Abstract
It is well documented that sex-dependent factors affect susceptibility to infection, with most mouse models demonstrating higher resistance in females. We made the unexpected observation that infection with the intracellular bacterium Listeria monocytogenes showed an opposite pattern in several commonly used inbred mouse strains: female C57BL/6J, BALB/c, C3H/HeN, and CBA/J mice were significantly more susceptible to Listeria infection. The pronounced sensitivity of females to Listeria, which was revealed by significantly higher lethality rates, correlated also with increased bacterial numbers in organ tissues (spleen and liver) and several immunological changes in peripheral blood samples. Surprisingly, increased severity of infection in females was associated with elevated interleukin-10 (IL-10) levels in plasma. Experiments using Il10 knockout mice, for which no differences between the susceptibilities of males and females to Listeria infection could be detected, confirmed the important role of this immunosuppressive cytokine for the outcome of disease. Our findings are likely to have clinical relevance, since similar sex differences with regard to infection with Listeria monocytogenes and other intracellular pathogens have been reported for humans.
Infectious diseases are a major cause of morbidity and mortality worldwide (52). It is well established that the sex of a host can significantly affect susceptibility to infection. A number of reports have shown that patients of one sex are more likely to get an infectious disease, and gender is often referred to as a risk factor for the severity and outcome of an illness; well-known examples from human pathology include tuberculosis (13), sepsis (7), invasive amebiasis (1), toxoplasmosis-related entities (8, 42), and listeriosis (21). The underlying molecular mechanisms of this predisposition are largely unknown.
Mice have been extensively used to study immune responses during infection, and it is not surprising that sex differences in susceptibility have also been observed in mouse models, further confirming clinical observations that female and male individuals handle infections differently. Most experimental settings examining a variety of different infectious agents have revealed a rather redundant susceptibility pattern, suggesting that female mice are in general more resistant to bacterial or viral diseases than males (4, 17, 27, 30, 35, 36, 53). In all these experimental models, increased resistance was associated with more vigorous and better-sustained immune responses in females (36, 41, 47, 51), which have been attributed to hormone-regulated dissimilarities in immune cell function and cytokine production (5, 44). Nevertheless, the intricate mechanisms of sex differences in infection susceptibility have remained obscure.
Listeria monocytogenes is an intracellular gram-positive bacterium that causes disease in immunocompromised individuals and pregnant women, often with deleterious consequences for the fetus (21). It is also one of the most widely used pathogens in experimental mouse studies that provided the basis for establishing major paradigms in contemporary immunology.
The origin of the gender “preference” of L. monocytogenes infection has never been clarified. We therefore analyzed sex-related susceptibility patterns for listeriosis in four commonly used inbred strains of mice. In contrast to most other infection models, we found that females of all mouse strains were more susceptible to L. monocytogenes than males. Interestingly, the increased severity of infection in females correlated with elevated interleukin-10 (IL-10) levels in plasma but not with gamma interferon (IFN-γ). L. monocytogenes infection experiments in Il10 knockout mice revealed a loss of sex dependence in the absence of this cytokine, demonstrating the important role of IL-10 in this model. We hypothesize that differential IL-10 production is a major factor in the observed sex dependence in susceptibility to L. monocytogenes infection.
MATERIALS AND METHODS
Mice.
Inbred, pathogen-free 12- to 14-week-old C57BL/6J, BALB/c, C3H/HeN, and CBA/J mice were purchased from Harlan-Winkelmann (Borchen, Germany) and housed under specific-pathogen-free conditions at the German Research Centre for Biotechnology (GBF). Il10tm1 Cgn knockout mice (which are deficient in IL-10 [34]), backcrossed for 10 generations onto the C57BL/6J background, were purchased from the Jackson Laboratory (Bar Harbor, Maine). These mice were sanitized via embryo transfer and imported into the specific-pathogen-free infection facility of the GBF. All subsequent experiments were performed in accordance with German laws after appropriate permission was obtained from the government (509.42502/07-06.02 from Bezirksregierung Braunschweig).
Infection and bacteria.
Age-matched groups of seven female and male mice from each strain were infected intravenously with 1.3 × 103 to 2.0 × 104 CFU of L. monocytogenes EGD or 1 × 105 CFU of Streptococcus pyogenes A20 (36). For survival experiments, mice were observed for 14 days after infection. Phosphate-buffered saline (PBS)-injected age-, sex-, and strain-matched animals served as controls.
For determination of organ bacterial numbers, mice were euthanized by CO2 inhalation, and livers and spleens were dissected, weighed, and put into 14-ml tubes containing 5 ml ice-cold PBS. Tissues were homogenized using an automatic homogenizer at maximum speed for 30 s. Serial dilutions were plated on brain heart infusion (Difco, N.J.) agar plates and incubated overnight at 37°C. After 24 h of growth, colonies were counted and the bacterial load of each organ was calculated.
FACS analysis.
All blood tests were performed at the German Mouse Clinic (20a). Peripheral blood lymphocytes (PBL) were isolated from 500 μl blood by erythrocyte lysis with NH4Cl (0.17 M)-Tris buffer (pH 7.45) directly into 96-well microtiter plates. After a subsequent wash with staining buffer (PBS, 0.5% bovine serum albumin, 0.02% sodium azide, pH 7.45), PBL were incubated for 20 min with 1 mM ethidium monoazide bromide (Molecular Probes, The Netherlands) and Fc block (clone 2.4G2; PharMingen, San Diego, Calif.). Ethidium monoazide bromide bound to the DNA of dead cells was photo-cross-linked by brief light exposure. Cells were then stained with fluorescence-conjugated monoclonal antibodies (PharMingen). The following main cell populations were analyzed: B cells (CD19+; clone 1D3), T cells (CD3+; clone 145-2C11), CD4+ T cells (clone RM4-5), CD8+ T cells (CD8α, clone 53-6.7; CD8β, clone H35-17.2), granulocytes (Gr-1+; clone RB6-8C5), and NK cells (CD49b+; clone DX5). Data were acquired on a FACSCalibur (Becton Dickinson, San Diego, Calif.) and analyzed using FlowJo software (Tree Star Inc., Ashland, Oreg.). All samples were acquired until a total number of 25,000 cells was reached.
