Abstract
Bacterial superantigen intoxication causes massive overactivation of T cells, which can result in potentially lethal toxic shock. Superantigens fall into two groups: superantigens such as staphylococcal enterotoxin B (SEB) that contain a single generic binding site for major histocompatibility complex class II (MHC-II) and more potent superantigens such as SEA with a second, zinc-dependent MHC-II binding site that enables them to cross-link adjacent MHC-II molecules. We found that although all superantigens bound rapidly to the surface of human B cells, zinc-binding superantigens largely remained at the cell surface for at least 40 h. In contrast, single-binding-site superantigens were greatly depleted from the surface by 4 h. Subcellular fractionation and confocal microscopy revealed that some SEB entered lysosomal compartments, but SEA remained almost undetectable inside cells at 20 h. SEA and SEB mutants that do not bind MHC-II were trafficked rapidly to lysosomal compartments. Our findings suggest that the persistence of SEA and other zinc-dependent, cross-linking superantigens on the surface of antigen-presenting cells contributes to their potency as T-cell activators.
Major histocompatibility complex class II (MHC-II) molecules are found on the surface of antigen-presenting cells (APCs) and contain peptide-binding sites that are occupied by fragments of protein antigens. These antigen fragments are recognized by a small number of T cells that are specific for the particular bound peptide in the context of the MHC-II binding site, resulting in T-cell activation. The half-life of MHC-II molecules on the surface of B cells is 36 to 44 h (9), although a small fraction appears to recycle rapidly (37).
Bacterial superantigens (SAgs) produced by Staphylococcus aureus and group A streptococci seriously disrupt the immune system by circumventing conventional routes of processing and presentation (23, 24). Intact bacterial SAgs bind to MHC-II, and the binary complexes activate a large fraction of T lymphocytes resulting in a massive release of cytokines that accounts for their acute toxicity. In addition, bacterial SAgs potently trigger T-cell proliferation, leading to expansion followed by clonal deletion and anergy of T cells bearing the variable (V) β chain elements recognized by the SAg. The structural basis for T-cell activation by bacterial SAgs resides in their ability to ligate MHC-II molecules on the surface of APCs to the Vβ chain of a large subset of T-cell receptors (20, 48). Staphylococcal enterotoxins (SEs) and streptoccoccal pyrogenic exotoxins (SPEs) are associated with serious diseases such as bacterial arthritis (10, 43) and other autoimmune disorders (33), as well as potentially life-threatening toxic shock (4, 40). When delivered by aerosol, SEs cause severe pulmonary distress and are highly lethal. Because of their extreme inhaled toxicity, these toxins are considered “dual-threat” (both lethality and incapacitation) agents and are of biodefense concern (6). Understanding how SAgs overstimulate the immune system is key to tailoring specific therapeutics.
Details of the interactions between SAgs and MHC-II molecules have been elucidated by X-ray crystallography (15, 16, 22, 29, 30). Many SAgs bind to the α chain of MHC-II via a “generic site” involving hydrophobic N-terminal residues, although important exceptions have been identified (2, 3, 22, 28, 29, 34, 39). Both SEB and toxic shock syndrome toxin 1 (TSST-1) lack zinc and have only this single, generic binding site for MHC-II. Other SAgs contain a bound zinc ion in their C-terminal domain that mediates binding, usually of high affinity, to the β chain of MHC-II (3, 22). SAgs such as SEA, SEE, and SPE-A1 that have two MHC-II-binding sites can cross-link adjacent molecules (3, 14, 51, 53, 54). SED has both binding sites and, in addition, it can also form homodimers and is thus capable of cross-linking MHC-II molecules in at least two distinct manners (1). Thus, different SAgs interact with MHC-II molecules in a variety of ways, utilizing the generic site, the Zn-dependent site, or both, and with or without SAg dimerization (2).
Little is known about the fate of MHC-II-SAg complexes after they are formed on the APC surface and how this relates to SAg potency. We have compared the time course of dissociation of cell surface-bound MHC-II complexes for the SAg subgroups that do or do not have zinc-mediated binding. In addition, we have used several approaches to compare the internalization of SEA to SEB and to that of a conventional vaccine antigen as well as to SEA and SEB mutants that do not bind MHC-II. Our data suggest that SAgs capable of cross-linking MHC-II further enhance T-cell activation via prolongation of cell-surface exposure to T cells, which may help to explain the potency of these SAgs.
MATERIALS AND METHODS
Cells and reagents.
LG2 cells (HLA-DR1 homozygous, human B-lymphoblastoid cells) were propagated and maintained at 37°C and 5% CO2 in Eagle modified essential medium containing 10% fetal bovine serum (FBS). Recombinant SEA, SEB, SED, SEE, and TSST-1 were purchased from Toxin Technology (Sarasota, FL). Genetically engineered recombinant SEA and SEB containing three site mutations, SEB L45R/Y89A/Y94A (referred to as rSEB vaccine) or SEA L48R/N70R/Y92A (referred to as rSEA vaccine), were cloned and expressed in our laboratory as described elsewhere (5, 8). Recombinant 50-kDa carboxyl-terminal portion of botulinum neurotoxin type A (BoNT/A HC) was a gift from Leonard Smith, U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). Affinity-purified rabbit anti-SEs and TSST-1 were purchased from Toxin Technology. The monoclonal antibodies 7G11 (specific for SEB) and 6C2-4 (specific for BoNT/A HC) were produced and characterized in our laboratory (32). Monoclonal anti-SEA was kindly provided by Bradley Stiles (USAMRIID). Except as noted, other reagents were from Sigma (St. Louis, MO).
Cell surface toxin detection by flow cytometry.
LG2 cells (2 × 105) were fixed with 4% paraformaldehyde, washed, and then incubated at 37°C for 45 min with 5 μg of SE or TSST-1 in 20 μl of flow medium (RPMI 1640 with 5% FBS), and unbound toxin was removed by several washes. Bound SAg was detected with 5 μg of affinity-purified anti-SAg antibody. After the cells were washed, bound antibody was detected with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit immunoglobulin G, and the cells were analyzed with a FACSort flow cytometer (Becton Dickinson, Mountain View, CA). To measure off rates, 1 or 5 μg of SE or TSST-1 was added to the fixed LG2 cells, followed by incubation for 1 h as described above, after which the unbound SAg was removed by washing. The cells were incubated for different times at 37°C in 3 ml of RPMI 1640 containing 10% FBS, and bound toxin was detected as described above. LG2 cells incubated with antibody specific for MHC-II, followed by FITC-labeled secondary antibody, gave a mean fluorescence intensity in the range of 350 to 450, and this level did not change significantly after incubation with SAgs.
