouse model of type 2 diabetes. We used two different age groups of mice to determine whether long-term exposure to the symptoms of diabetes affects the ability of diabetic mice to resist bacterial infections. The synergistic properties of the pathogens were different for young prediabetic mice and aged diabetic mice. This is reflected in the frequency with which age was a determining factor in the number of CFU isolated from most of the infections (Table 3).
Our results suggest that the highest level of synergy was provided by E. coli to B. fragilis (Fig. 2 and 3; Table 3). B. fragilis also received synergy from C. perfringens, but the effect was not as strong as that seen with E. coli. B. fragilis provided moderate levels of synergy to C. perfringens, but only in young mice at day 1 postinfection. It appeared that B. fragilis was the species that obtained the most benefit from the presence of the other bacteria, particularly in the young mice. This has been reported previously for abscess models using nondiabetic mice, and this ability may be a major factor in B. fragilis being identified at high frequencies in diabetic infections (5, 8, 9). While there are numerous reports of B. fragilis providing synergy to other organisms in cutaneous abscess models (5, 8, 42, 43), we did not observe this except in the case of C. perfringens in the young mice at day 1 postinfection (Fig. 2).
In young mice, the number of E. coli CFU at 22 days postinfection was reduced if B. fragilis or C. perfringens or both were also present. This effect has been observed in previous studies (5). We are calling this effect “anergy” to represent the effects that are opposite to those seen in synergistic interactions. This effect may be due to localized stimulation of the immune system by the other pathogens earlier in the infection, since neither B. fragilis nor C. perfringens was detected in these 22-day-old abscesses (Fig. 2). B. fragilis produces a proinflammatory capsule, which has been shown to be essential for abscess formation in animal models (34, 40). Pathogenic strains can produce up to eight types of capsular polysaccharides (PS), termed PS A to PS H (26). Phase switching of these polysaccharides occurs by inversion of a DNA region containing promoters lying upstream of the capsule biosynthesis genes (26). While both purified PS A and PS B can induce abscesses in the absence of bacteria (40), a mutation in the loci for genes involved in the biosynthesis of PS A led to a greatly decreased level of abscess formation by live bacteria in vivo (15), but mutations in the PS B (14) and PS C loci did not (13). It is possible that the immune response to the B. fragilis capsule, while favoring the formation of an abscess and persistence of B. fragilis in the wound, inhibited the survival of E. coli in the abscess environment.
The molecular mechanisms for the strong synergistic effect E. coli provided to B. fragilis may be due to a combination of environmental modulation effects and production of toxins. The facultative anaerobe E. coli has been hypothesized to lower the ambient oxygen concentration and redox potential via aerobic respiration, allowing growth of obligate anaerobes (36). The respiratory chain in E. coli has two major terminal oxidases, cytochrome bo3 and cytochrome bd, and a minor terminal oxidase, encoded by the genes cyxAB, that contributes only a small percentage of the aerobic respiratory activity (19). Oxygen depletion through the use of these terminal oxidases may be an important factor for the growth of B. fragilis in the abscesses in which E. coli was present. This hypothesis is currently being tested in our laboratory by introduction of mutations into the terminal oxidase-encoding genes and examination of synergy by the mutant E. coli strains.
Extraintestinal pathogenic E. coli, including the strain used in this study (360A), can produce a range of virulence factors, including the cytotoxins CNF1 and HlyA. CNF1 is cytotoxic due to its ability to deamidate glutamine residues in the small G proteins Rho, Rac, and Cdc42 (17, 28, 39). Deamidation results in constitutive activity of these G proteins and the formation of pronounced morphological changes due to aberrant actin polymerization control (4). Also, CNF1 has been shown to reduce transmigration of polymorphonuclear leukocytes across an epithelial-cell layer and inhibit bacterial phagocytosis by leukocytes (10, 21, 22). These effects on phagocytes may be important in providing protection for both E. coli and B. fragilis in a coinfection model. HlyA is a membrane binding toxin that forms small pores in the cytoplasmic membrane of host cells, leading to cell death (31). HlyA not only lyses red blood cells but also can prove very cytotoxic to leukocytes (31). Therefore, HlyA may provide dual functions in promoting synergy: protecting B. fragilis from phagocytic cells and lysing red blood cells, which release hemoglobin. The hemoglobin can then act as a source of hemin, a necessary growth factor for B. fragilis (35). Based on results with nonisogenic strains in a mouse model, Ushijima et al. (41) suggested that the production of hemolysin by E. coli might play a major role in the synergy during the formation of subcutaneous abscesses. The role that CNF1 and HlyA play in the contribution to synergy is currently under investigation.