Cytokine measurements.
Cytokines were measured on a Bioplex reader (Bio-Rad, Hercules, Calif.) using an 18-plex assay kit allowing simultaneous quantification of the following cytokines and chemokines in a single sample: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12 (p40), IL-12 (p70), IL-17, tumor necrosis factor alpha (TNF-α), IFN-γ, keratinocyte-derived chemokine (KC), macrophage inflammatory protein 1α (MIP-1α), granulocyte colony-stimulating factor (G-CSF), granulocyte-monocyte colony-stimulating factor (GM-CSF), and regulated on activation, normal T-cell expressed and secreted (RANTES). All procedures were carried out according to the manufacturer's specifications.
Statistical analysis.
Student's t test (with S-plus and SigmaPlot software) was used to establish the level of significance for comparisons of groups of infected and control animals as well as females and males. The log rank test (with continuity correction) was applied to evaluate data from survival experiments. Significance levels were defined and are marked in figures and tables as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Increased lethality of L. monocytogenes infection in female mice.
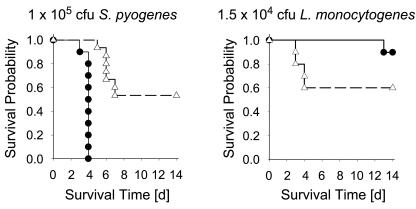
Although L. monocytogenes has been one of the most extensively used pathogens in mouse models for studying innate and adaptive immune responses, and in spite of the available clinical data providing evidence for the existence of sex-based susceptibility differences in listeriosis (21), the question of sex dependency has never been thoroughly elucidated in the mouse. To address this issue, we infected age-matched groups of male and female BALB/c mice with 1.5 × 104 CFU L. monocytogenes EGD, which was approximately 1 50% lethal dose for this mouse strain, and followed their survival for a period of 14 days. As a positive control, another group of mice from the same strain was infected with 1 × 105 CFU Streptococcus pyogenes, for which a clear sex dependency has been established (36). As expected, all S. pyogenes-infected male animals died by day 4, while half of the females survived until the end of the experiment (Fig. 1, left). Exactly the opposite pattern was observed after infection with L. monocytogenes: almost half of the female mice died by day 4 after infection, while males were clearly more resistant, and this difference was maintained until the end of the observation period (Fig. 1, right). This finding is contrary to what is known from most other bacterial and viral models in the mouse, where females are the more resistant sex (4, 17, 27, 30, 35, 36, 53).
FIG. 1.
Infection of BALB/c mice with L. monocytogenes reveals increased lethality in females. Kaplan-Meier survival curves for male (•) and female (▵) mice after infection with L. monocytogenes (right) or S. pyogenes (left) are shown. Seven mice per group were monitored for a period of 14 days. Representative data from one out of three independent experiments are shown.
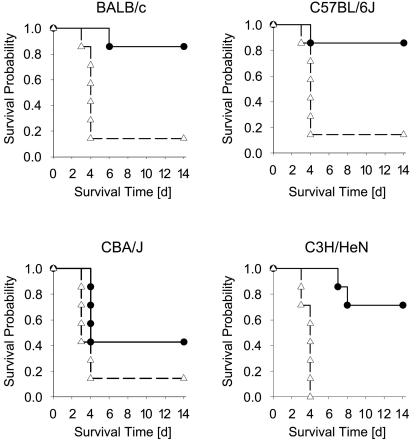
Because susceptibility to L. monocytogenes infection is partly genetically determined (9, 15, 18, 23, 46), the observed “reversed” sex-dependent sensitivity (Fig. 1) could just reflect a BALB/c-specific phenotype. We therefore determined lethality curves for a variety of commonly used inbred mouse strains; age- and sex-matched groups of C57BL/6J, C3H/HeN, BALB/c, and CBA/J mice were infected with 2 × 104 CFU L. monocytogenes, a slightly higher dose, since increased resistance to L. monocytogenes infection has been well documented for C57BL/6 mice (15). In findings similar to those from the first experiment (Fig. 1), female BALB/c mice showed increased susceptibility to L. monocytogenes, which was even more pronounced due to the higher infecting dose (Fig. 2) (P = 0.018). Female mice of the other three strains also had poorer survival rates than males (for C57BL/6J, P = 0.035; for C3H/HeN, P = 0.002; for CBA/J, P = 0.05).
FIG. 2.
Increased severity of L. monocytogenes infection in female mice is not dependent on the genetic background. Kaplan-Meier survival curves for L. monocytogenes-infected BALB/c (upper left), C57BL/6J (upper right), C3H/HeN (lower right), and CBA/J (lower left) male (•) and female (▵) mice are shown. Seven mice per group were monitored for 14 days. Representative data from one out of three independent experiments are shown.
Since male mice are usually slightly bigger than females, we needed to exclude the possibility that the differences observed were biased by sex-dependent differences in body weight. Therefore, we compared the survival data of mice infected with identical bacterial doses per gram of body weight (mean 680 CFU/g, which equals 2 × 104 CFU L. monocytogenes per mouse in males and 1.5 × 104 CFU in females). Under these conditions also, female mice continued always to be more susceptible, with higher lethality rates than males (data not shown). This series of experiments demonstrated that increased susceptibility of female mice is a general feature of this model. It also allowed us to estimate the effects of different genetic backgrounds on susceptibility to L. monocytogenes infection. Based on the data from males (since the majority of females died by day 4 after infection), BALB/c and C57BL/6J mice were most resistant, C3H/HeN mice showed an intermediate phenotype, and CBA/J mice were most susceptible to L. monocytogenes infection.
Pronounced severity of L. monocytogenes infection revealed by higher bacterial numbers in organ tissues and immunological changes in peripheral blood samples.