Microscopy and image analysis.
To detect and quantify surface label, samples were prepared for microscopy by incubating LG2 cells with 20 μg of SEA or SEB per ml for 15 min. Immediately after incubation with toxin or after 1, 6, or 20 h of subsequent incubation in medium (RPMI without FBS) without toxin, cells were treated with a 1:5 dilution of anti-SEA or anti-SEB polyclonal antibody for 15 min, washed, fixed with 3% formalin, washed, and incubated with Alexa 488 conjugated anti-rabbit secondary antibody (1:500; Molecular Probes, Eugene, OR) for 1 h. Cells were then rinsed in phosphate-buffered saline (PBS), mounted onto slides, and placed on the stage of a Nikon E600 microscope connected to a Bio-Rad Radiance 2000 MP confocal/multiphoton system (Hempstead Heath, United Kingdom). Cells were illuminated with 488-nm wavelength light from a krypton-argon laser, and optical sections of cells were acquired at 0.5-μm intervals. Pixel intensity measurements were made by using MetaMorph image analysis software (Universal Imaging Corp., Downingtown, PA). Summed pixel intensities were measured after applying a threshold that limited measurements to regions with fluorescent label.
To visualize internalized SEs in live cells, SEA and SEB were each tagged with Alexa 488 fluorescence (labeling kit from Molecular Probes, Eugene, OR). LG2 cells were grown in culture dishes coated with Cell-Tak (BD Biosciences, Bedford, MA) and were exposed to either SEA (1 μg/ml) or SEB (10 μg/ml) for 30 min. Cells examined immediately after exposure to toxin were incubated with 200 nM LysoTracker Red lysosomal dye and 0.4 μg of Hoechst nuclear stain (both from Molecular Probes, Eugene, OR)/ml during treatment with toxin. Cells examined 1, 6, or 20 h after exposure were incubated with these dyes for 30 min before viewing them. Images of live cells were scanned as described above by using 488- and 568-nm wavelength confocal laser and 800-nm wavelength multiphoton excitation.
T-cell activation assays.
To measure T-cell proliferation, human mononuclear cells were isolated by buoyant density centrifugation (Ficoll-Paque; Amersham Pharmacia Biotech AB, Piscataway, NJ) and washed three times. CD3+ T cells were obtained by negative selection to serve as responding cells. T cells were resuspended in medium containing 10% FBS, and 100 μl (5 × 104 cells) of the cell suspension was added to wells of a 96-well flat-bottom plate. Irradiated (12,000 rads) LG2 cells used as APCs were incubated (37°C for 45 min) with SEA or SEB, and the unbound toxin was removed by four washes. The LG2 cells were reincubated at 37°C in 3 ml of RPMI 1640 containing 10% FBS. At different times, aliquots of cells were removed, washed twice, and added in triplicate (each at 105 LG2 cells) to wells of the 96-well plate containing T cells. At 3 days after addition of LG2 cells, the cultures were pulsed (18 h) with 1 μCi of [3H]thymidine (Amersham, Arlington Heights, IL)/well, and the incorporated radioactivity was measured by liquid scintillation.
Internalization of bacterial SAgs.
SEA and SEB were biotinylated with N-hydroxysuccinimide-SS-biotin according to the manufacturer's instructions (Pierce, Rockford, IL). LG2 cells (106) were incubated with 1 to 10 μg of biotinylated SAg (b-SAg) in 20 μl of Hanks balanced salt solution containing 0.2% bovine serum albumin (30 min at 37°C), washed three times and then either harvested immediately or reincubated at 37°C for various times. A sample (105 cells) was removed to determine total cell-associated b-SAg. The cells were incubated with glutathione (250 mM, 2 h, 4°C) to cleave biotin from b-SAg exposed at the cell surface. After several washes, the cells were lysed, and b-SAg protected by internalization was captured on plates coated with streptavidin and measured by enzyme-linked immunosorbent assay (ELISA) as described below.
Subcellular fractionation by Percoll gradients.
For binding and internalization, 2 × 108 LG2 cells were incubated (50 × 106 cells/ml, 37°C, 30 min) with 2 μM SEA, 10 μM SEB, 10 μM rSEB or rSEA vaccine, or 10 μM BoNT/A HC in Hanks balanced salt solution containing 0.2% bovine serum albumin. The cells were washed four times in the same buffer and either harvested immediately or reincubated at 37°C for 1, 6, or 20 h at 5 × 106 cells/ml then washed once with PBS and once with homogenization buffer (0.25 M sucrose, 10 mM imidazole, 1 mM EDTA [pH 7.4], 2 mM phenylmethylsulfonyl fluoride, 100 μM leupeptin, 2 Ti units of aprotinin/ml). The cells were resuspended in 2 ml of homogenization buffer, broken by 10 to 15 passes through a 25-gauge needle, and centrifuged at 800 × g (10 min at 4°C). The pellet was washed once, and the supernatants were combined to yield postnuclear supernatant (PNS) free of nuclei and unbroken cells (visualized by microscopy). An aliquot (0.5 ml) of the PNS was layered over 9.5 ml of 17% Percoll (Pharmacia, Upsala, Sweden) containing 0.25 M sucrose and centrifuged for 50 min at 40,000 × g (27).
Latent β-hexosaminidase (total activity at pH 4.75 in the presence of 0.1% Triton X-100 minus activity in the absence of detergent) was used as a measure of intactness of internal organelles (47) and was always 65% or more. The density of Percoll fractions was determined by refractive index. Protein was assayed by using fluorescamine, total β-hexosaminidase was used as a marker for acidic compartments (endosomes and lysosomes), and α-mannosidase II (activity at pH 7.4 minus activity at pH 5.5) was used as a marker for Golgi apparatus (47). Activity of 5′-nucleotidase identified the plasma membrane (52).
ELISA procedures.