The diabetic mouse model we have used in these studies, which is homozygous for the leptin receptor (Leprdb or db/db), has been described as having defects in T-cell-mediated functions but is hypersensitive to monocyte/macrophage stimulation (16). However, to our knowledge, whether the age of the mice contributes to these immune effects has not been demonstrated. As measured by the number of WBC in the circulation in our experiments, the systemic immune responses to the infections differed between the young and aged mice. The young mice had a consistently higher level of WBC even in the absence of a bacterial challenge. The observation that infections with B. fragilis and C. perfringens actually lowered the number of WBC at 8 and 22 days postinfection in the young mice may be due to recruitment of the host immune cells to the site of infection, thereby leading to lower levels in the general circulation, but this remains to be proven. The aged diabetic mice responded to E. coli infections by raising the number of CFU in a typical response to a bacterial challenge. The high mortality rates due to E. coli infections in the young mice suggest that the E. coli strain we used has the ability to cause a systemic infection, so the response of the aged mice would be that expected if the bacteria enter the bloodstream.
The local immune responses to the infections also indicated that E. coli was responsible for eliciting the highest level of immune cell infiltration into the infected area. In fact, some of the abscesses formed granulomatous lesions, which indicates that a long-term infection state may become established in the abscesses formed in the diabetic mice. This would have consequences for infections of human diabetic patients, which have been shown to be chronic and last for months in some patients (1).
Supplementary Material
Acknowledgments
This work was supported by a grant from the State of Virginia ASPIRES program.
We thank L. Comstock for providing B. fragilis strain NCTC9343, S. Finegold for providing E. coli strain 360A, Rebecca Starr and Trevor Williams for technical assistance, and Daniel Ward for statistical analyses.
Editor: D. L. Burns
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Apelqvist, J., and J. Larsson. 2000. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes-Metab. Res. Rev. 16:S75-S83. [DOI] [PubMed] [Google Scholar]
- 2.Bessman, A. N., F. L. Sapico, M. Tabatabai, and J. Z. Montgomerie. 1986. Persistence of polymicrobial abscesses in the poorly controlled diabetic host. Diabetes 35:448-453. [DOI] [PubMed] [Google Scholar]
- 3.Bjornson, H. S., R. Colley, R. H. Bower, V. P. Duty, J. T. Schwartz-Fulton, and J. E. Fischer. 1982. Association between microorganism growth at the catheter insertion site and colonization of the catheter in patients receiving total parenteral nutrition. Surgery 92:720-727. [PubMed] [Google Scholar]
- 4.Boquet, P. 2001. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. Toxicon 39:1673-1680. [DOI] [PubMed] [Google Scholar]
- 5.Brook, I. 1985. Enhancement of growth of aerobic and facultative bacteria in mixed infections with Bacteroides species. Infect. Immun. 50:929-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook, I. 1987. Synergistic aerobic and anaerobic infections. Clin. Ther. 10(Suppl. A):19-35. [PubMed] [Google Scholar]
- 7.Brook, I., J. C. Coolbaugh, and R. I. Walker. 1983. Antibiotic and clavulanic acid treatment of subcutaneous abscesses caused by Bacteroides fragilis alone or in combination with aerobic bacteria. J. Infect. Dis. 148:156-159. [DOI] [PubMed] [Google Scholar]
- 8.Brook, I., V. Hunter, and R. I. Walker. 1984. Synergistic effect of bacteroides, Clostridium, Fusobacterium, anaerobic cocci, and aerobic bacteria on mortality and induction of subcutaneous abscesses in mice. J. Infect. Dis. 149:924-928. [DOI] [PubMed] [Google Scholar]
- 9.Brook, I., and R. I. Walker. 1986. Pathogenicity of Clostridium species with other bacteria in mixed infections. J. Infect. 13:245-253. [DOI] [PubMed] [Google Scholar]
- 10.Capo, C., S. Meconi, M. V. Sanguedolce, N. Bardin, G. Flatau, P. Boquet, and J. L. Mege. 1998. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton in human monocytes: role in the regulation of integrin-dependent phagocytosis. J. Immunol. 161:4301-4308. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2003. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2003. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga.