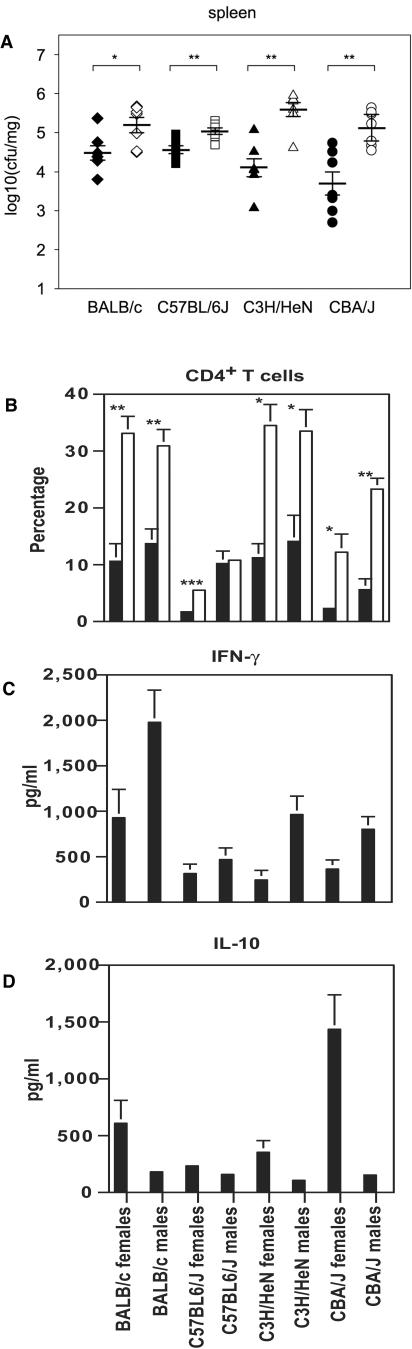
Our experiments established that female mice are more susceptible to L. monocytogenes infection, with poorer survival rates than males. To elucidate the underlying mechanisms of this “sex dimorphism,” we determined the bacterial loads in spleen and liver after infection. Although CFU from L. monocytogenes-infected animals are clearly affected by the genetic background, significantly higher numbers of bacteria were counted in spleens derived from female mice of all four mouse strains (Fig. 3A). Similar differences were also found in liver tissues (data not shown).
FIG.3.
Female mice show higher bacterial loads, more-pronounced lymphopenia, lower IFN-γ and higher IL-10 plasma concentrations than males on day 3 after L. monocytogenes infection. (A) CFU numbers in the spleen were calculated for male (closed symbols) and female (open symbols) BALB/c, C57BL/6J, CBA/J, and C3H/HeN mice. For each group, the mean CFU (horizontal lines) and standard error of the mean are indicated. (B though D) Relative proportions of CD4+ T cells (B) and plasma concentrations of IFN-γ (C) and IL-10 (D) were determined in peripheral blood samples from infected (black bars) and control (white bars) mice. In control groups, IFN-γ and IL-10 levels were below the detection limits of the assay. Means of results obtained from three to seven mice per group (± standard errors of the means) are shown. Significant differences are indicated as follows: *, P < 0.05; **, P < 0.01.
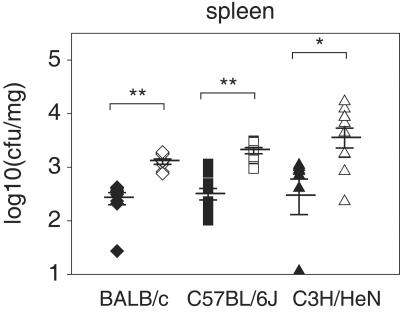
We also infected groups of female and male animals with a sublethal dose of L. monocytogenes (1 × 103 CFU). All mice survived until the end of the observation period (data not shown), but significantly higher bacterial numbers were again found in organ tissues of female mice (Fig. 4). These results support the interpretation that female mice have a reduced ability to control replicating bacteria during infection, which is achieved through the coordinated interplay of different immune system effectors (11, 12). As has been reported before (9, 15, 18, 23, 46), absolute numbers of viable bacteria recovered after infection from spleens or livers differed substantially between different mouse strains and did not necessarily correlate directly with lethality.
FIG. 4.
Female mice show increased bacterial loads after L. monocytogenes infection with a sublethal dose. CFU numbers in the spleen were calculated for male (closed symbols) and female (open symbols) BALB/c, C57BL/6J, and C3H/HeN mice. Mean CFU (horizontal lines) calculated for 9 or 10 mice per group and standard errors of the means are indicated. Significant differences are indicated as follows: *, P < 0.05; **, P < 0.01.
We performed extensive monitoring of immunological parameters during L. monocytogenes infection in order to examine sex-dependent differences in the immune response. Since most of the infected animals died by day 4 after infection, we decided to investigate changes in blood samples on day 3, when differences between male and female mice should already be detectable. For analysis of PBL, numbers of CD19+ cells (B cells), CD3+ cells (T cells), CD4+ T cells, CD8+ T cells, Gr-1+ cells (granulocytes), and CD49b+ (pan-NK) cells in all four inbred mouse strains were determined by flow cytometry. As reported previously, early L. monocytogenes infection is characterized by a severe reduction in the number of peripheral lymphocytes (37). This lymphopenia affected all lymphocyte subsets monitored and was found in both female and male mice of all mouse strains (Fig. 3B; Table 1). However, the extent of lymphopenia was more pronounced in female mice, inversely correlating with the increased bacterial load (Fig. 3A and B). Granulocyte frequencies were significantly elevated after L. monocytogenes infection, and this increase was stronger in males (with the exception of C57BL/6J mice, for which the increase in Gr-1+ cells was higher in females). In addition, we measured the levels of a variety of different cytokines and chemokines in plasma samples (Fig. 3C and D; Table 2). Our data revealed several changes in proinflammatory cytokines and chemokines that are typical for this infection model (39, 45) and that demonstrated some degree of sex dependency. Levels of IL-1α and IL-10 as well as IL-3, IL-6, IL-12 (p40 and p70), KC, MIP-1α (only in C3H/HeN and CBA/J mice), G-CSF, and RANTES (only in CBA/J mice) were more elevated in females. However, IFN-γ and IL-5 (only in C3H/HeN and CBA/J mice) were more strongly induced in males. Some cytokines did not demonstrate any sex-specific pattern (IL-1β, IL-17, and TNF-α), while others were hardly detectable (IL-2, IL-4, and GM-CSF) in the serum. However, all these changes could just reflect indirect effects mediated by the differences in bacterial loads (Fig. 3A). We were surprised, therefore, to find that plasma IFN-γ levels were significantly higher in males, although they had lower bacterial numbers in organ tissues than females. Another unexpected finding was that significantly higher levels of IL-10 (as much as threefold) were detected in the females of all four strains (Fig. 3D). Interestingly, a similar difference was observed in animals infected with a sublethal dose of L. monocytogenes (1.3 × 103 CFU), but due to the lower level of systemic infection under these conditions, the statistical significance of the difference was not as strong as that in the experiments where a higher infection dose was used (likewise for all other cytokines examined in the serum). IFN-γ is a crucial factor for the early control of L. monocytogenes infection, and IL-10 has been described as a major immunomodulatory cytokine that is able to down-regulate the production of proinflammatory cytokines such as IFN-γ (40). We therefore hypothesized that increased IL-10 production upon L. monocytogenes infection might be a crucial factor in mediating more-severe disease and increased lethality in female mice.
