Abstract
The ileal in vitro organ culture (IVOC) model using tissues originating from colostrum-deprived newborn piglets has proven to be an effective way to study the attaching and effacing (A/E) phenotype of porcine enteropathogenic Escherichia coli (EPEC) ex vivo. The aim of this study was to investigate the role of intimin subtype and Tir in the adherence of EPEC and Shiga-toxin-producing E. coli (STEC), isolated from different animal species, to porcine intestinal IVOC. Moreover, the role of intimin in Tir-independent adherence of the human EPEC strain E2348/69 was investigated using intimin and Tir-deficient derivatives. Our results demonstrated that A/E E. coli strains (AEEC) from various animal species and humans induce the A/E phenotype in porcine ileal IVOC and that intimin subtype influences intestinal adherence and tropism of AEEC strains. We also showed that a tir mutant of EPEC strain E2348/69 demonstrates close adherence to the epithelial cells of porcine ileal IVOC segments, with microvillous effacement but with no evidence of actin polymerization or pedestal formation, and that intimin seems to be involved in this phenotype. Overall, this study provides further evidence for the existence of one or more host-cell-encoded intimin receptor(s) in the pig gut.
Enteropathogenic Escherichia coli (EPEC) and Shiga-toxin-producing E. coli (STEC) are an important cause of enteric diseases in both humans and animals (42). EPEC are the most common bacterial cause of diarrhea in infants from developing countries, whereas STEC, especially those of serotype O157:H7, are important emerging pathogens causing food-borne infections leading to bloody diarrhea and hemolytic-uremic syndrome in developed countries. EPEC, and certain STEC, cause typical, intestinal attaching and effacing (A/E) lesions which are characterized by intimate bacterial adherence to intestinal epithelial cells, effacement of the brush border, F-actin rearrangement, and formation of a pedestal of polymerized F-actin and other cytoskeletal elements underneath the adherence site. Most A/E phenotype elements are encoded on a 35.6-kb (EPEC) to 43-kb (STEC of the O157:H7 serotype) pathogenicity island called locus of enterocytes effacement (LEE) (39, 40). The LEE contains genes encoding an outer membrane adhesin termed intimin (eae gene), a type III secretion system machinery (Esc and Sep proteins), chaperones (Ces proteins), and translocator (EspA, EspB, and EspD) and effector (EspF, EspG, and Map) proteins, as well as the translocated intimin receptor (Tir). The function of several open reading frames is still not known.
At least five distinct major intimin subtypes, designated α, β, δ, γ, and ɛ, have been identified to date (1, 43). Receptor binding activity of intimin is located in the C-terminal 280-amino-acid region (Int280) (22) that comprises three separate domains, two immunoglobulin-like domains, and a C-type lectin-like module (32). Intimin was shown to bind to Tir (13, 24, 33) and directly to uninfected host cells (2, 12, 22, 24, 41). The latter seems to be dependent on a disulfide bridge at the carboxy terminus of Int280 (24), which appears to be essential for correct folding of this domain and for carbohydrate binding by other C-type lectins (54). Some published data support the existence of host cell intimin receptors, such as β1-chain integrins recognized by the Int280 region of alpha intimin in human EPEC (23) and cell-surface-localized nucleolin recognized at least by alpha and beta intimins (50) and gamma intimin in STEC of the O157:H7 serotype (49). Some recent observations (8, 37) point to the lack of a Tir-independent receptor able to support intimin-mediated bacterial adhesion. Nevertheless, Shaw et al. (48) recently demonstrated that the mutant strain E2348/69Δtir, which lacks Tir but still possesses intimin α, adheres to human intestinal ileal in vitro organ culture (IVOC) in a manner independent of Tir but dependent on intimin, suggesting an interaction between surface intimin and a host cell intimin receptor.
The aim of this study was to investigate the role of intimin subtype and Tir in the adherence of EPEC and STEC strains isolated from different animal species to porcine intestinal IVOC, a model which more closely reflects in vivo conditions than in vitro cell culture. Moreover, the role of intimin in Tir-independent adherence of the human EPEC strain E2348/69 was investigated using intimin and Tir-deficient derivatives.
(This work was presented in part at the 102nd General Meeting of the American Society for Microbiology, Salt Lake City, Utah, May 2002.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. If not otherwise mentioned, bacteria were grown overnight in Dulbecco's modified Eagle's medium (DMEM; GibcoBRL, Burlington, Ontario, Canada) under agitation at 37°C and then transferred into fresh DMEM and grown to an optical density at 600 nm (OD600) of 0.7, corresponding to approximately 2.0 × 108 CFU, in the early-exponential phase prior to use. Bacteria grown for 3 h in DMEM express LEE genes and are primed ready to produce A/E lesions (9). Chloramphenicol was added to the broth culture at a final concentration of 30 μg ml−1 when using strains complemented with pICC55 or pCVD438 plasmids.
TABLE 1.
Bacterial strains and characteristics
| Bacterial strain | Description and origin | Serotype | Genetic characteristic(s) | Reference(s) or sourceb |
|---|---|---|---|---|
| M15 (pREP4) | E. coli host strain for recombinant proteins | NAa | NA | Qiagen (Mississauga, Ontario, Canada) |
| MG1655 | E. coli K-12 strain | NA | NA | 15 |
| ECL1001 (formerly 86-1390) | EPEC isolated from a pig | O45:H− | eaeβ+, espA+, tir+ | EcL, reference 26 |
| ECL1043 (formerly 89-4221) | EPEC isolated from a dog | O112ab | eaeα+, espA+, tir+ | EcL; reference 4 |
| ECL1019 (formerly 86-4225) | EPEC isolated from a dog | O49:H10 | eaeδ+, espA+, tir+ | EcL; reference 6 |
| RDEC-1 | EPEC isolated from a rabbit | O15:H− | eaeβ+, espA+, tir+ | 7 |
| E22 | EPEC isolated from a rabbit | O103:H2 | eaeβ+, espA+, tir+ | 38 |
| ECL1044 | EPEC isolated from a rabbit | Nontypable | eaeβ+, espA+, tir+ | EcL; this study |
| ECL1045 | EPEC isolated from a rabbit | Nonspecific typing | eaeβ+, espA+, tir+ | EcL; this study |
| ECL1046 | EPEC isolated from a rabbit | O55 | eaeβ+, espA+, tir+ | EcL; this study |
| E2348/69 | Prototypic EPEC isolated from a human | O127:H6 | eaeα+, espA+, tir+ | 36 |
| CVD206 | eae mutant of strain E2348/69 | O127:H6 | eae deficient, espA+, tir+ | 16 |
| EDL933 | STEC isolated from raw hamburger meat, implicated in a hemorrhagic colitis outbreak | O157:H7 | eaeγ+, espA+, tir+ | 46 |
| 85-170 | Derivative of STEC 84-289, isolated from a human, lacking the genes for Stx toxins | O157:H7 | eaeγ+, espA+, tir+ | 52 |
| ICC170 | eae mutant of strain 85-170 | O157:H7 | eae deficient, espA+, tir+ | 20 |
| STJ348 | STEC isolated from a human | O157:H7 | eaeγ+, espA+, tir+ | Ste-Justine's Hospital for Children, Montreal, Quebec, Canada; this study |
| STJ919 | STEC isolated from a human | O157:H7 | eaeγ+, espA+, tir+ | Ste-Justine's Hospital for Children, Montreal, Quebec, Canada; this study |
| STJ854 | STEC isolated from a human | O157:H7 | eaeγ+, espA+, tir+ | Ste-Justine's Hospital for Children, Montreal, Quebec, Canada; this study |
| 43888 | Strain lacking the genes of Stx toxins, isolated from human feces | O157:H7 | eaeγ+, espA+, tir+ | ATCC |
| ECL1047 | STEC isolated from a bovine | O157:H7 | eaeγ+, espA+, tir+ | EcL; this study |
| ECL1020 | STEC isolated from a bovine | O45 | eaeɛ+, espA+, tir+ | EcL; this study |
| ECL1106 | STEC isolated from a bovine | O26 | eaeβ+, espA+, tir+ | EcL; this study |
| ECL1197 | STEC isolated from a human | O26:H11 | eaeβ+, espA+, tir+ | EcL; this study |
| ECL1204 | STEC isolated from a human | O45:H2 | eaeɛ+, espA+, tir+ | EcL; this study |
| ECL1200 | STEC isolated from a human | O103:H2 | eaeɛ+, espA+, tir+ | EcL; this study |
| Intimin switches | ||||
| ICC170 (pCVD438) | Derivative of strain ICC170 harboring α intimin | O157:H7 | eaeα+, espA+, tir+ | 16, 32 |
| ICC170 (pICC55) | Derivative of strain ICC170 harboring a hybrid of γ intimin | O157:H7 | eaeγ, espA+, tir+ | 21, 25 |
| CVD206 (pCVD438) | Derivative of strain CDV206 harboring α intimin | O127:H6 | eaeα, espA+, tir+ | 30 |
| CVD206 (pICC55) | Derivative of strain CDV206 harboring a hybrid of γ intimin | O127:H6 | eaeγ, espA+, tir+ | 44 |
| Tir mutant E2348/69Δtir | tir mutant of strain E2348/69 | O127:H6 | eaeα, espA+, tir deficient | 33 |
Not applicable.