Wells of 96-well flat-bottom plates were coated (2 h at 37°C) with monoclonal antibody to SEA, SEB, or BoNT/A HC (1 μg/well in 100 μl of PBS). Binding of nonspecific proteins was blocked (overnight at 4°C) with 200 μl of 0.2% casein (Sigma) in PBS. Unbound proteins were removed by washing the plates four times with PBS containing 0.1% Tween 20 (Sigma). Gradient fractions (50 μl) were added in duplicate to the wells, and the plates were incubated (1.5 h at 37°C), washed as described above, and then incubated with rabbit anti-SEA, anti-SEB, or anti-BoNT/Hc antibody (1.0 μg/well). After four washes, bound antibody was detected with horseradish peroxidase-labeled goat anti-rabbit antibody (37°C, 1 h) and chromogen. To determine relative amounts of biotinylated proteins, the same procedure was used with microtiter plates coated with 1 μg of streptavidin/well and the bound biotinylated proteins were detected by using antibody specific for the protein, followed by horseradish peroxidase-labeled secondary antibody.
RESULTS
Binding of SE and TSST-1 to cell surface MHC-II and stability of the complexes.
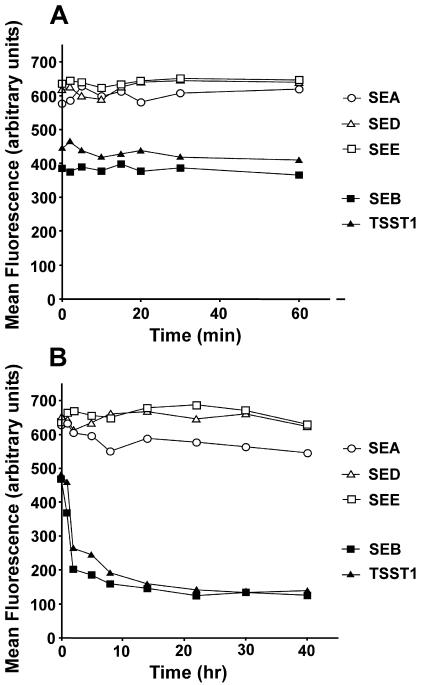
Human B lymphoblastoid LG2 cells were used for binding studies because these cells express very high quantities of HLA-DR1 (the human form of MHC-II) on their surface (11), and they can be fixed without disrupting bacterial SAg binding to MHC-II molecules (unpublished observations). Paraformaldehyde-fixed LG2 cells were incubated with SEA, SEB, SED, SEE, or TSST-1 for various times, and the surface-bound complexes were detected by flow cytometry. Figure 1A shows that at 250 μg/ml (10 μM) all SAgs attached to the LG2 cell surface and reached binding saturation within minutes. SEA, SED, and SEE bound to a similar extent, but TSST-1 and SEB produced a lower signal. Similar results were observed with fivefold-lower concentrations of SAgs (data not shown).
FIG. 1.
Binding and longevity of SEs and TSST-1 on the surface of B cells. (A) SEA, SEB, SED, SEE, or TSST1 (5 μg in 20 μl of RPMI 1640 containing 5% FBS) was added to 2 × 105 paraformaldehyde-fixed LG2 cells, and the toxin was washed away at different times. The amount of binding was measured by using anti-toxin antibody, followed by FITC-labeled secondary antibody and flow cytometry. The data are presented as mean fluorescence intensities. (B) Fixed LG2 cells were incubated as described above with SEA, SEB, SED, SEE, or TSST1 for 45 min and then washed and reincubated in 3 ml of medium containing 10% FBS for different times. Binding of the bacterial SAgs was determined as described above. The data are representative of four different experiments.
To study the stability of preformed SAg-MHC-II complexes, LG2 cells fixed with paraformaldehyde were washed, incubated with toxin, washed, and reincubated at 37°C for various times up to 40 h. Most of the cell-bound SEB and TSST-1 dissociated within 2 to 4 h, whereas less than 20% of the SEA, SED, or SEE was lost from the cell surface during this period (Fig. 1B). It should be emphasized that this dissociation was measured under nonequilibrium conditions since the cells were washed to remove the SAg and then reincubated in a large volume of medium. For all toxins, there was minimal dissociation after this initial loss. Experiments with lower concentrations of toxins gave similar results (data not shown). Therefore, the binding data provided strong evidence for the stability of cell surface zinc-binding SAg-MHC-II complexes. In addition, these data suggested that there are two populations of SEB- and TSST-1-MHC-II complexes, one that dissociates within the first few hours and a second, albeit small, population that remains stable for extended periods, possibly days.
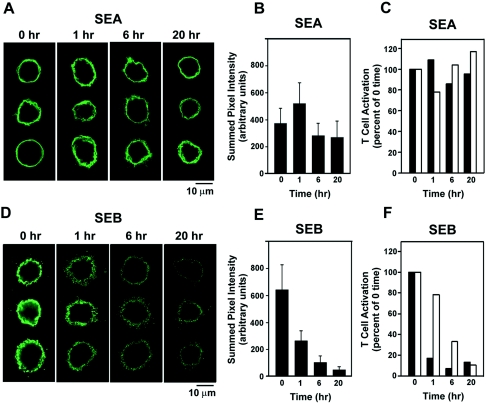
In the experiments above, the cells were fixed to block possible internalization of MHC-II molecules in order to focus on the stability of the SAg-MHC-II complexes. We next used live LG2 cells and studied the longevity of the toxins on the cell surface by confocal microscopy, with SEA and SEB as representatives for the zinc-binding, MHC-II cross-linking SAgs and the zinc-free, non-cross-linking SAgs, respectively. Confocal images of cells incubated with SEA or SEB and subsequently labeled with toxin-specific antibody showed that SEA immunofluorescence (Fig. 2A) on the cell surface remained high for 20 h after removal of SEA from the medium. In contrast, SEB immunofluorescence (Fig. 2D) on the cell surface rapidly decreased over time. Fluorescence intensities were measured for six to nine cells at each time point and are presented as graphs for SEA (Fig. 2B) and SEB (Fig. 2E). These results are in full agreement with T-cell proliferation studies, which showed that LG2 cells exposed to SEA remained biologically potent activators at 20 h (Fig. 2C), whereas T-cell activation by LG2 cells exposed to SEB fell sharply during this time interval (Fig. 2F).