TABLE 1.
Relative proportions of peripheral blood cell subsets in L. monocytogenes-infected and control mice
| Cell population | % of the indicated subset in the following straina:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BALB/c
|
C57BL/6J
|
C3H/HeN
|
CBA/J
|
|||||||||||||
| Male
|
Female
|
Male
|
Female
|
Male
|
Female
|
Male
|
Female
|
|||||||||
| Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | |
| CD3+ | 19.9 ± 4.0## | 52.9 ± 2.7 | 24.4 ± 3.5## | 55.8 ± 2.2 | 22.8 ± 2.3 | 26.6 ± 0.1 | 25.5 ± 8.3 | 27.8 ± 0.9 | 29.3 ± 6.6# | 60.5 ± 1.4 | 28.4 ± 4.1## | 63.7 ± 4.5 | 23.1 ± 2.2# | 38.5 ± 3.3 | 9.6 ± 2.3** | 18.1 ± 3.1 |
| CD4+ | 13.7 ± 2.6## | 30.9 ± 2.9 | 10.6 ± 3.1## | 33.1 ± 3.0 | 10.2 ± 2.2 | 10.8 ± 0.5 | 1.7 ± 0.3###** | 5.5 ± 0.0*** | 14.1 ± 4.6# | 33.5 ± 3.8 | 11.2 ± 2.5# | 34.5 ± 3.7 | 5.6 ± 1.9## | 23.3 ± 1.9 | 2.3 ± 0.5# | 12.2 ± 3.2 |
| CD8+ | 1.4 ± 0.2### | 9.5 ± 0.3 | 1.7 ± 0.6### | 9.2 ± 0.9 | 6.4 ± 2.1 | 9.2 ± 0.3 | 1.1 ± 0.3###* | 8.7 ± 1.8 | 2.6 ± 1.0# | 13.0 ± 0.9 | 3.3 ± 0.8### | 11.9 ± 0.6 | 3.1 ± 0.9# | 9.3 ± 0.3 | 1.8 ± 0.7 | 4.2 ± 0.6 |
| CD49b+ | 13.7 ± 2.9 | 23.2 ± 5.3 | 11.2 ± 2.7 | 19.9 ± 2.0 | 15.1 ± 4.7 | 16.6 ± 3.3 | 16.9 ± 2.1# | 31.4 ± 6.8 | 11.7 ± 4.5 | 18.5 ± 6.6 | 11.3 ± 2.9 | 21.8 ± 4.5 | 21.6 ± 3.6 | 30.4 ± 2.0 | 30.5 ± 4.9 | 22.3 ± 0.7* |
| Gr-1+ | 45.1 ± 7.1# | 4.7 ± 1.1 | 30.1 ± 7.6 | 4.2 ± 0.5 | 14.9 ± 4.1 | 2.9 ± 0.4* | 23.3 ± 4.0# | 6.4 ± 0.4 | 34.6 ± 7.0# | 3.2 ± 0.9 | 17.5 ± 3.7# | 3.3 ± 0.3 | 42.3 ± 9.2# | 6.9 ± 1.0 | 19.3 ± 4.7* | 4.4 ± 0.1 |
| CD19+ | 4.6 ± 1.3# | 12.6 ± 0.8 | 7.8 ± 2.2 | 11.8 ± 0.6 | 21.4 ± 3.4 | 30.5 ± 0.3 | 8.5 ± 2.5* | 19.3 ± 0.5* | 6.6 ± 2.4# | 18.7 ± 0.8 | 17.0 ± 4.5 | 17.2 ± 1.4 | 5.4 ± 1.5## | 14.3 ± 0.3 | 5.1 ± 1.1 | 4.0 ± 0.1*** |
Representative data from one out of three independent experiments are shown as mean values for three to seven mice per group ± standard errors of the means. Inf, infected mice; Con, control mice. For comparison between males and females, significantly lower values compared to those for the opposite sex are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. For comparison between L. monocytogenes-infected animals and sex-matched controls, significantly altered values compared to those for controls are indicated as follows: #, P < 0.05; ##, P < 0.01; ###, P < 0.001.
TABLE 2.