EcL, the E. coli Laboratory.
Primers and PCR-based amplification for detection of the LEE genes.
The primers used for detection of LEE genes in this study are listed in Table 2. Intimin subtype was determined as previously described (1, 43). The tir gene was amplified as follows: once at 94°C for 5 min and 25 times at 94°C for 30 s, 60°C for 30 s, 72°C for 30 s, followed by a final elongation step for 7 min at 72°C. The PCR products were further analyzed using agarose gel electrophoresis.
TABLE 2.
Primers used in this study
| Genes and subtypes | Sequence of primers (5′-3′) | Annealing temp (°C) | Amplicon size (bp) | Reference or source |
|---|---|---|---|---|
| eaeα | ||||
| int.α | CCTTAGGTAAGTTAAGT | 45 | 539 | 1 |
| int.Ru | TTTATTTGCAGCCCCCCAT | |||
| eaeβ | ||||
| int.β | TAAGGATTTTGGGACCC | 45 | 543 | 1 |
| int.Ru | ----------------------- | |||
| eaeγ | ||||
| int.γ | ACAAACTTTGGGATGTTC | 55 | 559 | 1 |
| int.Ru | ----------------------- | |||
| eaeδ | ||||
| int.δ | TACGGATTTTGGGGCAT | 45 | 548 | 1 |
| int.Ru | ----------------------- | |||
| eaeɛ | ||||
| SK1 | CCCGAATTCGGCACAAGCATAAGC | 55 | 2,608 | 43 |
| LP5 | AGCTCACTCGTAGATGACGGCAAGCG | |||
| tir | ||||
| tir for | GGATCCATGTCTCTTTCATCGGGGGTT | 60 | 650 | This study |
| tir rev | CTCGAGAACGAACGTGCGGGTCC | |||
| espA | ||||
| espA F | GGATCCATGGATACATCAACTGCA | 55 | 585 | This study |
| espA R | CTCGAGTTTACCAAGGGATA |
Production of antisera.
For the production of EspA polyclonal antisera in chickens and rabbits and of intimin polyclonal antiserum in chickens, the espA and eae (encoding intimin) genes from the porcine EPEC (PEPEC) strain ECL1001 were amplified by PCR using the primer pairs listed in Table 3. They were then cloned and expressed and the recombinant proteins purified as previously described for anti-Paa antiserum (3). Rabbits and/or laying hens were immunized with purified His-EspA or His-intimin fusion proteins. Some laying hens were immunized with a sonicate preparation from host strain M15 (pREP4) used for the production of recombinant protein as a control. Total antibodies were extracted from egg yolks as previously described (3), and the water-soluble fraction was lyophilized and stored at 4°C. Serum containing EspA-specific antibodies was recovered from rabbits by exsanguination and frozen at −20°C. The universal rabbit polyclonal anti-TirEPEC was obtained as previously described (17).
TABLE 3.
His-tagged fusion proteins used in this study
| Gene and primersa | Primer sequence (5′-3′) | Amplicon size (bp) | Predicted mol wt | Reference |
|---|---|---|---|---|
| eae | ||||
| F | GGATCCAATGGTGAAAAT | 2,712 | 95 | This study |
| R | AAGCTTTTTTACACAAACAGG | |||
| espA | ||||
| F | GGATCCATGGATACATCAACTGCA | 585 | 24 | This study |
| R | CTCGAGTTTACCAAGGGATA |
F, forward; R, reverse.
Collection and culture of porcine intestinal IVOC segments.
The porcine intestinal IVOC model was derived from the explant culture technique previously described by Zhu et al. (56) and Batisson et al. (3) with some modifications. Briefly, segments of the duodenum, jejunum, ileum, cecum, and colon were obtained from the colostrum-deprived newborn piglets of a conventional herd. Piglets were tranquilized with a mixture of 10 mg kg−1 of body weight of ketamine hydrochloride (Biomeda-MTC, Cambridge, Ontario, Canada) and 20 mg kg−1 of xylazine (Bayer, Toronto, Ontario, Canada) before being euthanized with an overdose of sodium pentobarbital (540 mg ml−1; Pharmacie, Faculté de Médecine Vétérinaire, Montreal, Quebec, Canada). Piglets were cared for in accordance with the Guidelines of the Canadian Council for Animal Care. Upon collection of the intestinal segments, the serosa was carefully removed with forceps and mucus was gently removed with a sterile swab. Tissues were then cut into 9-mm2 segments and placed mucosal side up onto biopsy foam pads (Curtin Matheson Scientific, Inc., Texas) in multidish four-well Nunclon Delta Surface tissue culture plates (Invitrogen life technologies, Burlington, Ontario, Canada), up to three segments being placed on each pad. Complete RPMI 1640 medium containing 10% fetal bovine serum (Gibco, NY), 0.25% lactalbumin hydrolysate, 0.2 μg ml−1 of hydrocortisone, 0.1 μg ml−1 of insulin, 75 mM β-mercaptoethanol, 1% d-mannose, and 2 mM concentrations of l-glutamate and l-aspartate (Sigma Chemical Co., St. Louis, MO) was added to each well. Segments were inoculated three times at hourly intervals with 50 μl of broth culture applied to the mucosal surface and incubated at 37°C on a rocker in a 95% O2 and 5% CO2 atmosphere for 8 h. To prevent bacterial overgrowth and acidic pH, hourly changes with sterile fresh complete RPMI 1640 medium were carried out during culture, starting 2 h after the initial inoculation of segments. Noninfected segments were also cultured in order to confirm that no external contamination occurred during the experimental process. In some experiments, broth cultures were incubated with lyophilized anti-intimin chicken antibodies previously reconstituted with sterile phosphate-buffered saline corresponding to approximately 5 mg ml−1 of total immunoglobulin Y (IgY) content at 37°C for 30 min prior to the first segment inoculation as previously described (3).