FIG. 2.
Levels of surface-bound SEA and SEB over time. Confocal images of immunostained LG2 cells were acquired immediately after (0 h) and at 1, 6, and 20 h after a 15-min incubation with SEA (A) or SEB (D). Three representative examples are shown for each time point. Each image represents four consecutive optical sections through the cell taken at 0.5-μm intervals. Settings were optimized separately for SEA and SEB experiments, but images within the SEA and SEB groups were acquired and processed identically for all time points. Summed pixel intensities were measured for fluorescent regions (six to nine cells per time point for each toxin) and graphed for SEA (B) and SEB (E). The ability of LG2 cells to activate T cells at times after incubation with SEA (C) or SEB (F) is shown for comparison with surface labeling. Open bars represent 100 ng/ml, and solid bars represent 10 ng of SAg/ml. The graphs are representative of three different experiments. Levels of T-cell activation were expressed as a percentage of time zero and were calculated as follows: ([3H]thymidine counts per minute for 1, 6, or 20 h divided by [3H]thymidine counts per minute at zero time) × 100. At time zero the counts per minute were 83,289 at 10 ng of SEA/ml, 128,547 at 100 ng of SEA/ml, 32,407 at 10 ng of SEB/ml, and 53,287 at 100 ng of SEB/ml.
Internalization of SAgs bound to the cell surface.
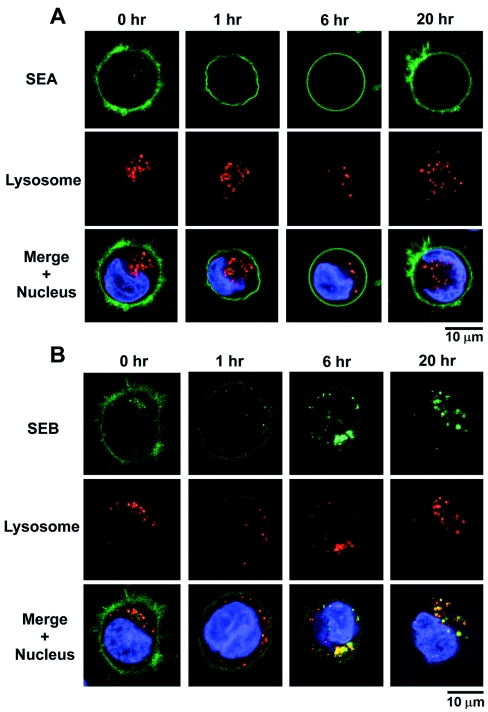
To determine whether the loss of SEB from the cell surface was due, at least in part, to internalization, we monitored the fate of fluorescent toxin for up to 20 h after binding to LG2 cells. As shown in Fig. 3, images of cells were acquired by confocal and multiphoton microscopy after incubation with Alexa 488-labeled SEA or SEB and reincubation with toxin-free medium for 0 to 20 h. In the case of SEB, significant fluorescence was detected within the cells after 6 h. Costaining with Lysotracker Red, a pH-dependent marker for lysosomes, suggested that internalized SEB was trafficked into lysosomal compartments. This is in stark contrast to SEA, which remained primarily on the cell surface throughout the experiment to 20 h.
FIG. 3.
Distribution of surface and internalized SEA (A) and SEB (B) over time. SEA remained primarily on the cell surface with little or no internalization for at least 20 h. In contrast, fluorescent SEB became apparent inside the cell as it disappeared from the cell surface. By 6 h most of the internalized SEB (green) had colocalized with a marker for lysosomes (red), and by 20 h internalized SEB was exclusively associated with the lysosomal compartment. The nucleus is stained blue. The pictures are representative of three different experiments.
To quantify the internalization of SAgs, we prepared biotin-SS-SAgs (i.e., b-SAgs) that can be cleaved at the disulfide bond by glutathione, a reagent that does not cross the plasma membrane and therefore leaves internalized b-SAgs intact (31). Cells were incubated with b-SAg for 30 min at 37°C, washed to remove unbound protein, and reincubated at 37°C for various times, and then cell surface-bound b-SAg was cleaved by glutathione. Cells were washed and lysed, and the total remaining b-SAg was measured by using streptavidin-coated wells and immunodetection. After the initial incubation and no chase, very little (3%) of the cell-associated biotin-SS-SEB was detected inside the cells, but this increased to 8 to 12% and 17 to 27% after chase periods of 6 and 20 h, respectively (Table 1). In contrast, even after a 20-h chase, only background levels of biotin-SS-SEA remained after glutathione treatment, indicating that nearly all of the SEA was exposed on the cell surface. For each time point, flow cytometry confirmed complete cleavage of surface biotinylated toxin by glutathione (data not shown). These data further support the conclusion that SEB was internalized from the plasma membrane at a slow but detectable rate but that virtually none of the surface-bound SEA was internalized.
TABLE 1.
Divergence of SEA and SEB internalization by human B cellsa
| Incubation time (h) | % Internalization
|
|||
|---|---|---|---|---|
| Expt 1
|
Expt 2
|
|||
| SEA | SEB | SEA | SEB | |
| 0 | 2 | 1 | 3 | 2 |
| 1 | 2 | 4 | 1 | 7 |
| 6 | 1 | 8 | 5 | 12 |
| 20 | 4 | 17 | 3 | 27 |
Biotin-SS-SEA or biotin-SS-SEB (20 μM) was incubated (45 min, 37°C) with LG2 cells, and the unbound toxin was removed by several washes. At various times, cell-surface-bound biotin-S-S-toxin was cleaved with glutathione (250 mM for 2 h at 4°C). After several washes, the cells were lysed, and internalized biotin-SS-toxin was measured by capture ELISA. Each time point represents the mean of four replicate samples, and the standard error of the mean was <10% in each case. The percent internalized was calculated relative to the total cell associated b-SAg at the same time. Flow cytometry with FITC-streptavidin confirmed that the glutathione treatment removed >95% of the b-SAgs from the cell surface.
Subcellular localization of SAgs.