Levels of 18 cytokines and chemokines in L. monocytogenes-infected and control mice
| Cytokine or chemokine | Level (pg/ml) of the indicated cytokine or chemokine in the following mouse straina:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BALB/c
|
C57BL/6J
|
C3H/HeN
|
CBA/J
|
|||||||||||||
| Male
|
Female
|
Male
|
Female
|
Male
|
Female
|
Male
|
Female
|
|||||||||
| Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | Inf | Con | |
| IL-1α | 625 ± 143* | 35 | 1,355 ± 283 | 62 ± 25 | 515 ± 118 | ND | 702 ± 85 | 17 | 353 ± 111*** | 50 | 1,699 ± 276 | 53 | 354 ± 106** | 45 | 1,964 ± 293 | ND |
| IL-1β | 87 ± 35 | ND | 686 ± 509 | 20 | 13 | ND | 96 | 93 | 47 ± 8 | ND | 199 | ND | 231 | 104 | 244 ± 71 | 20 |
| IL-2 | ND | ND | 19 | ND | ND | ND | ND | 19 | ND | ND | ND | ND | ND | 4 | ND | 1 |
| IL-3 | 73 ± 23 | 10 ± 4 | 72 ± 14 | 14 ± 1 | 54 ± 13 | 7 ± 0 | 45 ± 4 | 10 ± 4 | 19 ± 5*** | 13 ± 3 | 92 ± 14 | 10 ± 1 | 46 ± 8** | 10 ± 5 | 134 ± 24 | 11 ± 2 |
| IL-4 | 3 ± 1 | ND | 5 ± 1 | 5 ± 1 | ND | 3 | ND | 14 | 3 ± 1 | 3 | 1 ± 0 | 1 | 4 ± 1 | 7 | 3 ± 1 | 3 |
| IL-5 | 3 | ND | 5 | 18 ± 6 | ND | 17 | ND | 10 ± 4 | 30 ± 4 | 9 | 14 ± 4* | 8 | 30 ± 4 | ND | 9 ± 2*** | 4 ± 1 |
| IL-6 | 5,300 ± 1,869 | 552 ± 224 | 8,723 ± 2,009 | 436 ± 97 | 4,844 ± 1,311 | 306 ± 124 | 5,044 ± 676 | 661 | 1,184 ± 479** | 247 ± 73 | 9,206 ± 1,818 | 182 ± 69 | 2,682 ± 900* | 237 ± 90 | 26,455 ± 8,893 | 421 |
| IL-10 | 181 ± 30* | ND | 609 ± 201 | ND | 158 ± 32 | ND | 233 ± 36 | ND | 106 ± 33* | ND | 353 ± 103 | ND | 153 ± 39* | ND | 1,436 ± 303 | ND |
| IL-12 (p40) | 537 ± 134 | 55 ± 20 | 282 ± 32 | 152 ± 28 | 473 ± 100 | 111 ± 31 | 329 ± 42 | 138 ± 47 | 180 ± 28*** | 89 ± 22 | 432 ± 28 | 71 ± 4 | 446 ± 77 | 79 ± 21 | 521 ± 89 | 69 ± 30 |
| IL-12 (p70) | 415 ± 103 | 91 ± 63 | 329 ± 67 | 105 ± 18 | 192 ± 41 | 33 ± 1 | 157 ± 11 | 47 ± 11 | 130 ± 33*** | 129 ± 66 | 528 ± 48 | 93 ± 24 | 523 ± 137 | 70 ± 40 | 774 ± 138 | 58 ± 8 |
| IL-17 | 669 ± 169 | 158 ± 91 | 380 ± 88 | 195 ± 76 | 439 ± 107 | 194 ± 62 | 240 ± 48 | 185 ± 16 | 421 ± 131** | 685 ± 393 | 1,254 ± 187 | 517 ± 233 | 2,645 ± 753 | 706 | 1,463 ± 289 | 205 ± 55 |
| TNF-α | 15 ± 1 | ND | 106 | 22 | 36 | 57 | 30 ± 9 | 11 | ND | 22 | 323 | ND | 90 ± 30 | 66 | 83 ± 46 | 124 |
| IFN-γ | 1,977 ± 355 | ND | 929 ± 312 | ND | 468 ± 129 | ND | 314 ± 105 | ND | 963 ± 203 | ND | 244 ± 105 | ND | 801 ± 139 | ND | 363 ± 102* | ND |
| KC | 6,578 ± 1,939 | 114 ± 47 | 5,590 ± 2,743 | 814 ± 86 | 4,525 ± 818 | 148 ± 28 | 3,016 ± 397 | 417 ± 318 | 2,189 ± 1,091** | 190 ± 55 | 14,202 ± 2,915 | 369 ± 54 | 2,343 ± 803** | 311 | 19,676 ± 5,200 | 314 ± 104 |
| MIP-1α | 1,387 ± 589 | 60 ± 9 | 2,120 ± 566 | 173 ± 12 | 895 ± 217 | 80 ± 11 | 893 ± 95 | 92 ± 32 | 332 ± 116** | 86 ± 31 | 2,336 ± 478 | 99 ± 13 | 632 ± 132** | 64 ± 22 | 4,436 ± 924 | 85 ± 8 |
| G-CSF | 11,906 ± 7,142 | 268 | 33,419 ± 8,363 | 612 ± 158 | 8,120 ± 2,115 | 602 | 11,438 ± 2,331 | 436 | 2,742 ± 1,234 | 17 | 96,230 ± 64,452 | 394 | 2,750 ± 1,021** | 566 | 54,359 ± 14,854 | 447 |
| GM-CSF | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 17 | ND | ND | ND | 64 | ND |
| RANTES | 103 ± 12 | 18 ± 8 | 99 ± 16 | 132 ± 5 | 109 ± 8 | 55 ± 9 | 84 ± 6* | 82 ± 29 | 74 ± 7 | 44 ± 8 | 60 ± 18 | 74 ± 13 | 83 ± 10** | 48 ± 24 | 238 ± 47 | 66 ± 12 |
Representative data from one out of three independent experiments are shown as mean values (or a single value where cytokines could be detected in individual samples only) for three to seven mice per group ± standard errors of the means. Inf, infected mice; Con, control mice; ND, not detectable. For comparison between L. monocytogenes-infected males and females, significantly lower values compared to those for the opposite sex are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-10 is a crucial mediator of sex-dependent differences during L. monocytogenes infection.