Histopathology.
After culture, segments were rinsed thoroughly in sterile phosphate-buffered saline and fixed in 10% buffered formalin for microscopic examination. Formalin-fixed tissues were then placed into nylon tissue biopsy bags (Thermo Shandon, Pittsburgh, PA), processed, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin, phloxine, and safranine (HPS) according to standard techniques. Sections were examined by light microscopy for the presence of typical A/E lesions and intimately adhering bacteria on intestinal cells. Briefly, the mucosal epithelium located between adjacent crypts (designated the intercrypt mucosal epithelium [ICME]) was examined for the presence of intimate-adherent bacteria, either in large aggregates or a single layer, and the mean percentage of ICME demonstrating intimate-adherent bacteria per section was calculated. At least 20 ICME per IVOC segment were examined, and each result is based on at least 10 segments from at least two different pigs.
Detection of Tir and EspA secretion and intimin expression by immunoblotting.
To promote Tir and EspA secretion, PEPEC strain ECL1001, EPEC strain E2348/69, and its tir-deficient mutant strain E2348/69Δtir were grown overnight in minimal essential medium (GibcoBRL, Burlington, Ontario, Canada), transferred to 100 ml of fresh minimal essential medium, and incubated at 37°C with agitation to an OD600 of 1.0 as previously described (10, 29). Bacteria were pelleted by centrifugation, and phenylmethylsulfonyl fluoride (50 μg ml−1; Sigma Chemical Co., St. Louis, MO), aprotinin (0.5 μg ml−1; Roche Diagnostics GmbH, Mannheim, Germany), and EDTA (0.5 μM; Sigma Chemical Co., St. Louis, MO) were added to the supernatant. Proteins were precipitated by the addition of 10% trichloroacetic acid (Sigma Chemical Co., St. Louis, MO) overnight at 4°C. After centrifugation at 4,000 × g for 1 h, protein pellets were washed with cold 95% ethanol and recentrifuged at 4,000 × g for 1 h. Pellets were resuspended in 1.0 ml of Laemmli buffer and boiled for 5 min. Samples were further analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis.
To promote intimin expression, overnight Trypticase soy broth cultures were diluted 1:100 in fresh DMEM and incubated for 3 h at 37°C with agitation. An equivalent of an OD600 of 0.1 was loaded onto sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis as previously described (34).
The electrophoresed polypeptides were transferred onto a nitrocellulose membrane and examined by immunoblot analysis using universal rabbit polyclonal anti-TirEPEC (1:1,000) (17), chicken polyclonal anti-intimin, or anti-EspA antiserum (1:500) or rabbit polyclonal anti-EspA antiserum (1:500). This was followed by the addition of either a goat anti-rabbit IgG or goat anti-chicken IgY conjugated to horseradish peroxidase (Bio-Rad Laboratories, Hercules, CA). The reaction was visualized using 4-chloro-1-naphthol and H2O2 as substrate (Sigma Chemical Co., St. Louis, MO).
Electron microscopy.
Additional IVOC segments were processed for electron microscopy. Tissues were fixed for 2 h at room temperature in 2.5% glutaraldehyde and then rinsed in cacodylate buffer (0.1 M cacodylate, pH 7.3) for 1.5 h with regular changes. Samples for transmission electron microscopy (TEM) were postfixed for 1 h at room temperature in 2% osmium tetroxide (OsO4). Tissues were then rinsed in water for 1.5 h with regular changes and dehydrated in graded ethanol series.
For TEM, tissues were embedded in Spurr resin (Marivac, St-Laurent, Quebec, Canada). Thin sections were mounted on copper grids, stained with uranyl acetate and lead citrate, and examined for A/E lesions with a Philips 420 transmission electron microscope at 80 kV (Philips Electronics, Eindhover, The Netherlands).
For scanning electron microscopy (SEM), samples were critical-point dried, mounted, sputter coated (8 to 10 nm) with gold, and examined at 5 kV in an S-3000N scanning electron microscope (Hitachi, Rexdale, Ontario, Canada).
Image capture.
Images from HPS-stained sections were captured with a charge-coupled device CoolSNAP camera (RS Photometrics, California), and all images were processed with Adobe Photoshop 5.0 and Adobe Illustrator 8.0 software (Adobe Systems Incorporated, California).
Statistical analysis.
Results are presented as the means ± the standard deviations of the means. A Kruskal-Wallis test was performed with commercially available software (SAS 8.1, Cary, North Carolina), and post hoc 2-by-2 comparisons were done to assess differences between the groups; P < 0.05 was taken to be significant.
RESULTS
AEEC strains from various animal species induce A/E lesions in the porcine ileal IVOC model.
The ability of 21 A/E E. coli strain (AEEC) isolates of various origins, O serotypes, and intimin subtypes (Table 1) to adhere to and induce A/E lesions in the porcine ileal IVOC model was assessed as described for porcine AEEC by Zhu et al. (56). eae-Deficient mutants derived from the prototypic EPEC strain E2348/69 (CVD206) and STEC strain 85-170 (ICC170) were included as controls.
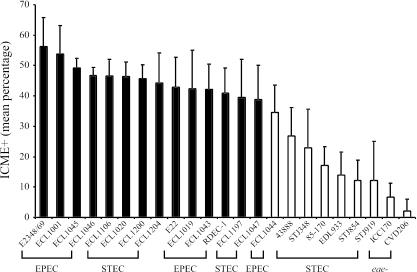
All of the EPEC and non-O157:H7 STEC strains, possessing eaeα, eaeβ, eaeδ, or eaeɛ genes, demonstrated a mean percentage of ICME with intimate-adherent bacteria of greater than 35%, being similar to that of the PEPEC strain ECL1001 and significantly different from that of the eae-deficient mutant strains CVD206 and ICC170 (Fig. 1), with the exception of one EPEC strain isolated from a rabbit. The O157:H7 STEC strains, possessing eaeγ, demonstrated a mean percentage of ICME with intimate-adherent bacteria of less than 28%, significantly different from that of the PEPEC strain ECL1001. Bacteria of the O157:H7 STEC strains were mostly found individually attached to the epithelial cells as previously described (14, 28) or in the intestinal lumen, whereas EPEC tended to pile up upon one another, forming larger bacterial aggregates at the surface of the cell. O157:H7 STEC strains possessing gamma intimin also showed a significant reduction in the proportion of epithelial surface covered by adherent bacteria on each ICME with intimate-adherent bacteria compared to other strains assessed, except for the eae-deficient mutant strains CVD206 and ICC170 (data not shown). Intestinal cells beneath intimate-adherent bacteria in all ileal segments observed were irregular (Fig. 2A), whereas a loose association of bacteria with the intestinal mucosa of some villi with no obvious change in associated epithelial cell morphology was observed for the eae-deficient mutants ICC170 and CVD206 (Fig. 2B). Neither intimate-adherent bacteria nor changes in epithelial cell morphology were observed on uninfected ileal segments (data not shown).