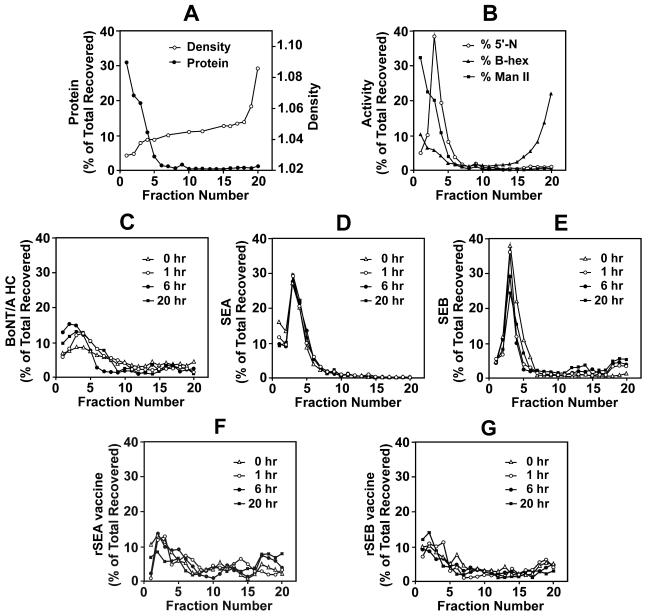
The intracellular trafficking of SAgs was studied by subcellular fractionations of SE-treated B cells using Percoll density gradients and compared to that of conventional vaccine antigens. To establish the distribution of subcellular organelles in the gradient, untreated LG2 cells were homogenized, and the PNS fraction was layered over 17% Percoll-0.25 M sucrose and centrifuged for 50 min at 40,000 × g. The density and distribution of protein (Fig. 4A) and enzymatic markers specific for various subcellular organelles (Fig. 4B) were determined. In this gradient, plasma membrane, Golgi apparatus, and endosomes were present in fractions one through five; processed antigens and MHC-II-enriched antigen loading compartments migrated just before or with the heavier lysosomal fraction at the bottom of the gradient. The profiles were similar to those described in other published reports (27, 35). Next, we compared the subcellular distributions of SAgs to those of a conventional vaccine antigen (BoNT/A HC) and mutated forms of SEA and SEB (rSEA and rSEB vaccine) that have no detectable binding to MHC-II (5). In all cases, cells were incubated with antigen or toxin for 30 min and then washed and harvested immediately or reincubated in medium for 1, 6, or 20 h before homogenization and gradient fractionation. After the initial binding period, BoNT/A HC was found throughout the gradient (Fig. 4C) in accord with previous studies showing that conventional antigens can reach early endosomes within 1 to 10 min and appear in acidic endocytic compartments after only 30 to 60 min (13, 46). Reincubation for 1, 6, or 20 h did not significantly change the distribution pattern of BoNT/A HC. Similar data were obtained when cells were incubated with the heavy chain of tetanus toxin (data not shown) or with highly attenuated vaccine forms of SAgs: rSEA (Fig. 4F) or rSEB (Fig. 4G). It should be noted that the mutated residues in both rSEA and rSEB are all in the N-terminal domain and would be expected to eliminate binding only to the generic site. The crystal structure of rSEA indicates distortions of the generic site but not of the Zn-dependent site, and so the inability of rSEA to bind to MHC-II via the latter site is not yet understood (18).
FIG. 4.
Comparison of the subcellular localization of bacterial SAgs and conventional antigen. The PNS fraction of treated or untreated LG2 cells was layered on 17% Percoll containing 0.25 M sucrose. After centrifugation, 0.50-ml fractions were collected, numbered from top (fraction 1) to bottom (fraction 20), and assayed for density and total protein (A); enzymatic activity of 5′-nucleotidase (5′-N; plasma membrane), β-hexosaminidase (B-hex; endosomes and lysosomes), and α-mannosidase II (Man II; Golgi) (B); BoNT/A HC (C); SEA (D); SEB (E); rSEA vaccine (E); and rSEB vaccine (G).
Totally different patterns were observed when LG2 cells were incubated with SEA or SEB. With no chase period and even after a 1-, 6-, or 20-h chase, more than 95% of the cell-associated SEA was detected in low-density fractions that included plasma membrane (Fig. 4D). These results are consistent with the data from our confocal microscopy and biotinylation studies, indicating that virtually no SEA was internalized up to 20 h. Immediately after incubating the cells with SEB, most of the SEB was also found near the top of the gradient (Fig. 4E), but after a 1-h chase period, SEB or SEB fragments could be detected in dense fractions containing lysosomes. By 6 or 20 h, a larger fraction sedimented with the lysosomes, and a third SEB-containing compartment was observed that migrated at intermediate density as expected for the MHC-II-enriched antigen loading compartment (55).
DISCUSSION
Binding of bacterial SAgs to MHC-II, and subsequent ligation of the TCR to this binary complex, sets in motion downstream signaling events that lead to proliferation of T cells and enormous release of inflammatory cytokines. These early biological events have been well documented and are clearly dependent on binding of the toxin to MHC-II molecules, but little is known regarding the stability or possible internalization of these bacterial SAg-MHC-II complexes. As a prelude to studying SAg binding stability and localization, we examined toxin binding to LG2 cells. Surprisingly, all SAgs tested bound to LG2 cells in amounts that reached a maximum within minutes, and similar results were observed with L cells transfected with HLA-DR1 (unpublished observations). Earlier kinetic studies reported much slower binding of TSST-1 and SEB (41, 42) and SEA (25) to the surface of B cells, perhaps due in part to the use of SAgs labeled with 125I, which is likely to alter tyrosine and/or primary amino groups implicated in SAg affinity for MHC-II. We observed differences in the extent of binding between the SAgs that were consistent with reported equilibrium constants of ca. 0.1 μM for cell surface-bound TSST1 and SEA (25) and 1 μM for SEB (41). The rapidity of binding was mirrored by vigorous T-cell responses to cell-bound SAgs as early as 1 min after the addition of SAg (data not shown).