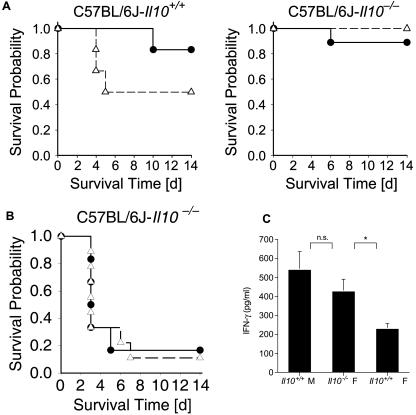
To test the hypothesis that the immunosuppressive cytokine IL-10 is involved in the increased susceptibility of female mice to L. monocytogenes infection, we infected Il10 knockout mice (congenic on a C57BL/6J genetic background) and wild-type control mice with 1.5 × 104 CFU of L. monocytogenes and determined their survival rates. As shown in the previous experiments (Fig. 2), female C57BL/6J mice are more susceptible to L. monocytogenes infection than males (Fig. 5). This sex difference disappeared in Il10 knockout mice, where female mice survived as well as their male littermates (Fig. 5A). Since Il10-deficient mice have been described as up to 50-fold more resistant to Listeria infection than wild-type controls (19), we performed similar survival experiments using higher infection doses. As shown in Fig. 5B, at higher doses also, no difference between the two groups could be observed. Furthermore, IFN-γ levels in female Il10-deficient animals were substantially higher than those in control females, reaching concentrations as high as those in control males (Fig. 5C). These data strongly support our interpretation that IL-10 is a key factor in sex-dependent differences during L. monocytogenes infection.
FIG. 5.
Absence of sex-specific susceptibility patterns in Il10 knockout mice after L. monocytogenes infection. (A) Kaplan-Meier survival curves for L. monocytogenes-infected (1.5 × 104 CFU) male (•) and female (▵) control (left) and Il10 knockout (Il10−/−) (right) mice. Seven mice per group were monitored for a period of 14 days. Representative data from one out of two independent experiments are shown. (B) Kaplan-Meier survival curves for L. monocytogenes-infected (2 × 106 CFU) male (•) and female (▵) Il10 knockout mice. The experimental setup was the same as that for panel A. Representative data from one out of three independent experiments are shown. (C) Female Il10 knockout mice show IFN-γ levels comparable to concentrations found in wild-type males after L. monocytogenes infection. Seven mice per group were sampled on day 3 postinfection. The level of significance is indicated as follows: *, P < 0.05; n.s., not significant.
DISCUSSION
Our experiments show for the first time that the outcome of disease after infection of mice with the intracellular pathogen Listeria monocytogenes is more severe in females than in male littermates. The sex-dependent difference was found in a variety of commonly used inbred mouse strains and is distinct from experimental data reported for several other bacterial pathogens. More-detailed analysis of the immune responses during L. monocytogenes infection resulted in the surprising finding that the relative increase in IFN-γ levels in the plasma, which have been reported to correlate directly with the severity of infection in experimental listeriosis (39, 45), is lower in female mice despite their higher bacterial load. In parallel, levels of the immunomodulatory cytokine IL-10 in plasma are highly elevated in infected female mice. The important role of IL-10 in mediating sex-dependent differences during L. monocytogenes infection could be directly demonstrated by the absence of this phenomenon in Il10 knockout mice.
Although the susceptibility pattern found for murine infection with L. monocytogenes is “inverse” compared to what is known for many other pathogens, a thorough review of the literature revealed similar experimental findings for other human pathogens including Leishmania, Toxoplasma, Babesia, and Pseudomonas spp., indicating that this phenotype might not be unique to L. monocytogenes infection. Ulcerations caused by Leishmania major, for example, healed faster in male B10 × 129 and DBA/2 mice, while females frequently developed nonhealing expanding ulcers (3, 24). Mapping experiments revealed a locus on chromosome 11 that contributed to the female-biased susceptibility to this pathogen (6). Babesia sp. is another parasite that caused increased mortality among female mice of several inbred and hybrid strains [AKR/J, 129/J, (B6 × 129)F1, C3B6F1 × C3H/HeN] (2). Other experimental studies revealed that male mice (BALB.K, C57BL/10ScSn, B6 × 129, C.B-17 scid/scid) can handle Toxoplasma infection better than females, and the differences described were attributed to different kinetics of IFN-γ (in spleen cell cultures) and IL-12 (in plasma) (43, 50). Female C57BL/6 mice were more susceptible to Pseudomonas aeruginosa infection, showing greater weight loss and higher bacterial loads in the lungs (26). Thus, our finding that females are more susceptible to L. monocytogenes infection seems to be part of a broader biological phenomenon. An interesting observation that further supports this interpretation can be found in human epidemiological studies that demonstrated higher incidence rates of toxoplasmosis-related lymphadenopathy in females than in males (8) as well as increased frequencies of Toxoplasma encephalitis and herpes simplex infection in female AIDS patients (42).
The gender differences observed during L. monocytogenes infection may differ from the situation for other bacterial pathogens due to specifics in the cell biology of this intracellular pathogen, which hides in the cytosol of infected cells. The reestablishment of immunological homeostasis during infection requires mobilization of various pathways leading to elimination of the causative pathogen (33). In the L. monocytogenes model, both innate and adaptive responses are necessary for the establishment of sterilizing immunity (28, 48). The dramatic susceptibility of female mice to L. monocytogenes during the first days of infection prompted us to look for differences in the innate immune response, which probably plays a crucial role in this discrepancy. Analysis of PBL subsets showed two major system changes during L. monocytogenes infection: lymphocyte numbers go down, most likely due to apoptosis (14, 37), and there is a “burst” of granulocytes, which are involved in the first line of defense against L. monocytogenes (16, 38). Although we could not detect clear-cut differences between males and females for the majority of cell populations analyzed, higher frequencies of Gr-1+ cells in males and more-pronounced lymphopenia in females were observed. Together with the lower bacterial numbers found in the spleens and livers of male mice, these data further characterize the nature of the increased resistance of male mice to L. monocytogenes infection, indicating that the quality of the induced immune response is the major cause of sex-dependent differences.