- 12.Chen, H., O. Charlat, L. A. Tartaglia, E. A. Woolf, X. Weng, S. J. Ellis, N. D. Lakey, J. Culpepper, K. J. Moore, R. E. Breitbart, G. M. Duyk, R. I. Tepper, and J. P. Morgenstern. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491-495. [DOI] [PubMed] [Google Scholar]
- 13.Comstock, L. E., M. J. Coyne, A. O. Tzianabos, A. Pantosti, A. B. Onderdonk, and D. L. Kasper. 1999. Analysis of a capsular polysaccharide biosynthesis locus of Bacteroides fragilis. Infect. Immun. 67:3525-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne, M. J., W. Kalka-Moll, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2000. Bacteroides fragilis NCTC9343 produces at least three distinct capsular polysaccharides: cloning, characterization, and reassignment of polysaccharide B and C biosynthesis loci. Infect. Immun. 68:6176-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyne, M. J., A. O. Tzianabos, B. C. Mallory, V. J. Carey, D. L. Kasper, and L. E. Comstock. 2001. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect. Immun. 69:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantuzzi, G., and R. Faggioni. 2000. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 68:437-446. [PubMed] [Google Scholar]
- 17.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 18.Fouad, A., J. Barry, J. Russo, J. Radolf, and Q. Zhu. 2002. Periapical lesion progression with controlled microbial inoculation in a type I diabetic mouse model. J. Endod. 28:8-16. [DOI] [PubMed] [Google Scholar]
- 19.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella, 2nd ed. ASM Press, Washington, D.C.
- 20.Ginunas, V. J., H. N. Canawati, and F. L. Sapico. 1984. Anaerobic bacteria isolated from foot infections in diabetic patients: in vitro susceptibility to nine antibiotics. Clin. Ther. 6:457-460. [PubMed] [Google Scholar]
- 21.Hofman, P., G. Flatau, E. Selva, M. Gauthier, G. Le Negrate, C. Fiorentini, B. Rossi, and P. Boquet. 1998. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect. Immun. 66:2494-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofman, P., G. Le Negrate, B. Mograbi, V. Hofman, P. Brest, A. Alliana-Schmid, G. Flatau, P. Boquet, and B. Rossi. 2000. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 68:522-528. [PubMed] [Google Scholar]
- 23.Ingham, H. R., P. R. Sisson, D. Tharagonnet, J. B. Selkon, and A. A. Codd. 1977. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet ii:1252-1254. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, J. L. 1978. Taxonomy of the Bacteroides. I. Deoxyribonucleic acid homologies among Bacteroides fragilis and other saccharolytic Bacteroides species. Int. J. Syst. Bacteriol. 28:245-268. [Google Scholar]
- 25.Kitahara, Y., T. Ishibashi, Y. Harada, M. Takamoto, and K. Tanaka. 1981. Reduced resistance to Pseudomonas septicaemia in diabetic mice. Clin. Exp. Immunol. 43:590-598. [PMC free article] [PubMed] [Google Scholar]
- 26.Krinos, C. M., M. J. Coyne, K. G. Weinacht, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414:555-558. [DOI] [PubMed] [Google Scholar]
- 27.Laing, P. 1998. The development and complications of diabetic foot ulcers. Am. J. Surg. 176:11S-19S. [DOI] [PubMed] [Google Scholar]