The kinetics of dissociation showed two markedly different patterns, depending on whether the toxin had a single, generic type MHC-II binding site or also contained a zinc-dependent MHC-II binding site. No more than 20% of the zinc-binding SAgs SEA, SED, or SEE disappeared from the cell surface within a period of about 2 days, as shown by flow cytometry analysis, and thus, these SAgs bound essentially irreversibly to MHC-II. For cells incubated with SEA, this conclusion was confirmed and extended to live cells by confocal microscopy and T-cell stimulation. We suggest that the irreversible binding of SEA (and probably of other cross-linking SAgs) is because its two MHC-II binding sites were both occupied and cross-linked adjacent MHC-II molecules (14, 51, 53, 54). For the mouse MHC class II molecule I-Ek, surface plasmon resonance indicated an extremely short half-life (2.9 min) for a complex between SEA and the immobilized, soluble fragment of MHC-II (36). However, these studies may not accurately reflect SEA binding to human MHC-II on the surface of APCs because chip-bound MHC-II may not have a sufficient density to allow simultaneous availability of two different MHC-II molecules. In contrast, the LG2 cells used in the present study have a very high density of MHC-II, some of which may have been clustered (whereas aggregates were specifically removed in the plasmon resonance study). Cross-linking of MHC-II by SAgs would still occur on APCs with a lower density of surface MHC-II molecules as a consequence of lateral mobility in the cell membrane (49), whereas MHC-II molecules immobilized on a chip have little or no lateral mobility.
Most of the TSST-1 and SEB, SAgs with only a single binding site for MHC-II, rapidly dissociated from the LG2 cell surface. However, both fluorescence-activated cell sorting analysis and confocal microscopy showed that a small amount of SEB persisted at the cell surface, suggesting a possible secondary interaction. Even proteins with low affinity for SAgs, such as MHC class I molecules (12, 38), might stabilize the surface binding of a minor fraction of SEB and TSST1. Such a “double receptor” model has been proposed for binding of other toxins to their receptors (26, 45). The absence of secondary binding sites in surface plasmon resonance studies might explain the very rapid off rates reported for soluble HLA-DR1 binding to immobilized SEB (44).
After incubation of live cells with SAgs and washing to remove unbound toxin, loss of SAg from the cell surface can occur by two means: dissociation of the toxin from MHC-II or internalization of the complex. A previous study using only confocal microscopy indicated accumulation within 30 min to 2 h of both SEA and SEB in 1-μm intracellular compartments of the B-cell line 721.221 transfected to express HLA-Cw6 (7). However, using both confocal microscopy and subcellular fractionation, we detected little or no internalization of either SEA or SEB in LG2 cells during the first 1 h after initial incubation. SEA continued to show little if any internalization throughout a 20-h chase. In contrast, significant internalization of SEB was observed after a 6- or 20-h chase period, and distinct peaks of toxin migrated with dense organelles, including lysosomes, on Percoll gradients. Similarly, fluorescently labeled SEB was seen to colocalize with a lysosomal marker at these later times in confocal microscopy experiments. This slow internalization of SEB, but not SEA, was further confirmed by experiments in which some biotinylated SEB became protected from cleavage by glutathione, which does not cross the plasma membrane.
A small fraction of surface MHC-II molecules are known to rapidly internalize and enter the endosomal compartment before recycling to the plasma membrane (31, 37). Binding of SEB to this rapidly recycling pool may account for some internalization of SEB, since it is likely to dissociate from MHC-II while within the cell and would eventually reach late endosomes and lysosomes. Any SEA bound to a rapidly recycling pool of MHC-II probably would not dissociate within the cell, but instead would recycle as a complex back to the plasma membrane because of its greater stability. The half-life of surface-bound MHC-II molecules was found to be approximately 35 h in LG2 cells (unpublished observation), which is similar to values of 36 to 44 h reported for other transformed B-lymphoblastoid cell lines (9, 19). Thus, ca. 30% of the MHC-II molecules would be internalized within 20 h and may carry some SEB to lysosomes. The virtual absence of internal SEA was surprising and suggests that SEA may extend the constitutive half-life of MHC-II on the surface. For many receptors such as the T-cell receptor, cross-linking promotes internalization, followed by degradation that causes downregulation of the receptor to limit cellular responses. However, most of the surface MHC-II on a variety of human B cells did not undergo endocytosis either in the presence or absence of bound antibody specific for MHC-II (9). Moreover, binding to multivalent ligands or a high degree of cross-linking has been reported to inhibit internalization of several receptors such as the B-cell receptor (50) and the transferrin receptor (21). Since SEA, SED, and SEE have two, noncompeting binding sites for two distinct regions of the MHC-II molecule, they can not only cross-link adjacent MHC-II molecules but may also mediate the formation of larger oligomers (17) that could stabilize their surface location and prolong the stimulation of T cells.
In conclusion, we report several observations that are new or diverge from the prior understanding about SAgs. First, bacterial SAgs bound much more rapidly to cell surface MHC-II molecules than previously thought. Second, SEB and TSST-1, which have a single binding site for MHC-II molecules, mostly dissociated from the APC surface within a few hours, but some remained surface bound for extended periods, and a small amount of SEB was internalized. Finally, SEA and other zinc-dependent, cross-linking SAgs bound almost irreversibly to the surface of fixed cells, and little or no SEA was internalized by live cells. Although the mechanism that dictates cell surface MHC-II turnover is not known, its finite half-life may serve as a key regulator for T-cell exposure to antigenic peptides. A substantial extension of this half-life would contribute to the pathology of the group of SAgs capable of cross-linking MHC-II.
Acknowledgments
We are grateful to Kathleen Callahan, Edna Torres-Melendez, and Jennifer Nolan for technical assistance and to Bradley Stiles and Leonard Smith for providing reagents.
The research described here was sponsored by the Defense Threat Reduction Agency JSTO-CB plan D-X015-04-RD-B and the Medical Research and Materiel Command Research.
The opinions, interpretations, conclusions, and recommendations included here are those of the authors and are not necessarily endorsed by the U.S. Army.