FIG. 1.
Adherence of various AEEC strains to the epithelial surface of porcine ileal segments. Whereas most of the strains demonstrated a mean percentage of intercrypt mucosal epithelium with bacterial adherence per examined IVOC segment similar to that of PEPEC strain ECL1001, STEC strains of the O157:H7 serotype possessing gamma intimin showed a significantly decreased adherence level compared to the latter PEPEC strain. White bars represent strains showing adherence significantly different (P < 0.0001) from that of PEPEC strain ECL1001 and not significantly different from that of both eae-deficient (eae-) mutants ICC170 (STEC) and CVD206 (EPEC). Results are presented as the means ± the standard deviations of the means, and P < 0.05 was taken to be significant.
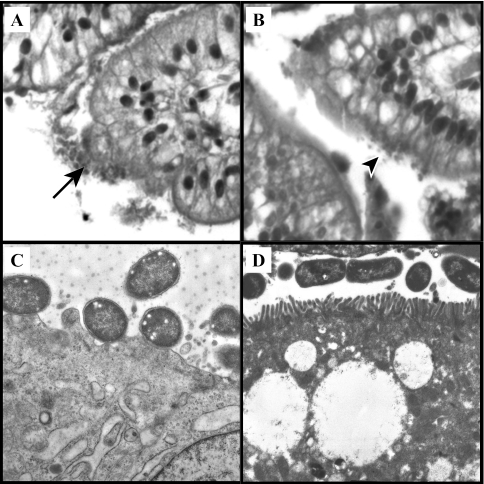
FIG. 2.
Micrographs of HPS-stained sections of porcine ileal segment inoculated with the PEPEC strain ECL1001 (A) (magnification, ×40) or with the eae-deficient mutant ICC170 (B) (magnification, ×40). Typical intimate-adherent bacteria and mucosal irregularities (arrow) were observed for ECL1001, whereas only a loose bacterial association with no obvious change in associated epithelial cells was observed for ICC170 (arrowhead). TEM micrographs showed attachment of bacteria with effacement of the brush border beneath the bacterial adherence site (magnification, ×10,500) after inoculation of a porcine ileal segment with PEPEC strain ECL1001 (C) and intact brush borders at the surface of epithelial cells of a porcine ileal segment inoculated with the eae-deficient mutant ICC170 (D) (magnification, ×7,000).
Further observation by TEM of tissues for which intimate-adherent bacteria were observed confirmed the presence of A/E lesions (Fig. 2C) for all the AEEC isolates examined in this study, including STEC of serotype O157:H7 and the absence of A/E lesions for ICC170 and CVD206 (Fig. 2D). CVD206 was often observed more closely associated with the brush border than ICC170, although neither intimate adherence nor evidence of effacement of the adjacent brush border was noted with CVD206 and ICC170.
Intimin subtype influences intestinal adherence and tropism of AEEC strains in porcine intestinal IVOC segments.
In order to further investigate the role of intimin subtype in the intestinal adherence and tissue tropism of AEEC strains, we examined the adherence to porcine duodenal, jejunal, and ileal IVOC of STEC strain 85-170 carrying intimin gamma, an eae-deficient mutant (ICC170) of this strain complemented in trans with pCVD438 plasmid encoding alpha intimin from EPEC strain E2348/69 (16), prototype EPEC strain E2348/69 carrying alpha intimin, and an eae-deficient mutant (CVD206) of this strain complemented in trans with the pICC55 plasmid encoding a hybrid intimin containing the receptor binding domain of gamma intimin on an alpha intimin backbone, which itself is 97% identical to alpha intimin (25).
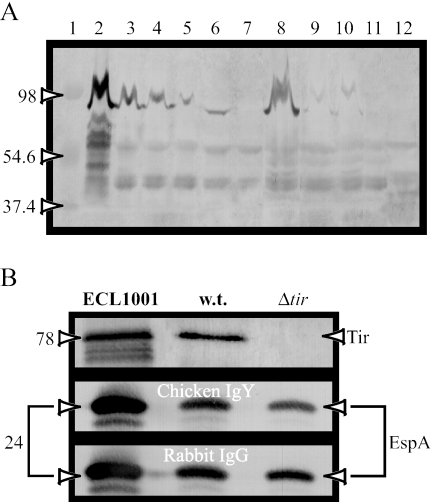
Before applying the strains to the porcine intestinal IVOC segments, we tested their intimin expression. Western blot analysis of intimin expression in whole-cell extracts demonstrated a band with an approximate molecular mass of 94 kDa corresponding to intimin for all the wild-type and complemented mutant EPEC and STEC A/E+ strains, but not for the eae-deficient mutant strains CVD206 and ICC170 (Fig. 3A). Nevertheless, the intimin expression level appeared to be low, but comparable, between complemented strains, compared to wild-type strains, as previously reported (34).
FIG. 3.
Western blot analysis of whole-cell extract (A) and secreted proteins from culture supernatants (B) probed with the anti-intimin, anti-EspA, or anti-Tir antisera. Chicken anti-intimin polyclonal antiserum reacted with a 94- to 97-kDa band corresponding to intimin in the whole-cell extract of PEPEC strain ECL1001 (lane 2), as well as in the whole-cell extract of the wild-type strains E2348/69 (lane 3) and 85-170 (lane 8) and of mutant E2348/69Δtir (lane 4) and of all their respective complemented mutant strains, except for eae-deficient mutants CVD206 (lane 7) and ICC170 (lane 11) and E. coli K-12 (MG1655, lane 12). Intimin expression appeared to be less high for EPEC-complemented mutant strains CVD206 (pCVD438) (lane 5) and CVD206 (pICC55) (lane 6), as well as for STEC A/E-positive complemented strains ICC170 (pCVD438) (lane 9) and ICC170 (pICC55) (lane 10), respectively. Rabbit and chicken polyclonal antisera (1:500) raised against the EspA protein of the PEPEC strain ECL1001 reacted with a 24-kDa protein in the supernatant of ECL1001, the wild-type EPEC strain E2348/69 (w.t.), and E2348/69Δtir (Δtir). On the other hand, universal rabbit polyclonal anti-TirEPEC (1:1,000) detected a single 78-kDa band only in the culture supernatants of ECL1001 and E2348/69. Lane 1 represents molecular mass markers, in kDa.