Several cytokines orchestrate effective immune responses during L. monocytogenes infection: IFN-γ, TNF-α, IL-1, IL-6, and IL-12 are intimately involved in the establishment of protection, while IL-10 exerts the opposite effect (20, 39). Interestingly, sex hormones can substantially affect the expression of those cytokines. Estrogen, for example, down-regulates IFN-γ and TNF-α but stimulates IL-10 production (25, 31, 32, 44). In our experimental system, IFN-γ was clearly increased to significantly higher levels in male mice than in females in all four strains after L. monocytogenes infection. Since IFN-γ seems to be essential for the resolution of L. monocytogenes infection (10), it is likely that the higher levels in males contributed to their increased resistance. In comparison, female mice up-regulated several cytokines, one of which (IL-10) might be crucial for their increased susceptibility. IL-10 is a potent immunosuppressor whose major effects may be summarized as suppression of Th1 differentiation and Th1-type cytokine synthesis, inhibition of macrophage effector function and antigen presentation, and repression of T-cell proliferation (40). These effects have also shown to be operative by interference with the prevalence of IL-10 in vivo during L. monocytogenes infection (19, 22, 29, 49), and it would therefore be expected that the increased levels of IL-10 in female mice would hamper the recovery of the animals and render them more susceptible to L. monocytogenes infection. This interpretation is convincingly supported by our finding that in Il10 knockout mice, females and males handle the infection equally well.
Infection of mice with Listeria monocytogenes has become one of the most commonly used infection models in immunological research. The dramatic sex-dependent differences in outcome of disease described in this study point out the importance of using sex-matched groups of animals for such studies. It is very likely that insufficient control for gender-influenced factors contributes substantially to controversial results generated by different laboratories using the same infection model.
A better understanding of the mechanisms determining susceptibility and resistance to infection is a prerequisite for future development of more-effective therapies for infectious diseases. Influences of gender on susceptibility to infection with distinct pathogens are also well known in humans (see also the introduction), giving our findings potential clinical relevance. If IL-10-mediated mechanisms are also involved in the epidemiological bias toward females found for tuberculosis, toxoplasmosis, and listeriosis as well as for localized infections of the urinary tract and the vagina, temporary neutralization of IL-10 or its functions could represent an interesting target for therapeutic interventions.
Acknowledgments
We thank Werner Müller (GBF) and Kristen Kerksiek for helpful and stimulating discussions and Stefanie Schiebe (GBF), Christine Fürmann, Kerstin Kutzner, and Florian Schleicher for excellent technical assistance.
This work was supported by the National Genome Research Network (NGFN) (KB-P5T0520, to D.H.B.; 01GR0102-KB-P5T0513, to A.L.). B.P. is supported by the EU project EUMORPHIA (QLG2-CT-2002-00930) and the Deutsche Forschungsgemeinschaft.
Editor: A. D. O'Brien
REFERENCES
- 1.Acuna-Soto, R., J. H. Maguire, and D. F. Wirth. 2000. Gender distribution in asymptomatic and invasive amebiasis. Am. J. Gastroenterol. 95:1277-1283. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Delfin, I., M. J. Homer, P. J. Wettstein, and D. H. Persing. 2001. Innate resistance to Babesia infection is influenced by genetic background and gender. Infect. Immun. 69:7955-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander, J. 1988. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology 96:297-302. [DOI] [PubMed] [Google Scholar]
- 4.Barna, M., T. Komatsu, Z. Bi, and C. S. Reiss. 1996. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67:31-39. [DOI] [PubMed] [Google Scholar]
- 5.Beagley, K. W., and C. M. Gockel. 2003. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38:13-22. [DOI] [PubMed] [Google Scholar]
- 6.Beebe, A. M., S. Mauze, N. J. Schork, and R. L. Coffman. 1997. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity 6:551-557. [DOI] [PubMed] [Google Scholar]
- 7.Beery, T. A. 2003. Sex differences in infection and sepsis. Crit. Care Nurs. Clin. N. Am. 15:55-62. [DOI] [PubMed] [Google Scholar]
- 8.Beverley, J. K., D. G. Fleck, W. Kwantes, and G. B. Ludlam. 1976. Age-sex distribution of various diseases with particular reference to toxoplasmic lymphadenopathy. J. Hyg. (London) 76:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyartchuk, V. L., K. W. Broman, R. E. Mosher, S. E. D'Orazio, M. N. Starnbach, and W. F. Dietrich. 2001. Multigenic control of Listeria monocytogenes susceptibility in mice. Nat. Genet. 27:259-260. [DOI] [PubMed] [Google Scholar]
- 10.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch, D. H., K. Kerksiek, and E. G. Pamer. 1999. Processing of Listeria monocytogenes antigens and the in vivo T-cell response to bacterial infection. Immunol. Rev. 172:163-169. [DOI] [PubMed] [Google Scholar]
- 12.Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353-362. [DOI] [PubMed] [Google Scholar]
- 13.Caracta, C. F. 2003. Gender differences in pulmonary disease. Mt. Sinai J. Med. 70:215-224. [PubMed] [Google Scholar]
- 14.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J. Immunol. 172:4866-4874. [DOI] [PubMed] [Google Scholar]
- 15.Cheers, C., and I. F. McKenzie. 1978. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect. Immun. 19:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlan, J. W., and R. J. North. 1991. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J. Exp. Med. 174:741-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curiel, R. E., M. H. Miller, R. Ishikawa, D. C. Thomas, and N. J. Bigley. 1993. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J. Interferon Res. 13:387-395. [DOI] [PubMed] [Google Scholar]
- 18.Czuprynski, C. J., P. M. Henson, and P. A. Campbell. 1984. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J. Leukoc. Biol. 35:193-208. [DOI] [PubMed] [Google Scholar]
- 19.Dai, W. J., G. Kohler, and F. Brombacher. 1997. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 158:2259-2267. [PubMed] [Google Scholar]