Editor: J. D. Clements
REFERENCES
- 1.Al-Daccak, R., K. Mehindate, F. Damdoumi, P. Etongue-Mayer, H. Nilsson, P. Antonsson, M. Sundstrom, M. Dohlsten, R. P. Sekaly, and W. Mourad. 1998. Staphylococcal enterotoxin D is a promiscuous superantigen offering multiple modes of interactions with the MHC class II receptors. J. Immunol. 160:225-232. [PubMed] [Google Scholar]
- 2.Baker, M. D., and K. R. Acharya. 2004. Superantigens: structure-function relationships. Int. J. Med. Microbiol. 293:529-537. [DOI] [PubMed] [Google Scholar]
- 3.Baker, M., D. M. Gutman, A. C. Papageorgiou, C. M. Collins, and K. R. Acharya. 2001. Structural features of a zinc binding site in the superantigen streptococcal pyrogenic exotoxin A (SpeA1): implications for MHC class II recognition. Protein Sci. 10:1268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohach, G. A., D. J. Fast, R. D. Nelson, and P. M. Schlievert. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251-272. [DOI] [PubMed] [Google Scholar]
- 5.Boles, J. W., M. L. Pitt, R. D. LeClaire, P. H. Gibbs, E. Torres, B. Dyas, R. G. Ulrich, and S. Bavari. 2003. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin. Immunol. 108:51-59. [DOI] [PubMed] [Google Scholar]
- 6.Burnett, J. C., E. A. Henchal, A. L. Schmaljohn, and S. Bavari. 2005. The evolving field of biodefense: therapeutic developments and diagnostics. Nat. Rev. Drug Discov. 4:281-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu, I., D. M. Davis, and J. L. Strominger. 1999. Trafficking of spontaneously endocytosed MHC proteins. Proc. Natl. Acad. Sci. USA 96:13944-13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffman, J. D., J. Zhu, J. M. Roach, S. Bavari, R. G. Ulrich, and S. L. Giardina. 2002. Production and purification of a recombinant Staphylococcal enterotoxin B vaccine candidate expressed in Escherichia coli. Protein Expr. Purif. 24:302-312. [DOI] [PubMed] [Google Scholar]
- 9.Davis, J. E., and P. Cresswell. 1990. Lack of detectable endocytosis of B lymphocyte MHC class II antigens using an antibody-independent technique. J. Immunol. 144:990-997. [PubMed] [Google Scholar]
- 10.Goldenberg, D. L., and J. I. Reed. 1985. Bacterial arthritis. N. Engl. J. Med. 312:764-771. [DOI] [PubMed] [Google Scholar]
- 11.Gorga, J. C., V. Horejsi, D. R. Johnson, R. Raghupathy, and J. L. Strominger. 1987. Purification and characterization of class II histocompatibility antigens from a homozygous human B-cell line. J. Biol. Chem. 262:16087-16094. [PubMed] [Google Scholar]
- 12.Haffner, A. C., K. Zepter, and C. A. Elmets. 1996. Major histocompatibility complex class I molecule serves as a ligand for presentation of the superantigen staphylococcal enterotoxin B to T cells. Proc. Natl. Acad. Sci. USA 93:3037-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding, C. V. 1996. Class II antigen processing: analysis of compartments and functions. Crit. Rev. Immunol. 16:13-29. [DOI] [PubMed] [Google Scholar]
- 14.Hudson, K. R., R. E. Tiedemann, R. G. Urban, S. C. Lowe, J. L. Strominger, and J. D. Fraser. 1995. Staphylococcal enterotoxin A has two cooperative binding sites on major histocompatibility complex class II. J. Exp. Med. 182:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jardetzky, T. S., J. H. Brown, J. C. Gorga, L. J. Stern, R. G. Urban, Y. I. Chi, C. Stauffacher, J. L. Strominger, and D. C. Wiley. 1994. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 368:711-718. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J., R. G. Urban, J. L. Strominger, and D. C. Wiley. 1994. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science 266:1870-1874. [DOI] [PubMed] [Google Scholar]
- 17.Kozono, H., D. Parker, J. White, P. Marrack, and J. Kappler. 1995. Multiple binding sites for bacterial superantigens on soluble class II MHC molecules. Immunity 3:187-196. [DOI] [PubMed] [Google Scholar]
- 18.Krupka, H. I., B. W. Segelke, R. G. Ulrich, S. Ringhofer, M. Knapp, and B. Rupp. 2002. Structural basis for abrogated binding between staphylococcal enterotoxin A superantigen vaccine and MHC-IIα. Protein Sci. 11:642-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanzavecchia, A., P. A. Reid, and C. Watts. 1992. Irreversible association of peptides with class II MHC molecules in living cells. Nature 357:249-252. [DOI] [PubMed] [Google Scholar]
- 20.Lavoie, P. M., J. Thibodeau, F. Erard, and R. P. Sekaly. 1999. Understanding the mechanism of action of bacterial superantigens from a decade of research. Immunol. Rev. 168:257-269. [DOI] [PubMed] [Google Scholar]
- 21.Lesley, J., R. Schulte, and J. Woods. 1989. Modulation of transferrin receptor expression and function by anti-transferrin receptor antibodies and antibody fragments. Exp. Cell Res. 182:215-233. [DOI] [PubMed] [Google Scholar]
- 22.Li, Y., H. Li, N. Dimasi, J. K. McCormick, R. Martin, P. Schuck, P. M. Schlievert, and R. A. Mariuzza. 2001. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity 14:93-104. [DOI] [PubMed] [Google Scholar]
- 23.Llewelyn, M., and J. Cohen. 2002. Superantigens: microbial agents that corrupt immunity. Lancet Infect. Dis. 2:156-162. [DOI] [PubMed] [Google Scholar]
- 24.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 25.Mollick, J. A., M. Chintagumpala, R. G. Cook, and R. R. Rich. 1991. Staphylococcal exotoxin activation of T cells. Role of exotoxin-MHC class II binding affinity and class II isotype. J. Immunol. 146:463-468. [PubMed] [Google Scholar]
- 26.Montecucco, C., G. Schiavo, Z. Gao, E. Bauerlein, P. Boquet, and B. R. DasGupta. 1988. Interaction of botulinum and tetanus toxins with the lipid bilayer surface. Biochem. J. 251:379-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton, P. A., M. L. Zacheis, K. S. Giacoletto, J. A. Manning, and B. D. Schwartz. 1995. Delivery of nascent MHC class II-invariant chain complexes to lysosomal compartments and proteolysis of invariant chain by cysteine proteases precedes peptide binding in B-lymphoblastoid cells. J. Immunol. 154:137-150. [PubMed] [Google Scholar]