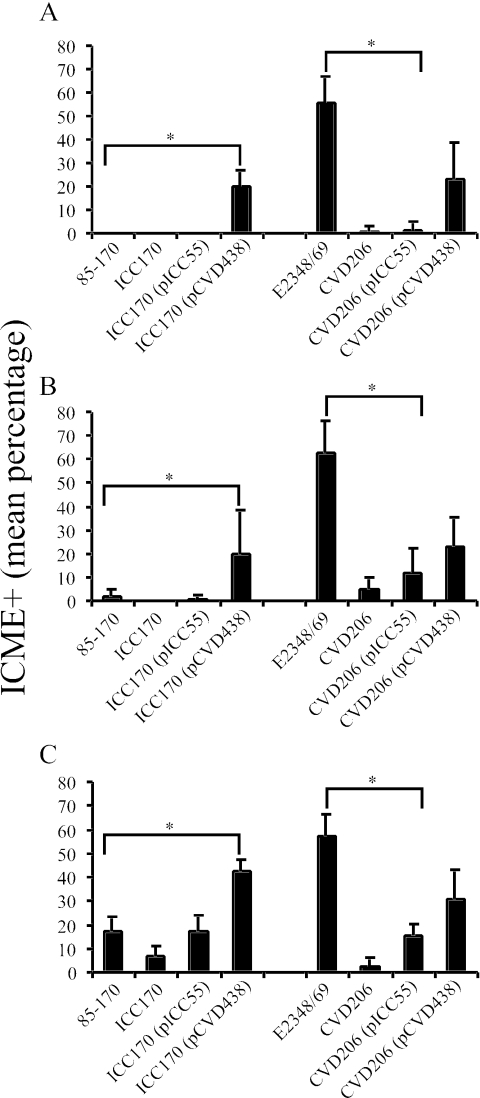
Adherence of STEC strain 85-170 was restricted to the ileum, whereas adherence of EPEC strain E2348/69 was observed to the same extent in the ileum, jejunum, and duodenum (Fig. 4). The absence of intimin in the eae-deficient mutants of the STEC and EPEC strains resulted in a significant decrease in bacterial adherence. In the ileal IVOC segments, trans complementation of the STEC eae-deficient mutant ICC170 with the pICC55 plasmid encoding gamma intimin restored the adherence capacity of ICC170 to a level similar to that of the wild-type STEC strain 85-170. In contrast, trans complementation of the eae-deficient mutant with the pCVD438 plasmid encoding alpha intimin significantly enhanced bacterial adherence in the ileal, jejunal, and duodenal IVOC segments to a level comparable to that of the trans-complemented derivative CVD206 (pCVD438) derived from EPEC strain E2348/69 (Fig. 4). Inversely, trans complementation of EPEC eae-deficient mutant CVD206 with the pICC55 plasmid encoding gamma intimin significantly reduced the bacterial adherence, compared to the wild-type EPEC strain, to a level similar to that of the STEC strain 85-170 (Fig. 4C).
FIG. 4.
Intimin subtype influences the bacterial adherence and intestinal tropism of STEC and EPEC in porcine intestinal IVOC segments. Whereas adherence of STEC strain 85-170 is restricted to the ileum, EPEC strain E2348/69 extensively adhered to the duodenum (A), jejunum (B), and ileum (C). Complementation of the eae-deficient mutant ICC170 with pCVD438 plasmid encoding alpha intimin significantly enhanced (P < 0.0001 in duodenum and ileum; P = 0,0025 in the jejunum) the adherence level in all intestinal segments assessed, and inversely, trans complementation of the EPEC eae-deficient mutant CVD206 with the pICC55 plasmid significantly reduced (P < 0.0001) bacterial intimate adherence to a level similar to that of the STEC strain 85-170. Results are presented as the means ± the standard deviations of the means, and P < 0.05 was taken to be significant (*).
When cecal and colonic IVOC segments were cultured and infected, only a few, scattered and mostly individual, intimate-adherent bacteria were observed and for EPEC strain E2348/69 only (data not shown).
Tissues on which intimate-adherent bacteria were observed were processed for TEM, and the presence of A/E lesions was confirmed for all EPEC and STEC derivatives in all IVOC segments (data not shown), whereas no A/E lesions were observed for the eae-deficient mutants ICC170 and CVD206 (data not shown).
A tir mutant of EPEC strain E2348/69 adheres closely to the epithelial cells of porcine IVOC, with microvillous effacement but no pedestal formation.
Previous studies demonstrated that, although intimin can bind to uninfected host cells in vitro (2, 22, 24), binding to the receptor Tir is essential for triggering the focused accumulation of host cytoskeletal proteins beneath the adherent bacteria in pedestal-like structures (33, 47). In this study, we investigated the capacity of the mutant strain E2348/69Δtir, which lacks Tir but still possesses intimin, to adhere to the epithelial surface in a Tir-independent fashion.
Before applying the strains to the porcine intestinal IVOC, we tested their protein expression profiles. Western blot analysis of secreted proteins in culture supernatants demonstrated a single 78-kDa band corresponding to Tir protein for E2348/69, but not for the mutant strain E2348/69Δtir (Fig. 3B), whereas a 24-kDa protein corresponding to the EspA protein was detected at a similar intensity in the supernatants of ECL1001, E2348/69, and E2348/69Δtir (Fig. 3B). Finally, Western blot analysis of whole-cell extracts of all of these strains showed a band of similar intensity with an approximate molecular mass of 94 kDa corresponding to intimin (Fig. 3A).
Compared to the wild-type strain E2348/69, the mutant strain E2348/69Δtir demonstrated significantly less (P < 0.0001) bacterial adherence to epithelial cells in all small intestinal segments assessed, most notably in the duodenum (data not shown). Nevertheless, the bacterial adherence of E2348/69Δtir was greater than that of the eae-deficient mutant CVD206, although the difference was not significant by statistical analysis (data not shown). Foci of large aggregates of adherent bacteria were observed for both the wild-type EPEC strains E2348/69 (Fig. 5) and E2348/69Δtir (Fig. 6) in all HPS-stained sections examined, although adjacent epithelial cells appeared to be more irregular in IVOC segments inoculated with E2348/69 (Fig. 5 and 6). As previously described for the eae-deficient mutant CVD206, the occasional foci of bacteria in a loose association with the intestinal mucosa, with no obvious change in adjacent epithelial cell morphology, were observed in all HPS-stained sections examined (Fig. 7).
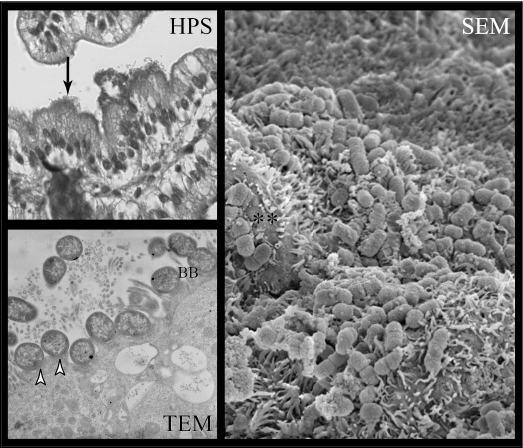
FIG. 5.
Wild-type human EPEC strain E2348/69 adheres to small intestinal epithelial cells and induces typical A/E lesions in porcine ileal IVOC segments. HPS-stained sections showed the extensive foci of intimately adhered bacteria (HPS, arrow), as well as typical effacement of the brush border (**) and A/E lesions on TEM (arrowheads) and SEM ileal sections. BB, brush border. Magnifications: HPS, ×40; TEM, ×10,500; SEM, ×6,000.
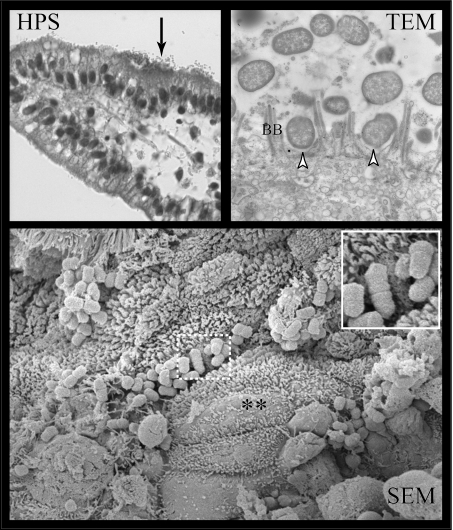
FIG. 6.