- 20.Ehlers, S., C. Holscher, S. Scheu, C. Tertilt, T. Hehlgans, J. Suwinski, R. Endres, and K. Pfeffer. 2003. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J. Immunol. 170:5210-5218. [DOI] [PubMed] [Google Scholar]
- 20a.Gailus-Durner, V., H. Fuchs, L. Becker, I. Bolle, M. Brielmeier, J. Calzada-Wack, R. Elvert, N. Ehrhardt, C. Dalke, T. J. Franz, et al. 2005. Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nat. Methods 2:403-404. [DOI] [PubMed] [Google Scholar]
- 21.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 22.Genovese, F., G. Mancuso, M. Cuzzola, C. Biondo, C. Beninati, D. Delfino, and G. Teti. 1999. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 163:2777-2782. [PubMed] [Google Scholar]
- 23.Gervais, F., M. Stevenson, and E. Skamene. 1984. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J. Immunol. 132:2078-2083. [PubMed] [Google Scholar]
- 24.Giannini, M. S. 1986. Sex-influenced response in the pathogenesis of cutaneous leishmaniasis in mice. Parasite Immunol. 8:31-37. [DOI] [PubMed] [Google Scholar]
- 25.Giron-Gonzalez, J. A., F. J. Moral, J. Elvira, D. Garcia-Gil, F. Guerrero, I. Gavilan, and L. Escobar. 2000. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 143:31-36. [DOI] [PubMed] [Google Scholar]
- 26.Guilbault, C., P. Stotland, C. Lachance, M. Tam, A. Keller, L. Thompson-Snipes, E. Cowley, T. A. Hamilton, D. H. Eidelman, M. M. Stevenson, and D. Radzioch. 2002. Influence of gender and interleukin-10 deficiency on the inflammatory response during lung infection with Pseudomonas aeruginosa in mice. Immunology 107:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han, X., P. Lundberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75:3048-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harty, J. T., L. L. Lenz, and M. J. Bevan. 1996. Primary and secondary immune responses to Listeria monocytogenes. Curr. Opin. Immunol. 8:526-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa, S., T. Miura, S. Sasaki, H. Madarame, and A. Nakane. 2002. Dysregulation of interleukin-10 and interleukin-12 are involved in the reduced host resistance to Listeria monocytogenes infection in alymphoplastic aly mutant mice. FEMS Immunol. Med. Microbiol. 32:111-117. [DOI] [PubMed] [Google Scholar]
- 30.Huber, S. A., and B. Pfaeffle. 1994. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J. Virol. 68:5126-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito, A., B. F. Bebo, Jr., A. Matejuk, A. Zamora, M. Silverman, A. Fyfe-Johnson, and H. Offner. 2001. Estrogen treatment down-regulates TNF-α production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J. Immunol. 167:542-552. [DOI] [PubMed] [Google Scholar]
- 32.Karpuzoglu-Sahin, E., Y. Zhi-Jun, A. Lengi, N. Sriranganathan, and S. Ansar Ahmed. 2001. Effects of long-term estrogen treatment on IFN-γ, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine 14:208-217. [DOI] [PubMed] [Google Scholar]
- 33.Kimbrell, D. A., and B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2:256-267. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 35.Lortholary, O., L. Improvisi, C. Fitting, J. M. Cavaillon, and F. Dromer. 2002. Influence of gender and age on course of infection and cytokine responses in mice with disseminated Cryptococcus neoformans infection. Clin. Microbiol. Infect. 8:31-37. [DOI] [PubMed] [Google Scholar]
- 36.Medina, E., O. Goldmann, M. Rohde, A. Lengeling, and G. S. Chhatwal. 2001. Genetic control of susceptibility to group A streptococcal infection in mice. J. Infect. Dis. 184:846-852. [DOI] [PubMed] [Google Scholar]
- 37.Merrick, J. C., B. T. Edelson, V. Bhardwaj, P. E. Swanson, and E. R. Unanue. 1997. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 151:785-792. [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto, M., M. Emoto, Y. Emoto, V. Brinkmann, I. Yoshizawa, P. Seiler, P. Aichele, E. Kita, and S. H. Kaufmann. 2003. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J. Immunol. 170:5228-5234. [DOI] [PubMed] [Google Scholar]
- 39.Mocci, S., S. A. Dalrymple, R. Nishinakamura, and R. Murray. 1997. The cytokine stew and innate resistance to L. monocytogenes. Immunol. Rev. 158:107-114. [DOI] [PubMed] [Google Scholar]
- 40.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 41.Morrel, V. 1995. Zeroing on how hormones affect the immune system. Science 269:773-775. [DOI] [PubMed] [Google Scholar]
- 42.Phillips, A. N., F. Antunes, G. Stergious, A. Ranki, G. F. Jensen, Z. Bentwich, T. Sacks, C. Pedersen, J. D. Lundgren, A. M. Johnson, et al. 1994. A sex comparison of rates of new AIDS-defining disease and death in 2554 AIDS cases. AIDS 8:831-835. [DOI] [PubMed] [Google Scholar]
- 43.Roberts, C. W., S. M. Cruickshank, and J. Alexander. 1995. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect. Immun. 63:2549-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salem, M. L. 2004. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr. Drug Targets Inflamm. Allergy 3:97-104. [DOI] [PubMed] [Google Scholar]
- 45.Serbina, N., and E. G. Pamer. 2003. Quantitative studies of CD8+ T-cell responses during microbial infection. Curr. Opin. Immunol. 15:436-442. [DOI] [PubMed] [Google Scholar]
- 46.Skamene, E., P. A. Kongshavn, and D. H. Sachs. 1979. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J. Infect. Dis. 139:228-231. [DOI] [PubMed] [Google Scholar]
- 47.Terres, G., S. L. Morrison, and G. S. Habicht. 1968. A quantitative difference in the immune response between male and female mice. Proc. Soc. Exp. Biol. Med. 127:664-667. [DOI] [PubMed] [Google Scholar]
- 48.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11-25. [DOI] [PubMed] [Google Scholar]
- 49.Wagner, R. D., N. M. Maroushek, J. F. Brown, and C. J. Czuprynski. 1994. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect. Immun. 62:2345-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker, W., C. W. Roberts, D. J. Ferguson, H. Jebbari, and J. Alexander. 1997. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect. Immun. 65:1119-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinstein, Y., S. Ran, and S. Segal. 1984. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 132:656-661. [PubMed] [Google Scholar]
- 52.World Health Organization. 2003. The World Health Report 2003: shaping the future. World Health Organization, Geneva, Switzerland.
- 53.Yancey, A. L., H. L. Watson, S. C. Cartner, and J. W. Simecka. 2001. Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. Infect. Immun. 69:2865-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]