- 28.Papageorgiou, A. C., and K. R. Acharya. 2000. Microbial superantigens: from structure to function. Trends Microbiol. 8:369-375. [DOI] [PubMed] [Google Scholar]
- 29.Petersson, K., M. Hakansson, H. Nilsson, G. Forsberg, L. A. Svensson, A. Liljas, and B. Walse. 2001. Crystal structure of a superantigen bound to MHC class II displays zinc and peptide dependence. EMBO J. 20:3306-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersson, K., M. Thunnissen, G. Forsberg, and B. Walse. 2002. Crystal structure of a SEA variant in complex with MHC class II reveals the ability of SEA to crosslink MHC molecules. Structure 10:1619-1626. [DOI] [PubMed] [Google Scholar]
- 31.Pinet, V., M. Vergelli, R. Martin, O. Bakke, and E. O. Long. 1995. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature 375:603-606. [DOI] [PubMed] [Google Scholar]
- 32.Pless, D. D., E. R. Torres, E. K. Reinke, and S. Bavari. 2001. High-affinity, protective antibodies to the binding domain of botulinum neurotoxin type A. Infect. Immun. 69:570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posnett, D. N. 1993. Do superantigens play a role in autoimmunity? Semin. Immunol. 5:65-72. [DOI] [PubMed] [Google Scholar]
- 34.Proft, T., V. L. Arcus, V. Handley, E. N. Baker, and J. D. Fraser. 2001. Immunological and biochemical characterization of streptococcal pyrogenic exotoxins I and J (SPE-I and SPE-J) from Streptococcus pyogenes. J. Immunol. 166:6711-6719. [DOI] [PubMed] [Google Scholar]
- 35.Qiu, Y., X. Xu, A. Wandinger-Ness, D. P. Dalke, and S. K. Pierce. 1994. Separation of subcellular compartments containing distinct functional forms of MHC class II. J. Cell Biol. 125:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redpath, S., S. M. Alam, C. M. Lin, A. M. O'Rourke, and N. R. J. Gascoigne. 1999. Trimolecular interaction of TCR with MHC class II and bacterial superantigen shows a similar affinity to MHC-peptide ligands. J. Immunol. 163:6-10. [PubMed] [Google Scholar]
- 37.Roche, P. A., C. L. Teletski, E. Stang, O. Bakke, and E. O. Long. 1993. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc. Natl. Acad. Sci. USA 90:8581-8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers, T. J., and L. Zhang. 1997. Structural basis for the interaction of superantigen with the alternative superantigen-binding receptor p85. Mol. Immunol. 34:263-272. [DOI] [PubMed] [Google Scholar]
- 39.Roussel, A., B. F. Anderson, H. M. Baker, J. D. Fraser, and E. N. Baker. 1997. Crystal structure of the streptococcal superantigen SPE-C: dimerization and zinc binding suggest a novel mode of interaction with MHC class II molecules. Nat. Struct. Biol. 4:635-643. [DOI] [PubMed] [Google Scholar]
- 40.Schlievert, P. M. 1986. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet i:1149-1150. [DOI] [PubMed] [Google Scholar]
- 41.Scholl, P. R., A. Diez, and R. S. Geha. 1989. Staphylococcal enterotoxin B and toxic shock syndrome toxin-1 bind to distinct sites on HLA-DR and HLA-DQ molecules. J. Immunol. 143:2583-2588. [PubMed] [Google Scholar]
- 42.Scholl, P., A. Diez, W. Mourad, J. Parsonnet, R. S. Geha, and T. Chatila. 1989. Toxic shock syndrome toxin 1 binds to major histocompatibility complex class II molecules. Proc. Natl. Acad. Sci. USA 86:4210-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwab, J. H., R. R. Brown, S. K. Anderle, and P. M. Schlievert. 1993. Superantigen can reactivate bacterial cell wall-induced arthritis. J. Immunol. 150:4151-4159. [PubMed] [Google Scholar]
- 44.Seth, A., L. J. Stern, T. H. Ottenhoff, I. Engel, M. J. Owen, J. R. Lamb, R. D. Klausner, and D. C. Wiley. 1994. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature 369:324-327. [DOI] [PubMed] [Google Scholar]
- 45.Singh, B. R. 2000. Intimate details of the most poisonous poison. Nat. Struct. Biol. 7:617-619. [DOI] [PubMed] [Google Scholar]
- 46.Song, W., H. Cho, P. Cheng, and S. K. Pierce. 1995. Entry of B cell antigen receptor and antigen into class II peptide-loading compartment is independent of receptor cross-linking. J. Immunol. 155:4255-4263.7594583 [Google Scholar]
- 47.Storrie, B., and E. A. Madden. 1990. Isolation of subcellular organelles. Methods Enzymol. 182:203-225. [DOI] [PubMed] [Google Scholar]
- 48.Sundberg, E. J., Y. Li, and R. A. Mariuzza. 2002. So many ways of getting in the way: diversity in the molecular architecture of superantigen-dependent T-cell signaling complexes. Curr. Opin. Immunol. 14:36-44. [DOI] [PubMed] [Google Scholar]
- 49.Tang, Q., and M. Edidin. 2003. Lowering the barriers to random walks on the cell surface. Biophys. J. 84:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thyagarajan, R., N. Arunkumar, and W. J. Song. 2003. Polyvalent antigens stabilize B-cell antigen receptor surface signaling microdomains. J. Immunol. 170:6099-6106. [DOI] [PubMed] [Google Scholar]
- 51.Tiedemann, R. E., and J. D. Fraser. 1996. Cross-linking of MHC class II molecules by staphylococcal enterotoxin A is essential for antigen-presenting cell and T-cell activation. J. Immunol. 157:3958-3966. [PubMed] [Google Scholar]
- 52.Touster, O., N. N. Aronson, Jr., J. T. Dulaney, and H. Hendrickson. 1970. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J. Cell Biol. 47:604-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulrich, R. G., S. Bavari, and M. A. Olson. 1995. Bacterial superantigens in human disease: structure, function, and diversity. Trends Microbiol. 3:463-468. [DOI] [PubMed] [Google Scholar]
- 54.Ulrich, R. G., S. Bavari, and M. A. Olson. 1995. Staphylococcal enterotoxins A and B share a common structural motif for binding class II major histocompatibility complex molecules. Nat. Struct. Biol. 2:554-560. [DOI] [PubMed] [Google Scholar]
- 55.West, M. A., J. M. Lucocq, and C. Watts. 1994. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature 369:147-151. [DOI] [PubMed] [Google Scholar]