E2348/69Δtir mutant strain shows reduced adherence capacity to small intestinal epithelial cells on HPS-stained sections (HPS, arrow), although with a loss of A/E capacity but a close attachment with effacement of the brush border (**) with no evidence of actin polymerization and pedestal formation (arrowheads) on TEM and SEM sections. Inset demonstrates the close attachment with the effacement phenotype observed with E2348/69Δtir on SEM. BB, brush border. Magnifications: HPS, ×40; TEM, ×10,500; SEM, ×8,000.
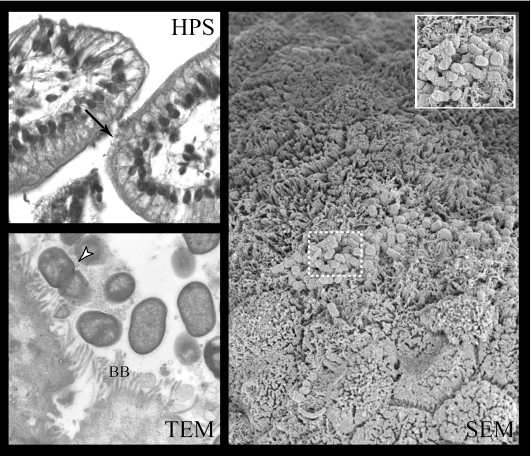
FIG. 7.
For the CVD206 mutant strain, only a loose association of bacteria with no obvious change in associated epithelial cells was observed on HPS-stained sections (HPS, arrow), whereas bacteria closely associated to the brush border (BB) without effacement (TEM, arrowhead) or in the intestinal lumen were observed on TEM. SEM sections show only rare, small foci of loosely associated bacteria (inset), although areas of mucosa with elongated brush border microvilli could be seen, particularly around the periphery of bacterial microcolonies. Magnifications: HPS, ×40; TEM, ×10,500; SEM, ×3,000; inset, ×7,000.
On TEM, typical A/E lesions were observed in all intestinal segments examined for the wild-type strain E2348/69 (Fig. 5), whereas effacement of the brush border beneath closely, but not intimately, adherent bacteria and the absence of pedestal was observed for E2348/69Δtir (Fig. 6). Only a few bacteria of the eae-deficient mutant CVD206 were found associated with the intestinal epithelial cells, with neither intimate adherence nor effacement of the adjacent brush border (Fig. 7).
SEM analysis of the mucosal surface of whole ileal IVOC segments demonstrated highly colonized mucosa with typical A/E lesions and gross microvillous elongation for the wild-type strain E2348/69 (Fig. 5). In contrast, relatively large areas showing clearly obvious effacement and microvillous elongation were found associated with closely, but not intimately, adherent bacteria and the absence of pedestal for strain E2348/69Δtir (Fig. 6). As previously shown by TEM, the mucosa of IVOC segments inoculated with CVD206 were only slightly colonized by bacteria loosely attached to the healthy brush border, although rare, small mucosal areas seemed to show effacement of the brush border (Fig. 7).
Anti-intimin antibodies reduce adherence of strain E2348/69Δtir in porcine IVOC.
Chicken antibodies specific for the intimin of PEPEC strain ECL1001 significantly reduce intimate adherence of the wild-type EPEC strain E2348/69 in the porcine ileal IVOC model, suggesting the expression of intimin during the development of A/E lesions (Girard et al., unpublished data). In order to investigate the involvement of intimin in the close adherence phenotype observed for the mutant strain E2348/69Δtir, we performed blocking experiments using chicken polyclonal anti-intimin antiserum in porcine ileal segments inoculated with E2348/69Δtir.
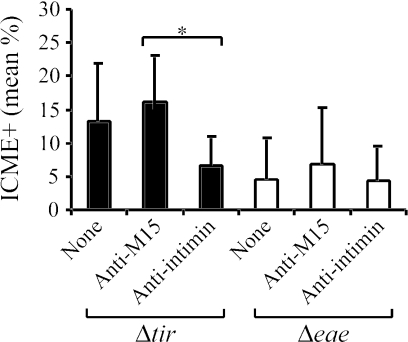
The presence of the anti-intimin antibodies resulted in a significant reduction (P = 0.017) in the number of closely adherent bacteria (Fig. 8) observed for E2348/69Δtir in the ileum compared to the adherence observed in the absence of antibodies or in the presence of antibodies from hens immunized with a sonicated preparation from the host strain M15 (pREP4), the latter being used as a control (Fig. 8). No significant reduction in the number of closely adherent bacteria was observed when anti-intimin antibodies were added to the eae-deficient strain CVD206 (Fig. 8).
FIG. 8.
Reduction of the mean percentage of intercrypt mucosal epithelium with bacterial adherence (ICME+) per examined porcine ileal segment inoculated with mutant E2348/69Δtir (Δtir) or CVD206 (Δeae), following treatment with chicken anti-intimin antibodies. Treatment with anti-intimin antibodies resulted in a significantly reduced (*, P = 0.017) mean percentage of intercrypt mucosal epithelium with bacterial adherence per examined ileal segment (ICME+) inoculated with E2348/69Δtir, whereas the latter antibodies did not affect mean percentage ICME+ per examined porcine ileal segment inoculated with CVD206. Porcine ileal segments inoculated with either bacteria of the E2348/69Δtir or CVD206 mutants alone (None) or with E2348/69Δtir or CVD206 mutants previously incubated with antibodies from hens immunized with a sonicate preparation from host strain (Anti-M15) were used as controls. Results are presented as the means ± the standard deviations of the means, and P < 0.05 was taken to be significant.
DISCUSSION
In this study, we have shown that the porcine ileal IVOC model is an effective model to the study pathogenesis of AEEC infections. All the wild-type strains tested were able to induce typical A/E lesions. The use of quantitative parameters, based on the mean percentage of intercrypt mucosal epithelium demonstrating intimate-adherent bacteria, allowed us show differences between the AEEC isolates. Previously, the ileal IVOC model using tissues originating from colostrum-deprived newborn piglets had proved to be an effective way to study A/E of PEPEC (56). This approach was more practical than the inoculation of tissues from gnotobiotic animals, which are expensive to maintain and time consuming and require sophisticated facilities (53, 55). We have now demonstrated that this model may be used for the quantitative study of the development of A/E lesions and the role of various effector molecules in a wide range of AEEC strains in conditions as close to in vivo as possible but using a minimum of animals. Importantly, our results confirmed that intimin is essential for the development of A/E lesions (31), as demonstrated by the impaired ability of the two eae-deficient mutants, ICC170 (STEC) and CVD206 (EPEC), to develop A/E lesions.