- 28.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 67:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahony, D. E., and T. I. Moore. 1976. Stable L-forms of Clostridium perfringens and their growth on glass surfaces. Can. J. Microbiol. 22:953-959. [DOI] [PubMed] [Google Scholar]
- 30.Melville, S. B., R. Labbe, and A. L. Sonenshein. 1994. Expression from the Clostridium perfringens cpe promoter in C. perfringens and Bacillus subtilis. Infect. Immun. 62:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menestrina, G., C. Moser, S. Pellet, and R. Welch. 1994. Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology 87:249-267. [DOI] [PubMed] [Google Scholar]
- 32.Obana, Y., T. Nishino, and T. Tanino. 1985. Therapeutic efficacy of beta-lactam and aminoglycoside antibiotics on experimental pneumonia caused by Klebsiella pneumoniae B-54 in diabetic mice. J. Antibiot. (Tokyo) 38:941-947. [DOI] [PubMed] [Google Scholar]
- 33.Obana, Y., K. Shibata, and T. Nishino. 1991. Adherence of Serratia marcescens in the pathogenesis of urinary tract infections in diabetic mice. J. Med. Microbiol. 35:93-97. [DOI] [PubMed] [Google Scholar]
- 34.Onderdonk, A. B., D. L. Kasper, R. L. Cisneros, and J. G. Bartlett. 1977. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J. Infect. Dis. 136:82-89. [DOI] [PubMed] [Google Scholar]
- 35.Otto, B. R., M. Sparrius, A. M. Verweij-van Vught, and D. M. MacLaren. 1990. Iron-regulated outer membrane protein of Bacteroides fragilis involved in heme uptake. Infect. Immun. 58:3954-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotstein, O. D., T. L. Pruett, and R. L. Simmons. 1985. Mechanisms of microbial synergy in polymicrobial surgical infections. Rev. Infect. Dis. 7:151-170. [DOI] [PubMed] [Google Scholar]
- 37.Sapico, F. L., H. N. Canawati, J. L. Witte, J. Z. Montgomerie, F. W. Wagner, Jr., and A. N. Bessman. 1980. Quantitative aerobic and anaerobic bacteriology of infected diabetic feet. J. Clin. Microbiol. 12:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sapico, F. L., J. L. Witte, H. N. Canawati, J. Z. Montgomerie, and A. N. Bessman. 1984. The infected foot of the diabetic patient: quantitative microbiology and analysis of clinical features. Rev. Infect. Dis. 6(Suppl. 1):S171-S176. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 40.Tzianabos, A. O., A. B. Onderdonk, B. Rosner, R. L. Cisneros, and D. L. Kasper. 1993. Structural features of polysaccharides that induce intra-abdominal abscesses. Science 262:416-419. [DOI] [PubMed] [Google Scholar]
- 41.Ushijima, T., M. Takahashi, and A. Seto. 1990. The role of Escherichia coli haemolysin in the pathogenic synergy of colonic bacteria in subcutaneous abscess formation in mice. J. Med. Microbiol. 33:17-22. [DOI] [PubMed] [Google Scholar]
- 42.Verweij-van Vught, A. M., F. Namavar, M. Sparrius, W. A. Vel, and D. M. MacLaren. 1985. Pathogenic synergy between Escherichia coli and Bacteroides fragilis: studies in an experimental mouse model. J. Med. Microbiol. 19:325-331. [DOI] [PubMed] [Google Scholar]
- 43.Verweij-van Vught, A. M., F. Namavar, W. A. Vel, M. Sparrius, and D. M. MacLaren. 1986. Pathogenic synergy between Escherichia coli and Bacteroides fragilis or B. vulgatus in experimental infections: a nonspecific phenomenon. J. Med. Microbiol. 21:43-47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.