In addition, we confirmed that the intimin subtype influences intestinal tropism in a Tir-subtype independent pattern (19, 21, 25, 45), a feature phenomenon previously observed in gnotobiotic piglets challenged with an STEC A/E-positive strain of O157:H7 serotype with or without a switch from gamma to alpha intimin (51). The use of IVOC allowed us to more quantitatively and hence more convincingly demonstrate the role of intimin subtype in intestinal tropism. Indeed, change in intimin subtype influenced the overall bacterial adherence even though the Tir remained the same, strengthening the argument that intimin may interact with other, possibly eukaryotic, host intimin receptors (HIR). Whereas intimin expression appeared to be lower in complemented mutant strains than in wild-type strains, it is likely that its expression was consistent between these complemented mutant strains. The reduced intensity of the band corresponding to intimin in whole-cell extracts of STEC strain 85-170 could be explained, in part, by differences in specificity of the different intimin subtypes of PEPEC, EPEC, and STEC (1, 43). Nevertheless, preliminary results using porcine ileal IVOC demonstrated that complemented mutant strains behaved statistically in a similar manner to their respective parental strains, strongly suggesting that intimin expression in complemented strains is sufficient to promote the adherence to and development of A/E lesions. Moreover, it appears that this intestinal tropism is species independent, at least between human and porcine intestinal tissues (19, 20, 27). The limited capacity of strains harboring gamma intimin to adhere to the epithelial cells of porcine ileal segments may explain, in part, the relatively low prevalence of STEC strains of O157:H7 serotype in pigs (18) compared to that in cattle. The latter are an important reservoir of these strains which are responsible for hemorrhagic colitis and hemolytic uremic syndrome in humans (11).
The intimin switch from gamma to alpha in STEC, and inversely in EPEC, had shown that the adhesion of STEC expressing gamma intimin is restricted to the porcine ileum. In contrast, the presence of alpha intimin permitted bacterial adherence in the duodenum, jejunum, and ileum. Similarly, in a human pediatric IVOC model, STEC adherence was found to be restricted to the follicle-associated epithelium doming the Peyer's patches, whereas alpha intimin-harboring strains adhered to both the small intestine and follicle-associated epithelium (19). The presence of more extensive Peyer's patches in the ileum in comparison to the jejunum and duodenum of the pig may explain our findings. Nevertheless, we did not note any particular tropism for these structures in our study.
In porcine intestinal IVOC, deletion of the eae gene, encoding intimin, from wild-type EPEC strain E2348/69 resulted in a significant reduction of the adherence capacity of CVD206 and a loss of the A/E phenotype, as previously described (31). Remarkably, although a tir deletion resulted in a significantly reduced adherence and absence of pedestal formation and of the condensation of cytoskeletal elements beneath adherent bacteria, as has been previously described in vitro (33), bacteria were frequently observed demonstrating a close, but not intimate, adherence pattern, accompanied by effacement of the brush border adjacent to the closely adherent bacteria. We termed this phenotype “close attachment and effacement”. As this phenotype was not observed following infection with CVD206, which expresses Tir but lacks the intimin, we therefore believe that alpha intimin of the human EPEC strain E2348/69 can interact directly with HIR of the intestinal epithelial cell surface and that the effacement of the brush border in the absence of typical pedestal formation is Tir independent, at least in the pig gut.
Shaw et al. (48) recently demonstrated a similar phenomenon in human IVOC, using SEM. Indeed, they showed that bacteria of the mutant strain E2348/69Δtir adhere to the brush border and induce remodeling, including brush border effacement at the site of bacterial attachment. Some of the bacteria, however, appeared to have come away from the brush border surface, leaving a bacterial footprint termed the “effacing bacterial footprint”. This footprint phenotype was shown to be independent of Tir but consistent with a requirement for effector protein translocation to induce effacement of the brush border and for the presence of intimin. We have confirmed these findings in porcine ileal IVOC, using both SEM and TEM, the latter allowing us to more thoroughly investigate the close relationship between adherent bacteria and the adjacent intestinal epithelial cells. In our study, the presence of areas showing effacement of the brush border in the absence of adhered bacteria led us to speculate that the relatively low number of adhering bacteria observed with E2348/69Δtir could be explained by a low-affinity intimin-HIR interaction, as previously proposed (24), or by a bundle-forming pilus (BFP)-mediated bacterial dispersal (5, 35). Indeed, it has been demonstrated that deletion of the tir gene in E2348/69Δtir does not affect either secreted proteins or intimin expression (33). As rare, small mucosal areas seemed to show effacement of the brush border on intestinal segments inoculated with CVD206 in our study, it is unlikely that intimin alone mediates such remodeling.
The decrease of bacterial adherence of E2348/69Δtir in the presence of anti-intimin antiserum strengthens the argument for a role of intimin in the Tir-independent adherence of EPEC to the host cell. Recently, Cleary et al. (8) showed that a double mutant of E2348/69 (UMD883; bfp deficient, espA deficient, eae positive) did not adhere to Caco2 and HEp-2 cells in vitro. These authors interpreted this inability to adhere to be due to the absence of a receptor able to support intimin-mediated bacterial adhesion in the absence of Tir in vitro. Considering that BFP and EspA contribute to initial adherence, we believe that these factors may be required to bring EPEC bacteria close enough to the brush border to permit intimin to bind to the HIR in the IVOC model. In this study, the argument for the involvement of intimin in the Tir-independent adherence of EPEC to the host cell is also strengthened by the observation of the expression of intimin at the surface of bacteria of strain E2349/69Δtir adhered to epithelial cells as demonstrated by immunolabeling in porcine IVOC (data not shown).
In conclusion, we have demonstrated that the use of porcine ileal IVOC is a suitable, quantitative way to study the A/E phenotype of AEEC isolates from various animal species, including human EPEC and STEC. We also showed that the bacterial adherence is influenced by the intimin subtype, this being responsible for intestinal tropism in a Tir-subtype independent fashion, as previously described in vivo in gnotobiotic piglets (51). We also provided evidence for the interaction of the alpha intimin from EPEC strain E2348/69 with a eukaryotic HIR other than Tir and propose that this HIR may be common to the human and pig gut. In light of the growing evidence for the existence of other eukaryotic HIR (12, 23, 37, 48-50), investigation of the eukaryotic receptor candidates for alpha intimin from EPEC, as well as the role of such interactions in the pathogenesis of AEEC infections, will be the challenge of future studies.
Acknowledgments
We thank Eric Oswald for kindly providing strain E22, Sylvie Pérès, Chengru Zhu, and Pierre Hélie for helpful scientific discussions, Guy Beauchamp for statistical analysis, and Diane Montpetit for electron microscopy.
This work was supported by the Fond Québécois de la Recherche sur la Nature et les Technologies grant 0214 (J.M.F. and J.H.), by the Natural Sciences Engineering Research Council of Canada strategic grant 215841-98 and discovery grant 2294 (J.M.F.), and by EU-Community Quality of Life project QLK2-2000-00600.
Editor: A. D. O'Brien
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, C. Bain, A. G. Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, H., J. M. Fairbrother, J. D. Dubreuil, and J. Harel. 1997. Cloning and characterization of the eae gene from dog attaching and effacing Escherichia coli strain 4221. FEMS Microbiol. Lett. 148:239-245. [DOI] [PubMed] [Google Scholar]
- 3.Batisson, I., M.-P. Guimond, F. Girard, H. An, C. Zhu, E. Oswald, J. M. Fairbrother, M. Jacques, and J. Harel. 2003. Characterization of the novel factor Paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect. Immun. 71:4516-4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudry, M., C. Zhu, J. M. Fairbrother, and J. Harel. 1996. Genotypic and phenotypic characterization of Escherichia coli isolates from dogs manifesting attaching and effacing lesions. J. Clin. Microbiol. 34:144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 6.Broes, A., R. Drolet, M. Jacques, J. M. Fairbrother, and W. M. Johnson. 1988. Natural infection with an attaching and effacing Escherichia coli in a diarrheic puppy. Can. J. Vet. Res. 52:280-282. [PMC free article] [PubMed] [Google Scholar]
- 7.Cantey, J. R., L. R. Inman, and R. K. Blake. 1989. Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J. Infect. Dis. 160:136-141. [DOI] [PubMed] [Google Scholar]
- 8.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 9.Collington, G. K., I. W. Booth, and S. Knutton. 1998. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut 42:200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford, J. A., and J. B. Kaper. 2002. The N-terminus of enteropathogenic Escherichia coli (EPEC) Tir mediates transport across bacterial and eukaryotic cell membranes. Mol. Microbiol. 46:855-868. [DOI] [PubMed] [Google Scholar]
- 11.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1997. Pathogenesis of O157:H7 Escherichia coli infection in neonatal calves. Adv. Exp. Med. Biol. 412:47-51. [DOI] [PubMed] [Google Scholar]
- 12.Deibel, C., P. Dersch, and F. Ebel. 2001. Intimin from Shiga toxin-producing Escherichia coli and its isolated C-terminal domain exhibit different binding properties for Tir and a eukaryotic surface receptor. Int. J. Med. Microbiol. 290:683-691. [DOI] [PubMed] [Google Scholar]
- 13.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 14.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dezfulian, H., D. Tremblay, and J. Harel. 2004. Molecular characterization of extraintestinal pathogenic Escherichia coli (ExPEC) pathogenicity islands in F165-positive E. coli strain from a diseased animal. FEMS Microbiol. Lett. 238:321-332. [DOI] [PubMed] [Google Scholar]
- 16.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott, S. J., S. W. Hutcheson, M. S. Dubois, J. L. Mellies, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176-1189. [DOI] [PubMed] [Google Scholar]
- 18.Feder, I., F. M. Wallace, J. T. Gray, P. Fratamico, P. J. Fedorka-Cray, R. A. Pearce, J. E. Call, R. Perrine, and J. B. Luchansky. 2003. Isolation of Escherichia coli O157:H7 from intact colon fecal samples of swine. Emerg. Infect. Dis. 9:380-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2003. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzhenry, R. J., S. Reece, L. R. Trabulsi, R. Heuschkel, S. Murch, M. Thomson, G. Frankel, and A. D. Phillips. 2002. Tissue tropism of enteropathogenic Escherichia coli strains belonging to the O55 serogroup. Infect. Immun. 70:4362-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzhenry, R. J., M. P. Stevens, C. Jenkins, T. S. Wallis, R. Heuschkel, S. Murch, M. Thomson, G. Frankel, and A. D. Phillips. 2003. Human intestinal tissue tropism of intimin epsilon O103 Escherichia coli. FEMS Microbiol. Lett. 218:311-316. [DOI] [PubMed] [Google Scholar]
- 22.Frankel, G., D. C. Candy, P. Everest, and G. Dougan. 1994. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect. Immun. 62:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 24.Hartland, E. L., M. Batchelor, R. M. Delahay, C. Hale, S. Matthews, G. Dougan, S. Knutton, I. Connerton, and G. Frankel. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol. Microbiol. 32:151-158. [DOI] [PubMed] [Google Scholar]
- 25.Hartland, E. L., V. Huter, L. M. Higgins, N. S. Goncalves, G. Dougan, A. D. Phillips, T. T. MacDonald, and G. Frankel. 2000. Expression of intimin γ from enterohemorrhagic Escherichia coli in Citrobacter rodentium. Infect. Immun. 68:4637-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hélie, P., M. Morin, M. Jacques, and J. M. Fairbrother. 1991. Experimental infection of newborn pigs with an attaching and effacing Escherichia coli O45: K“E65” strain. Infect. Immun. 59:814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismaili, A., D. J. Philpott, M. T. Dytoc, and P. M. Sherman. 1995. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect. Immun. 63:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis, K. G., and J. B. Kaper. 1996. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect. Immun. 64:4826-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly, G., S. Prasannan, S. Daniell, K. Fleming, G. Frankel, G. Dougan, I. Connerton, and S. Matthews. 1999. Structure of the cell-adhesion fragment of intimin from enteropathogenic Escherichia coli. Nat. Struct. Biol. 6:313-318. [DOI] [PubMed] [Google Scholar]
- 33.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 34.Knutton, S., J. Adu-Bobie, C. Bain, A. D. Phillips, G. Dougan, and G. Frankel. 1997. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun. 65:1644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knutton, S., R. K. Shaw, R. P. Anantha, M. S. Donnenberg, and A. A. Zorgani. 1999. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33:499-509. [DOI] [PubMed] [Google Scholar]
- 36.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 37.Liu, H., L. Magoun, and J. M. Leong. 1999. β1-chain integrins are not essential for intimin-mediated host cell attachment and enteropathogenic Escherichia coli-induced actin condensation. Infect. Immun. 67:2045-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchès, O., J.-P. Nougayrède, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 41.McKee, M. L., and A. D. O'Brien. 1996. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect. Immun. 64:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marches, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 181:1496-1500. [DOI] [PubMed] [Google Scholar]
- 45.Reece, S., C. P. Simmons, R. J. Fitzhenry, S. Matthews, A. D. Phillips, G. Dougan, and G. Frankel. 2001. Site-directed mutagenesis of intimin alpha modulates intimin-mediated tissue tropism and host specificity. Mol. Microbiol. 40:86-98. [DOI] [PubMed] [Google Scholar]
- 46.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 47.Rosenshine, I., S. Ruschkowski, M. Stein, D. J. Reinscheid, S. D. Mills, and B. B. Finlay. 1996. A pathogenic bacterium triggers epithelial signals to form a functionnal bacterial receptor that mediates actin pseudopod formation. EMBO J. 15:2613-2624. [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw, R. K., J. Cleary, M. S. Murphy, G. Frankel, and S. Knutton. 2005. Interaction of enteropathogenic Escherichia coli with human intestinal mu-cosa: role of effector proteins in brush border remodeling and formation of attaching and effacing lesions. Infect. Immun. 73:1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 277:2876-2885. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair, J. F., and A. D. O'Brien. 2004. Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J. Biol. Chem. 279:33751-33758. [DOI] [PubMed] [Google Scholar]
- 51.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immun. 63:3621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzipori, S., R. M. Robins-Browne, G. Gonis, J. Hayes, M. Withers, and E. McCartney. 1985. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut 26:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weis, W. I., and K. Drickamer. 1996. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 65:441-473. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, C., J. Harel, M. Jacques, C. Desautels, M. S. Donnenberg, M. Beaudry, and J. M. Fairbrother. 1994. Virulence properties and attaching-effacing activity of Escherichia coli O45 from swine postweaning diarrhea. Infect. Immun. 62:4153-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu, C., J. Harel, M. Jacques, and J. M. Fairbrother. 1995. Interaction with pig ileal explants of Escherichia coli O45 isolates from swine with postweaning diarrhea. Can. J. Vet. Res. 59:118-123. [PMC free article] [PubMed] [Google Scholar]